Author Contributions
Conceptualization, M.C.; methodology, M.C.; software, M.C. and S.H.L.; validation, M.C. and S.H.L.; formal analysis, S.H.L., K.-T.C., N.C., B.W., H.P., and C.C.; investigation, S.H.L., K.-T.C., N.C., B.W., H.P., and C.C.; resources, M.C.; data curation, S.H.L.; writing—original draft preparation, M.C. and S.L.; writing—review and editing, M.C. and S.L.; visualization, M.C., K.C., and S.H.L.; supervision, M.C.; project administration, M.C.; funding acquisition, M.C.
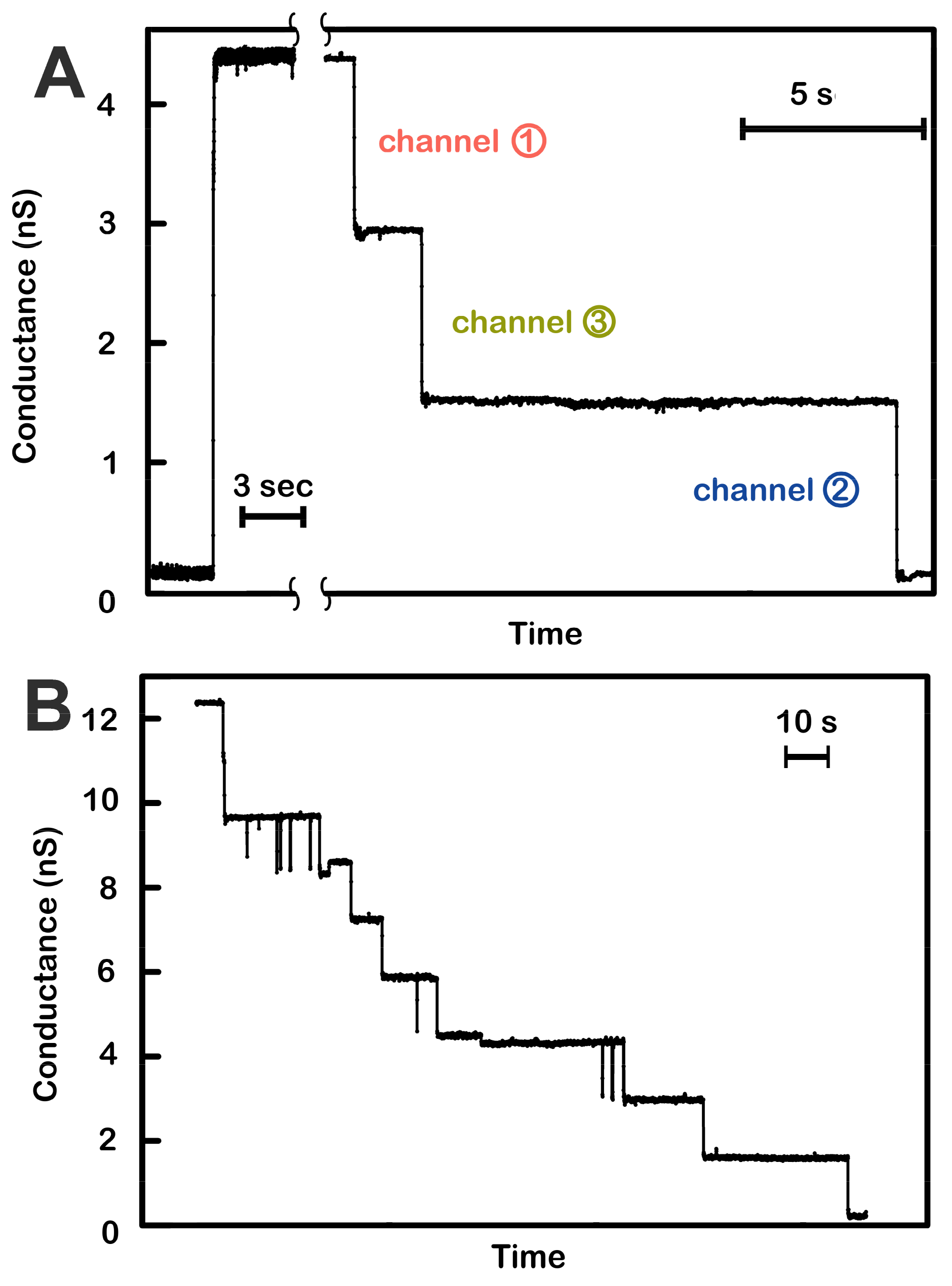
Figure 1.
Voltage-dependent channel closure of triplins inserted into planar phospholipid membranes. (A) Insertion of a single triplin into a planar phospholipid membrane. The single insertion event (left) took place under a +10 mV applied potential. When the potential was raised to +70 mV, three conductance drops took place (right). The indicated order of closure (channel 1, 3, then 2) is based on the model presented in Figure 7. (B) A +70 mV transmembrane potential was applied to a planar membrane containing three triplins. Nine conductance decrements took place, equivalent to the closure of all the channels formed by the three triplins.
Figure 1.
Voltage-dependent channel closure of triplins inserted into planar phospholipid membranes. (A) Insertion of a single triplin into a planar phospholipid membrane. The single insertion event (left) took place under a +10 mV applied potential. When the potential was raised to +70 mV, three conductance drops took place (right). The indicated order of closure (channel 1, 3, then 2) is based on the model presented in Figure 7. (B) A +70 mV transmembrane potential was applied to a planar membrane containing three triplins. Nine conductance decrements took place, equivalent to the closure of all the channels formed by the three triplins.
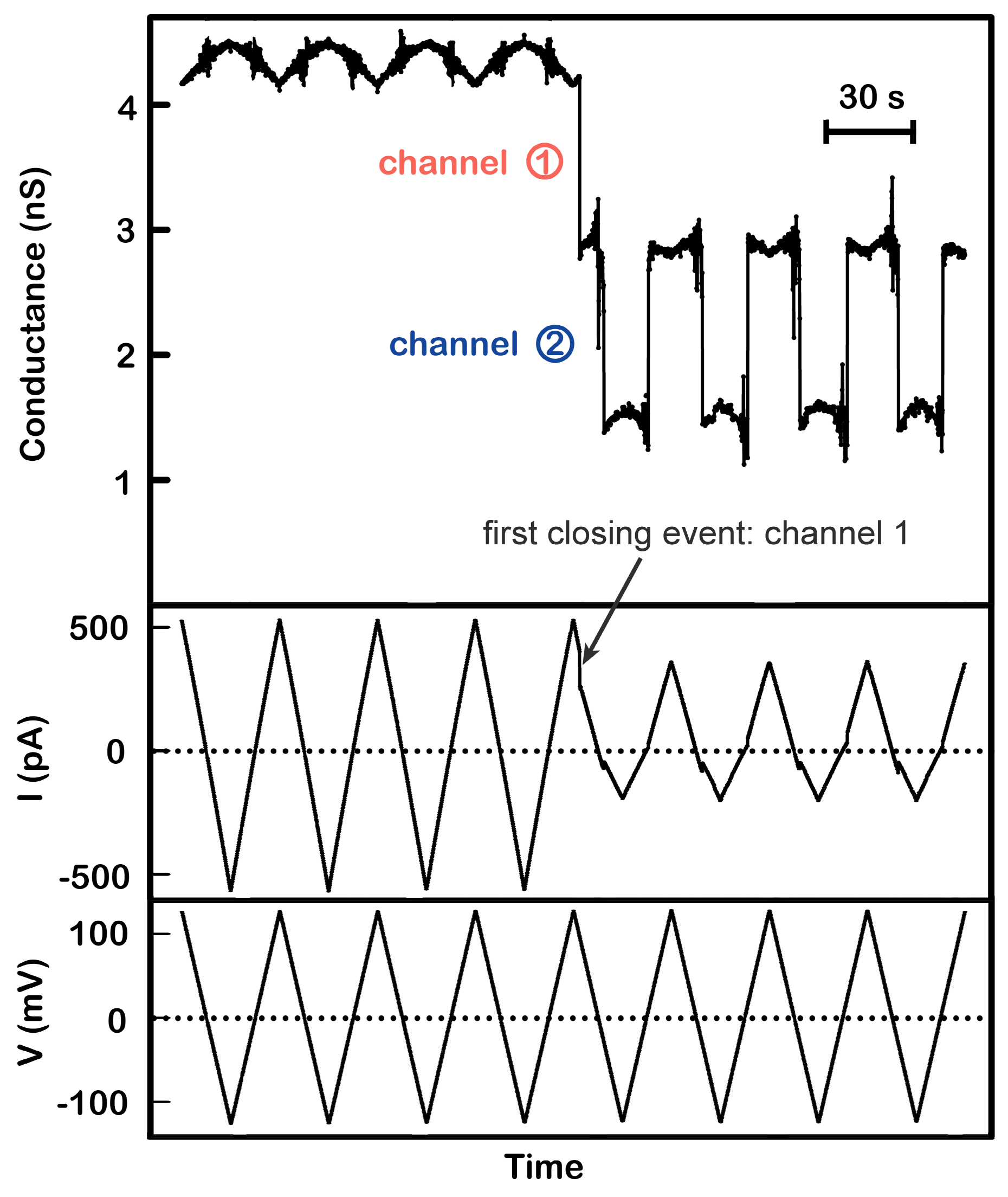
Figure 2.
Positive cooperativity between channel 1 and 2. A single triplin (4.4 nS) was probed using a 30 mHz (7.4 mV/s) triangular voltage ramp (±124 mV). From top to bottom, the panels are the calculated conductance, the current (in picoAmperes, pA), and the applied voltage traces. Closure of channel 2 at negative potentials only occurred after closure of channel 1 at a high positive potential (here at +98 mV). Channel 2 closure and reopening occurred in all subsequent voltage ramps. Note that in the absence of gating the conductance varies somewhat with voltage due to a small amount of rectification in these channels.
Figure 2.
Positive cooperativity between channel 1 and 2. A single triplin (4.4 nS) was probed using a 30 mHz (7.4 mV/s) triangular voltage ramp (±124 mV). From top to bottom, the panels are the calculated conductance, the current (in picoAmperes, pA), and the applied voltage traces. Closure of channel 2 at negative potentials only occurred after closure of channel 1 at a high positive potential (here at +98 mV). Channel 2 closure and reopening occurred in all subsequent voltage ramps. Note that in the absence of gating the conductance varies somewhat with voltage due to a small amount of rectification in these channels.
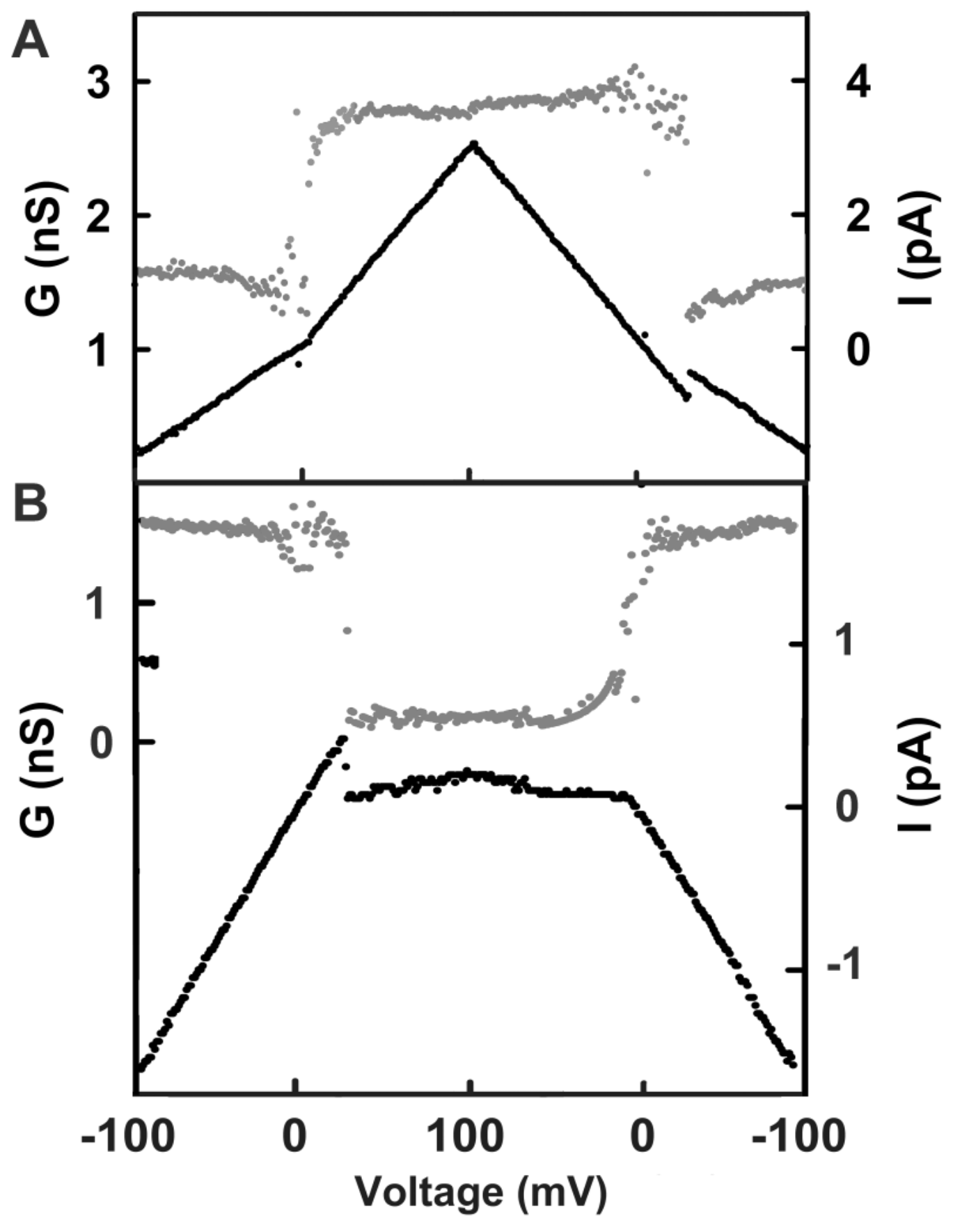
Figure 3.
Gating pattern of channels 2 and 3. The membrane contained a single triplin, and by the time these segments were recorded, channel 1 had closed. Illustrated in each panel is the current recorded (lower trace, using the scale at the right) during one cycle of a triangular voltage ramp (31 mHz, ± 100 mV). The upper trace (gray dots) is the calculated conductance, using the scale at the left. (A) Opening and closing of channel 2. Note that channel 2 closure took place at negative potentials, whereas the reopening, being kinetically delayed, occurred at a slightly positive voltage. (B) Closing and opening of channel 3 when channels 1 and 2 remained closed. Channel 3 closed at positive voltages, and the remaining conductance was close to zero.
Figure 3.
Gating pattern of channels 2 and 3. The membrane contained a single triplin, and by the time these segments were recorded, channel 1 had closed. Illustrated in each panel is the current recorded (lower trace, using the scale at the right) during one cycle of a triangular voltage ramp (31 mHz, ± 100 mV). The upper trace (gray dots) is the calculated conductance, using the scale at the left. (A) Opening and closing of channel 2. Note that channel 2 closure took place at negative potentials, whereas the reopening, being kinetically delayed, occurred at a slightly positive voltage. (B) Closing and opening of channel 3 when channels 1 and 2 remained closed. Channel 3 closed at positive voltages, and the remaining conductance was close to zero.
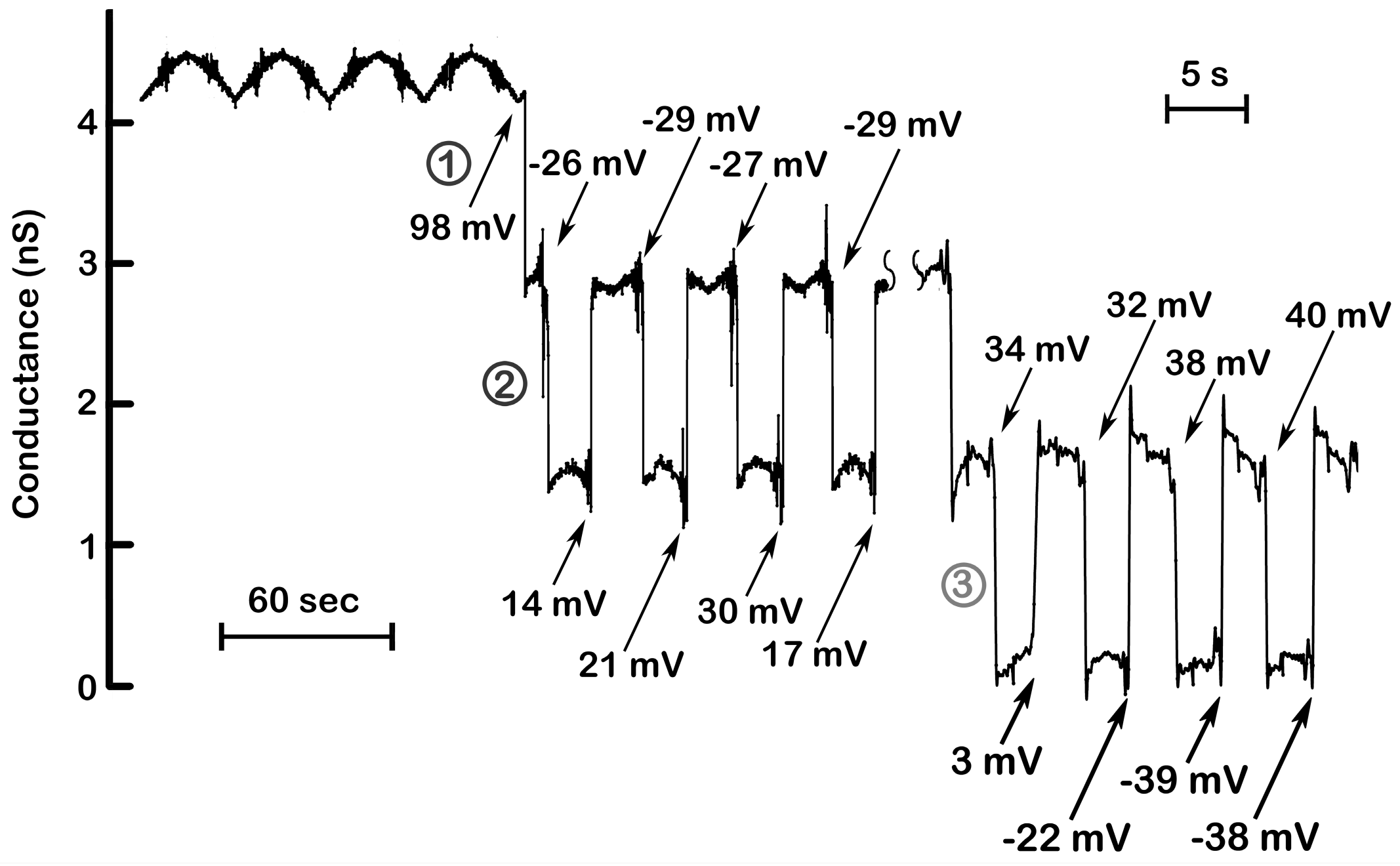
Figure 4.
Steep voltage dependence and cooperativity of channel 2 and 3. The results illustrated in
Figure 2 were supplemented with those collected at a later time in the same experiment but recorded while applying triangular ramps at a higher frequency (175 mHz; 43.4 mV/s). The numbers indicate the voltages at which the channels closed or opened. Channel 2 closed at nearly identical voltages (−26, −29, −27, −29 mV), whereas reopening was delayed to positive voltages due to slower kinetics of channel reopening. (Note the wide variation in the voltages at which channel 2 reopened) At higher ramp frequencies (right), the slow reopening kinetics of channel 2 resulted in its remaining closed at the positive potentials needed to close channel 3. Again, note that the voltages at which channel 3 closed are in a narrow range whereas the voltages at which opening occurred varied widely.
Figure 4.
Steep voltage dependence and cooperativity of channel 2 and 3. The results illustrated in
Figure 2 were supplemented with those collected at a later time in the same experiment but recorded while applying triangular ramps at a higher frequency (175 mHz; 43.4 mV/s). The numbers indicate the voltages at which the channels closed or opened. Channel 2 closed at nearly identical voltages (−26, −29, −27, −29 mV), whereas reopening was delayed to positive voltages due to slower kinetics of channel reopening. (Note the wide variation in the voltages at which channel 2 reopened) At higher ramp frequencies (right), the slow reopening kinetics of channel 2 resulted in its remaining closed at the positive potentials needed to close channel 3. Again, note that the voltages at which channel 3 closed are in a narrow range whereas the voltages at which opening occurred varied widely.
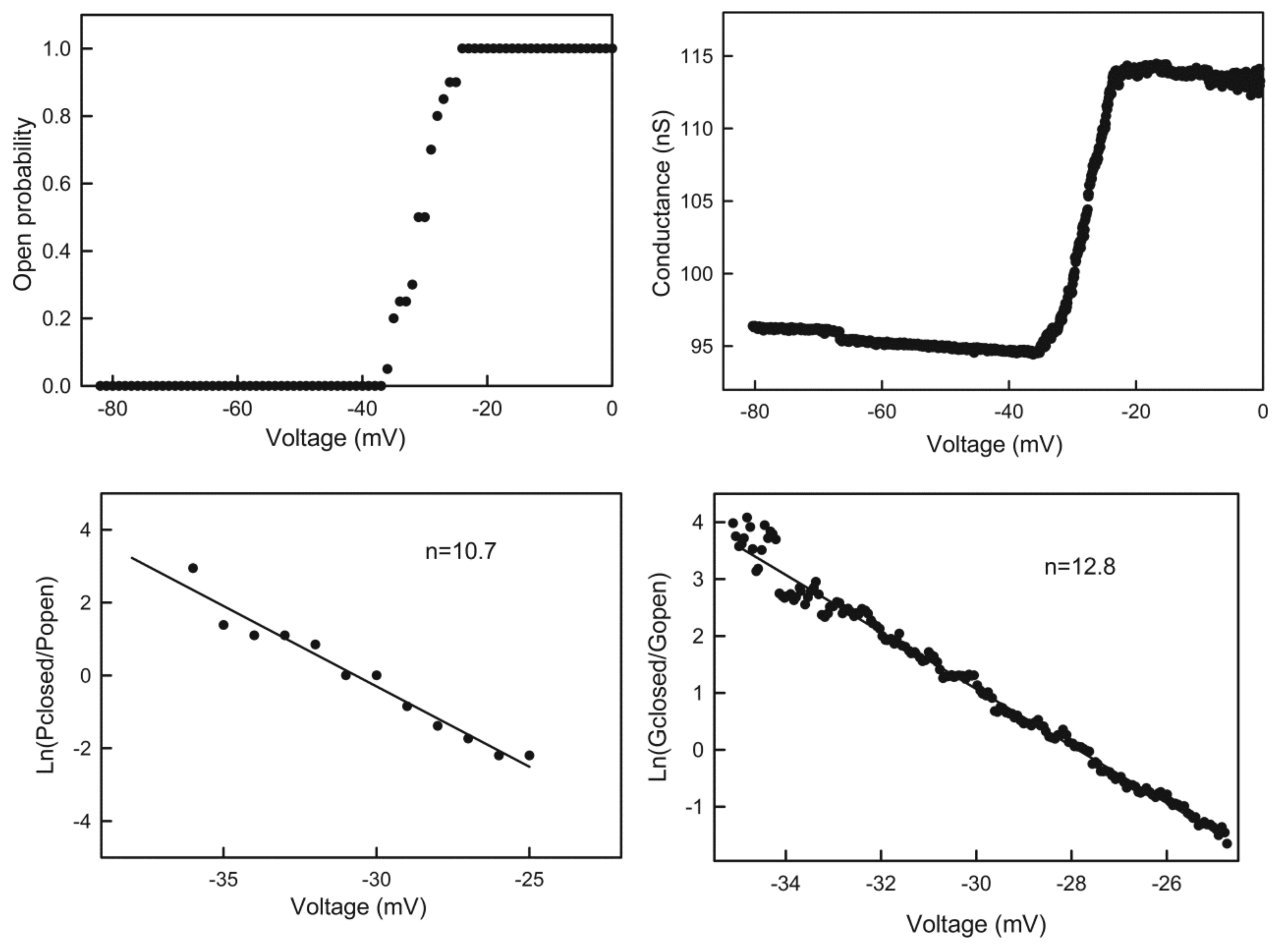
Figure 5.
Quantitation of the voltage dependence of triplin. Upper left: the data was collected from an experiment with a single triplin. Twenty consecutive records of the current response to a 30 mHz triangular voltage ramp (±82mV) were analyzed to determine the probability of finding channel 2 open as the voltage declined from 0 to −82 mV. This is the channel closing process. This data was log transformed as described in Methods to yield the plot in the lower left panel. The line is the least squares fit through the data. From the slope of the line, the voltage dependence parameters n and V0 were determined, and the n values are indicated. The right panels are similar except that in this experiment the membrane contained many triplins (perhaps 28) only some of which were gating. The triangular voltage ramp was run at 9 mHz (±80 mV).
Figure 5.
Quantitation of the voltage dependence of triplin. Upper left: the data was collected from an experiment with a single triplin. Twenty consecutive records of the current response to a 30 mHz triangular voltage ramp (±82mV) were analyzed to determine the probability of finding channel 2 open as the voltage declined from 0 to −82 mV. This is the channel closing process. This data was log transformed as described in Methods to yield the plot in the lower left panel. The line is the least squares fit through the data. From the slope of the line, the voltage dependence parameters n and V0 were determined, and the n values are indicated. The right panels are similar except that in this experiment the membrane contained many triplins (perhaps 28) only some of which were gating. The triangular voltage ramp was run at 9 mHz (±80 mV).
Figure 6.
Estimate of the effective number of gating charges, n. The values plotted were the results of Boltzmann distribution fits to the gating of channel 2 as the voltage was varied at different rates. The data originated from ten separate experiments. The solid line is the least squares fit, and the dotted lines show the 95% confidence limits. Extrapolation to zero rate of voltage change yields a value for n (equilibrium value) of 14 ± 2.5.
Figure 6.
Estimate of the effective number of gating charges, n. The values plotted were the results of Boltzmann distribution fits to the gating of channel 2 as the voltage was varied at different rates. The data originated from ten separate experiments. The solid line is the least squares fit, and the dotted lines show the 95% confidence limits. Extrapolation to zero rate of voltage change yields a value for n (equilibrium value) of 14 ± 2.5.
Figure 7.
Proposed voltage-gating model of triplin channels. Each triplin consists of three cylindrical channels arranged in a row. The orientation of each channel is indicated by the graded shading. Thus, the middle cylinder (channel 2) has an orientation opposite to that of its neighbors. Each channel has its own gating charge whose asymmetric distribution results in a dipole (arrow). The tip of the arrow is the negative end. An open channel is designated by a white circle on top, a closed one has a colored circle. Channel closure translocates the charged voltage sensor through the membrane thus inverting the dipole moment (i.e. inverting the arrow). The orientation of the channel is not changed. Adjacent channels influence each other through dipole-dipole interactions. The applied voltage is indicated by the symbol at the upper right corner of each triplin. All three channels are open when no potential is applied (upper left). From an all channels open state, a high positive voltage induces closure of channel 1 whereas a negative voltage has no effect. Snail path: with a constant voltage, adjacent dipoles stabilize the structure thus inhibiting channel closure resulting in slow rates of closure. With time a high positive voltage closes both channel 1 and 3. Cooperation with its neighbors forces channel 2 to close despite the wrong sign of the applied voltage. Rabbit path: using triangular voltage ramps, the closure of channel 1 favors closure of channel 2 due to favorable dipole-dipole interaction. Channel 2 closure occurs at negative voltages due to its opposite orientation. Dipole-dipole interaction between 2 and 3 requires 2 to be closed for 3 to close. When fast triangular voltage ramps are applied, the slow rate of opening of channel 2 results in 2 remaining closed at positive potentials long enough for channel 3 to close. This leads to the all-closed structure at the lower right. As the voltage is reduced there are only two outcomes: either channel 3 reopens followed by channel 2 or channel 3 and 2 can reopen simultaneously (one-way arrow).
Figure 7.
Proposed voltage-gating model of triplin channels. Each triplin consists of three cylindrical channels arranged in a row. The orientation of each channel is indicated by the graded shading. Thus, the middle cylinder (channel 2) has an orientation opposite to that of its neighbors. Each channel has its own gating charge whose asymmetric distribution results in a dipole (arrow). The tip of the arrow is the negative end. An open channel is designated by a white circle on top, a closed one has a colored circle. Channel closure translocates the charged voltage sensor through the membrane thus inverting the dipole moment (i.e. inverting the arrow). The orientation of the channel is not changed. Adjacent channels influence each other through dipole-dipole interactions. The applied voltage is indicated by the symbol at the upper right corner of each triplin. All three channels are open when no potential is applied (upper left). From an all channels open state, a high positive voltage induces closure of channel 1 whereas a negative voltage has no effect. Snail path: with a constant voltage, adjacent dipoles stabilize the structure thus inhibiting channel closure resulting in slow rates of closure. With time a high positive voltage closes both channel 1 and 3. Cooperation with its neighbors forces channel 2 to close despite the wrong sign of the applied voltage. Rabbit path: using triangular voltage ramps, the closure of channel 1 favors closure of channel 2 due to favorable dipole-dipole interaction. Channel 2 closure occurs at negative voltages due to its opposite orientation. Dipole-dipole interaction between 2 and 3 requires 2 to be closed for 3 to close. When fast triangular voltage ramps are applied, the slow rate of opening of channel 2 results in 2 remaining closed at positive potentials long enough for channel 3 to close. This leads to the all-closed structure at the lower right. As the voltage is reduced there are only two outcomes: either channel 3 reopens followed by channel 2 or channel 3 and 2 can reopen simultaneously (one-way arrow).
![Ijms 20 04501 g007 Ijms 20 04501 g007]()
Figure 8.
The virtually simultaneous reopening of channel 2 and 3 demonstrates strong cooperative behavior. A single triplin was probed with a 71 mHz (13.6 mV/s) triangular voltage ramp. (A) A portion of the recording is shown whereby three successive voltage ramps show essentially simultaneous reopening of channels 2 and 3. (B) A conductance vs. voltage plot of the data in the shaded area in (A) was generated and annotated to indicate which channels are gating. Both (A) and (B) show channel 2 closure at a negative voltage, then channel 3 closing as the potential became positive, followed by simultaneous reopening of channels 2 and 3. (C) Expanded view of the circled area in (A) showing that the current increased within 5 ms.
Figure 8.
The virtually simultaneous reopening of channel 2 and 3 demonstrates strong cooperative behavior. A single triplin was probed with a 71 mHz (13.6 mV/s) triangular voltage ramp. (A) A portion of the recording is shown whereby three successive voltage ramps show essentially simultaneous reopening of channels 2 and 3. (B) A conductance vs. voltage plot of the data in the shaded area in (A) was generated and annotated to indicate which channels are gating. Both (A) and (B) show channel 2 closure at a negative voltage, then channel 3 closing as the potential became positive, followed by simultaneous reopening of channels 2 and 3. (C) Expanded view of the circled area in (A) showing that the current increased within 5 ms.
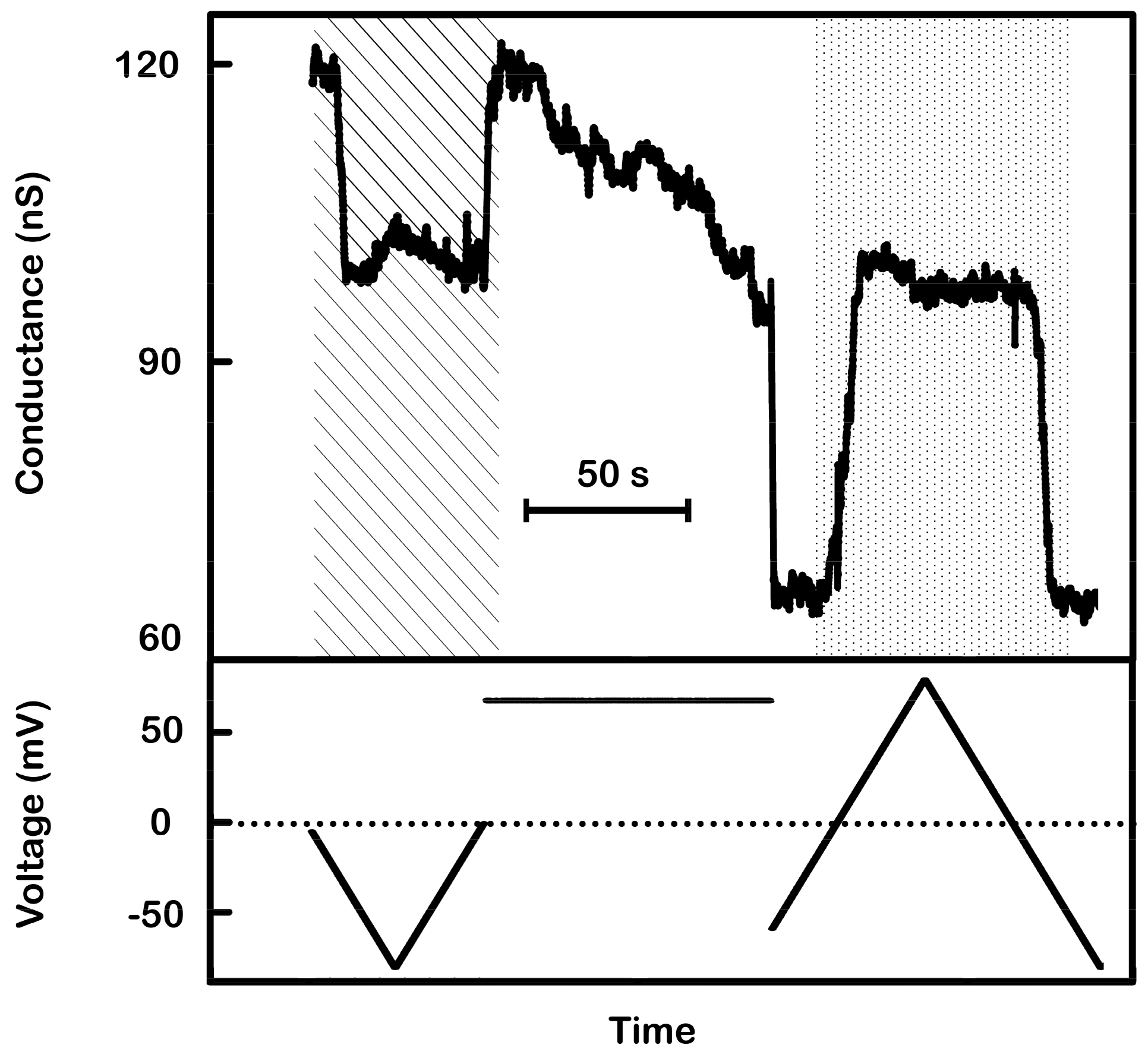
Figure 9.
Model tested by voltage pulse sequence. This membrane contained two conducting triplins (total conductance a little over the expected 6 nS due to a small background conductance). First the voltage was stepped from +69 to −45 mV in order to close the two channel 2. A rapid drop in conductance (<47 ms) of ~3.4 nS was observed, consistent with about two channel 2 closures. Then the voltage was stepped back to +69 mV to close channel 3 before channel 2 could reopen. An immediate decrement of conductance from 3.3 to 0.5 nS was seen, indicating the closure of two channel 3. Soon after the drop, two increments, each of about 3 nS, were detected. This was interpreted to be two synchronized reopenings of channels 2 and 3. This recording is typical of many observations.
Figure 9.
Model tested by voltage pulse sequence. This membrane contained two conducting triplins (total conductance a little over the expected 6 nS due to a small background conductance). First the voltage was stepped from +69 to −45 mV in order to close the two channel 2. A rapid drop in conductance (<47 ms) of ~3.4 nS was observed, consistent with about two channel 2 closures. Then the voltage was stepped back to +69 mV to close channel 3 before channel 2 could reopen. An immediate decrement of conductance from 3.3 to 0.5 nS was seen, indicating the closure of two channel 3. Soon after the drop, two increments, each of about 3 nS, were detected. This was interpreted to be two synchronized reopenings of channels 2 and 3. This recording is typical of many observations.
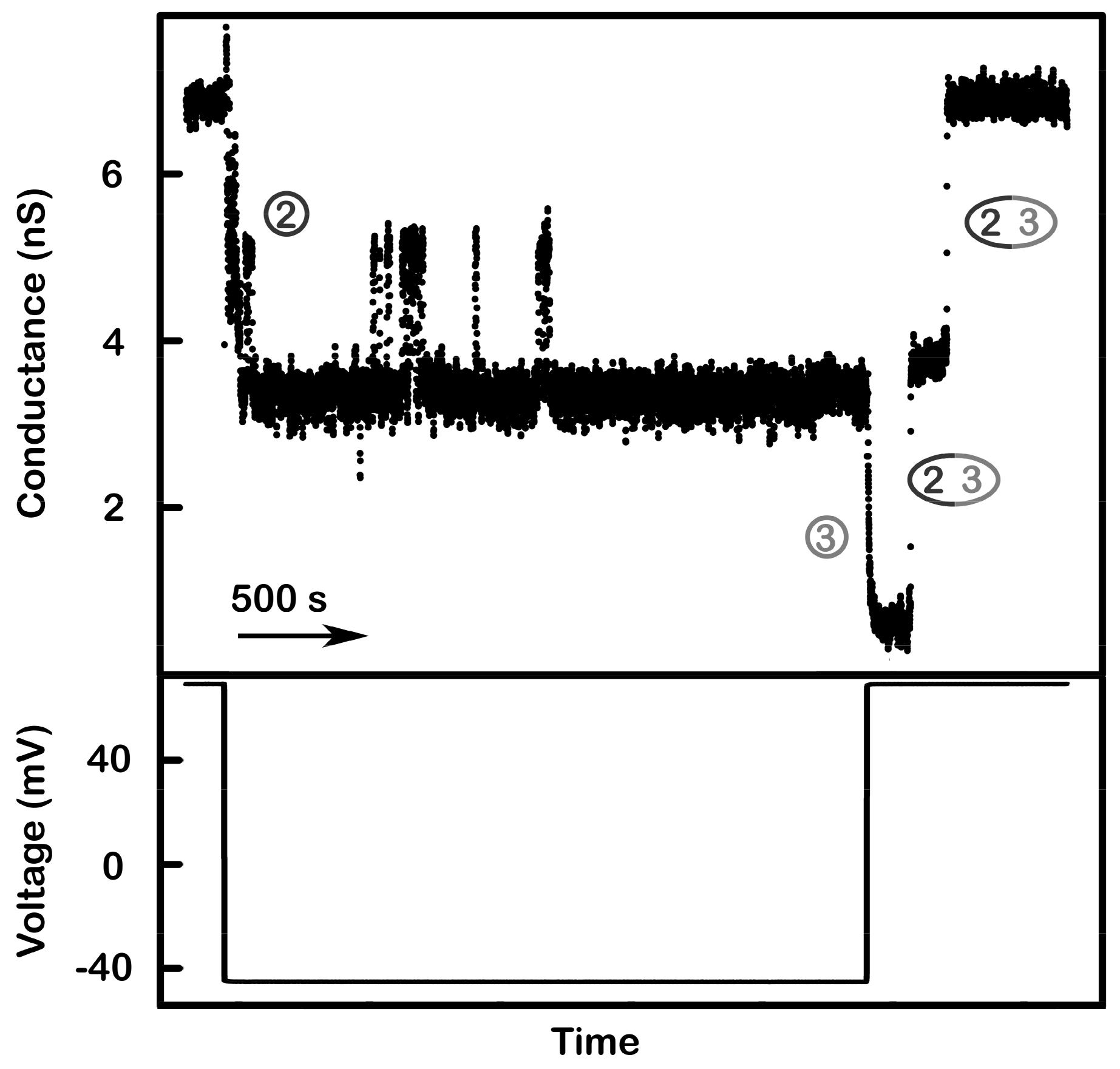
Figure 10.
Supportive evidence of the model: multi-triplin containing membranes. A 2 mHz (0.28 mV/s) triangular voltage ramp (± 70 mV) was applied to a membrane. In the illustrated segment the voltage changed from positive to negative values and at −20 mV, four decrements are visible indicating the closure of four channel 2s. Of the remaining conductance, only 6 nS could be due to the remain channel 3s of the active triplins. The remaining nine nS (total of 15 nS) are consistent with the two non-gating triplins. Therefore, at positive voltages the conductance was composed of two non-gating triplins and four gating triplins i.e. with their channel 1s closed (3.0 nS per triplin) for a total of 21 nS.
Figure 10.
Supportive evidence of the model: multi-triplin containing membranes. A 2 mHz (0.28 mV/s) triangular voltage ramp (± 70 mV) was applied to a membrane. In the illustrated segment the voltage changed from positive to negative values and at −20 mV, four decrements are visible indicating the closure of four channel 2s. Of the remaining conductance, only 6 nS could be due to the remain channel 3s of the active triplins. The remaining nine nS (total of 15 nS) are consistent with the two non-gating triplins. Therefore, at positive voltages the conductance was composed of two non-gating triplins and four gating triplins i.e. with their channel 1s closed (3.0 nS per triplin) for a total of 21 nS.
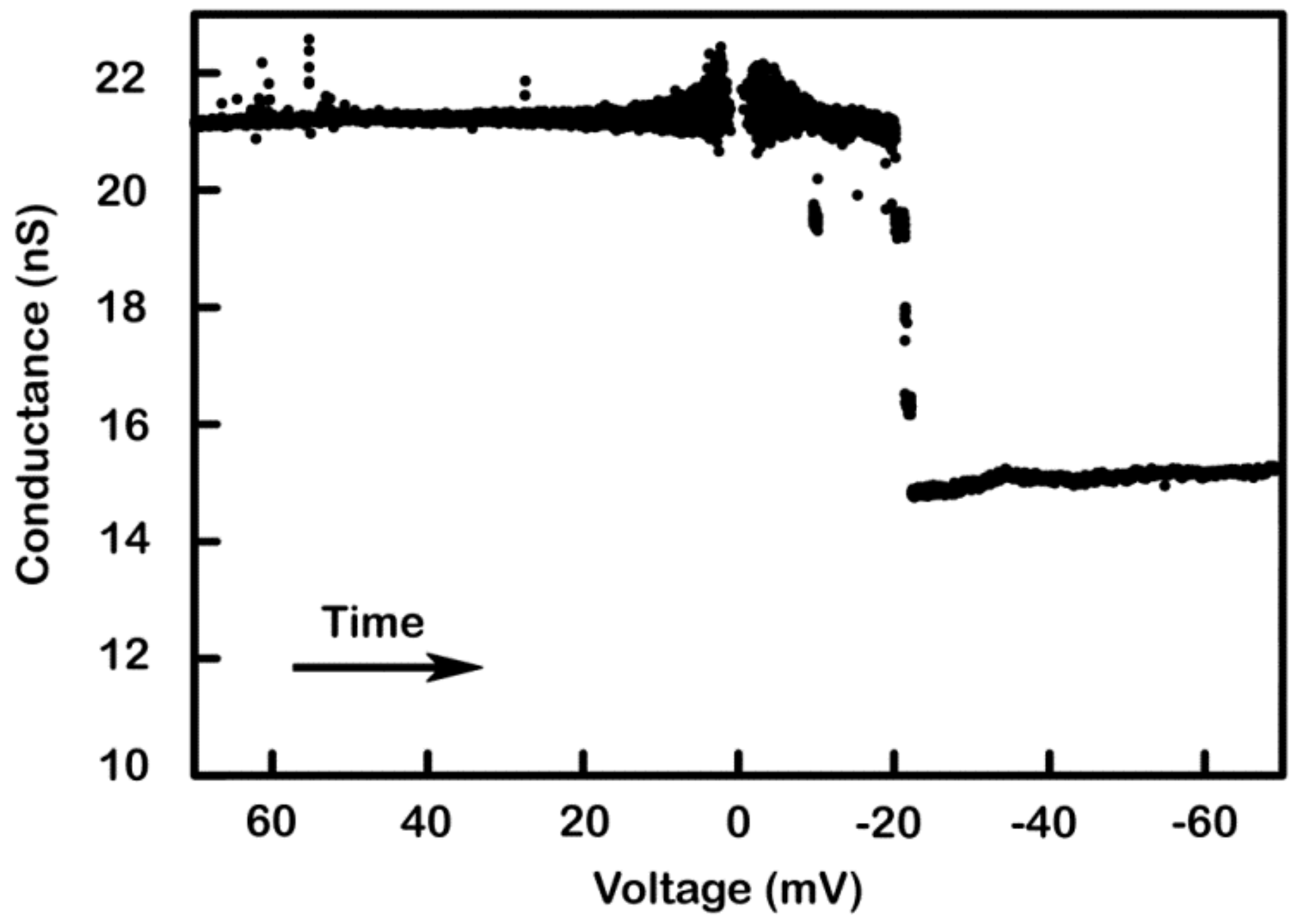
Figure 11.
Supportive evidence of the model: activation of non-responding triplins by closing channel 1s. A 9 mHz (1.44 mV/s) triangular voltage ramp (± 80 mV) was applied to a membrane containing many triplins. As the voltage became more negative the conductance decreased rapidly by 17 nS, equivalent to the closure of 11 channel 2’s (each 1.5 nS) (in striped region). As the voltage moved toward zero, that same conductance increased once again indicating the reopening of those channels. Thus, only 11 triplins had a closed channel 1 and were capable of gating. The subsequent application of +70 mV for 85 s produced a conductance decrement of 24 nS, corresponding to the closure of 16 channel 1s. Reapplication of the voltage ramp (dotted region) showed conductance changes at negative potentials of 38 nS, equivalent to the closure and reopening of 25 to 27 channel 2’s.
Figure 11.
Supportive evidence of the model: activation of non-responding triplins by closing channel 1s. A 9 mHz (1.44 mV/s) triangular voltage ramp (± 80 mV) was applied to a membrane containing many triplins. As the voltage became more negative the conductance decreased rapidly by 17 nS, equivalent to the closure of 11 channel 2’s (each 1.5 nS) (in striped region). As the voltage moved toward zero, that same conductance increased once again indicating the reopening of those channels. Thus, only 11 triplins had a closed channel 1 and were capable of gating. The subsequent application of +70 mV for 85 s produced a conductance decrement of 24 nS, corresponding to the closure of 16 channel 1s. Reapplication of the voltage ramp (dotted region) showed conductance changes at negative potentials of 38 nS, equivalent to the closure and reopening of 25 to 27 channel 2’s.