Local Growth Hormone Therapy for Pressure Ulcer Healing on a Human Skin Mouse Model
Abstract
1. Introduction
2. Results
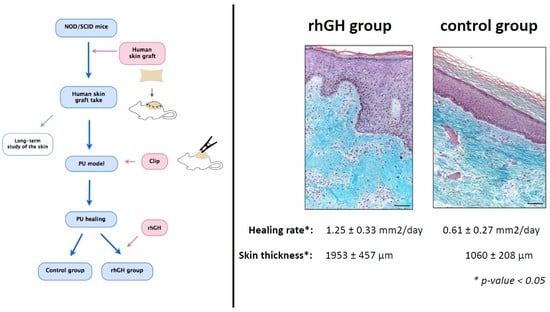
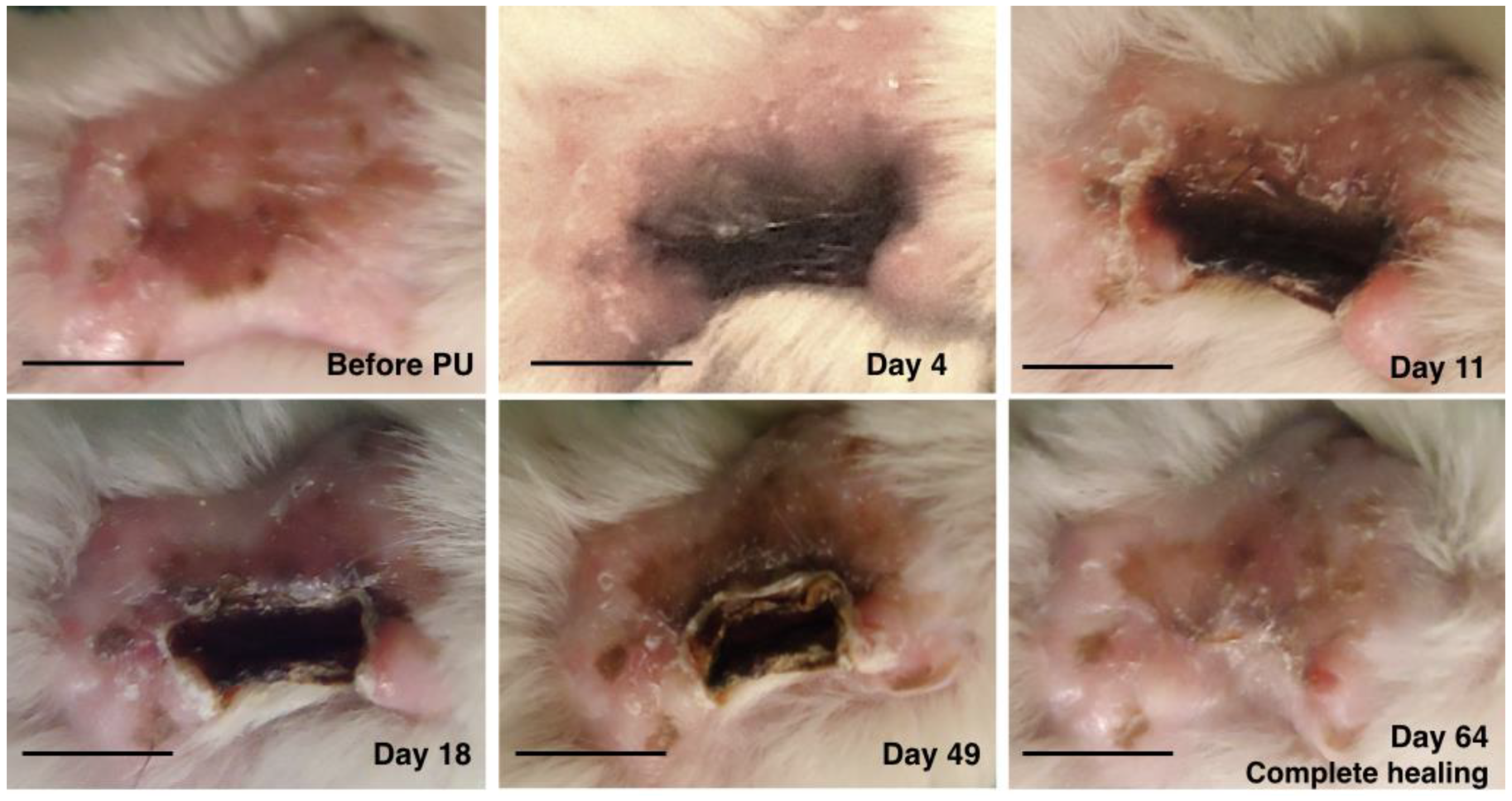
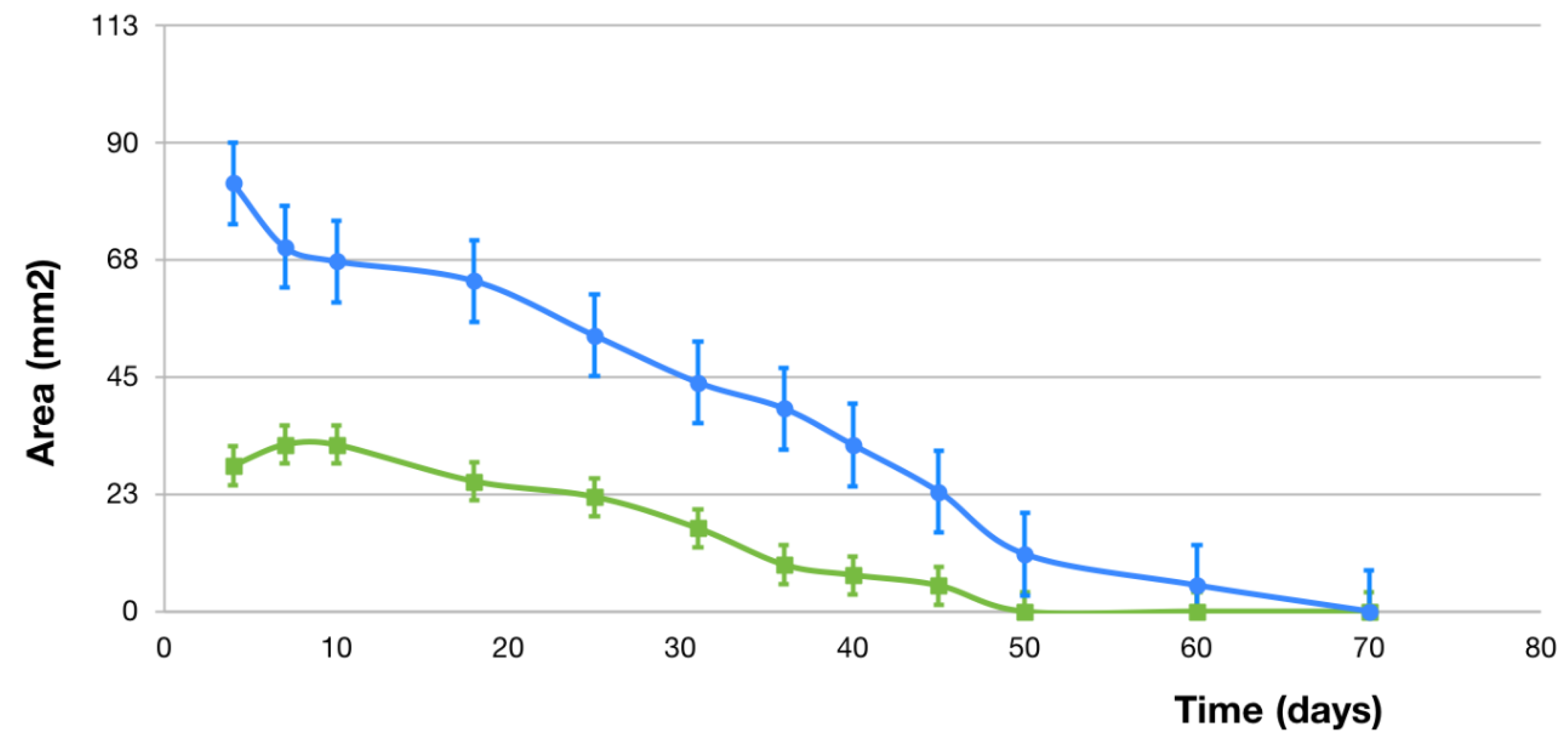
2.1. Macroscopic Evaluation
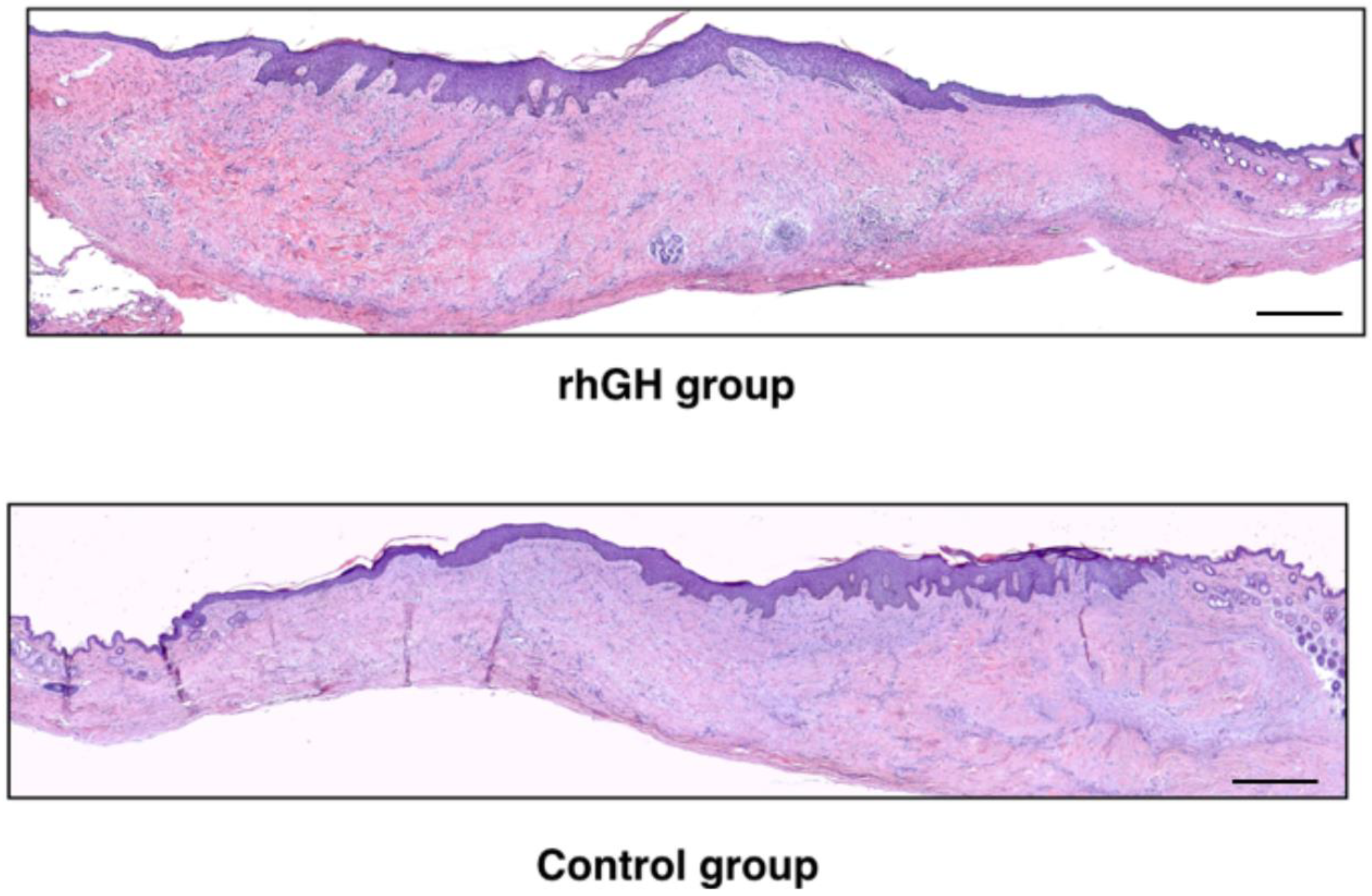
2.2. Microscopic Evaluation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. PU model on Human Skin
4.3. Recombinant Human Growth Hormone (rhGH)
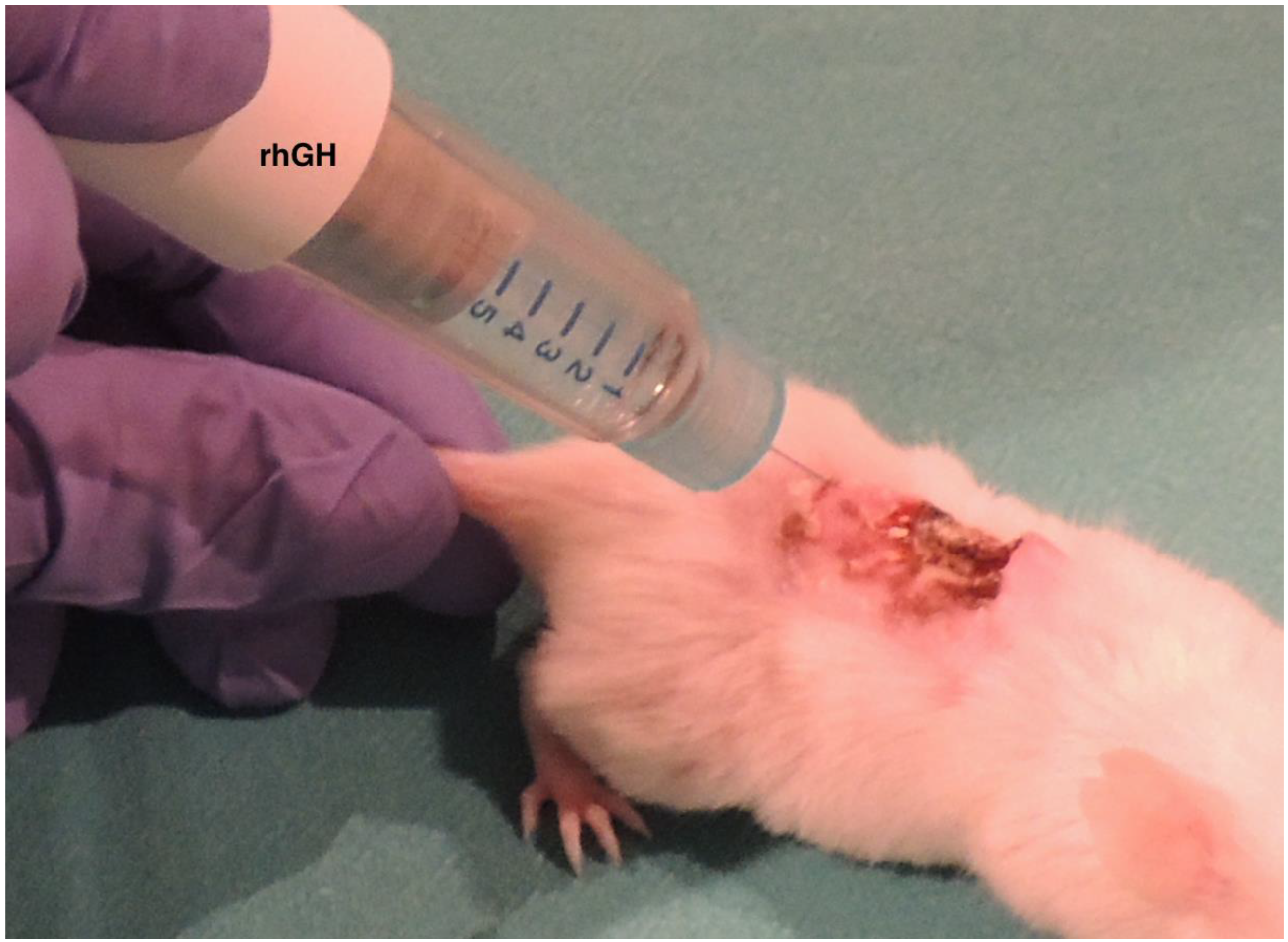
4.4. Local Treatment of the PU with rhGH
4.5. Macroscopic Analysis
4.6. Microscopic Evaluation
4.7. Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyder, C.H. Pressure ulcer prevention and management. JAMA 2003, 289, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Salcido, R.; Popescu, A.; Ahn, C. Animal models in pressure ulcer research. J. Spinal Cord Med. 2007, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R. Does pressure cause pressure ulcers? An inquiry into the etiology of pressure ulcers. J. Am. Med. Dir. Assoc. 2010, 11, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Ichioka, S.; Sekiya, N.; Nakatsuka, T. Analysis of ischemia- reperfusion injury in a microcirculatory model of pressure ulcers. Wound Repair Regen. 2005, 13, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.M.; Sinno, S.; Levine, J.P.; Saadeh, P.B. Current thoughts for the prevention and treatment of pressure ulcers: Using the evidence to determine fact or fiction. Ann. Surg. 2013, 257, 603–608. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Ulcer Advisory Panel. European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. In Prevention and Treatment of Pressure Ulcers: Quick Reference Guide; Haesler, E., Ed.; Cambridge Media: Osborne Park, Western Australia, 2014. [Google Scholar]
- Rees, R.S.; Robson, M.C.; Smiell, J.M.; Perry, B.H. Becaplermin gel in the treatment of pressure ulcers: A phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen. 1999, 7, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kallianinen, L.K.; Hirshberg, J.; Marchant, B.; Rees, R.S. Role of platelet derived growth factor as an adjunct to surgery in the management of pressure ulcers. Plast. Reconstr. Surg. 2000, 106, 1243–1248. [Google Scholar] [CrossRef]
- Green, H.; Morikawa, M.; Nixon, T. A dual effector theory of growth-hormone action. Differentiation 1985, 29, 195–198. [Google Scholar] [CrossRef]
- Strobl, J.S.; Thomas, M.J. Human growth hormone. Pharmacol. Rev. 1994, 46, 1–34. [Google Scholar]
- Edmondson, S.R.; Thumiger, S.P.; Werther, G.A.; Wraight, C.J. Epidermal homeostasis: The role of the growth hormone and insulin-like growth factor systems. Endocr. Rev. 2003, 24, 737–764. [Google Scholar] [CrossRef]
- Bhora, F.Y.; Dunkin, B.J.; Batzri, S.; Aly, H.M.; Bass, B.L.; Sidawy, A.N.; Harmon, J.W. Effect of growth factors on cell proliferation and epithelialization in human skin. J. Surg. Res. 1995, 59, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.M.; Lehnhardt, M.; Wagner, D.; Jansen, W.; Krieg, M.; Steinau, H.U. Determination of endogenous growth factors in human wound fluid: Temporal presence and profiles of secretion. Plast. Reconstr. Surg. 1998, 102, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Garbarsch, C.; Schuppan, D.; Moe, D.; Horslev-Pedersen, K.; Gottrup, F.; Steenfos, H. Influence of human growth hormone on granulation tissue formation, collagen deposition, and the aminoterminal propeptide of collagen type III in wound chambers in rats. Wound Repair Regen. 1994, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.H.; Bang, C.; Andreassen, T.T.; Flyvbjerg, A.; Orskov, H. Dose-response study of the effect of growth hormone on mechanical properties of skin graft wounds. J. Surg. Res. 1995, 58, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.H.; Oxlund, H. Growth hormone increases the biomechanical strength and collagen deposition rate during the early phase of skin wound healing. Wound Repair Regen. 1996, 4, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Thorey, I.S.; Hinz, B.; Hoeflich, A.; Kaesler, S.; Elmlinger, M.; Wanke, R.; Wolf, E.; Werner, S. Transgenic Mice Reveal Novel Activities of Growth Hormone in Wound Repair, Angiogenesis, and Myofibroblast Differentiation. J. Biol. Chem. 2004, 279, 26674–26684. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, M.J.; García-Esteo, F.; García-Honduvilla, N.; San Román, J.; Bellón, J.M.; Buján, J. A novel controlled drug-delivery system for growth hormone applied to healing skin wounds in diabetic rats. J. Biomater. Sci. Polym. Ed. 2003, 14, 821–835. [Google Scholar] [CrossRef][Green Version]
- García-Esteo, F.; Pascual, G.; García-Honduvilla, N.; Gallardo, A.; San Román, J.; Bellón, J.M.; Buján, J. Histological evaluation of scar tissue inflammatory response: The role of hGH in diabetic rats. Histol. Histopathol. 2005, 20, 53–57. [Google Scholar]
- García-Esteo, F.; Pascual, G.; Gallardo, A.; San Román, J.; Buján, J.; Bellón, J.M. A biodegradable copolymer for the slow release of growth hormone expedites scarring in diabetic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 291–304. [Google Scholar] [CrossRef]
- Bowers, D.; McKenzie, D.; Dutta, D.; Wheeless, C.R.; Cohen, W.R. Growth hormone treatment after cesarean delivery in rats increases the strength of the uterine scar. Am. J. Obstet. Gynecol. 2001, 185, 614–617. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.H.; Kim, J.Y.; Lee, Y. The effect of growth hormone on fibroblast proliferation and keratinocyte migration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, e364–e369. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The roles of growth factors in keratinocyte migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef]
- Meazza, C.; Pagani, S.; Travaglino, P.; Bozzola, M. Effect of growth hormone (GH) on the immune system. Pediatr. Endocrinol. Rev. 2004, 1, 490–495. [Google Scholar]
- Breederveld, R.S.; Tuinebreijer, W.E. Recombinant human growth hormone for treating burns and donor sites. Cochrane Database Syst. Rev. 2014, 9, CD008990. [Google Scholar] [CrossRef]
- Krysiak, R.; Gdula-Dymek, A.; Bednarska-Czerwińska, A.; Okopien, B. Growth hormone therapy in children and adults. Pharmacol. Rep. 2007, 59, 500–516. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative skin wound healing in mammals: State-of-the-Art on growth factor and stem cell based treatments. Cell Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef]
- Akita, S.; Akino, K.; Imaizumi, T.; Hirano, A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008, 16, 635–641. [Google Scholar] [CrossRef]
- Steenfos, H.H.; Jansson, J.O. Growth hormone stimulates granulation tissue formation and insulin-like growth factor-I gene expression in wound chambers in the rat. J. Endocrinol. 1992, 132, 293–298. [Google Scholar] [CrossRef]
- Tuffaha, S.H.; Budihardjo, J.D.; Sarhane, K.A.; Khusheim, M.; Song, D.; Broyles, J.M.; Salvatori, R.; Means, K.R., Jr.; Higgins, J.P.; Shores, J.T.; et al. Growth hormone therapy accelerates axonal regeneration, promotes motor reinnervation, and reduces muscle atrophy following peripheral nerve injury. Plast. Reconstr. Surg. 2016, 137, 1771–1780. [Google Scholar] [CrossRef]
- Andreassen, T.T.; Oxlund, H. Local anabolic effects of growth hormone on intact bone and healing fractures in rats. Calcif. Tissue Int. 2003, 73, 258–264. [Google Scholar] [CrossRef]
- Wirostko, B.; Rafii, M.; Sullivan, D.A.; Morelli, J.; Ding, J. Novel therapy to treat corneal epithelial defects: A hypothesis with Growth Hormone. Ocul. Surf. 2015, 13, 204–212. [Google Scholar] [CrossRef]
- Ding, J.; Wirostko, B.; Sullivan, D.A. Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea 2015, 34, 686–692. [Google Scholar] [CrossRef]
- Rudman, D.; Feller, A.G.; Nagraj, H.S.; Gergans, G.A.; Lalitha, P.Y.; Goldberg, A.F.; Schlenker, R.A.; Cohn, L.; Rudman, I.W.; Mattson, D.E. Effects of human growth hormone in men over 60 years old. N. Engl. J. Med. 1990, 323, 1–6. [Google Scholar] [CrossRef]
- Jørgensen, J.O.; Pedersen, S.A.; Thuesen, L.; Jørgensen, J.; Ingemann-Hansen, T.; Skakkebaek, N.E.; Christiansen, J.S. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1989, 1, 1221–1225. [Google Scholar] [CrossRef]
- Conte, F.; Diridollou, S.; Jouret, B.; Turlier, V.; Charveron, M.; Gall, Y.; Rochiccioli, P.; Bieth, E.; Tauber, M. Evaluation of cutaneous modifications in seventy-seven growth hormone-deficient children. Horm. Res. 2000, 54, 92–97. [Google Scholar] [CrossRef]
- Messias de Lima, C.F.; De Araujo Vieira, L.F.; de Carvalho Wanderley, L.A.; de Souza Ferro, J.N.; Smaniotto, S. Topical Growth Hormone Accelerates Wound Healing in Mice. Wounds 2017, 29, 387–392. [Google Scholar]
- Theocharidis, G.; Connelly, J.T. Minor collagens of the skin with not so minor functions. J. Anat. 2019, 235, 418–429. [Google Scholar] [CrossRef]
- Mecham, R.P. The Extracellullar Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef]
- Peeters, E.; De Hertogh, G.; Junge, K.; Klinge, U.; Miserez, M. Skin as marker for collagen type I/III ratio in abdominal wall fascia. Hernia 2014, 18, 519–525. [Google Scholar] [CrossRef]
- Ortega, M.A.; Asúnsolo, A.; Álvarez-Rocha, M.J.; Romero, B.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Remodelling of collagen fibres in the placentas of women with venous insufficiency during pregnancy. Histol. Histopathol. 2018, 33, 567–576. [Google Scholar]
- Cristóbal, L.; Ortega, M.A.; Asúnsolo, Á.; Romero, B.; Álvarez-Mon, M.; Buján, J.; Maldonado, A.A.; García-Honduvilla, N. Human skin model for mimic dermal studies in pathology with a clinical implication in pressure ulcers. Histol. Histopathol. 2018, 33, 959–970. [Google Scholar]
- Thum, T.; Hoeber, S.; Froese, S.; Klink, I.; Stichtenoth, D.O.; Galuppo, P.; Jakob, M.; Tsikas, D.; Anker, S.D.; Poole-Wilson, P.A.; et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1. Circ. Res. 2007, 100, 434–443. [Google Scholar] [CrossRef]
- Herndon, D.N.; Barrow, R.E.; Kunkel, K.R.; Broemeling, L.; Rutan, R.L. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann. Surg. 1990, 212, 424. [Google Scholar] [CrossRef]
- Massey, K.A.; Blakeslee, C.; Pitkow, H.S. Possible therapeutic effects of growth hormone on wound healing in the diabetic patient. J. Am. Podiatr. Med. Assoc. 1998, 88, 25–29. [Google Scholar] [CrossRef]
- Barret, J.P.; Dziewulski, P.; Jeschke, M.G.; Wolf, S.E.; Herndon, D.N. Effects of recombinant human growth hormone on the development of burn scarring. Plast. Reconstr. Surg. 1999, 104, 726–729. [Google Scholar] [CrossRef]
- Branski, L.K.; Herndon, D.N.; Barrow, R.E.; Kulp, G.A.; Suman, O.E.; Przkora, R.; Meyer, W., 3rd; Huang, T.; Lee, J.O.; Chinkes, D.L.; et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann. Surg. 2009, 250, 514–523. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and Physiology of Wound Healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef]
- Auernhammer, C.J.; Strasburger, C.J. Effects of growth hormone and insulin-like growth factor I on the immune system. Eur. J. Endocrinol. 1995, 133, 635–645. [Google Scholar] [CrossRef]
- Gala, R.R. Prolactin and growth hormone in the regulation of the immune system. Proc. Soc. Exp. Biol. Med. 1991, 198, 513–527. [Google Scholar] [CrossRef]
- Naing, C.; Whittaker, M.A. Anabolic steroids for treating pressure ulcers. Cochrane Wounds Group, editor. Cochrane Database Syst. Rev. 2017, 158, 718–735. [Google Scholar]
- Rasmussen, L.H.; Garbarsch, C.; Schuppan, D.; Moe, D.; Horslev-Pedersen, K.; Gottrup, F.; Steenfos, H. Dose response profiles of human growth hormone in subcutaneous wound chambers in rats. Eur. J. Surg. 1995, 161, 157–162. [Google Scholar]
- Kim, S.H.; Heo, E.J.; Lee, S.W. The effect of topically applied recombinant human growth hormone on wound healing in pigs. Wounds 2009, 21, 158–163. [Google Scholar]
- Ravina, A.; Pestel, M.; Dayras, J.C. Action de l’hormone somatotrope sur les escarres de decubitus et les troubles neurotrophiques. Presse Med. 1955, 63, 305–308. [Google Scholar]
- Waago, H. Local treatment of ulcers in diabetic foot with human growth hormone. Lancet 1987, 1, 1485. [Google Scholar] [CrossRef]
- Maldonado, A.A.; Cristóbal, L.; Martín-López, J.; Mallén, M.; García-Honduvilla, N.; Buján, J. A novel model of human skin pressure ulcers in mice. PLoS ONE 2014, 9, e109003. [Google Scholar] [CrossRef]
- Gerbault, O. Cicatrización cutánea. In Enciclopedia Médico-Quirúrgica. Técnicas Quirúgicas—Cirugía Plástica Reconstructiva y Estética; Elsevier: Paris, France, 2000. [Google Scholar]
- Carriel, V.S.; Aneiros-Fernandez, J.; Arias-Santiago, S.; Garzón, I.J.; Alaminos, M.; Campos, A. A novel histochemical method for a simultaneous staining of melanin and collagen fibers. J. Histochem. Cytochem. 2011, 59, 270–277. [Google Scholar] [CrossRef]
- Shultz. Multiple Defects in Innate and Adaptive Immunologic Function in NOD/LtSz-scid Mice’. J. Immunol. 1995, 154, 180–191. [Google Scholar]
- Stadler, I.; Zhang, R.Y.; Oskoui, P.; Whittaker, M.B.S.; Lanzafame, R.J. Development of a Simple, Noninvasive, Clinically Relevant Model of Pressure Ulcers in the Mouse. J. Investig. Surg. 2004, 17, 221–227. [Google Scholar] [CrossRef]
- Ortega, M.A.; Asúnsolo, Á.; Leal, J.; Romero, B.; Alvarez-Rocha, M.J.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Implication of the PI3K/Akt/mTOR Pathway in the Process of Incompetent Valves in Patients with Chronic Venous Insufficiency and the Relationship with Aging. Oxid. Med. Cell Longev. 2018, 2018, 1495170. [Google Scholar] [CrossRef]
- Remmele, W.; Schicketanz, K.H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef]



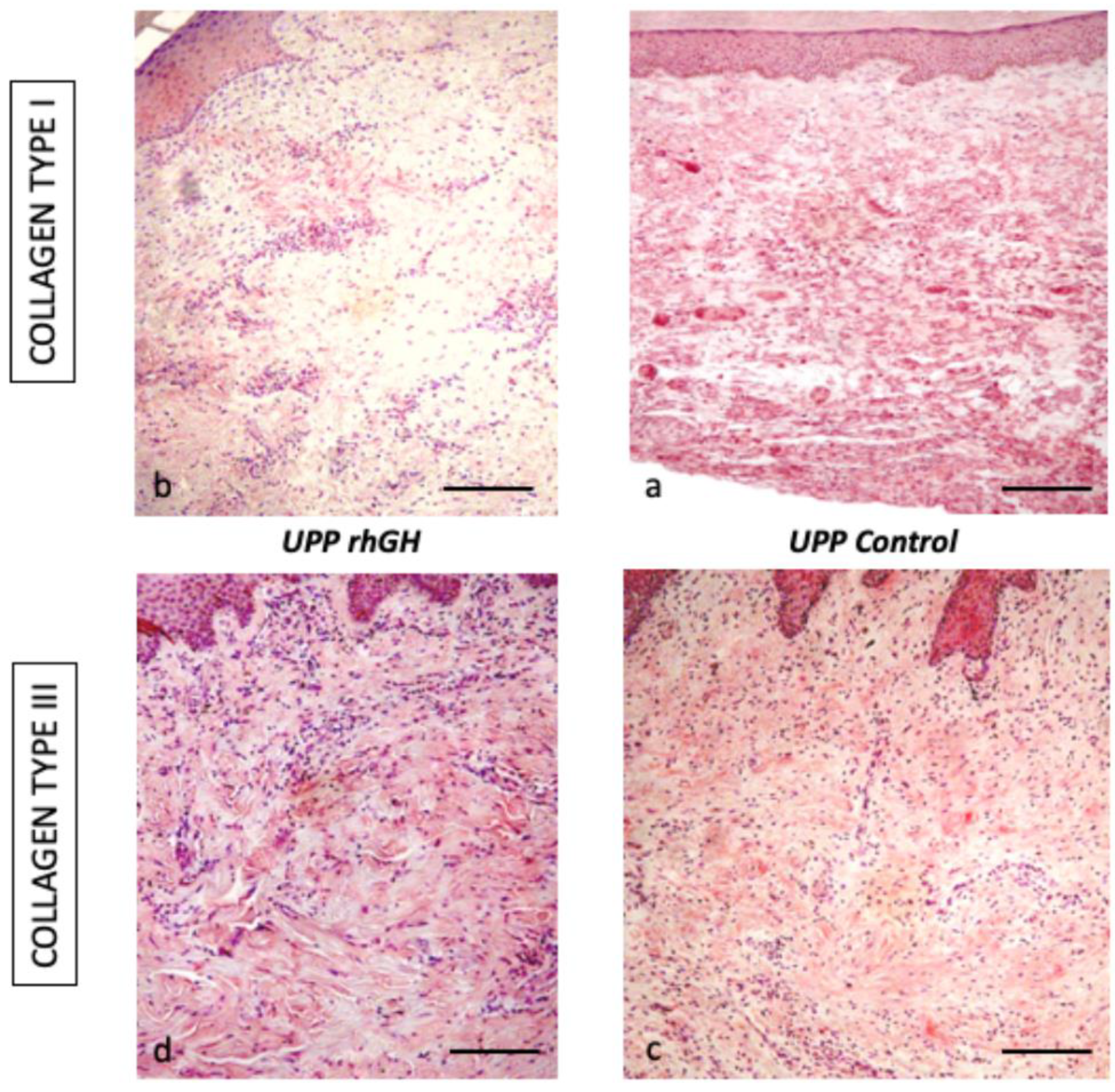
| Collagen type I | Collagen type III | |
|---|---|---|
| rhGH group | 16.8 % ± 4.4 | 70.8 % ± 2.8 |
| Control group | 76.8 % ± 16.5 | 64.4 % ± 3.8 |
| p-value | <0.01 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóbal, L.; de los Reyes, N.; Ortega, M.A.; Álvarez-Mon, M.; García-Honduvilla, N.; Buján, J.; Maldonado, A.A. Local Growth Hormone Therapy for Pressure Ulcer Healing on a Human Skin Mouse Model. Int. J. Mol. Sci. 2019, 20, 4157. https://doi.org/10.3390/ijms20174157
Cristóbal L, de los Reyes N, Ortega MA, Álvarez-Mon M, García-Honduvilla N, Buján J, Maldonado AA. Local Growth Hormone Therapy for Pressure Ulcer Healing on a Human Skin Mouse Model. International Journal of Molecular Sciences. 2019; 20(17):4157. https://doi.org/10.3390/ijms20174157
Chicago/Turabian StyleCristóbal, Lara, Nerea de los Reyes, Miguel A. Ortega, Melchor Álvarez-Mon, Natalio García-Honduvilla, Julia Buján, and Andrés A. Maldonado. 2019. "Local Growth Hormone Therapy for Pressure Ulcer Healing on a Human Skin Mouse Model" International Journal of Molecular Sciences 20, no. 17: 4157. https://doi.org/10.3390/ijms20174157
APA StyleCristóbal, L., de los Reyes, N., Ortega, M. A., Álvarez-Mon, M., García-Honduvilla, N., Buján, J., & Maldonado, A. A. (2019). Local Growth Hormone Therapy for Pressure Ulcer Healing on a Human Skin Mouse Model. International Journal of Molecular Sciences, 20(17), 4157. https://doi.org/10.3390/ijms20174157