Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali
Abstract
1. Introduction
2. Results
2.1. Production of Transgenic Wheat TdPIP2;1 Plants
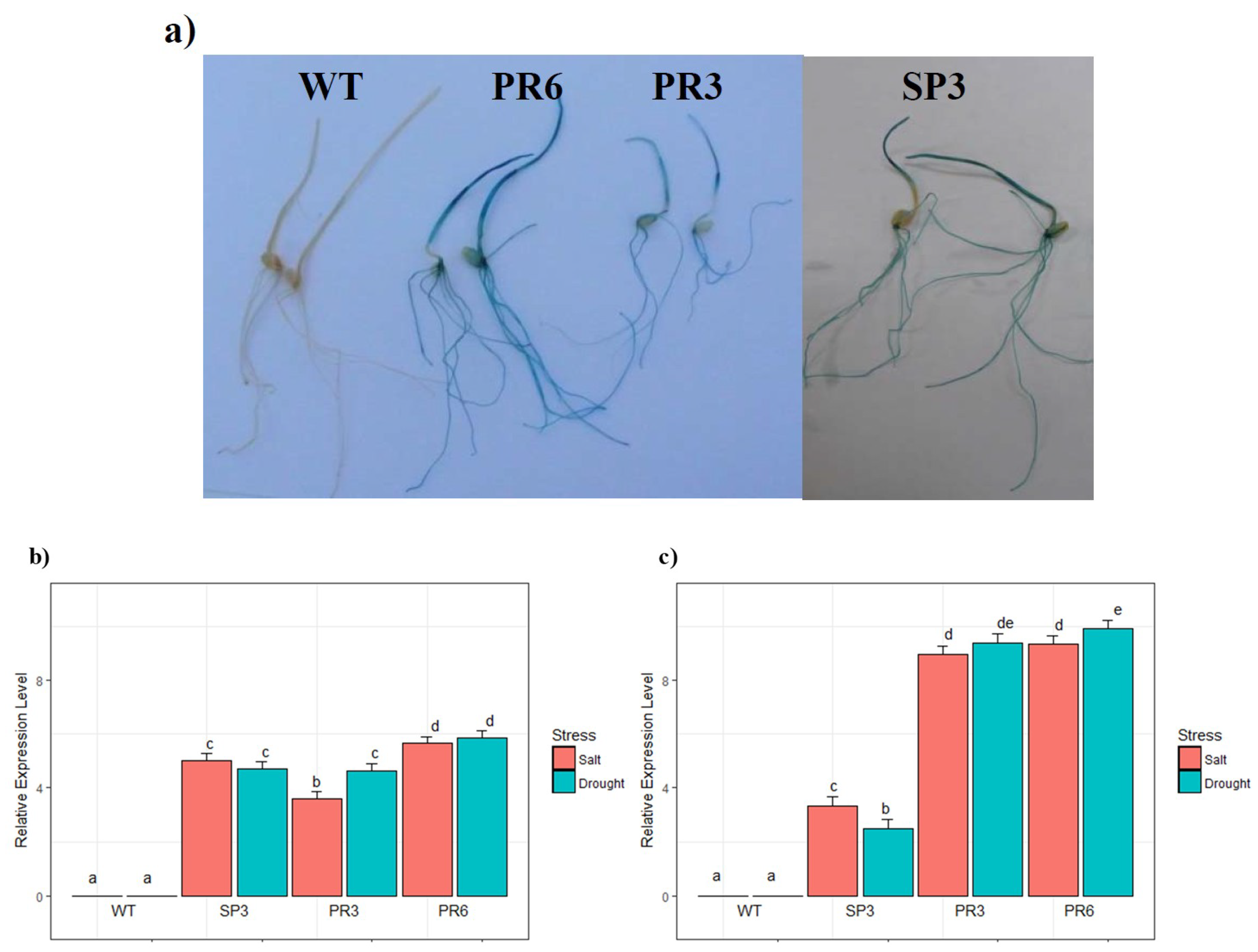
2.2. Analysis of GUS Activity in Relation to Stress Treatment
2.3. Evaluation of the Wheat TdPIP2;1 Gene for in Vitro Stress Tolerance
2.3.1. Effect of Salt Stress on Seed Germination
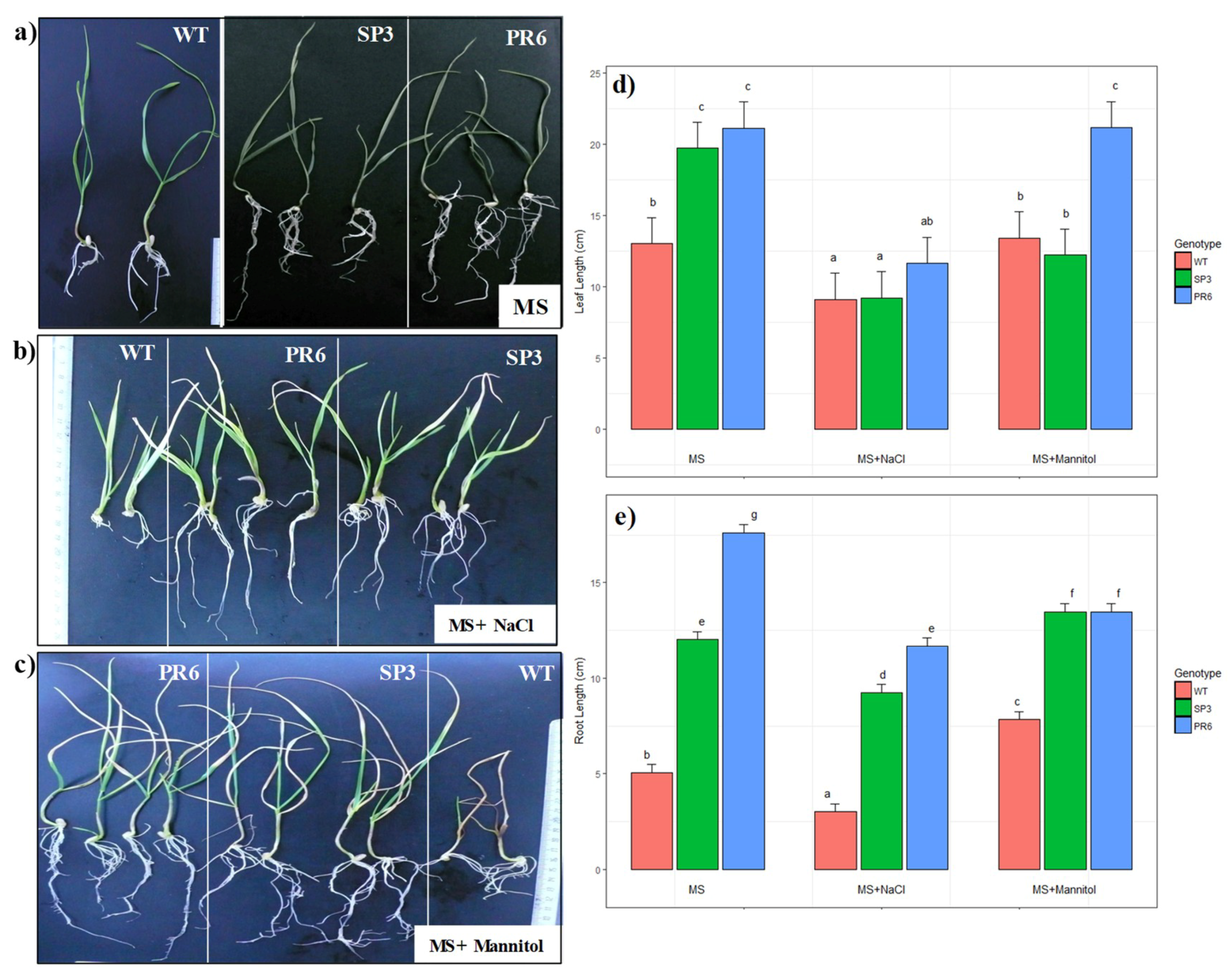
2.3.2. Effect of Salt and Osmotic Stresses on Root and Leaf Length
2.4. Evaluation of the Wheat TdPIP2;1 Gene for Stress Tolerance under Greenhouse Conditions
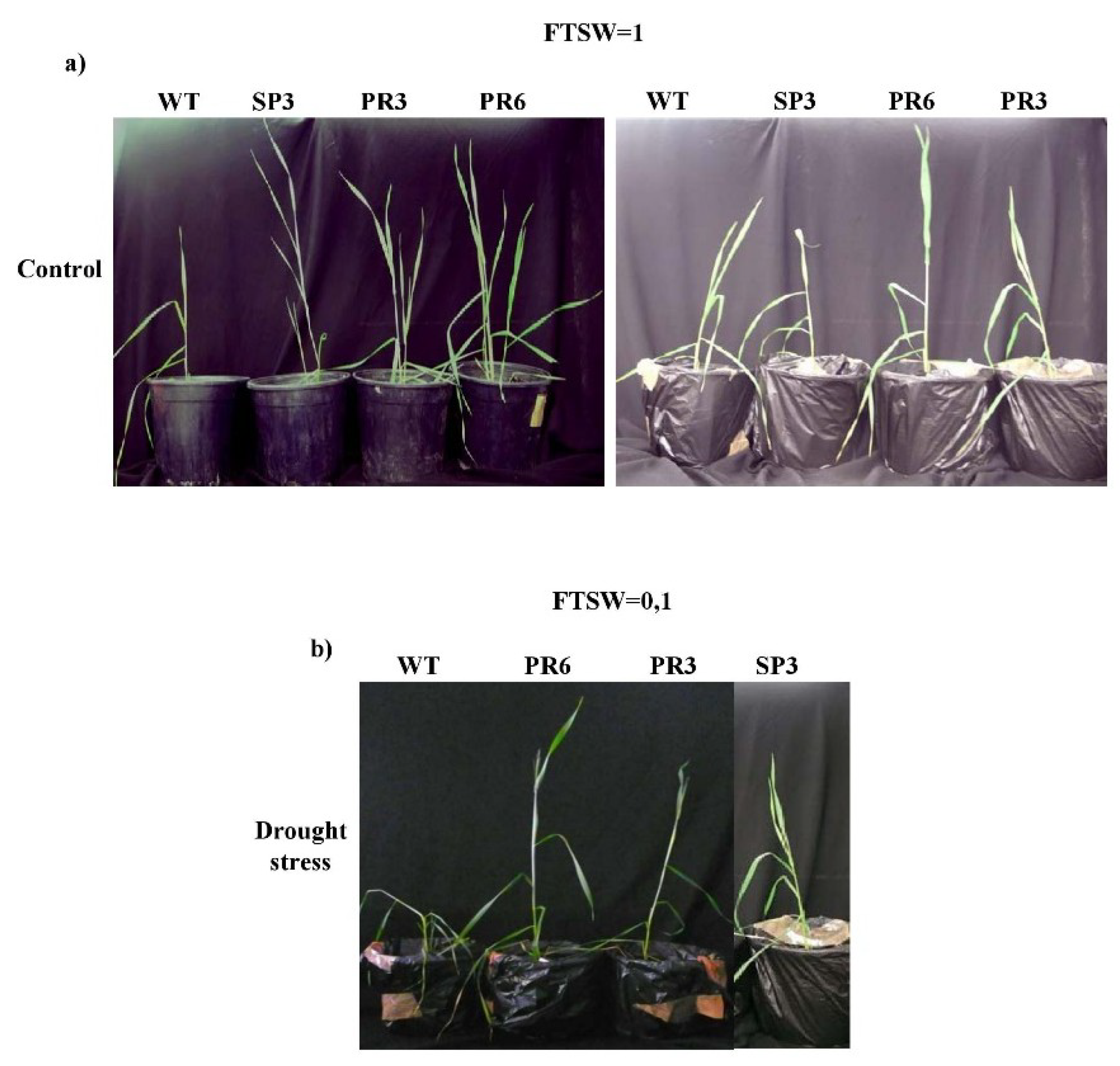
2.4.1. Fraction of Transpirable Soil Water (FTSW)
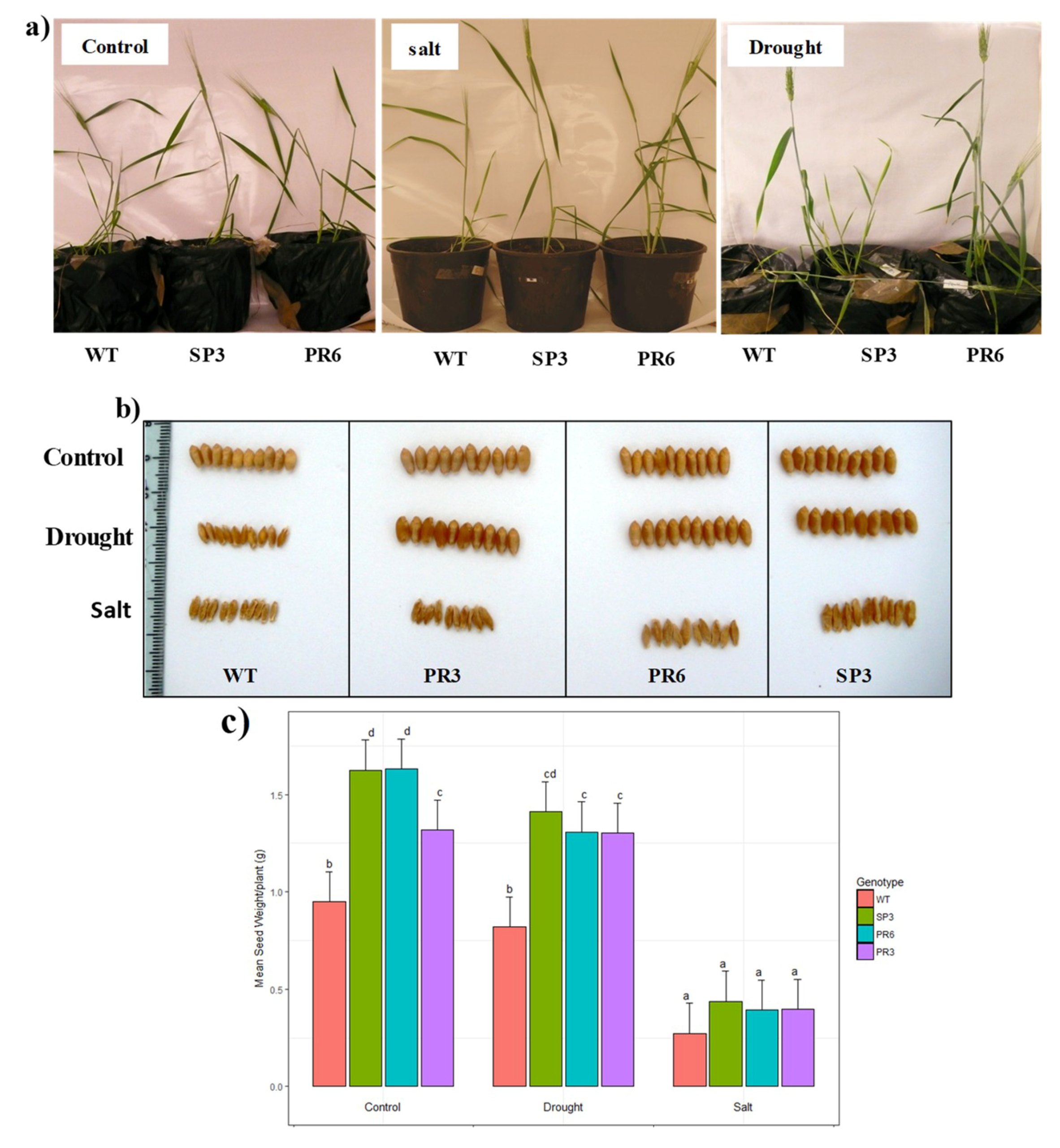
2.4.2. Effect on Biomass and Grain Production
2.5. Na+ and K+ Accumulation in Transgenic Wheat TdPIP2;1 Plants under Salt Stress Treatment
2.6. Oxidative Stress Evaluation of Transgenic Wheat TdPIP2;1 Plants
3. Discussion
4. Materials and methods
4.1. Plant Material
4.2. Construction of the Binary Vector and Wheat Transformation Procedure
4.3. Histochemical GUS Staining
4.4. GusA Expression Analysis by the Quantitative Real-Time qPCR
4.5. Evaluation of Transgenic Wheat Plants for Abiotic Stress Tolerance
4.5.1. In Vitro Assays
4.5.2. Greenhouse Assays
4.5.3. Water Treatments
4.5.4. Plant Transpiration Rate
4.6. Whole Plant Response Modelling to Increasing Water Deficit
y = 1;
else y = 1/FTSWt × FTSW;
y being NTR
4.7. Lipid Peroxidation
4.8. Quantitative H2O2 Measurement
4.9. Enzyme Assays
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant response to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Jubany-Mari, T.; Alegre, L. Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant Cell Environ. 2001, 24, 1319–1327. [Google Scholar] [CrossRef]
- Jubany-Mari, T.; Munne-Bosch, S.; Alegre, L. Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol. Biochem. 2010, 48, 351–358. [Google Scholar] [CrossRef]
- Kyparissis, A.; Drilias, P.; Manetas, Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Funct. Plant Biol. 2000, 27, 265–272. [Google Scholar] [CrossRef]
- Kyparissis, A.; Petropoulou, Y.; Manetas, Y. Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: Avoidance of photoinhibitory damage through decreased chlorophyll contents. J. Exp. Bot. 2000, 46, 1825–1831. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Aust. J. Plant Physiol. 1999, 26, 569–578. [Google Scholar] [CrossRef]
- Beck, P.A.; Hutchison, S.; Gunter, A.S.; Losi, C.T.; Stewart, B.C.; Capps, K.P.; Phillips, J.M. Chemical composition and in situ dry matter and fiber disappearance of sorghum x Sudangrass hybrids. J. Anim. Sci. 2007, 85, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Pelleschi, S.; Rocher, J.P.; Prioul, J.L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M.; Ricardo, C.P. Alterations in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus albus L. J. Exp. Bot. 2001, 52, 1063–1070. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Remobilization of carbon reserves in response to water-deficit during grain filling of rice. Field Crops Res. 2001, 71, 47–55. [Google Scholar] [CrossRef]
- Toumi, I.; Gargouri, M.; Nouairi, I.; Moschou, P.; Salem-Fnayou, A.; Mliki, A.; Zarrouk, M.; Ghorbel, A. Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol. Plant. 2008, 52, 161–164. [Google Scholar] [CrossRef]
- Passioura, J. Increasing crop productivity when water is scarce- from breeding to field management. In Proceedings of the 4th International Crop Science Congress: New Directions for a Diverse Planet, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef]
- Pandey, B.; Sharma, P.; Pandey, D.M.; Sharma, I.; Chatrath, R. Identification of New Aquaporin Genes and Single Nucleotide Polymorphism in Bread Wheat. Evol. Bioinform. 2013, 9, 437–452. [Google Scholar] [CrossRef]
- Zhuang, L.; Liu, M.; Yuan, X.; Yang, Z.; Huang, B. Physiological Effects of Aquaporin in Regulating Drought Tolerance through Overexpressing of Festuca arundinacea Aquaporin Gene FaPIP2;1. J. Am. Soc. Hort. Sci. 2015, 140, 404–412. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Kei, L.; Uehlein, N. Aquaporin and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta-Gen. Subj. 2013, 1840, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta- Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Ann. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Fetter, K.; van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane Aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Moshelion, M.; Daniels, M.J. Regulation of plant aquaporin activity. Biol. Cell 2005, 97, 749–764. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Javot, H.; Lauvergeat, V.; Gerbeau, P.; Tournaire, C.; Santoni, V. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. 2002, 215, 105–148. [Google Scholar]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Zelazny, E.; Chaumont, F. Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1142–1156. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.H.; Chen, Z.L.; Qu, L.J.; Gu, H.Y. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Kitagawa, Y. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Overexpression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef]
- Cui, X.H.; Hao, F.S.; Chen, H.; Chen, J.; Wang, X.C. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves drought resistance. J. Plant Res. 2003, 121, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Alavilli, H.; Awasthi, J.P.; Rout, G.R.; Sahoo, L.; Lee, B.H.; Panda, S.K. Overexpression of a Barley Aquaporin gene, HvPIP2;5 confers salt and osmotic stress tolerance in yeast and plants. Front. Plant Sci. 2016, 7, 1566. [Google Scholar] [CrossRef]
- Sreedharm, S.; Shekhawat, U.K.; Gamapathi, T.R. Transgenic Banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 2013, 8, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Wang, T.; Guo, Z.; Lu, S. Overexpression of MfPIP2-7 from Medicago falcata promotes cold tolerance and growth under NO−3 deficiency in transgenic tobacco plants. BMC Plant Biol. 2016, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, M.; Zhou, L.; Quan, T.; Xia, G. Heterologous expression of the wheat Aquaporin gene TaPIP2;2 compromises the abiotic stress tolerance of Arabidopsis thaliana. PLoS ONE 2013, 8, e79618. [Google Scholar]
- Aharon, R.; Shahak, Y.; Winniger, S.; Bendov, R.; Kapulink, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigour under favourable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Cavez, D.; Miled, N.; Chaumont, F.; Masmoudi, K. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. subsp. durum) and their role in abiotic stress tolerance. Plant Physiol. Biochem. 2011, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Mieulet, D.; Fabre, D.; Verdeil, J.L.; Vernet, A.; Guiderdoni, E.; Masmoudi, K. Functional analysis of the durum wheat gene TdPIP2; 1 and its promoter region in response to abiotic stress in rice. Plant Physiol. Biochem. 2014, 79, 98–108. [Google Scholar] [CrossRef]
- Gago, J.; Grima-Pettenati, J.; Gallego, P.P. Vascular-specific expression of GUS and GFP reporter genes in transgenic grapevine (Vitis vinifera L. cv. Albariño) conferred by the EgCCR promoter of Eucalyptus gunnii. Plant Physiol. Biochem. 2011, 49, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Galle, A.; Flexas, J.; Lerner, S.; Peleg, G.; Yaaran, A.; Moshelion, M. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 2013, 239, 357–366. [Google Scholar] [CrossRef]
- Lu, L.; Dong, C.; Liu, R.; Zhou, B.; Wang, C.; Shou, H. Roles of soybean plasma membrane intrinsic protein GmPIP2;9 in drought tolerance and seed development. Front. Plant Sci. 2018, 9, 530. [Google Scholar] [CrossRef]
- Forrest, K.L.; Bhave, M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct. Integr. Genom. 2008, 8, 115e133. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 2003, 26, 845–856. [Google Scholar] [CrossRef]
- Deng, L.; Chen, F.; Jiang, L.; Lam, H.M.; Xiao, G. Ectopic expression of GmPAP3 enhances salt tolerance in rice by alleviating oxidative damage. Plant Breed. 2014, 133, 348–355. [Google Scholar] [CrossRef]
- Hernández, I.; Alegrel, L.; Munné-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef]
- Ben Amor, N.; Ben Hamed, K.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedling. Biol. Plant. 2006, 227, 227–231. [Google Scholar] [CrossRef]
- Koca, H.; Bor, M.; Ozdemir, F.; Turkan, I. Effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munne’-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.E.; Gelvin, S.B.; Melchers, S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plant. Transgenic Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Chen, H.; Nelson, R.S.; Sherwood, J.L. Enhanced recovery of transformants of Agrobacterium tumifaciens after freeze–thaw transformation and drug selection. Biotechniques 1994, 16, 664–668. [Google Scholar] [PubMed]
- Supartana, P.; Shimizu, T.; Nogawa, M.; Shioiri, H.; Nakajima, T.; Haramoto, N.; Nozue, M.; Kojima, M. Development of simple and efficient in-planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J. Biosci Bioeng. 2006, 102, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, D.J.; Nester, E.W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J. Bacteriol. 1980, 144, 732–774. [Google Scholar] [PubMed]
- Ohira, K.; Ojima, K.; Fujiwara, A. Studies on the nutrition of rice cell culture 1: A simple defined medium for rapid growth in suspension culture. Plant Cell Physiol. 1973, 14, 1113–1121. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 DDCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Zwieniecki, M.A.; Holbrook, N.M. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiol. Plant. 2008, 132, 446–451. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ludlow, M.M. Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust. J. Plant Physiol. 1986, 13, 329–341. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant system in acid rain treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Starr, D.; Su, X.; Tang, G.; Chen, Z.; Carter, J.; Wittich, P.E.; Dong, S.; Green, J.; Burch, E.; et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 2019, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]

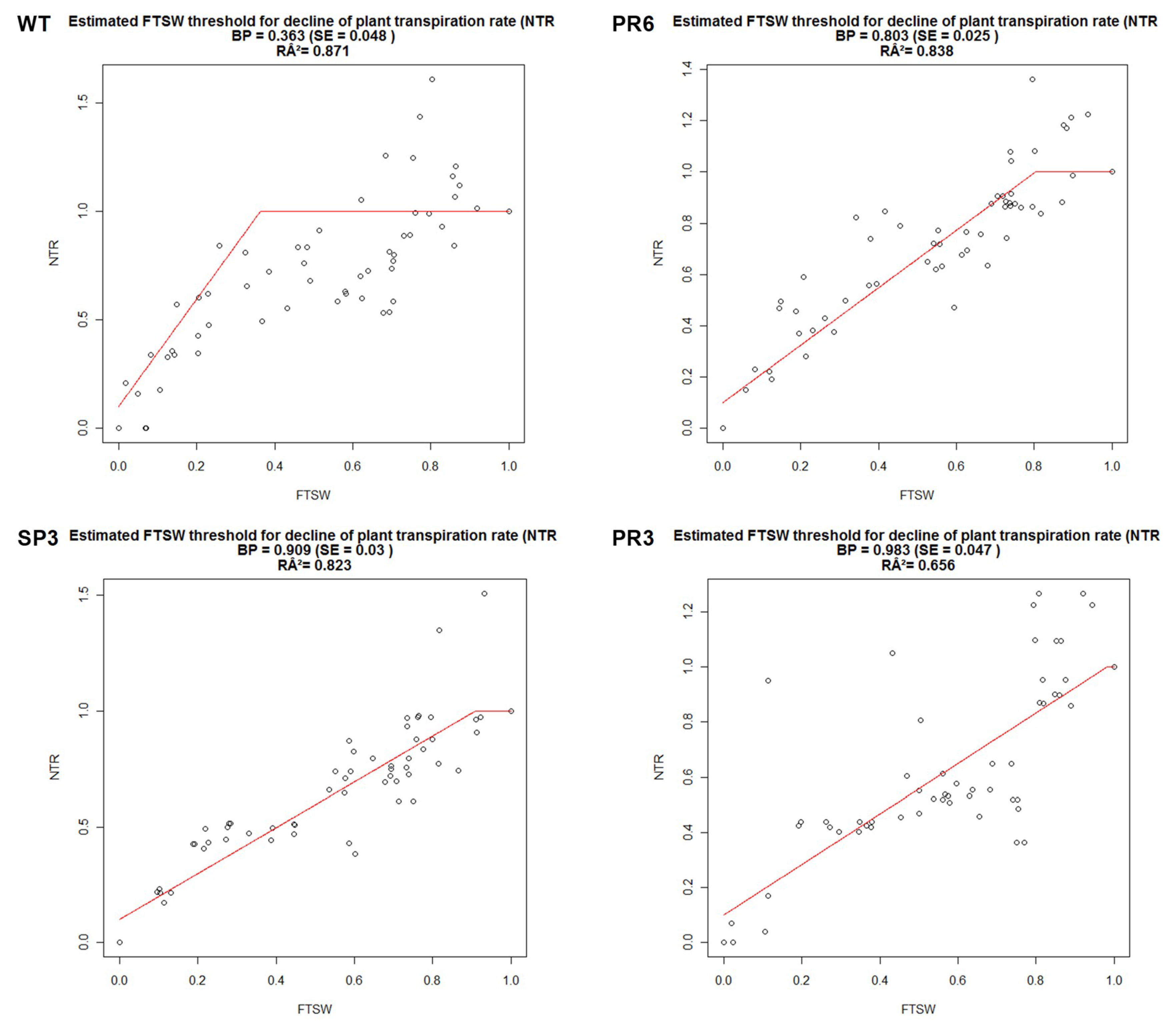


| BP | (R2) | |
|---|---|---|
| WT | 0.363 | 0.871 |
| SP3 | 0.909 | 0.823 |
| PR3 | 0.983 | 0.656 |
| PR6 | 0.803 | 0.838 |
| Primers | Nucleotides Sequences (5′-3′) |
|---|---|
| Act F: | TGCATAGAGGGAAAGCACG |
| Act R: | AACCCAAAAGCCAACAACAGAGA |
| q-GusF: | CACGCCGTATGTTATTGCCG |
| q-GusR: | TCTTGCCGTTTTCGTGGGTA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayadi, M.; Brini, F.; Masmoudi, K. Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali. Int. J. Mol. Sci. 2019, 20, 2389. https://doi.org/10.3390/ijms20102389
Ayadi M, Brini F, Masmoudi K. Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali. International Journal of Molecular Sciences. 2019; 20(10):2389. https://doi.org/10.3390/ijms20102389
Chicago/Turabian StyleAyadi, Malika, Faiçal Brini, and Khaled Masmoudi. 2019. "Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali" International Journal of Molecular Sciences 20, no. 10: 2389. https://doi.org/10.3390/ijms20102389
APA StyleAyadi, M., Brini, F., & Masmoudi, K. (2019). Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali. International Journal of Molecular Sciences, 20(10), 2389. https://doi.org/10.3390/ijms20102389






