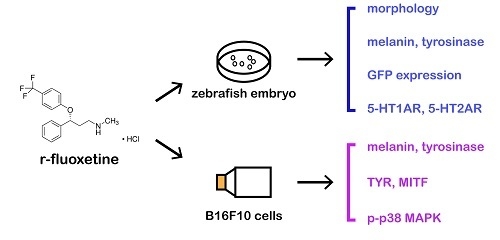
R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways
Abstract
1. Introduction
2. Results
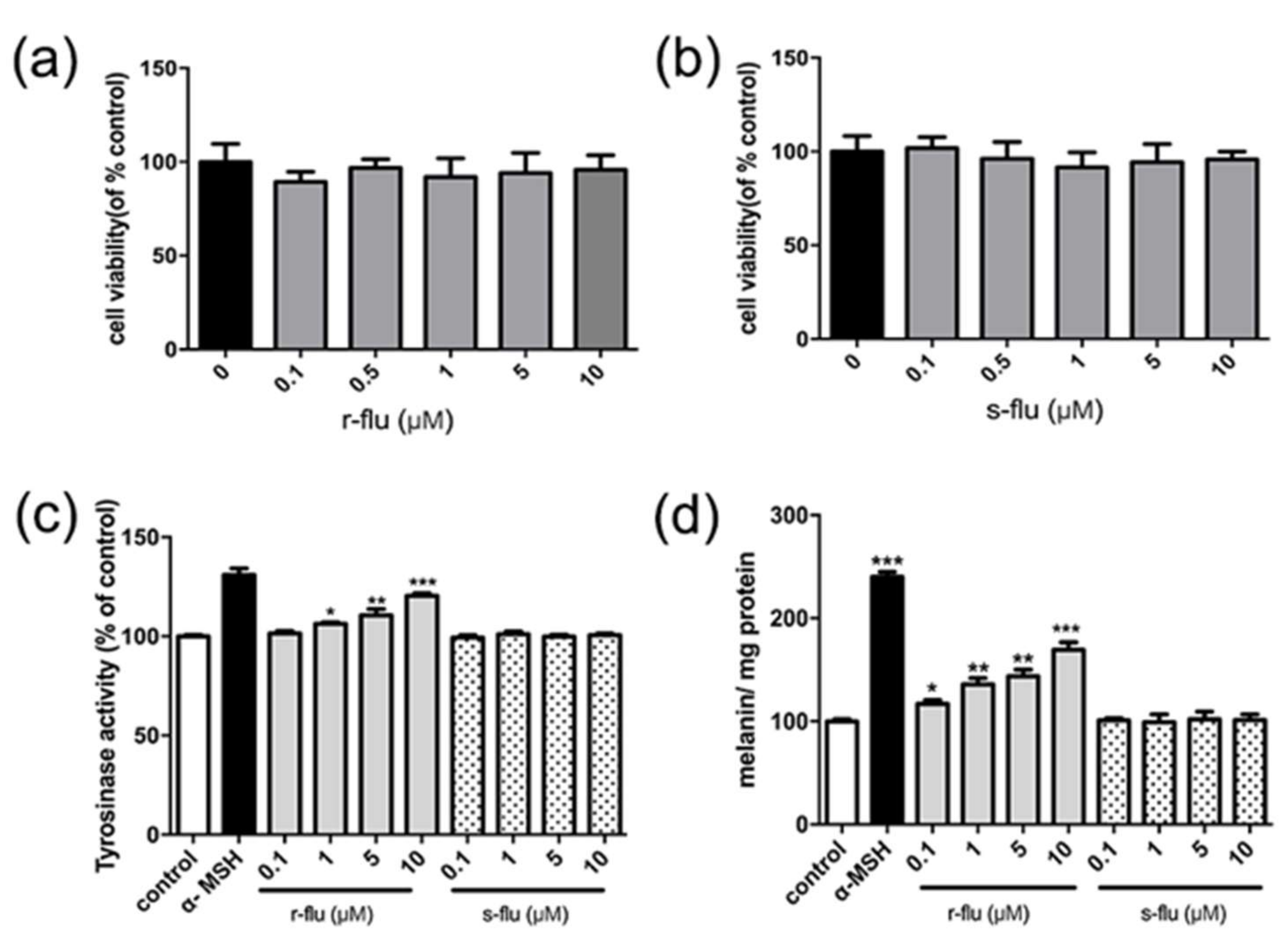
2.1. Effect of R/S-Fluoxetine on Tyrosinase Activity and Melanin Synthesis in B16F10 Cells
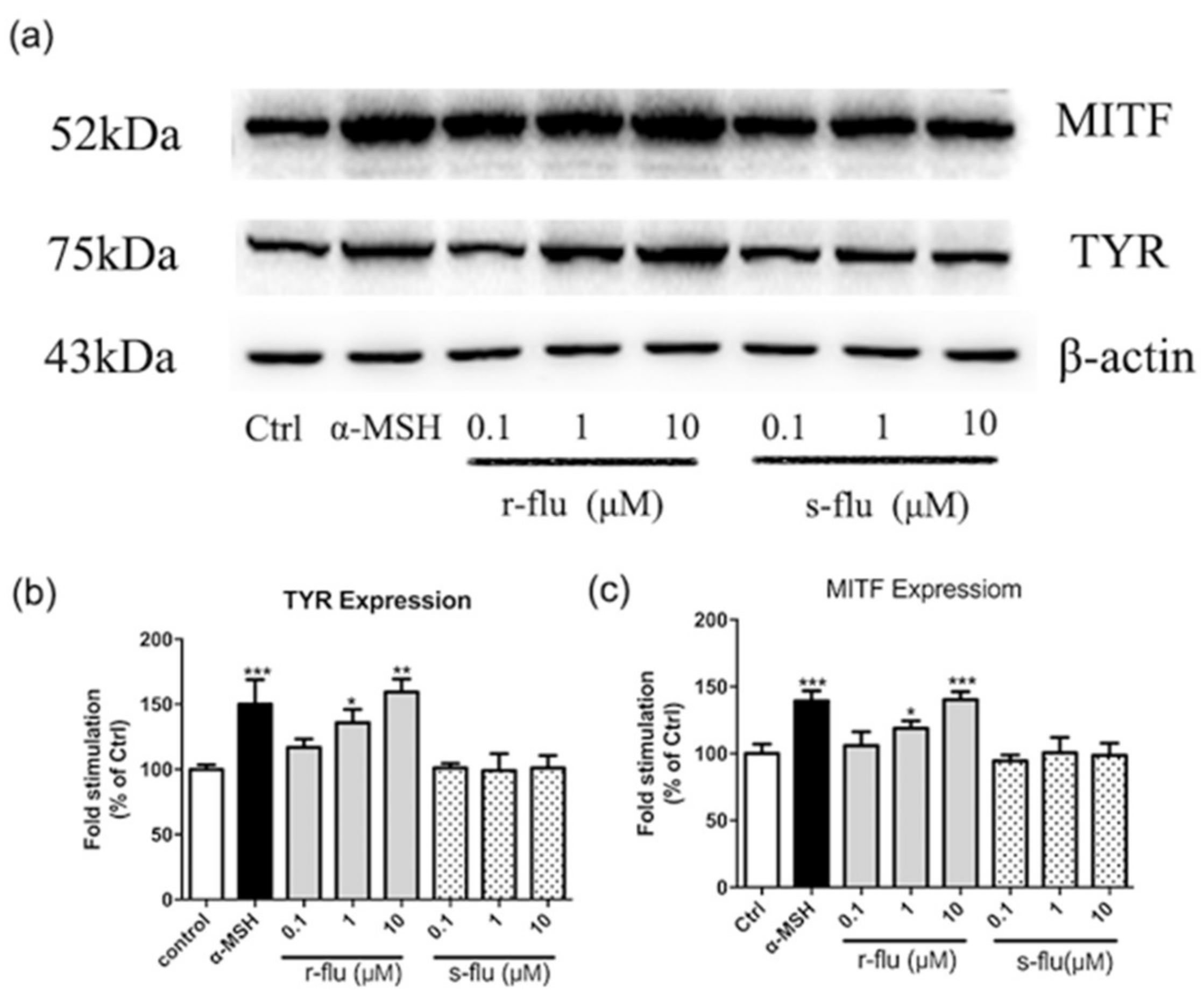
2.2. Effect of R/S-Fluoxetine on MITF and Tyrosinase Protein Expression in B16F10 Cells
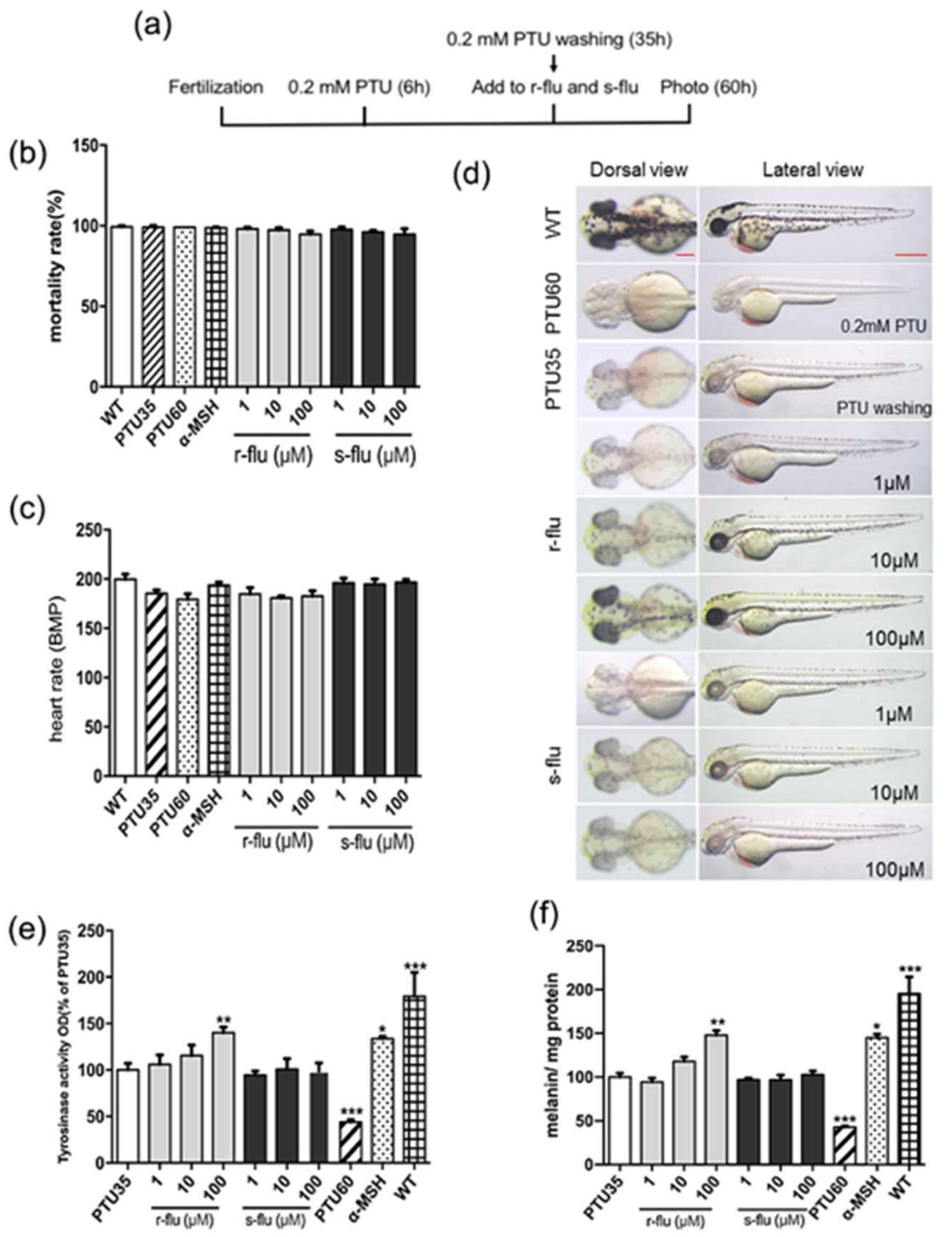
2.3. Effect of R/S-Fluoxetine on Tyrosinase Activity and Melanin Synthesis in Zebrafish
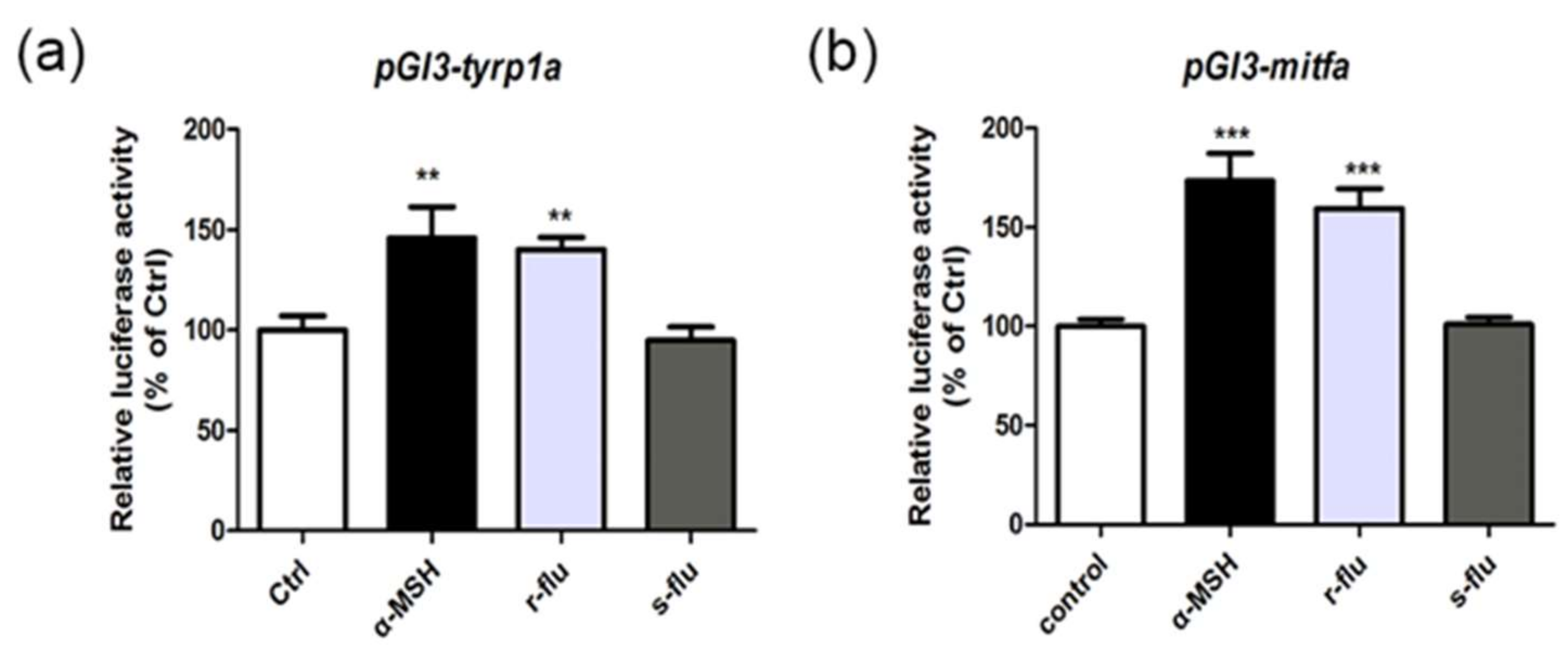
2.4. Effect of R/S-Fluoxetine on the Promoter Activities of Mitfa and Tyrp1a in the Zebrafish
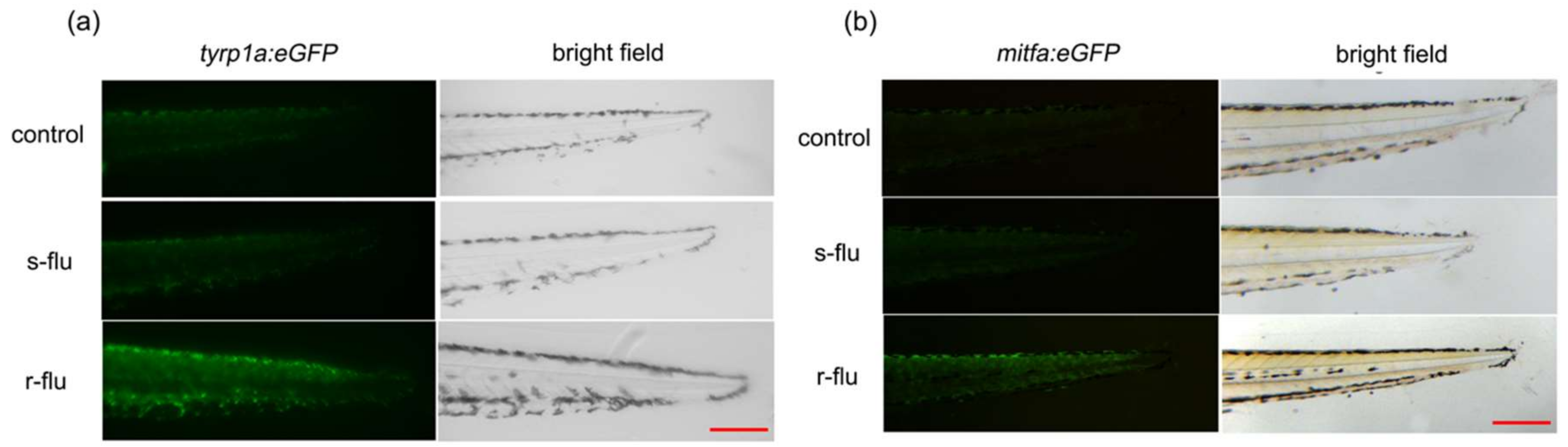
2.5. Effect of R/S-Fluoxetine on the GFP (Green Fluorescent Protein) Expression in the Tyrp1a:eGFP and Mitfa:eGFP Zebrafish
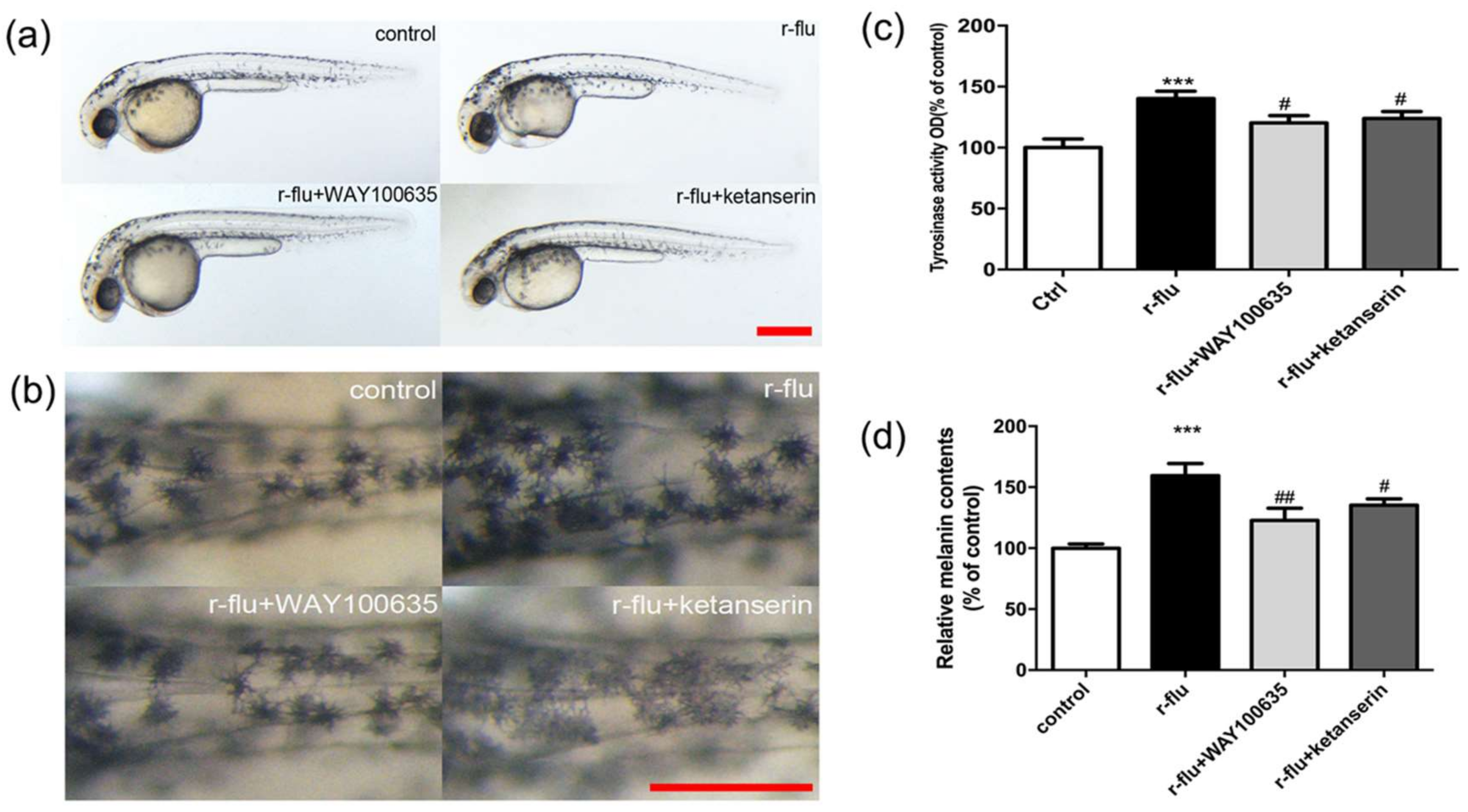
2.6. R-Fluoxetione Induce Melanin Synthesis through 5-HT1A Receptor and 5-HT2A Receptor
2.7. Effect of the R-Fluoxetine on Phosphorylation of the p38 MAPK, ERK1/2, and JNK in the B16F10 Cells
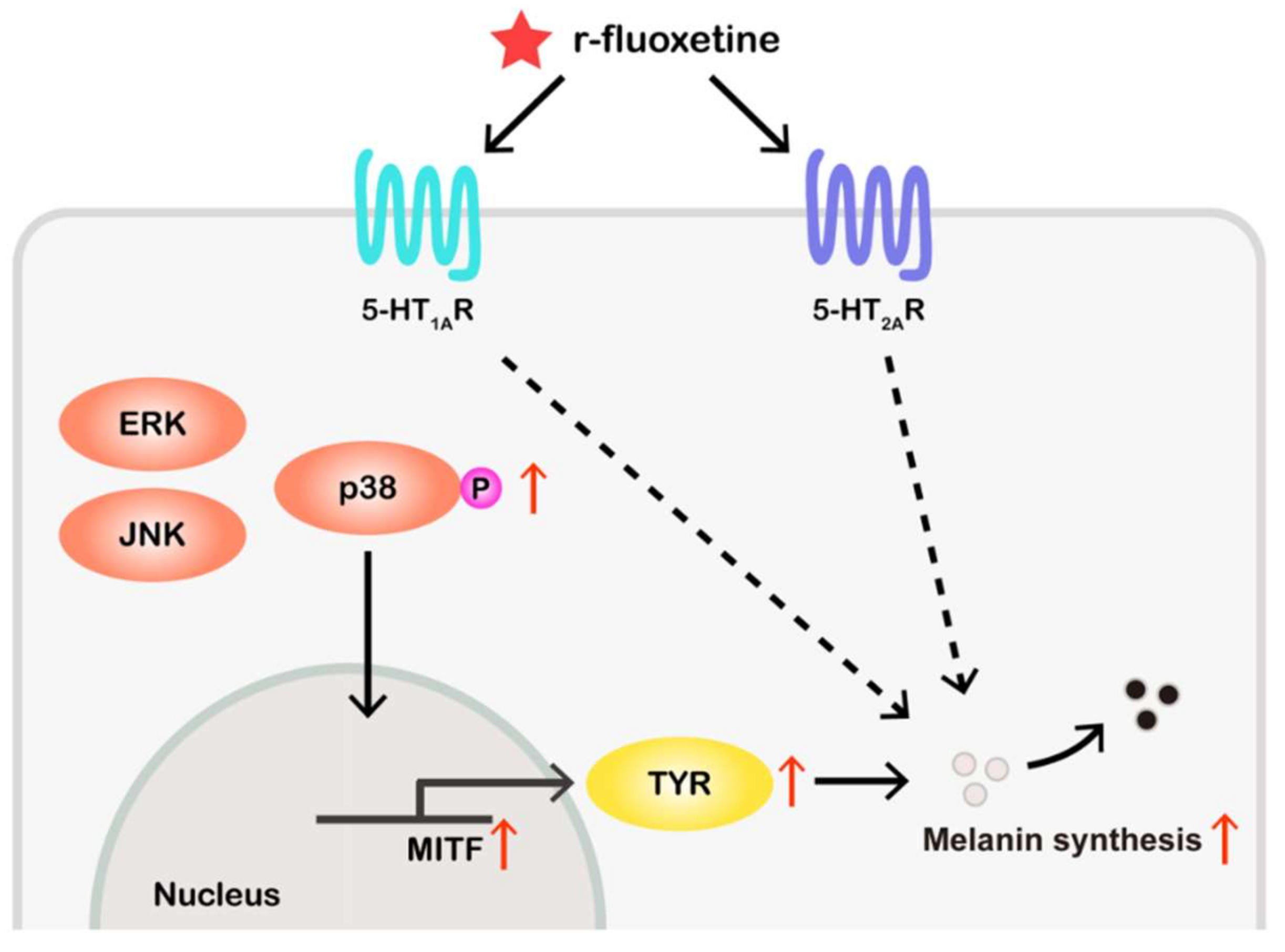
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Materials
4.2. Cell Viability Assay
4.3. Melanin Measurement and Tyrosinase Assay
4.4. Western Blot Analysis
4.5. Zebrafish Maintenance
4.6. Measurement of the Pigmenting Activity in the Zebrafish
4.7. The Luciferase Assay
4.8. Measurement of the Heart-Beating Rate and the Mortality Rate
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TYR | tyrosinase |
| MITF | microphthalmia-associated transcription factor |
| 5-HT1AR | serotonin1A receptor |
| 5-HT2AR | serotonin 2A receptor |
| PTU | 1-phenyl-2-thiourea |
| PTU35 | 1-phenyl-2-thiourea 35 hpf |
| PTU60 | 1-phenyl-2-thiourea 60 hpf |
| MAPK | mitogen-activated protein kinase |
| TBS | tris- buffered saline |
| V/V | volume/volume |
| DMSO | dimethyl sulfoxide |
| hpf | hours post-fertilization |
| MTT | 3-(4, 5-dimethylthiazol)-2, 5-diphenyl tetrazolium bromide |
References
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis--controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, K.; Kobayashi, Y.; Sunuma, T.; Asahida, T.; Saito, Y.; Takahashi, A. Inhibiting roles of melanin-concentrating hormone for skin pigment dispersion in barfin flounder, Verasper moseri. Gen. Comp. Endocrinol. 2011, 171, 75–81. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Yu, Z.L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional Analysis of Microphthalmia-associated Transcription Factor in Pigment Cell-specific Transcription of the Human Tyrosinase Family Genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef]
- Tachibana, M. Cochlear melanocytes and MITF signaling. J. Investig. Dermatol. Sympos. Proc. 2001, 6, 95–98. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Den Hertog, J. Chemical genetics: Drug screens in Zebrafish. Biosci. Rep. 2005, 25, 289–297. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Serotoninergic system in hamster skin. J. Investig. Dermatol. 2002, 119, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.J.; Kauser, S.; Wortsman, J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 2003, 196, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Costa, E.; Guidotti, A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc. Natl. Acad. Sci. USA 2004, 101, 6222–6225. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.E.; Schmidt, M.E.; Hennen, J.; Villafuerte, R.A.; Butman, M.L.; Tran, P.; Kerner, L.T.; Cohen, B.; Renshaw, P.F. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: A 19-F MRS study. Neuropsychopharmacology 2005, 30, 1576–1583. [Google Scholar] [CrossRef]
- Liao, S.; Shang, J.; Tian, X.; Fan, X.; Shi, X.; Pei, S.; Wang, Q.; Yu, B. Up-regulation of melanin synthesis by the antidepressant fluoxetine. Exp. Dermatol. 2012, 21, 635–637. [Google Scholar] [CrossRef]
- Wong, D.T.; Bymaster, F.P.; Engleman, E.A. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sci. 1995, 57, 411–441. [Google Scholar] [CrossRef]
- Warnock, J.K.; Morris, D.W. Adverse cutaneous reactions to antidepressants. Am. J. Clin. Dermatol. 2002, 3, 329–339. [Google Scholar] [CrossRef]
- Koch, S.; Perry, K.W.; Nelson, D.L.; Conway, R.G.; Threlkeld, P.G.; Bymaster, F.P. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: An in vivo microdialysis and receptor binding study. Neuropsychopharmacology 2002, 27, 949–959. [Google Scholar] [CrossRef]
- Robertson, D.W.; Krushinski, J.H.; Fuller, R.W.; Leander, J.D. Absolute configurations and pharmacological activities of the optical isomers of fluoxetine, a selective serotonin-uptake inhibitor. J. Med. Chem. 1988, 31, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Michael Lemming, O.; Bang Hedegaard, K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int. Clin. Psychopharmacol. 2002, 17, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.; Vigneri, S.; Savarino, V.; Scarpignato, C. Clinical pharmacology and safety profile of esomeprazole, the first enantiomerically pure proton pump inhibitor. Digest. Liver Dis. 2001, 33, 600–606. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeong, Y.M.; Park, I.K.; Hahn, H.G.; Lee, H.K.; Kwon, S.B.; Jeong, J.H.; Yang, S.J.; Sohn, U.D.; Park, K.C. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse B16 melanoma cells. Biol. Ppharm. Bull. 2007, 30, 180–183. [Google Scholar] [CrossRef]
- Fang, D.; Tsuji, Y.; Setaluri, V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002, 30, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Moellmann, G.; Kuklinska, E. l-tyrosine, l-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in Bomirski Ab amelanotic melanoma cells. Pigment Cell Res. 1989, 2, 109–116. [Google Scholar] [CrossRef]
- Soboleski, M.R.; Oaks, J.; Halford, W.P. Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB J. 2005, 19, 440–442. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Liang, F.; Li, S.; Zhong, H.; Lin, S. Characterization of two novel small molecules targeting melanocyte development in zebrafish embryogenesis. Pigment Cell Melanoma Res. 2012, 25, 446–453. [Google Scholar] [CrossRef]
- Zou, J.; Beermann, F.; Wang, J.; Kawakami, K.; Wei, X. The Fugu tyrp1 promoter directs specific GFP expression in zebrafish: Tools to study the RPE and the neural crest-derived melanophores. Pigment Cell Res. 2006, 19, 615–627. [Google Scholar] [CrossRef]
- Curran, K.; Raible, D.W.; Lister, J.A. Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev. Biol. 2009, 332, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, M.K.; Kim, S.Y.; Park Choo, H.Y.; Lee, A.Y.; Lee, C.H. Serotonin induces melanogenesis via serotonin receptor 2A. Br. J. Dermatol. 2011, 165, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Zhou, J.; Song, J.; Ping, F.; Shang, J. Enhancement of the p38 MAPK and PKA signaling pathways is associated with the pro-melanogenic activity of Interleukin 33 in primary melanocytes. J. Dermatol. Sci. 2014, 73, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shang, J.; Song, J.; Ping, F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-κB pathway. Int. J. Biochem. Cell Biol. 2013, 45, 308–316. [Google Scholar] [CrossRef]
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dynamics 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Fu, M.; Pei, S.; Zhou, L.; Shang, J. R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 80. https://doi.org/10.3390/ijms20010080
Liu L, Fu M, Pei S, Zhou L, Shang J. R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways. International Journal of Molecular Sciences. 2019; 20(1):80. https://doi.org/10.3390/ijms20010080
Chicago/Turabian StyleLiu, Li, Mengsi Fu, Siran Pei, Liangliang Zhou, and Jing Shang. 2019. "R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways" International Journal of Molecular Sciences 20, no. 1: 80. https://doi.org/10.3390/ijms20010080
APA StyleLiu, L., Fu, M., Pei, S., Zhou, L., & Shang, J. (2019). R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways. International Journal of Molecular Sciences, 20(1), 80. https://doi.org/10.3390/ijms20010080