Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease
Abstract
1. Introduction
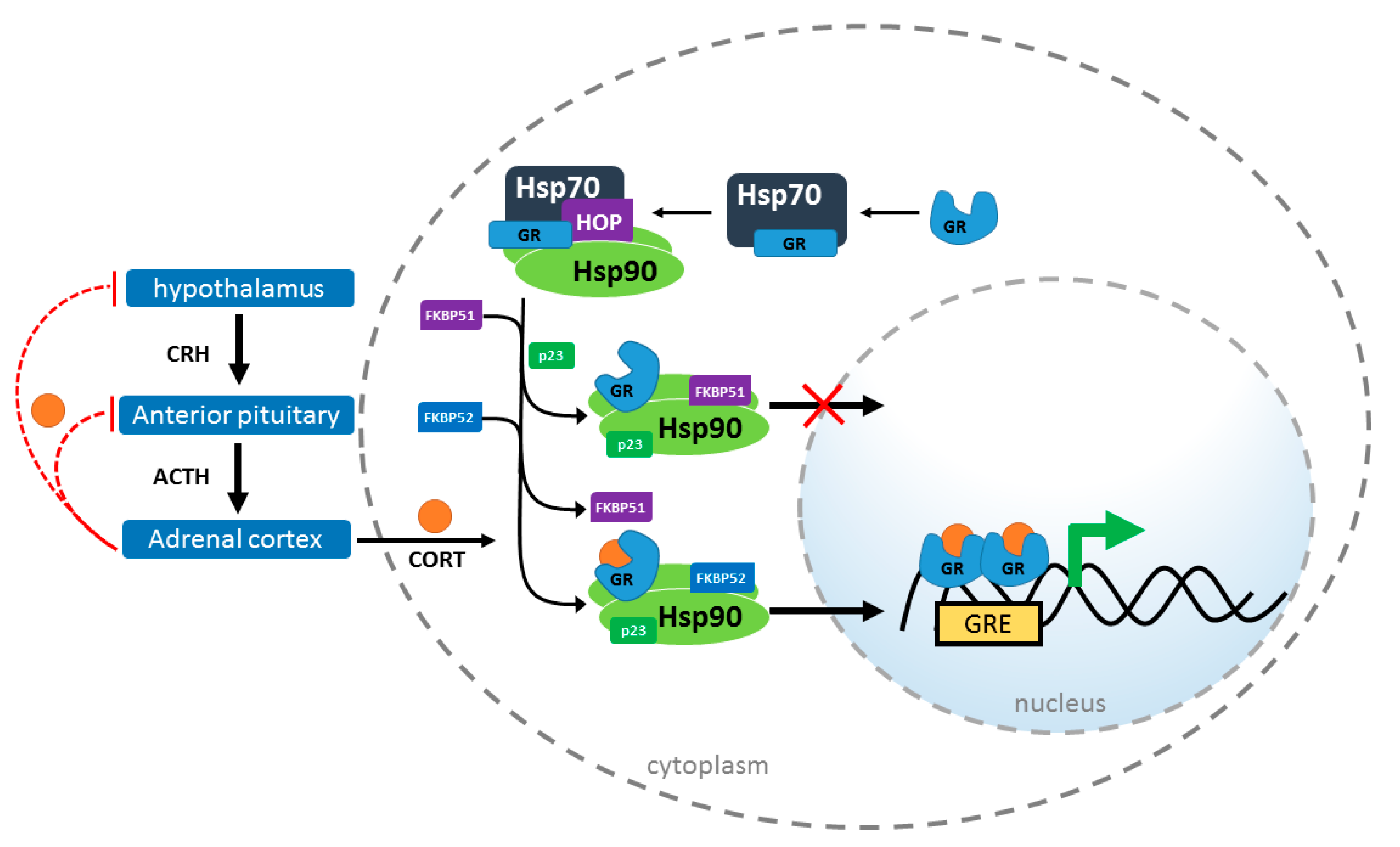
2. Chaperones in GR Signaling
Hsp90 Heterocomplex
3. Chaperones Implicated in Psychiatric Disorders
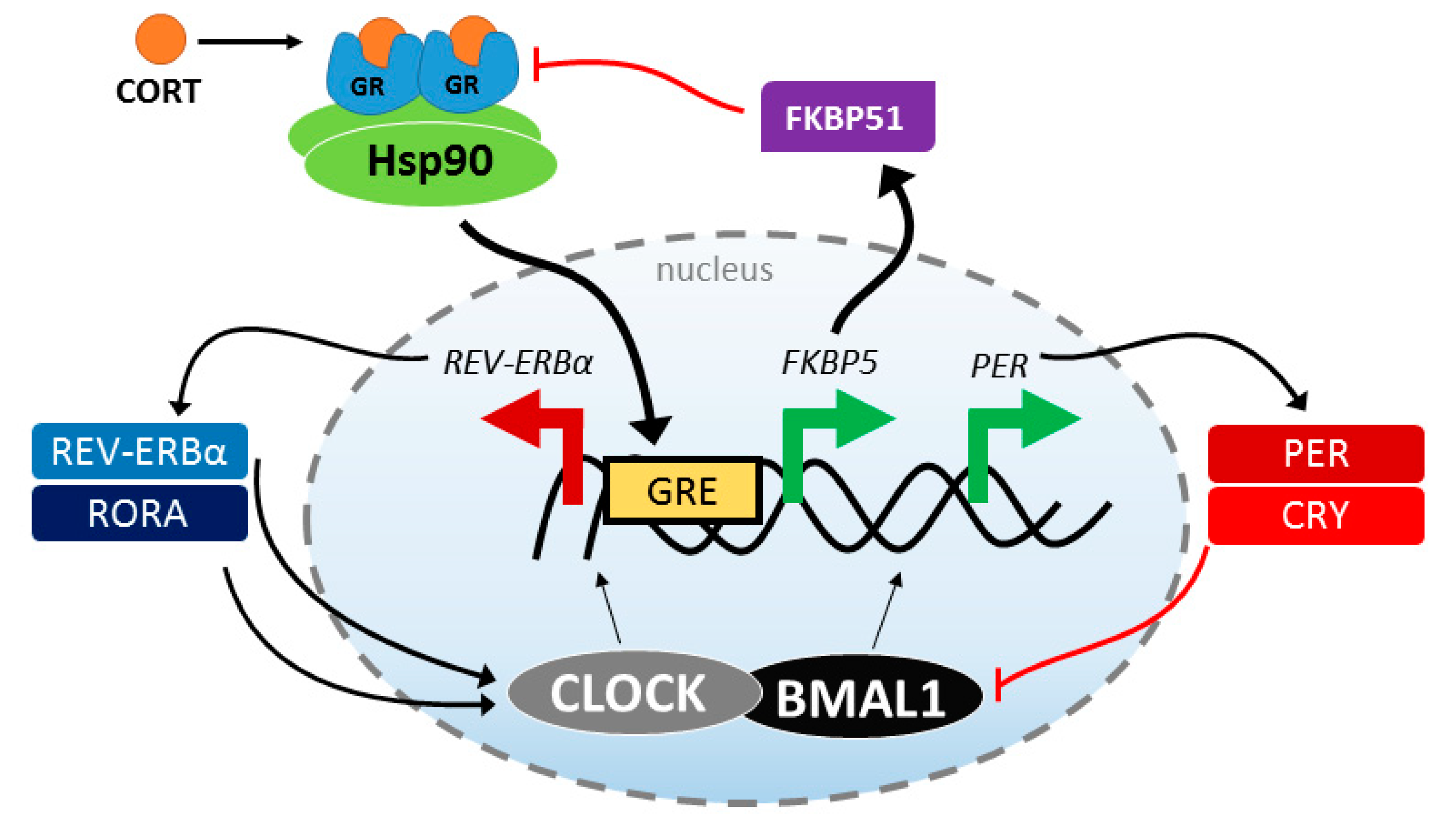
4. Stress Response and Circadian Rhythmicity
5. Therapeutic Progress
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| ATP | adenosine triphosphate |
| CORT | cortisol |
| CRH | corticotropin-releasing hormone |
| CaN/PP2B | calcineurin |
| CyP40 | cyclophilin 40 |
| FKBP | FK506-binding protein |
| GR | glucocorticoid receptor |
| GRE | glucocorticoid response element |
| HPA | hypothalamic-pituitary-adrenal |
| Hsp90 | heat shock protein 90 |
| LBD | ligand-binding domain |
| MDD | major depressive disorder |
| MEEVD | binding motif in Hsp90 that binds TPR domain |
| MR | mineralocorticoid receptors |
| N2A | Neuro2A, a mouse neuroblastoma cell line |
| PTSD | post-traumatic stress disorder |
| SNP | single nucleotide polymorphism |
| TPR | tetratricopeptide repeat |
References
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Eisenlohr-Moul, T.A.; Miller, A.B.; Giletta, M.; Hastings, P.D.; Rudolph, K.D.; Nock, M.K.; Prinstein, M.J. HPA axis response and psychosocial stress as interactive predictors of suicidal ideation and behavior in adolescent females: A multilevel diathesis-stress framework. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Lehrner, A.; Yehuda, R. Endocrine Aspects of Post-traumatic Stress Disorder and Implications for Diagnosis and Treatment. Endocrinol. Metab. Clin. N. Am. 2013, 42, 503–513. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A.C.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol. Res. Curr. Rev. 2012, 34, 468–483. [Google Scholar]
- Rose, A.K.; Shaw, S.G.; Prendergast, M.A.; Little, H.J. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcohol. Clin. Exp. Res. 2010, 34, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- Keller-Wood, M. Hypothalamic-Pituitary—Adrenal Axis-Feedback Control. Compr. Physiol. 2015, 5, 1161–1182. [Google Scholar] [CrossRef]
- Zhe, D.; Fang, H.; Yuxiu, S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem. Cytochem. 2008, 41, 89–95. [Google Scholar] [CrossRef]
- Herman, J.P.; Patel, P.D.; Akil, H.; Watson, S.J. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. (Baltimore MD.) 1989, 3, 1886–1894. [Google Scholar] [CrossRef]
- Qin, D.-D.; Rizak, J.; Feng, X.-L.; Yang, S.-C.; Lü, L.-B.; Pan, L.; Yin, Y.; Hu, X.-T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Vasconcelos-Moreno, M.P.; Gubert, C.; dos Santos, B.T.M.Q.; Sartori, J.; Eisele, B.; Ferrari, P.; Fijtman, A.; Rüegg, J.; Gassen, N.C.; et al. Hypothalamic-pituitary-adrenal axis dysfunction and illness progression in bipolar disorder. Int. J. Neuropsychopharmacol. 2014, 18, pyu043. [Google Scholar] [CrossRef] [PubMed]
- Meewisse, M.L.; Reitsma, J.B.; de Vries, G.J.; Gersons, B.P.; Olff, M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br. J. Psychiatry J. Ment. Sci. 2007, 191, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. (1957) 2014, 87, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- de Quervain, D.J.; Roozendaal, B.; McGaugh, J.L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998, 394, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Wolf, O.T.; Kuhlmann, S.; Buss, C.; Hellhammer, D.H.; Kirschbaum, C. Cortisol and memory retrieval in humans: Influence of emotional valence. Ann. N. Y. Acad. Sci. 2004, 1032, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Dedovic, K.; Ngiam, J. The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 1181–1189. [Google Scholar] [CrossRef]
- Dougherty, L.R.; Klein, D.N.; Olino, T.M.; Dyson, M.; Rose, S. Increased waking salivary cortisol and depression risk in preschoolers: The role of maternal history of melancholic depression and early child temperament. J. Child Psychol. Psychiatry Allied Discip. 2009, 50, 1495–1503. [Google Scholar] [CrossRef]
- Otte, C.; Hart, S.; Neylan, T.C.; Marmar, C.R.; Yaffe, K.; Mohr, D.C. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology 2005, 30, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Chaudieu, I.; Beluche, I.; Norton, J.; Boulenger, J.P.; Ritchie, K.; Ancelin, M.L. Abnormal reactions to environmental stress in elderly persons with anxiety disorders: Evidence from a population study of diurnal cortisol changes. J. Affect. Disord. 2008, 106, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, P.; Gelber, S.; Kin, F.N.; Nair, V.N.; Schwartz, G. Circadian secretion of cortisol in bipolar disorder. J. Psychiatry Neurosci. JPN 2001, 26, 411–416. [Google Scholar] [PubMed]
- Joyce, P.R.; Donald, R.A.; Elder, P.A. Individual differences in plasma cortisol changes during mania and depression. J. Affect. Disord. 1987, 12, 1–5. [Google Scholar] [CrossRef]
- Wand, G. The influence of stress on the transition from drug use to addiction. Alcohol. Res. Health J. Natl. Inst. Alcohol Abuse Alcohol. 2008, 31, 119–136. [Google Scholar]
- Meijsing, S.H. Mechanisms of Glucocorticoid-Regulated Gene Transcription. Adv. Exp. Med. Biol. 2015, 872, 59–81. [Google Scholar] [CrossRef]
- Echeverría, P.C.; Mazaira, G.; Erlejman, A.; Gomez-Sanchez, C.; Piwien Pilipuk, G.; Galigniana, M.D. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol. Cell. Biol. 2009, 29, 4788–4797. [Google Scholar] [CrossRef]
- Pratt, W.B.; Galigniana, M.D.; Morishima, Y.; Murphy, P.J. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004, 40, 41–58. [Google Scholar] [CrossRef]
- Noguchi, T.; Makino, S.; Matsumoto, R.; Nakayama, S.; Nishiyama, M.; Terada, Y.; Hashimoto, K. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology 2010, 151, 4344–4355. [Google Scholar] [CrossRef]
- Lorenz, O.R.; Freiburger, L.; Rutz, D.A.; Krause, M.; Zierer, B.K.; Alvira, S.; Cuellar, J.; Valpuesta, J.M.; Madl, T.; Sattler, M.; et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol. Cell 2014, 53, 941–953. [Google Scholar] [CrossRef]
- Picard, D.; Khursheed, B.; Garabedian, M.J.; Fortin, M.G.; Lindquist, S.; Yamamoto, K.R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 1990, 348, 166. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Radanyi, C.; Renoir, J.M.; Housley, P.R.; Pratt, W.B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 2001, 276, 14884–14889. [Google Scholar] [CrossRef] [PubMed]
- Storer, C.L.; Dickey, C.A.; Galigniana, M.D.; Rein, T.; Cox, M.B. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. TEM 2011, 22, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Cox, M.B.; Tardif, H.L.; Hessling, M.; Buchner, J.; Smith, D.F. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol. Cell. Biol. 2007, 27, 8658–8669. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Periyasamy, S.; Wolf, I.M.; Hinds, T.D., Jr.; Yong, W.; Shou, W.; Sanchez, E.R. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry 2008, 47, 10471–10480. [Google Scholar] [CrossRef]
- Johnson, J.L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 607–613. [Google Scholar] [CrossRef]
- Liu, X.D.; Liu, P.C.; Santoro, N.; Thiele, D.J. Conservation of a stress response: Human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997, 16, 6466–6477. [Google Scholar] [CrossRef]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. CMLS 2002, 59, 1640–1648. [Google Scholar] [CrossRef]
- Zhao, R.; Davey, M.; Hsu, Y.-C.; Kaplanek, P.; Tong, A.; Parsons, A.B.; Krogan, N.; Cagney, G.; Mai, D.; Greenblatt, J.; et al. Navigating the Chaperone Network: An Integrative Map of Physical and Genetic Interactions Mediated by the Hsp90 Chaperone. Cell 2005, 120, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gholami, A.M.; Kuster, B. Systematic identification of the HSP90 candidate regulated proteome. Mol. Cell. Proteomics MCP 2012, 11, M111.016675. [Google Scholar] [CrossRef] [PubMed]
- Mailhos, C.; Howard, M.K.; Latchman, D.S. Heat shock proteins hsp90 and hsp70 protect neuronal cells from thermal stress but not from programmed cell death. J. Neurochem. 1994, 63, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 2011, 286, 30152–30160. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S. Protein quality control: Knowing when to fold. Nat. Cell Biol. 2005, 7, 647. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid Receptor Function Regulated by Coordinated Action of the Hsp90 and Hsp70 Chaperone Cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Buchner, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef]
- Oroz, J.; Chang, B.J.; Wysoczanski, P.; Lee, C.-T.; Pérez-Lara, Á.; Chakraborty, P.; Hofele, R.V.; Baker, J.D.; Blair, L.J.; Biernat, J.; Urlaub, H.; et al. Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex. Nat. Commun. 2018, 9, 4532. [Google Scholar] [CrossRef]
- Hellenkamp, B.; Wortmann, P.; Kandzia, F.; Zacharias, M.; Hugel, T. Multidomain structure and correlated dynamics determined by self-consistent FRET networks. Nat. Methods 2017, 14, 174–180. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Mayer, M.P.; Le Breton, L. Hsp90: Breaking the symmetry. Mol. Cell 2015, 58, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, Z.L.; Molugu, S.K.; Herrera, N.; Ramirez, C.; Xiao, C.; Bernal, R.A. Hsp90 can accommodate the simultaneous binding of the FKBP52 and HOP proteins. Oncotarget 2011, 2, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Harst, A.; Lin, H.; Obermann, W.M.J. Aha1 competes with Hop, p50 and p23 for binding to the molecular chaperone Hsp90 and contributes to kinase and hormone receptor activation. Biochem. J. 2005, 387 Pt 3, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.; Johnson, J.L. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 2010, 93, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, P.; Rohrberg, J.; Biebl, M.M.; Rutz, D.A.; Buchner, J. The Plasticity of the Hsp90 Co-chaperone System. Mol. Cell 2017, 67, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.D.; Hutchison, K.A.; Owens-Grillo, J.K.; Pratt, W.B. Reconstitution of the steroid receptor.hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J. Biol. Chem. 1996, 271, 12833–12839. [Google Scholar] [CrossRef]
- Röhl, A.; Wengler, D.; Madl, T.; Lagleder, S.; Tippel, F.; Herrmann, M.; Hendrix, J.; Richter, K.; Hack, G.; Schmid, A.B.; et al. Hsp90 regulates the dynamics of its cochaperone Sti1 and the transfer of Hsp70 between modules. Nat. Commun. 2015, 6, 6655. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Kino, T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann. N. Y. Acad. Sci. 2009, 1179, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Grenert, J.P.; Johnson, B.D.; Toft, D.O. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J. Biol. Chem. 1999, 274, 17525–17533. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.C.; Picard, D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 2010, 1803, 641–649. [Google Scholar] [CrossRef]
- Morishima, Y.; Kanelakis, K.C.; Murphy, P.J.; Lowe, E.R.; Jenkins, G.J.; Osawa, Y.; Sunahara, R.K.; Pratt, W.B. The hsp90 cochaperone p23 is the limiting component of the multiprotein hsp90/hsp70-based chaperone system in vivo where it acts to stabilize the client protein: Hsp90 complex. J. Biol. Chem. 2003, 278, 48754–48763. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Genest, O.; Mollapour, M. The multiple facets of the Hsp90 machine. Nat. Struct. Mol. Biol. 2018, in press. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry 2005, 44, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.M.; Periyasamy, S.; Hinds, T., Jr.; Yong, W.; Shou, W.; Sanchez, E.R. Targeted ablation reveals a novel role of FKBP52 in gene-specific regulation of glucocorticoid receptor transcriptional activity. J. Steroid Biochem. Mol. Biol. 2009, 113, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Harrell, J.M.; Murphy, P.J.; Chinkers, M.; Radanyi, C.; Renoir, J.M.; Zhang, M.; Pratt, W.B. Binding of hsp90-associated immunophilins to cytoplasmic dynein: Direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry 2002, 41, 13602–13610. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Galigniana, M.D.; Chen, M.S.; Owens-Grillo, J.K.; Chinkers, M.; Pratt, W.B. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J. Biol. Chem. 1997, 272, 16224–16230. [Google Scholar] [CrossRef]
- Golden, T.; Swingle, M.; Honkanen, R.E. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008, 27, 169–178. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Kono, E.; Dang, T.; Garabedian, M.J. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol. Endocrinol. 2007, 21, 625–634. [Google Scholar] [CrossRef]
- O’Leary, J.C., 3rd; Zhang, B.; Koren, J., 3rd; Blair, L.; Dickey, C.A. The role of FKBP5 in mood disorders: Action of FKBP5 on steroid hormone receptors leads to questions about its evolutionary importance. CNS Neurol. Disord. Drug Targets 2013, 12, 1157–1162. [Google Scholar]
- Jaaskelainen, T.; Makkonen, H.; Palvimo, J.J. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr. Opin. Pharmacol. 2011, 11, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Rein, T.; Binder, E.B.; Porter, J.T.; Koren, J.; Blair, L.J. Hsp90 and FKBP51: Complex regulators of psychiatric diseases. Philos. Trans. R. Soc. B Biol.Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Kranzler, H.R.; Poling, J.; Stein, M.B.; Anton, R.F.; Farrer, L.A.; Gelernter, J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 1684–1692. [Google Scholar] [CrossRef]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, J.J.; Cordova, R.A.; Zheng, D.; Criado-Marrero, M.; Lemus, A.; Li, P.; Baker, J.D.; Nordhues, B.A.; Darling, A.L.; Martinez-Licha, C.; et al. Targeting the FKBP51/GR/Hsp90 Complex to Identify Functionally Relevant Treatments for Depression and PTSD. ACS Chem. Biol. 2018, 13, 2288–2299. [Google Scholar] [CrossRef]
- O’Leary, J.C., 3rd; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A new anti-depressive strategy for the elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6, e24840. [Google Scholar] [CrossRef]
- Touma, C.; Gassen, N.C.; Herrmann, L.; Cheung-Flynn, J.; Bull, D.R.; Ionescu, I.A.; Heinzmann, J.M.; Knapman, A.; Siebertz, A.; Depping, A.M.; et al. FK506 binding protein 5 shapes stress responsiveness: Modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 2011, 70, 928–936. [Google Scholar] [CrossRef]
- Young, M.J.; Geiszler, P.C.; Pardon, M.C. A novel role for the immunophilin FKBP52 in motor coordination. Behav. Brain Res. 2016, 313, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Gunduz-Cinar, O.; Brockway, E.; Lederle, L.; Wilcox, T.; Halladay, L.R.; Ding, Y.; Oh, H.; Busch, E.F.; Kaugars, K.; Flynn, S.; et al. Identification of a novel gene regulating amygdala-mediated fear extinction. Mol. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.G.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef]
- Varghese, F.P.; Brown, E.S. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Primary Care Companion J. Clin. Psychiatry 2001, 3, 151–155. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Zschocke, J.; Stepan, J.; Hafner, K.; Zellner, A.; Kirmeier, T.; Kollmannsberger, L.; Wagner, K.V.; Dedic, N.; et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: Evidence in cells, mice, and humans. PLoS Med. 2014, 11, e1001755. [Google Scholar] [CrossRef] [PubMed]
- Pöhlmann, M.L.; Häusl, A.S.; Harbich, D.; Balsevich, G.; Engelhardt, C.; Feng, X.; Breitsamer, M.; Hausch, F.; Winter, G.; Schmidt, M.V. Pharmacological Modulation of the Psychiatric Risk Factor FKBP51 Alters Efficiency of Common Antidepressant Drugs. Front. Behav. Neurosci. 2018, 12, 262. [Google Scholar] [CrossRef]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Pütz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319. [Google Scholar] [CrossRef]
- Lekman, M.; Laje, G.; Charney, D.; Rush, A.J.; Wilson, A.F.; Sorant, A.J.M.; Lipsky, R.; Wisniewski, S.R.; Manji, H.; McMahon, F.J.; et al. The FKBP5-gene in depression and treatment response—An association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol. Psychiatry 2008, 63, 1103–1110. [Google Scholar] [CrossRef]
- Sarginson, J.E.; Lazzeroni, L.C.; Ryan, H.S.; Schatzberg, A.F.; Murphy, G.M., Jr. FKBP5 polymorphisms and antidepressant response in geriatric depression. Ame. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 554–560. [Google Scholar] [CrossRef]
- Gassen, N.C.; Fries, G.R.; Zannas, A.S.; Hartmann, J.; Zschocke, J.; Hafner, K.; Carrillo-Roa, T.; Steinbacher, J.; Preissinger, S.N.; Hoeijmakers, L.; et al. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 2015, 8, ra119. [Google Scholar] [CrossRef]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress 2017, 6, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Pilz, L.K.; Carissimi, A.; Oliveira, M.A.B.; Francisco, A.P.; Fabris, R.C.; Medeiros, M.S.; Scop, M.; Frey, B.N.; Adan, A.; Hidalgo, M.P. Rhythmicity of Mood Symptoms in Individuals at Risk for Psychiatric Disorders. Sci. Rep. 2018, 8, 11402. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Ghobadi, C.; Rostami-Hodjegan, A.; Huatan, H.; Campbell, M.J.; Newell-Price, J.; Darzy, K.; Merke, D.P.; Arlt, W.; Ross, R.J. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 2009, 94, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Chabot, C.C.; Taylor, D.H. Daily rhythmicity of the rat acoustic startle response. Physiol. Behav. 1992, 51, 885–889. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, E.R.; Chun, L.E.; Hinds, L.R.; Spencer, R.L. Diurnal Corticosterone Presence and Phase Modulate Clock Gene Expression in the Male Rat Prefrontal Cortex. Endocrinology 2016, 157, 1522–1534. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Dardente, H.; Giguere, V.; Cermakian, N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythms 2005, 20, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Larkin, D.W.; Albrecht, U.; Sun, Z.S.; Sage, M.; Eichele, G.; Lee, C.C.; Bradley, A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999, 400, 169–173. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Müller, M.B. Expression and Regulation of the Fkbp5 Gene in the Adult Mouse Brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C., III; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; Cotman, C.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, J.J.; O‘Leary, J.C., 3rd; Blair, L.J.; Klengel, T.; Nordhues, B.A.; Fontaine, S.N.; Binder, E.B.; Dickey, C.A. Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PLoS ONE 2014, 9, e107241. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.L.; Badia, P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neurosci. Biobehav. Rev. 1995, 19, 553–571. [Google Scholar] [CrossRef]
- Albu, S.; Romanowski, C.P.; Letizia Curzi, M.; Jakubcakova, V.; Flachskamm, C.; Gassen, N.C.; Hartmann, J.; Schmidt, M.V.; Schmidt, U.; Rein, T.; et al. Deficiency of FK506-binding protein (FKBP) 51 alters sleep architecture and recovery sleep responses to stress in mice. J. Sleep Res. 2014, 23, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Yuno, A.; Lee, M.J.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J.B. Clinical Evaluation and Biomarker Profiling of Hsp90 Inhibitors. Methods Mol. Biol. 2018, 1709, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Ishii, H.; Hyodo, F.; Samuni, U.; Krishna, M.C.; Goldstein, S.; Mitchell, J.B. Reactive oxygen species mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin and its analogs. Free Radic. Biol. Med. 2010, 48, 1559–1563. [Google Scholar] [CrossRef]
- Amin, K.; Ip, C.; Jimenez, L.; Tyson, C.; Behrsing, H. In vitro detection of differential and cell-specific hepatobiliary toxicity induced by geldanamycin and 17-allylaminogeldanamycin using dog liver slices. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 87, 442–450. [Google Scholar] [CrossRef]
- Rajan, A.; Kelly, R.J.; Trepel, J.B.; Kim, Y.S.; Alarcon, S.V.; Kummar, S.; Gutierrez, M.; Crandon, S.; Zein, W.M.; Jain, L.; et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin. Cancer Res. 2011, 17, 6831–6839. [Google Scholar] [CrossRef]
- Xie, Q.; Wondergem, R.; Shen, Y.; Cavey, G.; Ke, J.; Thompson, R.; Bradley, R.; Daugherty-Holtrop, J.; Xu, Y.; Chen, E.; et al. Benzoquinone ansamycin 17AAG binds to mitochondrial voltage-dependent anion channel and inhibits cell invasion. Proc. Natl. Acad. Sci. USA 2011, 108, 4105–4110. [Google Scholar] [CrossRef]
- Gadelle, D.; Bocs, C.; Graille, M.; Forterre, P. Inhibition of archaeal growth and DNA topoisomerase VI activities by the Hsp90 inhibitor radicicol. Nucleic Acids Res. 2005, 33, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Kijima, T.; Prince, T.L.; Tigue, M.L.; Yim, K.H.; Schwartz, H.; Beebe, K.; Lee, S.; Budzynski, M.A.; Williams, H.; Trepel, J.B.; et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci. Rep. 2018, 8, 6976. [Google Scholar] [CrossRef] [PubMed]
- Gaali, S.; Kirschner, A.; Cuboni, S.; Hartmann, J.; Kozany, C.; Balsevich, G.; Namendorf, C.; Fernandez-Vizarra, P.; Sippel, C.; Zannas, A.S.; et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 2015, 11, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sabbagh, J.J.; Blair, L.J.; Darling, A.L.; Wen, X.; Dickey, C.A. MicroRNA-511 Binds to FKBP5 mRNA, Which Encodes a Chaperone Protein, and Regulates Neuronal Differentiation. J. Biol. Chem. 2016, 291, 17897–17906. [Google Scholar] [CrossRef]
- Guy, N.C.; Garcia, Y.A.; Cox, M.B. Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease. Curr. Mol. Pharmacol. 2015, 9, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Flynn, J.; Prapapanich, V.; Cox, M.B.; Riggs, D.L.; Suarez-Quian, C.; Smith, D.F. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol. Endocrinol. 2005, 19, 1654–1666. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, J.D.; Ozsan, I.; Rodriguez Ospina, S.; Gulick, D.; Blair, L.J. Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease. Int. J. Mol. Sci. 2019, 20, 79. https://doi.org/10.3390/ijms20010079
Baker JD, Ozsan I, Rodriguez Ospina S, Gulick D, Blair LJ. Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease. International Journal of Molecular Sciences. 2019; 20(1):79. https://doi.org/10.3390/ijms20010079
Chicago/Turabian StyleBaker, Jeremy D., Ilayda Ozsan, Santiago Rodriguez Ospina, Danielle Gulick, and Laura J. Blair. 2019. "Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease" International Journal of Molecular Sciences 20, no. 1: 79. https://doi.org/10.3390/ijms20010079
APA StyleBaker, J. D., Ozsan, I., Rodriguez Ospina, S., Gulick, D., & Blair, L. J. (2019). Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease. International Journal of Molecular Sciences, 20(1), 79. https://doi.org/10.3390/ijms20010079




