Cooperative Binding of KaiB to the KaiC Hexamer Ensures Accurate Circadian Clock Oscillation in Cyanobacteria
Abstract
1. Introduction
2. Results and Discussion
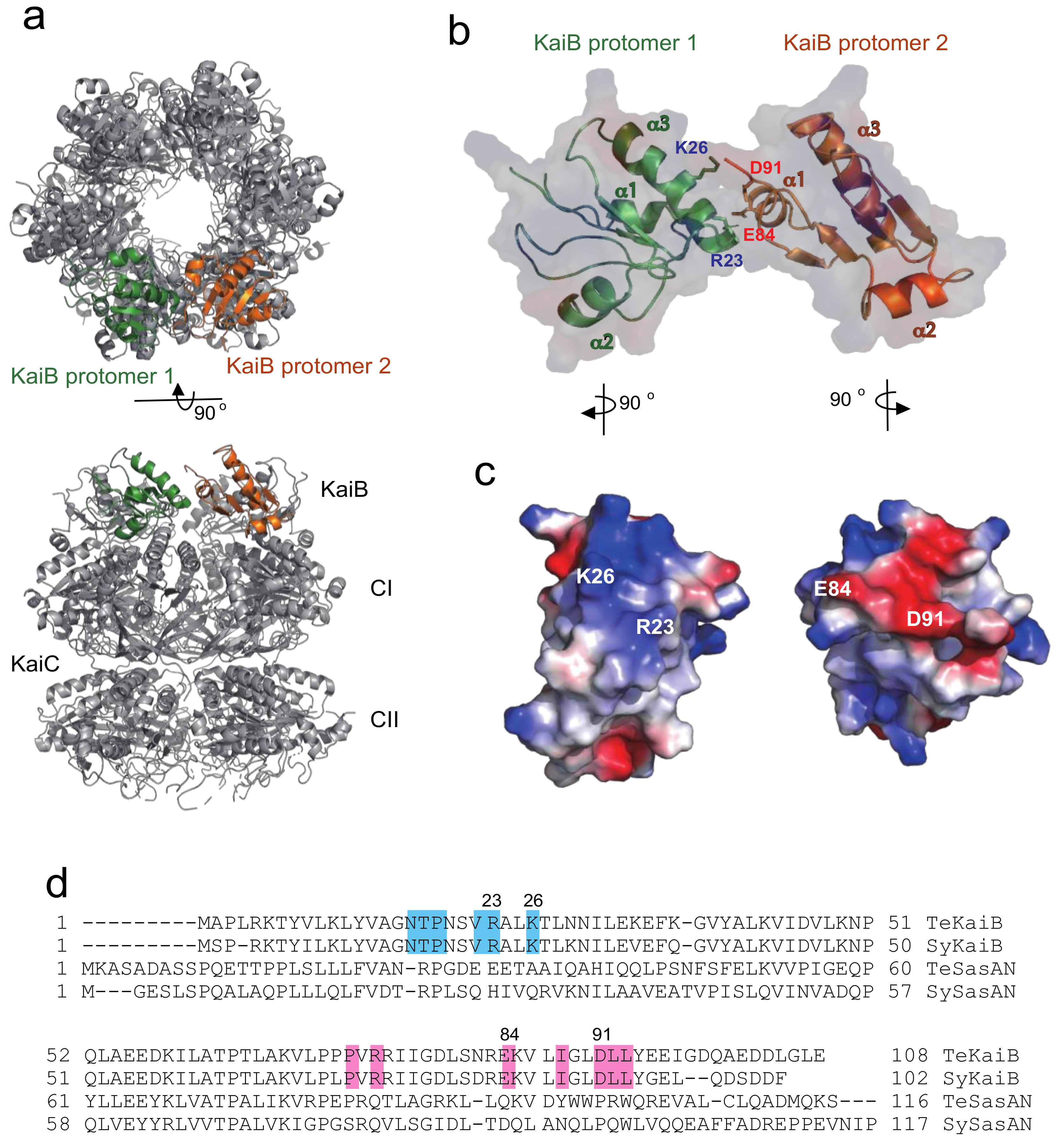
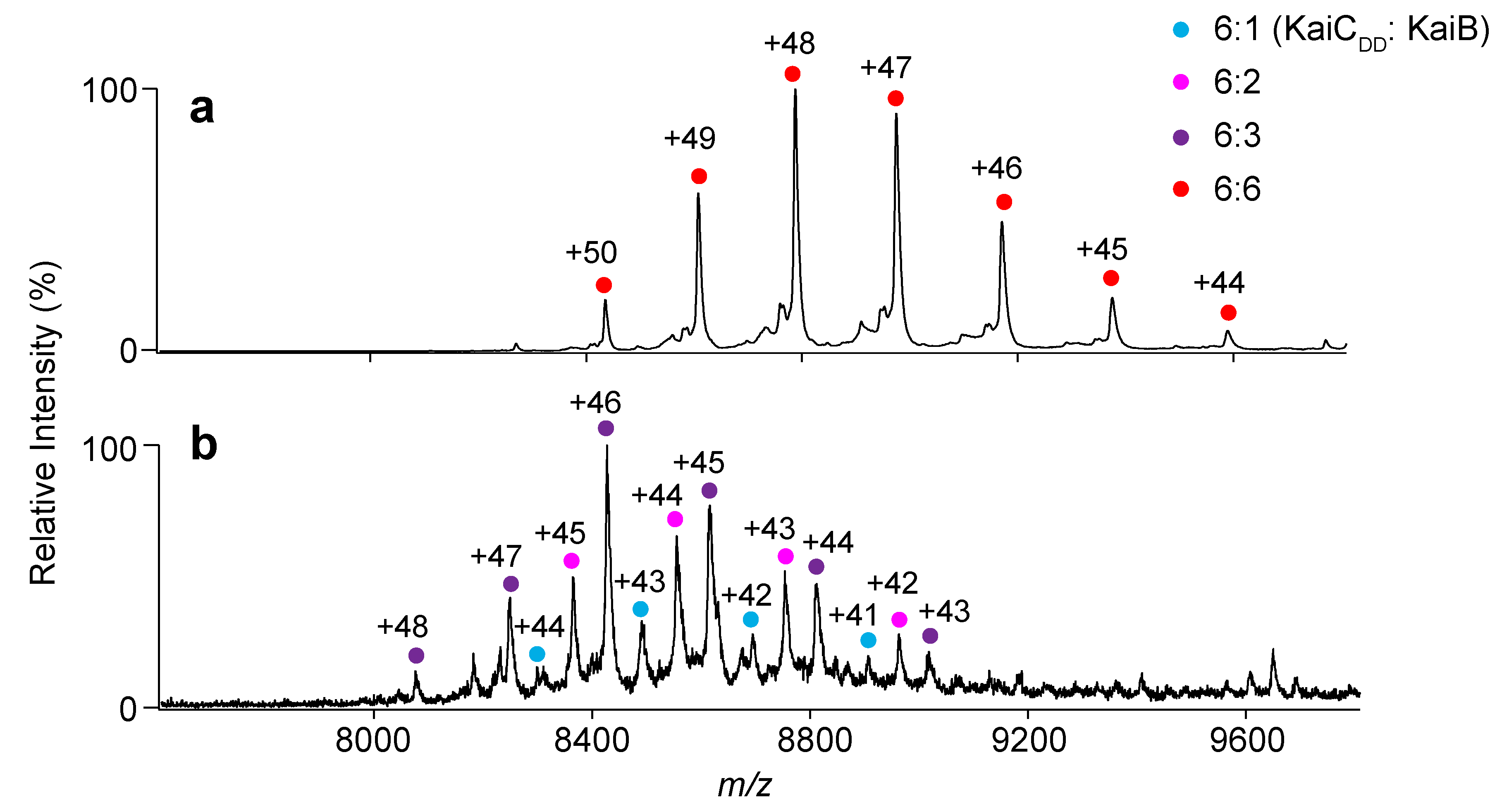
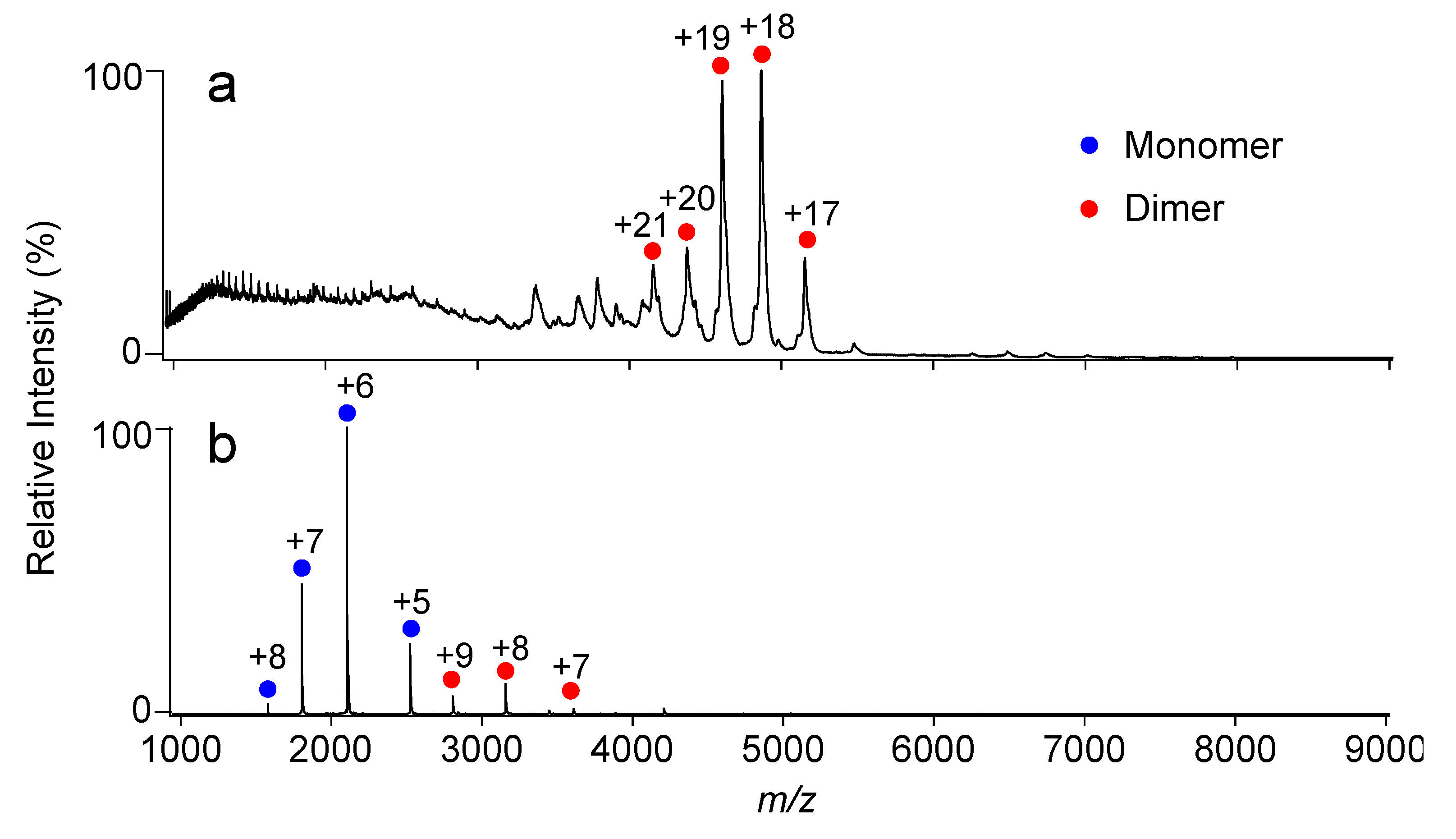
2.1. Complex Formation of KaiC with KaiB Mutants
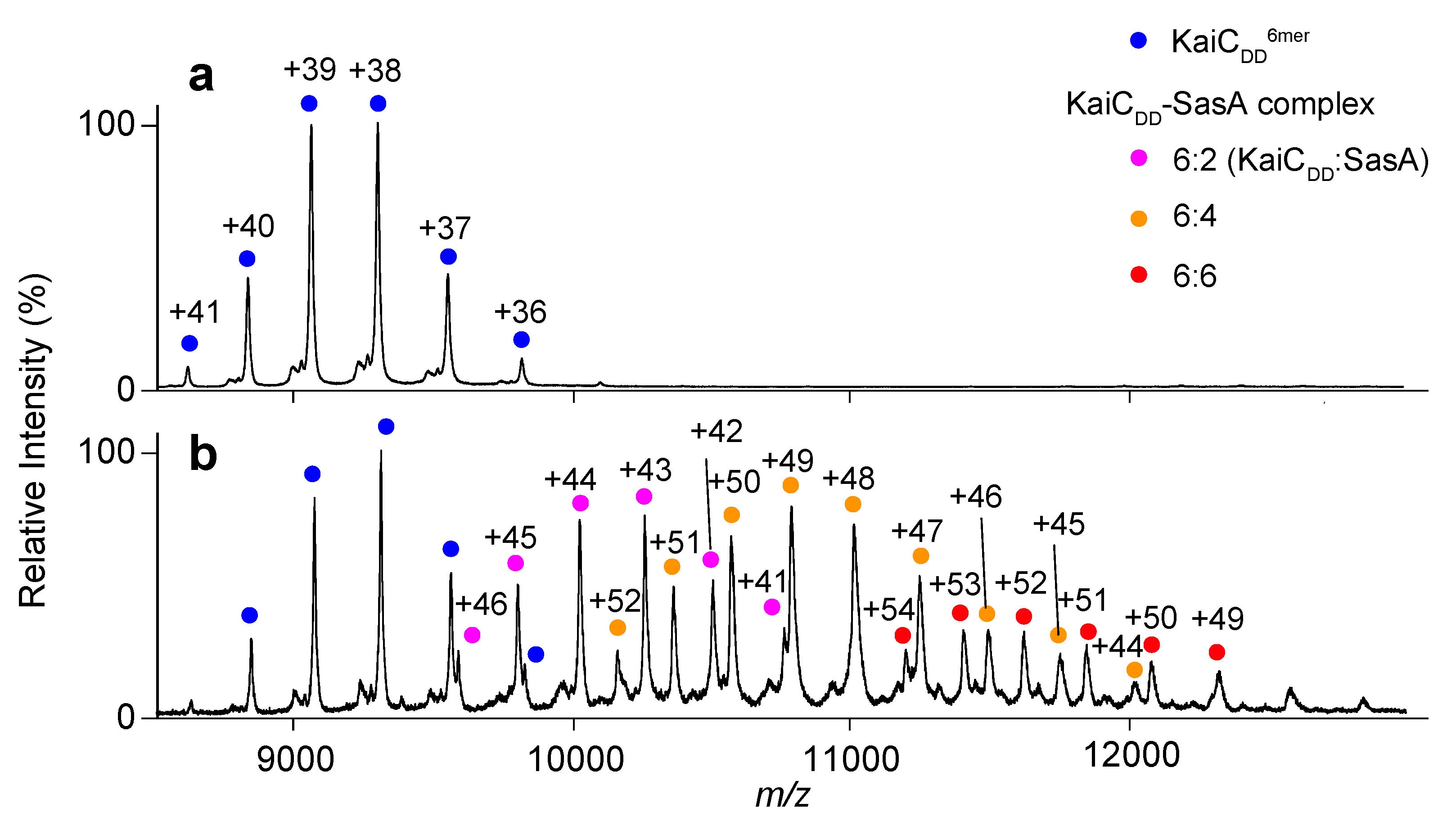
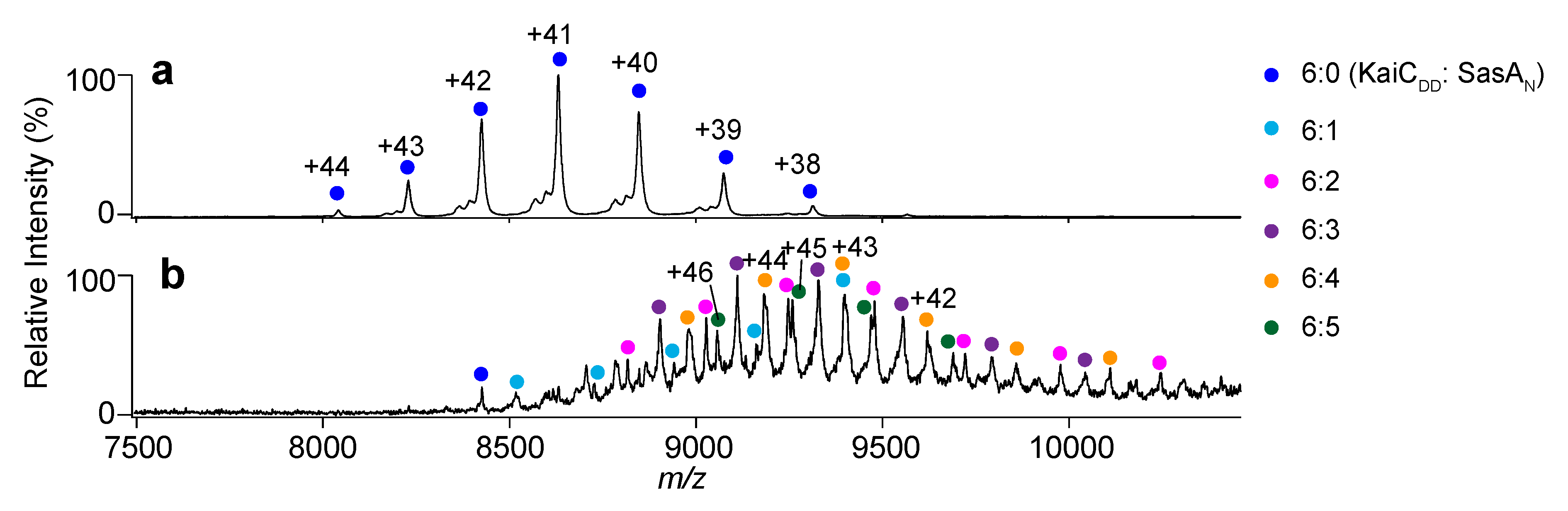
2.2. Complex Formation of KaiC with SasA
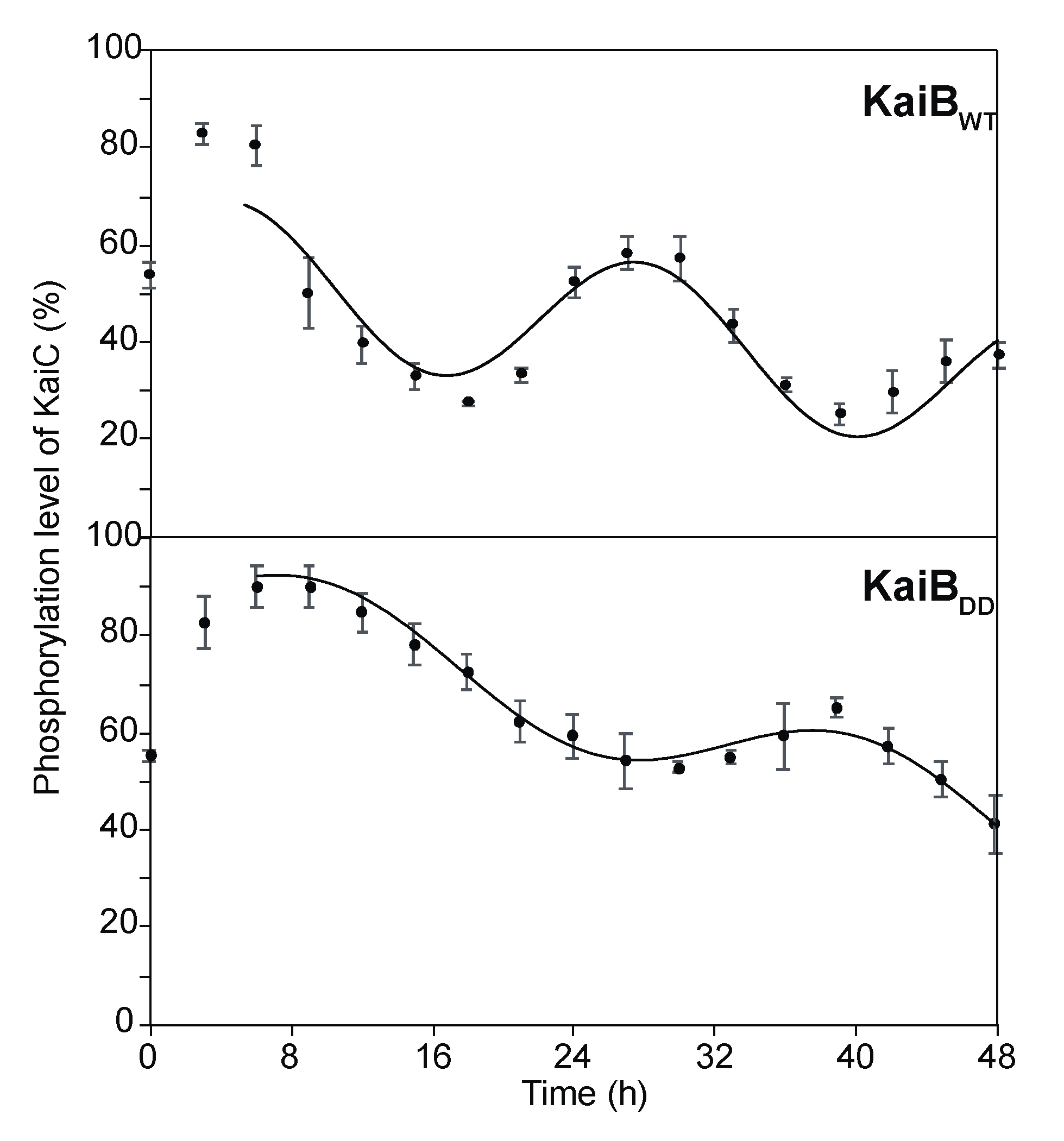
2.3. Effects of KaiB Mutantation on Circadian Oscillations
3. Materials and Methods
3.1. Protein Expression and Purification
3.2. Size Exclusion Chormatography
3.3. Circular Dichroism Spectra
3.4. Native MS Analysis
3.5. Measurement of Time-dependent Phosphorylated KaiC Levels
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine 5′-triphosphate |
| p-KaiC | phosphorylated KaiC |
| MS | mass spectrometry |
| Te | Thermosynechococcus elongatus (strain BP-I) |
| Sy | Synechococcus elongatus (strain PCC 7942) |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
References
- Bünning, E. The Physiological Clock, 3rd ed.; English Universities Press: New York, NY, USA; Springer: London, UK, 1973. [Google Scholar]
- Ishiura, M.; Kutsuna, S.; Aoki, S.; Iwasaki, H.; Andersson, C.R.; Tanabe, A.; Golden, S.S.; Johnson, C.H.; Kondo, T. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 1998, 281, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Taniguchi, Y.; Ishiura, M.; Kondo, T. Physical interactions among circadian clock proteins KaiA, KaiB and KaiC in cyanobacteria. EMBO J. 1999, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Suzuki, H.; Iwase, R.; Uzumaki, T.; Miyake, A.; Shen, J.R.; Imada, K.; Furukawa, Y.; Yonekura, K.; Namba, K.; et al. ATP-Induced hexameric ring structure of the cyanobacterial circadian clock protein KaiC. Genes Cells 2003, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Pattanayek, R.; Wang, J.; Mori, T.; Xu, Y.; Johnson, C.H.; Egli, M. Visualizing a circadian clock protein: Crystal structure of KaiC and functional insights. Mol. Cell 2004, 15, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, T.; Satomi, Y.; Kitayama, Y.; Terauchi, K.; Kiyohara, R.; Takao, T.; Kondo, T. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 2007, 26, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Dong, G.; Carruthers, C.W., Jr.; Golden, S.S.; LiWang, A. The day/night switch in KaiC, a central oscillator component of the circadian clock of cyanobacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 12825–12830. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Nishiwaki, T.; Kitayama, Y.; Nakajima, M.; Kondo, T. KaiA-Stimulated KaiC phosphorylation in circadian timing loops in cyanobacteria. Proc. Natl. Acad. Sci. USA 2002, 99, 15788–15793. [Google Scholar] [CrossRef]
- Kageyama, H.; Nishiwaki, T.; Nakajima, M.; Iwasaki, H.; Oyama, T.; Kondo, T. Cyanobacterial circadian pacemaker: Kai protein complex dynamics in the KaiC phosphorylation cycle in vitro. Mol. Cell 2006, 23, 161–171. [Google Scholar] [CrossRef]
- Williams, S.B.; Vakonakis, I.; Golden, S.S.; LiWang, A.C. Structure and function from the circadian clock protein KaiA of Synechococcus elongatus: A potential clock input mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 15357–15362. [Google Scholar] [CrossRef]
- Kitayama, Y.; Iwasaki, H.; Nishiwaki, T.; Kondo, T. KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacterial circadian clock system. EMBO J. 2003, 22, 2127–2134. [Google Scholar] [CrossRef]
- Iwasaki, H.; Williams, S.B.; Kitayama, Y.; Ishiura, M.; Golden, S.S.; Kondo, T. A kaiC-Interacting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell 2000, 101, 223–233. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-Component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Nakajima, M.; Oyama, T.; Kito, R.; Sugita, C.; Sugita, M.; Kondo, T.; Iwasaki, H. A KaiC-Associating SasA-RpaA two-Component regulatory system as a major circadian timing mediator in cyanobacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 12109–12114. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.G.; Cohen, S.E.; Phong, C.; Myers, W.K.; Kim, Y.I.; Tseng, R.; Lin, J.; Zhang, L.; Boyd, J.S.; Lee, Y.; et al. Circadian rhythms. A protein fold switch joins the circadian oscillator to clock output in cyanobacteria. Science 2015, 349, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Mutoh, R.; Iwase, R.; Furukawa, Y.; Imada, K.; Onai, K.; Morishita, M.; Yasui, S.; Ishii, K.; Valencia Swain, J.O.; et al. The roles of the dimeric and tetrameric structures of the clock protein KaiB in the generation of circadian oscillations in cyanobacteria. J. Biol. Chem. 2012, 287, 29506–29515. [Google Scholar] [CrossRef] [PubMed]
- Valencia, S.J.; Bitou, K.; Ishii, K.; Murakami, R.; Morishita, M.; Onai, K.; Furukawa, Y.; Imada, K.; Namba, K.; Ishiura, M. Phase-Dependent generation and transmission of time information by the KaiABC circadian clock oscillator through SasA-KaiC interaction in cyanobacteria. Genes Cells 2012, 17, 398–419. [Google Scholar] [CrossRef] [PubMed]
- Garces, R.G.; Wu, N.; Gillon, W.; Pai, E.F. Anabaena circadian clock proteins KaiA and KaiB reveal a potential common binding site to their partner KaiC. EMBO J. 2004, 23, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; Oyama, T.; Han, S.; Arvai, A.S.; Getzoff, E.D. Tetrameric architecture of the circadian clock protein KaiB. A novel interface for intermolecular interactions and its impact on the circadian rhythm. J. Biol. Chem. 2005, 280, 19127–19135. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.; Goularte, N.F.; Chavan, A.; Luu, J.; Cohen, S.E.; Chang, Y.G.; Heisler, J.; Li, S.; Michael, A.K.; Tripathi, S.; et al. Structural basis of the day-Night transition in a bacterial circadian clock. Science 2017, 355, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Yagi, H.; Ishii, K.; Porcar, L.; Martel, A.; Oyama, K.; Noda, M.; Yunoki, Y.; Murakami, R.; Inoue, R.; et al. Structural characterization of the circadian clock protein complex composed of KaiB and KaiC by inverse contrast-matching small-Angle neutron scattering. Sci. Rep. 2016, 6, 35567. [Google Scholar] [CrossRef]
- Iida, T.; Mutoh, R.; Onai, K.; Morishita, M.; Furukawa, Y.; Namba, K.; Ishiura, M. Importance of the monomer-Dimer-Tetramer interconversion of the clock protein KaiB in the generation of circadian oscillations in cyanobacteria. Genes Cells 2015, 20, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Snijder, J.; Burnley, R.J.; Wiegard, A.; Melquiond, A.S.; Bonvin, A.M.; Axmann, I.M.; Heck, A.J. Insight into cyanobacterial circadian timing from structural details of the KaiB-KaiC interaction. Proc. Natl. Acad. Sci. USA 2014, 111, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Vakonakis, I.; Klewer, D.A.; Williams, S.B.; Golden, S.S.; LiWang, A.C. Structure of the N-Terminal domain of the circadian clock-Associated histidine kinase SasA. J. Mol. Biol. 2004, 342, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Imai, K.; Ito, H.; Nishiwaki, T.; Murayama, Y.; Iwasaki, H.; Oyama, T.; Kondo, T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 2005, 308, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Snijder, J.; Schuller, J.M.; Wiegard, A.; Lossl, P.; Schmelling, N.; Axmann, I.M.; Plitzko, J.M.; Forster, F.; Heck, A.J. Structures of the cyanobacterial circadian oscillator frozen in a fully assembled state. Science 2017, 355, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Onai, K.; Ishiura, M. RAP, an integrated program for monitoring bioluminescence and analyzing circadian rhythms in real time. Anal. Biochem. 2005, 340, 193–200. [Google Scholar] [CrossRef]
- Oyama, K.; Azai, C.; Nakamura, K.; Tanaka, S.; Terauchi, K. Conversion between two conformational states of KaiC is induced by ATP hydrolysis as a trigger for cyanobacterial circadian oscillation. Sci. Rep. 2016, 6, 32443. [Google Scholar] [CrossRef]
- Hayashi, F.; Itoh, N.; Uzumaki, T.; Iwase, R.; Tsuchiya, Y.; Yamakawa, H.; Morishita, M.; Onai, K.; Itoh, S.; Ishiura, M. Roles of two ATPase-Motif-Containing domains in cyanobacterial circadian clock protein KaiC. J. Biol. Chem. 2004, 279, 52331–52337. [Google Scholar] [CrossRef]
- Murakami, R.; Miyake, A.; Iwase, R.; Hayashi, F.; Uzumaki, T.; Ishiura, M. ATPase activity and its temperature compensation of the cyanobacterial clock protein KaiC. Genes Cells 2008, 13, 387–395. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, R.; Yunoki, Y.; Ishii, K.; Terauchi, K.; Uchiyama, S.; Yagi, H.; Kato, K. Cooperative Binding of KaiB to the KaiC Hexamer Ensures Accurate Circadian Clock Oscillation in Cyanobacteria. Int. J. Mol. Sci. 2019, 20, 4550. https://doi.org/10.3390/ijms20184550
Murakami R, Yunoki Y, Ishii K, Terauchi K, Uchiyama S, Yagi H, Kato K. Cooperative Binding of KaiB to the KaiC Hexamer Ensures Accurate Circadian Clock Oscillation in Cyanobacteria. International Journal of Molecular Sciences. 2019; 20(18):4550. https://doi.org/10.3390/ijms20184550
Chicago/Turabian StyleMurakami, Reiko, Yasuhiro Yunoki, Kentaro Ishii, Kazuki Terauchi, Susumu Uchiyama, Hirokazu Yagi, and Koichi Kato. 2019. "Cooperative Binding of KaiB to the KaiC Hexamer Ensures Accurate Circadian Clock Oscillation in Cyanobacteria" International Journal of Molecular Sciences 20, no. 18: 4550. https://doi.org/10.3390/ijms20184550
APA StyleMurakami, R., Yunoki, Y., Ishii, K., Terauchi, K., Uchiyama, S., Yagi, H., & Kato, K. (2019). Cooperative Binding of KaiB to the KaiC Hexamer Ensures Accurate Circadian Clock Oscillation in Cyanobacteria. International Journal of Molecular Sciences, 20(18), 4550. https://doi.org/10.3390/ijms20184550