Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice
Abstract
1. Introduction
2. Results
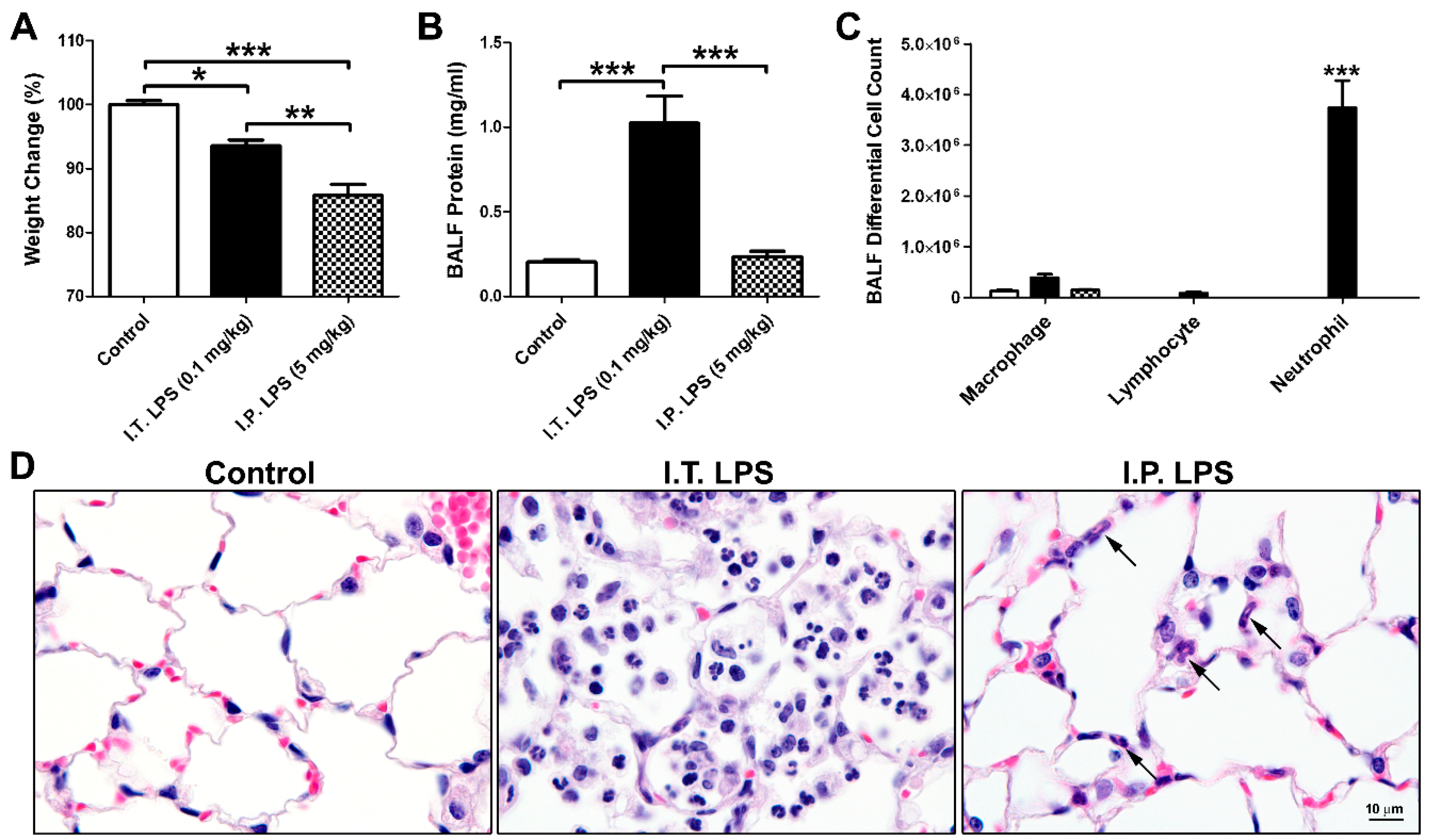
2.1. LPS-Induced Direct and Indirect Lung Injury in Mice
2.2. BALF Proteomic Analysis
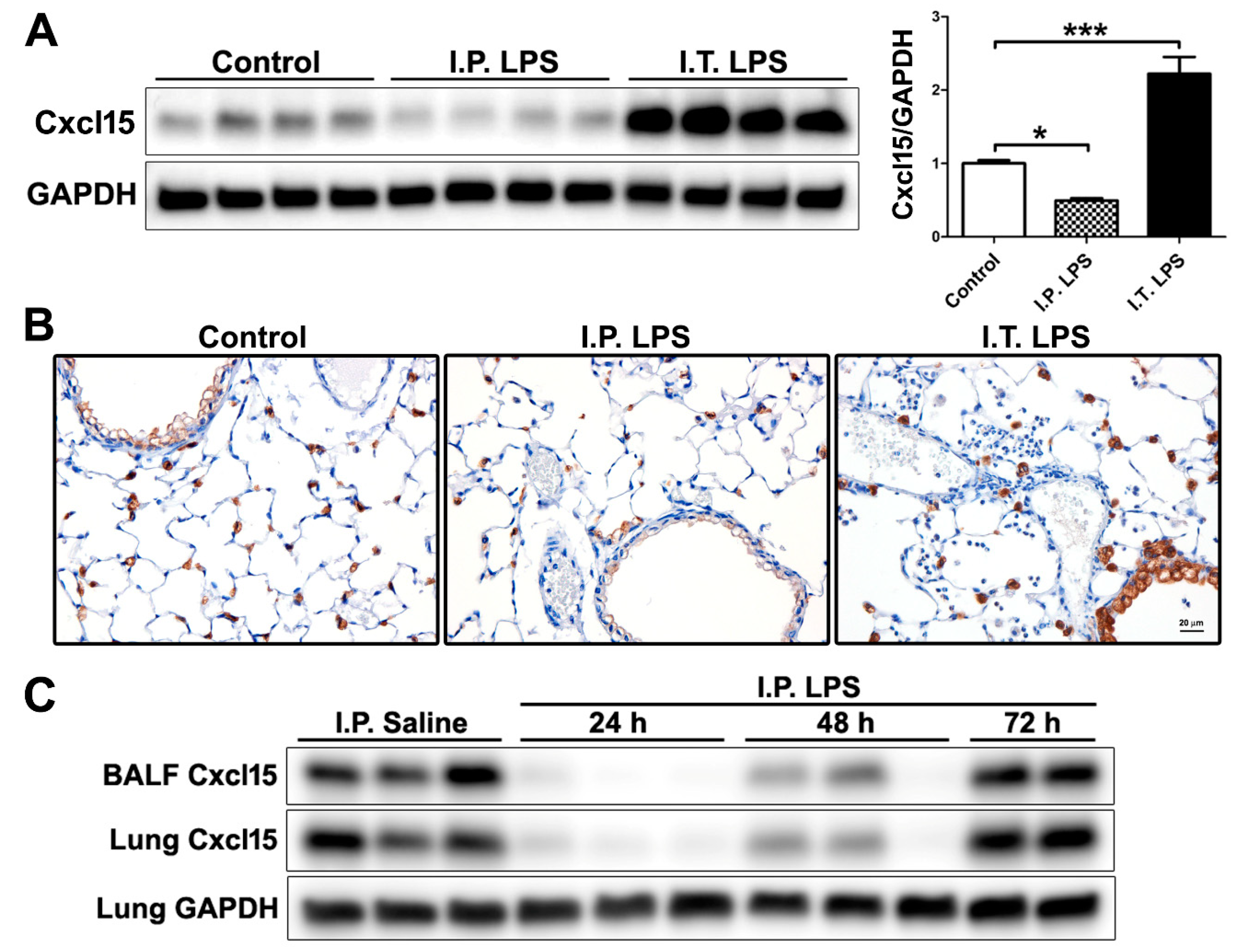
2.3. Differential Regulation of Cxcl15 by Direct and Indirect Lung Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. LPS Administration
4.3. Tissue Harvest, Collection and BALF Analysis
4.4. Protein Preparation and Discovery-Based Quantitative Shotgun Proteomics
4.5. Western Blotting
4.6. Immunohistochemistry
4.7. Statistical Analysis and Bioinformatics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ALI | Acute lung injury |
| Apo | Apolipoprotein |
| ARDS | Acute respiratory distress syndrome |
| BALF | Bronchoalveolar lavage fluid |
| Cadm1 | Cell adhesion molecule 1 |
| Clic5 | Chloride intracellular channel protein 5 |
| FA | Formic acid |
| FDR | False discovery rate |
| H&E | Hematoxylin and eosin |
| H2afz | Histone H2A.Z |
| IGF | Insulin-like growth factor |
| I.P. | Intraperitoneal |
| IPA | Ingenuity Pathway Analysis |
| LPS | Lipopolysaccharide |
| I.T. | Intratracheal |
| Ltf | Lactotransferrin |
| LXR/RXR | Liver X receptor/retinoid X receptor |
| NO | Nitric oxide |
| Pon1 | Paraoxonase 1 |
| PPBP | Pro-platelet basic protein |
| Retnla | Resistin-like alpha |
| ROS | Reactive oxygen species |
| Sftpb | Surfactant protein B |
| TMT | Tandem Mass Tag |
| Tnfrsf1B | Tumor necrosis factor receptor superfamily member 1B |
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; D’Onofrio, D.; Chiumello, D.; Paolo, S.; Chiara, G.; Capelozzi, V.L.; Barbas, C.S.; Chiaranda, M.; Gattinoni, L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur. Respir. J. Suppl. 2003, 42, 48s–56s. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Janz, D.R.; Bernard, G.R.; May, A.K.; Kangelaris, K.N.; Matthay, M.A.; Ware, L.B. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015, 147, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, K.; Fujitani, S.; Taira, Y.; Kushimoto, S.; Kitazawa, Y.; Okuchi, K.; Ishikura, H.; Sakamoto, T.; Tagami, T.; Yamaguchi, J.; et al. Difference in pulmonary permeability between indirect and direct acute respiratory distress syndrome assessed by the transpulmonary thermodilution technique: A prospective, observational, multi-institutional study. J. Intensive Care 2014, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann. Intensive Care 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Li, G.; Li, L.; Fu, L.; Yang, Y.; Overdier, K.H.; Douglas, I.S.; Linhardt, R.J. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J. Biol. Chem. 2014, 289, 8194–8202. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Laorden, M.I.; Lorente, J.A.; Flores, C.; Slutsky, A.S.; Villar, J. Biomarkers for the acute respiratory distress syndrome: How to make the diagnosis more precise. Ann. Transl. Med. 2017, 5, 283. [Google Scholar] [CrossRef]
- Norman, K.C.; Moore, B.B.; Arnold, K.B.; O’Dwyer, D.N. Proteomics: Clinical and research applications in respiratory diseases. Respirology 2018, 23, 993–1003. [Google Scholar] [CrossRef]
- Chang, D.W.; Hayashi, S.; Gharib, S.A.; Vaisar, T.; King, S.T.; Tsuchiya, M.; Ruzinski, J.T.; Park, D.R.; Matute-Bello, G.; Wurfel, M.M.; et al. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2008, 178, 701–709. [Google Scholar] [CrossRef]
- Ren, S.; Chen, X.; Jiang, L.; Zhu, B.; Jiang, Q.; Xi, X. Deleted in malignant brain tumors 1 protein is a potential biomarker of acute respiratory distress syndrome induced by pneumonia. Biochem. Biophys. Res. Commun. 2016, 478, 1344–1349. [Google Scholar] [CrossRef]
- Bhargava, M.; Becker, T.L.; Viken, K.J.; Jagtap, P.D.; Dey, S.; Steinbach, M.S.; Wu, B.; Kumar, V.; Bitterman, P.B.; Ingbar, D.H.; et al. Proteomic profiles in acute respiratory distress syndrome differentiates survivors from non-survivors. PLoS ONE 2014, 9, e109713. [Google Scholar] [CrossRef] [PubMed]
- Schnapp, L.M.; Donohoe, S.; Chen, J.; Sunde, D.A.; Kelly, P.M.; Ruzinski, J.; Martin, T.; Goodlett, D.R. Mining the acute respiratory distress syndrome proteome: Identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am. J. Pathol. 2006, 169, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.; Rietschel, E.T.; Ulmer, A.J. The biology of endotoxin. Mol. Biotechnol. 2001, 19, 279–296. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Aeffner, F.; Bolon, B.; Davis, I.C. Mouse Models of Acute Respiratory Distress Syndrome: A Review of Analytical Approaches, Pathologic Features, and Common Measurements. Toxicol. Pathol. 2015, 43, 1074–1092. [Google Scholar] [CrossRef]
- Rocco, P.R.; Nieman, G.F. ARDS: What experimental models have taught us. Intensive Care Med. 2016, 42, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. Acute Lung Injury in Animals Study, G., An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Ward, P.A.; Grailer, J.J. Acute lung injury and the role of histones. Transl. Respir. Med. 2014, 2, 1. [Google Scholar] [CrossRef]
- Bosmann, M.; Grailer, J.J.; Ruemmler, R.; Russkamp, N.F.; Zetoune, F.S.; Sarma, J.V.; Standiford, T.J.; Ward, P.A. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013, 27, 5010–5021. [Google Scholar] [CrossRef]
- Rossi, D.L.; Hurst, S.D.; Xu, Y.; Wang, W.; Menon, S.; Coffman, R.L.; Zlotnik, A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J. Immunol. 1999, 162, 5490–5497. [Google Scholar]
- de Torre, C.; Ying, S.X.; Munson, P.J.; Meduri, G.U.; Suffredini, A.F. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics 2006, 6, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shan, Q.; Jiang, L.; Zhu, B.; Xi, X. Quantitative proteomic analysis by iTRAQ for identification of candidate biomarkers in plasma from acute respiratory distress syndrome patients. Biochem. Biophys. Res. Commun. 2013, 441, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M.; van der Hoeven, J.G.; Netea, M.G.; Pickkers, P. Immunotherapy for the adjunctive treatment of sepsis: From immunosuppression to immunostimulation. Time for a paradigm change? Am. J. Respir. Crit. Care Med. 2013, 187, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- van Vught, L.A.; Wiewel, M.A.; Hoogendijk, A.J.; Frencken, J.F.; Scicluna, B.P.; Klein Klouwenberg, P.M.C.; Zwinderman, A.H.; Lutter, R.; Horn, J.; Schultz, M.J.; et al. The Host Response in Patients with Sepsis Developing Intensive Care Unit-acquired Secondary Infections. Am. J. Respir. Crit. Care Med. 2017, 196, 458–470. [Google Scholar] [CrossRef] [PubMed]
- van Vught, L.A.; Klein Klouwenberg, P.M.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nurnberg, P.; Bonten, M.J.; Cremer, O.L.; et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 2016, 315, 1469–1479. [Google Scholar] [CrossRef]
- Wu, A.; Hinds, C.J.; Thiemermann, C. High-density lipoproteins in sepsis and septic shock: Metabolism, actions, and therapeutic applications. Shock 2004, 21, 210–221. [Google Scholar] [CrossRef]
- Mackall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef]
- Unsinger, J.; Burnham, C.A.; McDonough, J.; Morre, M.; Prakash, P.S.; Caldwell, C.C.; Dunne, W.M., Jr.; Hotchkiss, R.S. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012, 206, 606–616. [Google Scholar] [CrossRef]
- Venet, F.; Foray, A.P.; Villars-Mechin, A.; Malcus, C.; Poitevin-Later, F.; Lepape, A.; Monneret, G. IL-7 restores lymphocyte functions in septic patients. J. Immunol. 2012, 189, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Mehrad, B.; Deng, J.C.; Vassileva, G.; Manfra, D.J.; Cook, D.N.; Wiekowski, M.T.; Zlotnik, A.; Standiford, T.J.; Lira, S.A. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J. Immunol. 2001, 166, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Caramori, G.; Gnemmi, I.; Contoli, M.; Bristot, L.; Capelli, A.; Ricciardolo, F.L.; Magno, F.; D’Anna, S.E.; Zanini, A.; et al. Association of increased CCL5 and CXCL7 chemokine expression with neutrophil activation in severe stable COPD. Thorax 2009, 64, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Rios, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, 11033. [Google Scholar] [CrossRef]
- Lu, J.; Auduong, L.; White, E.S.; Yue, X. Up-regulation of heparan sulfate 6-O-sulfation in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 106–114. [Google Scholar] [PubMed]
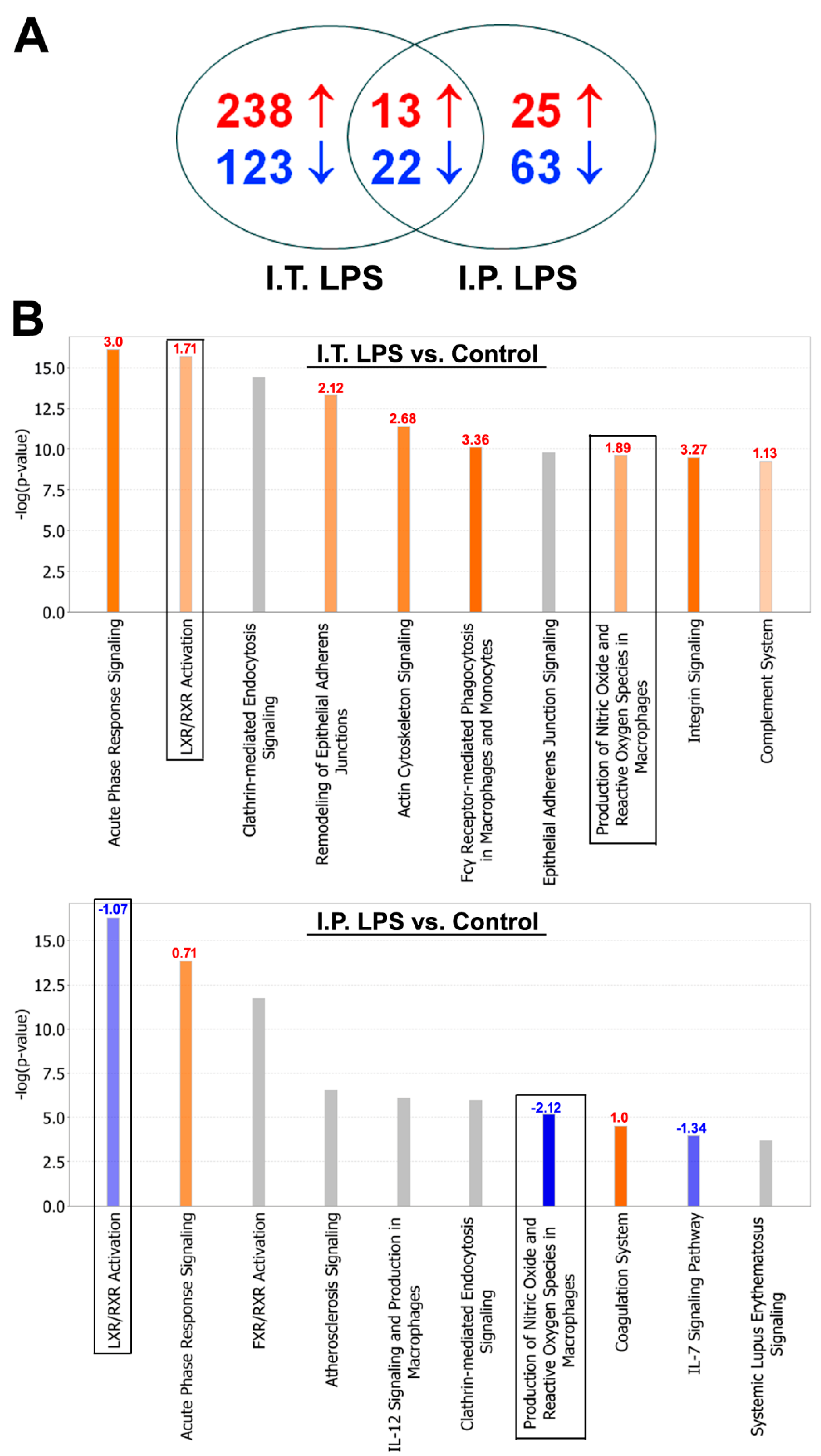
| Groups | Differentially Regulated Proteins |
|---|---|
| ↑ in Both (13) | Apcs, H2afz, Hba-a1, Hist2h2aa2, Hmgn2, Itih3, Lcat, Ltf, Nucks1, Retnla, Serpina3m, Serpina3n, Tmsb4x |
| ↓ in Both (22) | Anpep, Anxa5, AU021092, Cadm1, Cd200, Chia, Chmp5, Clic5, Cst3, Ctsc, Cyp2f2, Fam3c, Fgfr2, Il6st, Lyz1, Lyz2, Mme, Mup11, Npc2, Nrp1, Serpina7, Sftpb |
| ↑ in I.T. and ↓ in I.P. LPS (10) | Adpgk, Apoa4, Apoe, Cxcl15, Hist1h2bp, Ighm, Igkv6-17, Lcp1, Nap1l1, Pon1 |
| ↓ in I.T. and ↑ in I.P. LPS (4) | Gsta4, Pebp1, Tppp3, Vars |
| Pathways | I.T. LPS | I.P. LPS | ||
|---|---|---|---|---|
| z-Score | Differentially Regulated Proteins | z-Score | Differentially Regulated Proteins | |
| LXR/RXR Activation | 1.71 | Alb, ApoA1, ApoA2, ApoA4, ApoD, ApoE, C3, C4A/C4B, CD14, Clu, Fga, Gc, Il1rn, Itih4, Kng1, Lbp, Lcat, Lyz2, Mmp9, Pon1, S100A8, Tnfrsf1B | −1.07 | ApoA4, ApoE, Hpx, Il1rap, Lcat, Ldlr, Lpl, Lyz1, Pltp, Pon1, Rbp4, Saa1, Serpina1, Serpinf1, Ttr |
| NO & ROS Production in Macrophages | 1.89 | Alb, ApoA1, ApoA2, ApoA4, ApoD, ApoE, Clu, Fgfr2, Lyz2, Mapk14, Mpo, Ncf1, Ncf4, Pon1, Ppp1CA, Ppp2R1A, Ptpn6, Rhog, S100A8, Tnfrsf1B | −2.12 | ApoA4, ApoE, Fgfr2, Lyz1, Mark3, Pon1, Rbp4, Serpina1 |
| Up-Regulated by I.T. LPS | Down-Regulated by I.T. LPS |
|---|---|
| Histone H2A.Z (H2afz, ↑87.10) | Cell adhesion molecule 1 (Cadm1, ↓16.39) |
| Small nuclear ribonucleoprotein E (Snrpe, ↑55.57) | Eosinophil cationic protein 1 (Ear1, ↓15.15) |
| Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 (Nucks1, ↑44.40) | WAP four-disulfide core domain protein 2 (Wfdc2, ↓14.93) |
| Histone H3.1 (Hist1h3a, ↑37.41) | LIM zinc-binding domain-containing Nebulette (Nebl, ↓14.09) |
| Serine/threonine-protein kinase VRK1 (Vrk1, ↑30.05) | SEC14-like protein 2 (Sec14l2, ↓12.50) |
| Histone H2A type 2-A (Hist2h2aa1, ↑26.25) | Major urinary protein 18 (Mup18, ↓-10.99) |
| Inhibin beta A chain (Inhba, ↑23.52) | CD166 antigen (Alcam, ↓10.75) |
| Olfactomedin-4 (Olfm4, ↑21.75) | Lysozyme c-2 (Lyz2, ↓10.20) |
| Histone H3.3C (H3f3c, ↑21.04) | Cytochrome P450 2F2 (Cyp2f2, ↓9.80) |
| Histone H2B type 1-M (Hist1h2bm, ↑19.92) | Four and a half LIM domains protein 1 (Fhl1, ↓9.709) |
| Up-Regulated by I.P. LPS | Down-Regulated by I.P. LPS |
|---|---|
| Histone H2A.Z (H2afz, ↑15.03) | Lysozyme c-1 (Lyz1, ↓11.36) |
| Serum amyloid P-component (Apcs, ↑8.80) | Beta-mannosidase (Manba, ↓8.40) |
| Indolethylamine N-methyltransferase (Inmt, ↑8.30) | Thyroxine-binding globulin (Serpina7, ↓7.14) |
| Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 (Nucks1, ↑6.46) | Neuropilin-1 (Nrp1, ↓6.10) |
| Serum amyloid A-1 protein (Saa1, ↑6.13) | Calpain small subunit 1 (Capns1, ↓5.88) |
| Serine protease inhibitor A3n (Serpina3n, ↑5.62) | Ig heavy chain V region 5-84 (Ighv5-12, ↓5.75) |
| Resistin-like alpha (Retnla, ↑4.65) | Alpha-N-acetylgalactosaminidase (Naga, ↓5.41) |
| Thioredoxin-like protein 1 (Txnl1, ↑4.44) | Zinc transporter ZIP4 (Slc39a4, ↓-5.38) |
| Ig kappa chain V-VI region XRPC 44 (Igkv4-86, ↑4.21) | Complement C5 (C5, ↓5.38) |
| Acidic leucine-rich nuclear phosphoprotein 32 family member A (Anp32a, ↑4.08) | NPC intracellular cholesterol transporter 2 (Npc2, ↓5.35) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Guidry, J.J. Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice. Int. J. Mol. Sci. 2019, 20, 3401. https://doi.org/10.3390/ijms20143401
Yue X, Guidry JJ. Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice. International Journal of Molecular Sciences. 2019; 20(14):3401. https://doi.org/10.3390/ijms20143401
Chicago/Turabian StyleYue, Xinping, and Jessie J. Guidry. 2019. "Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice" International Journal of Molecular Sciences 20, no. 14: 3401. https://doi.org/10.3390/ijms20143401
APA StyleYue, X., & Guidry, J. J. (2019). Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice. International Journal of Molecular Sciences, 20(14), 3401. https://doi.org/10.3390/ijms20143401




