LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks
Abstract
1. Introduction
2. Results
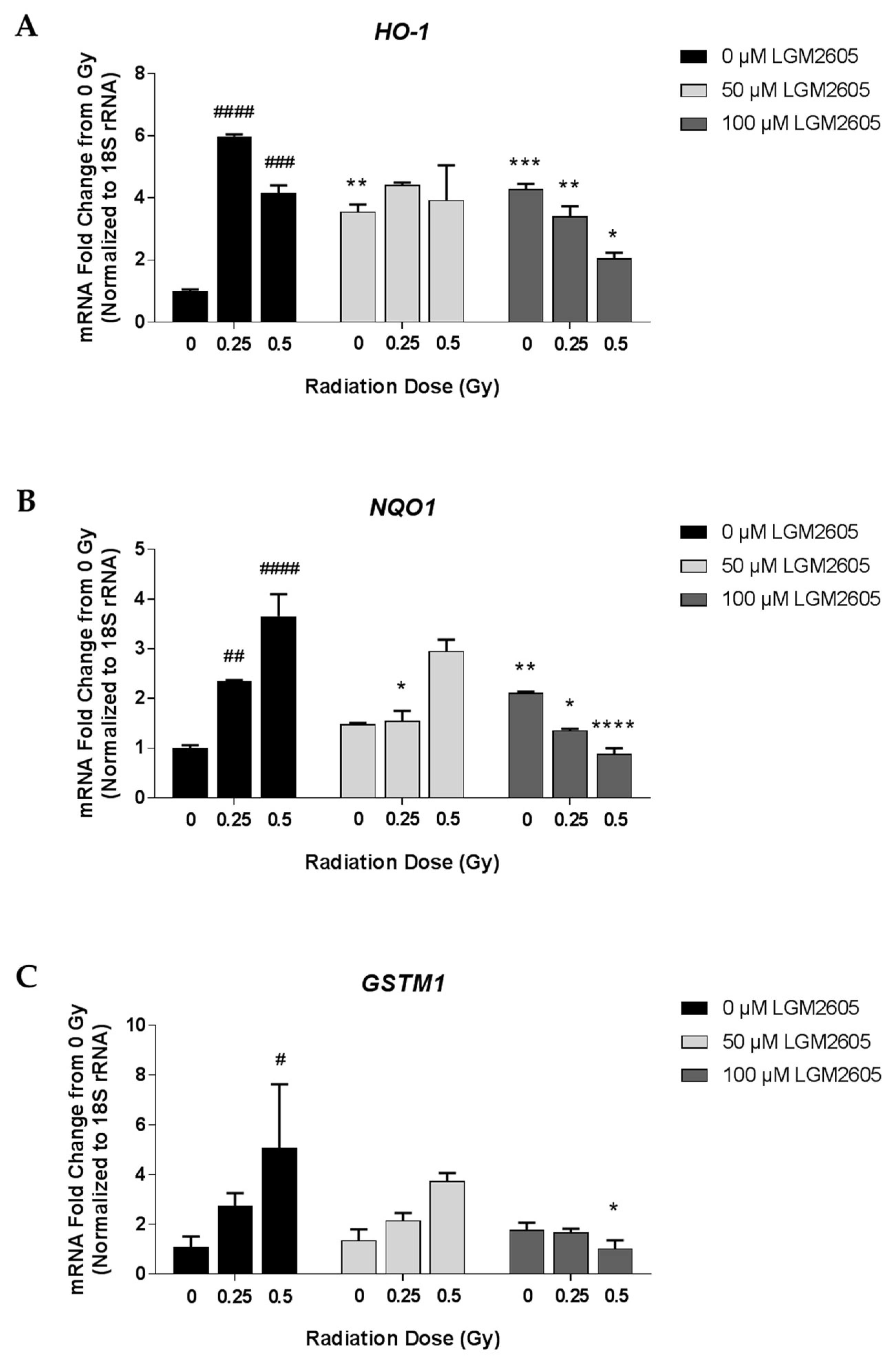
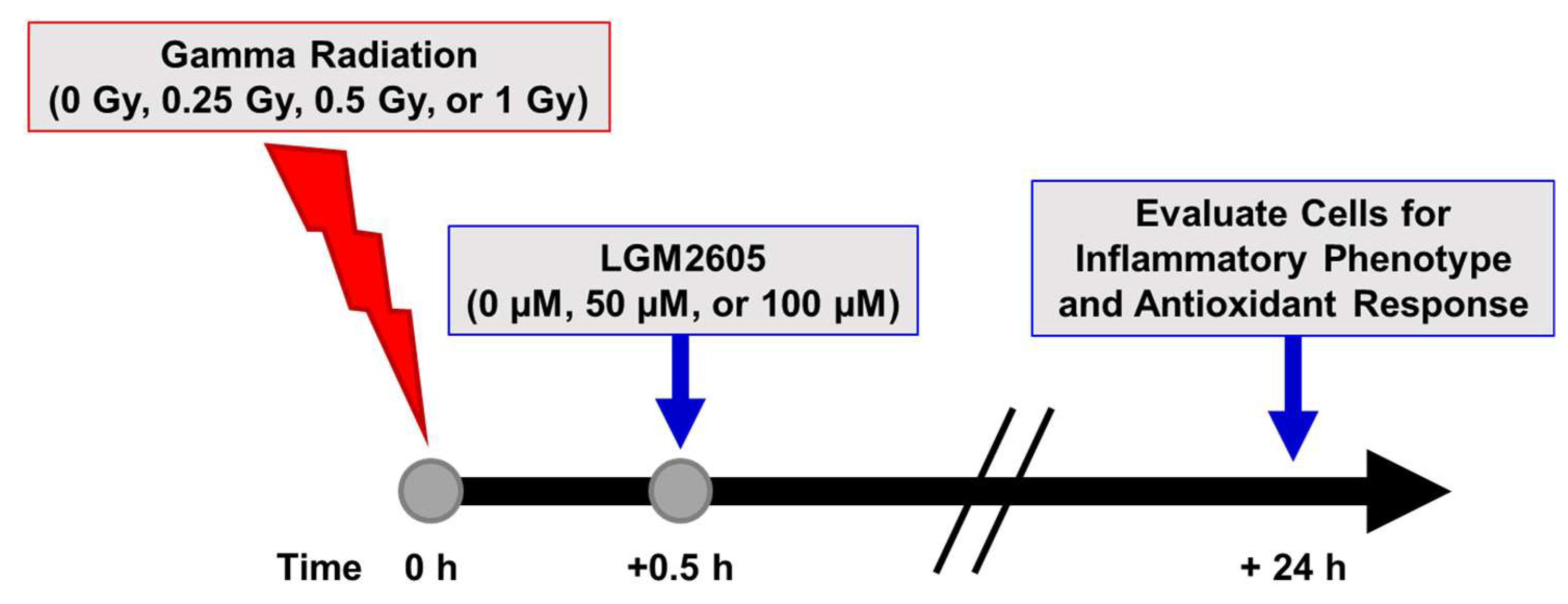
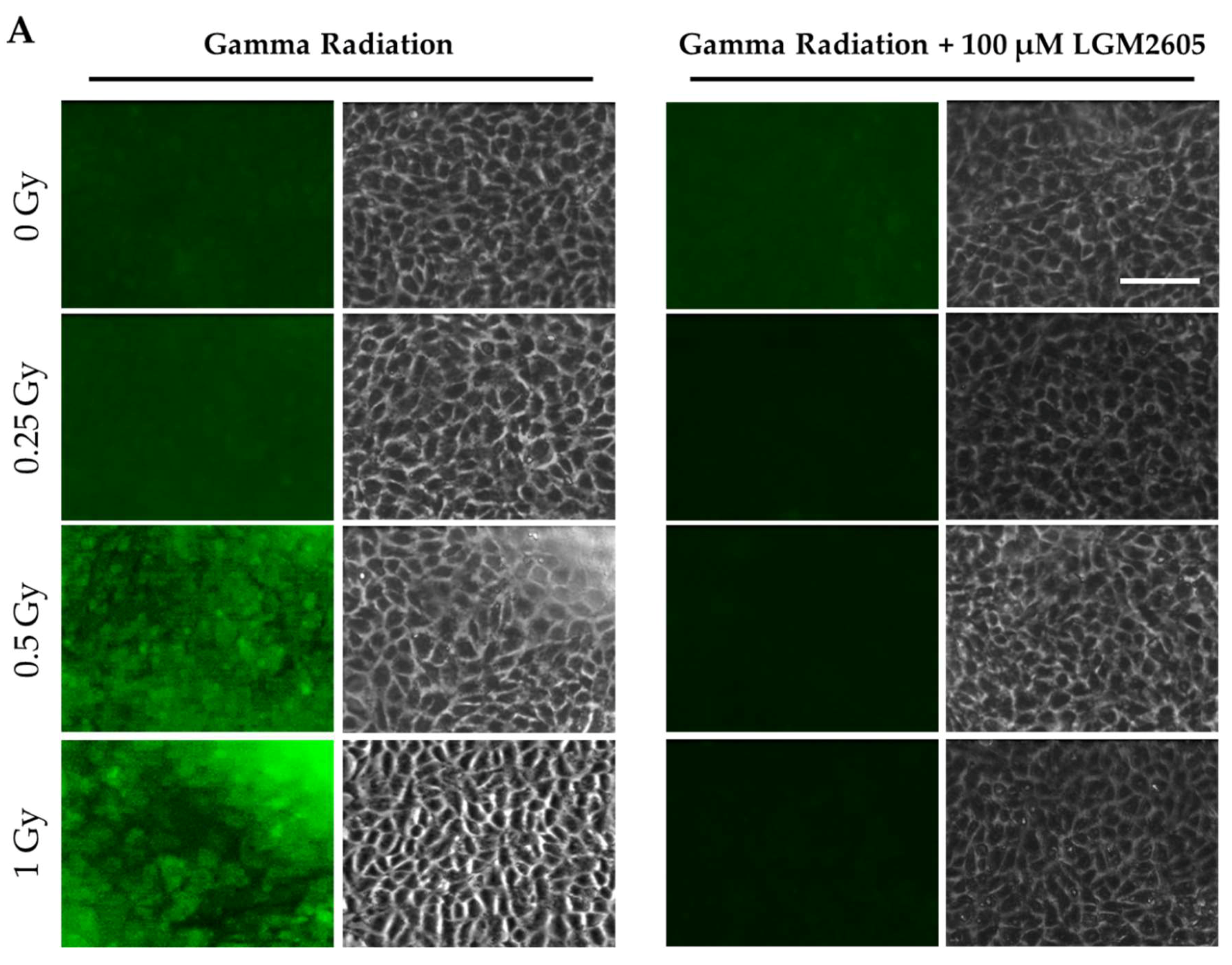
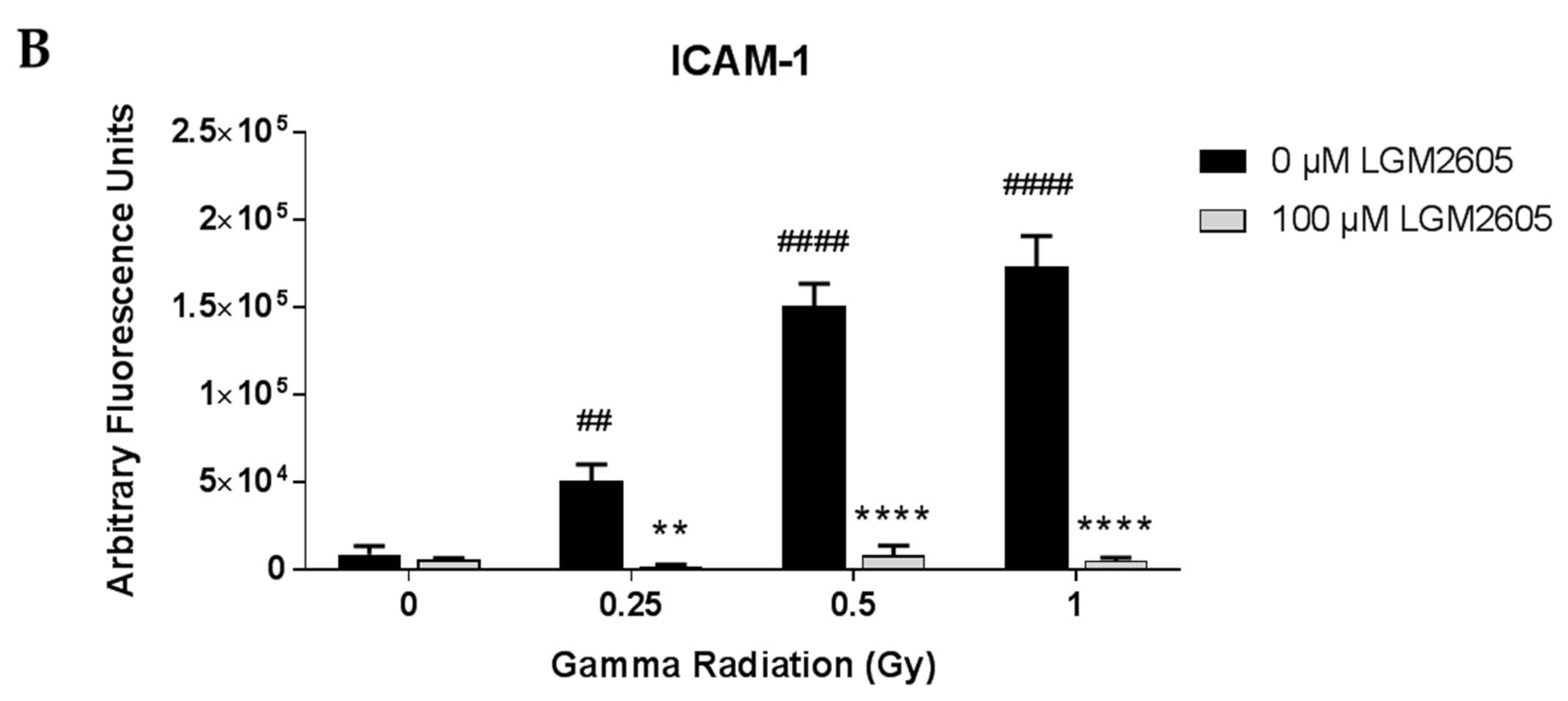
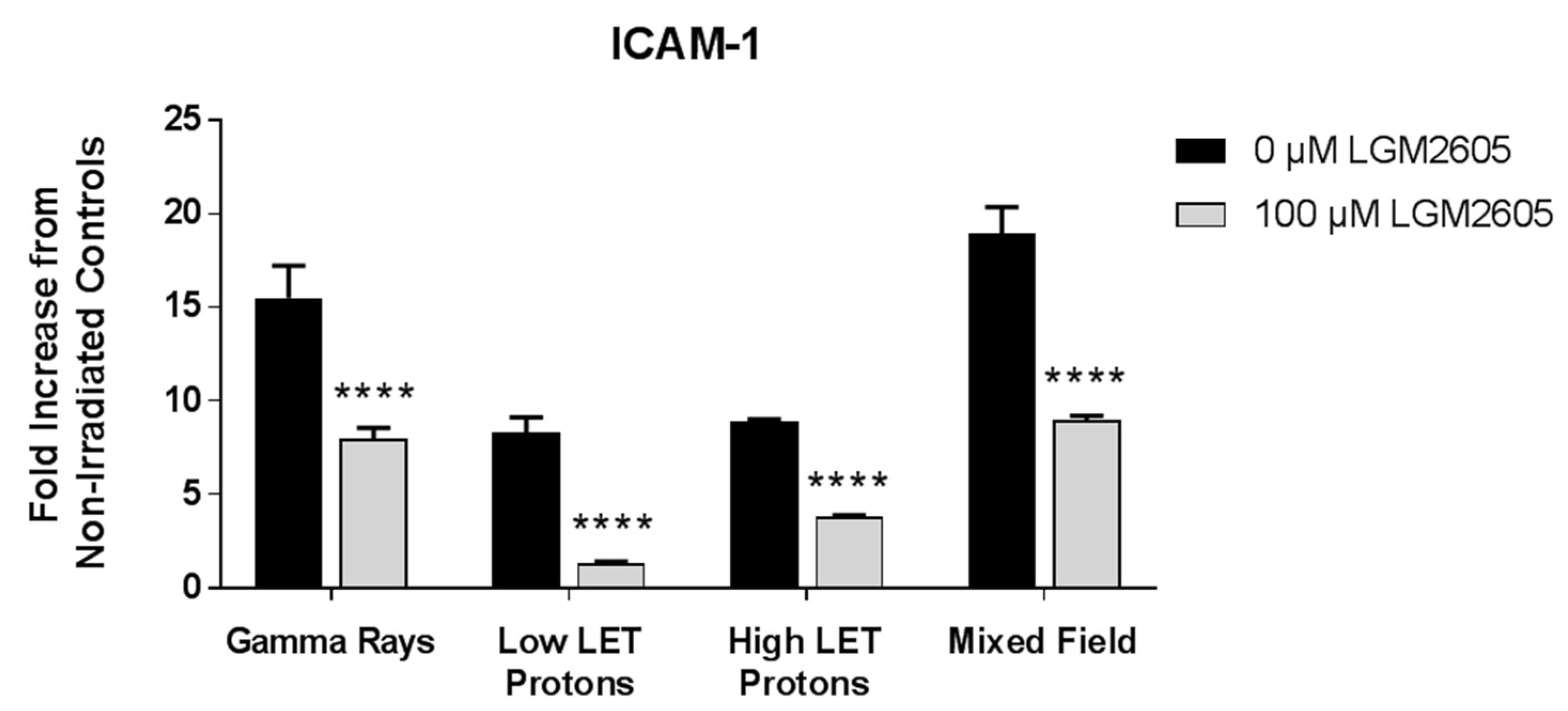
2.1. LGM2605 Alters the Antioxidant Response in In Vitro Lung Vascular Networks and Mitigates Gamma Radiation-Induced Inflammatory Phenotype
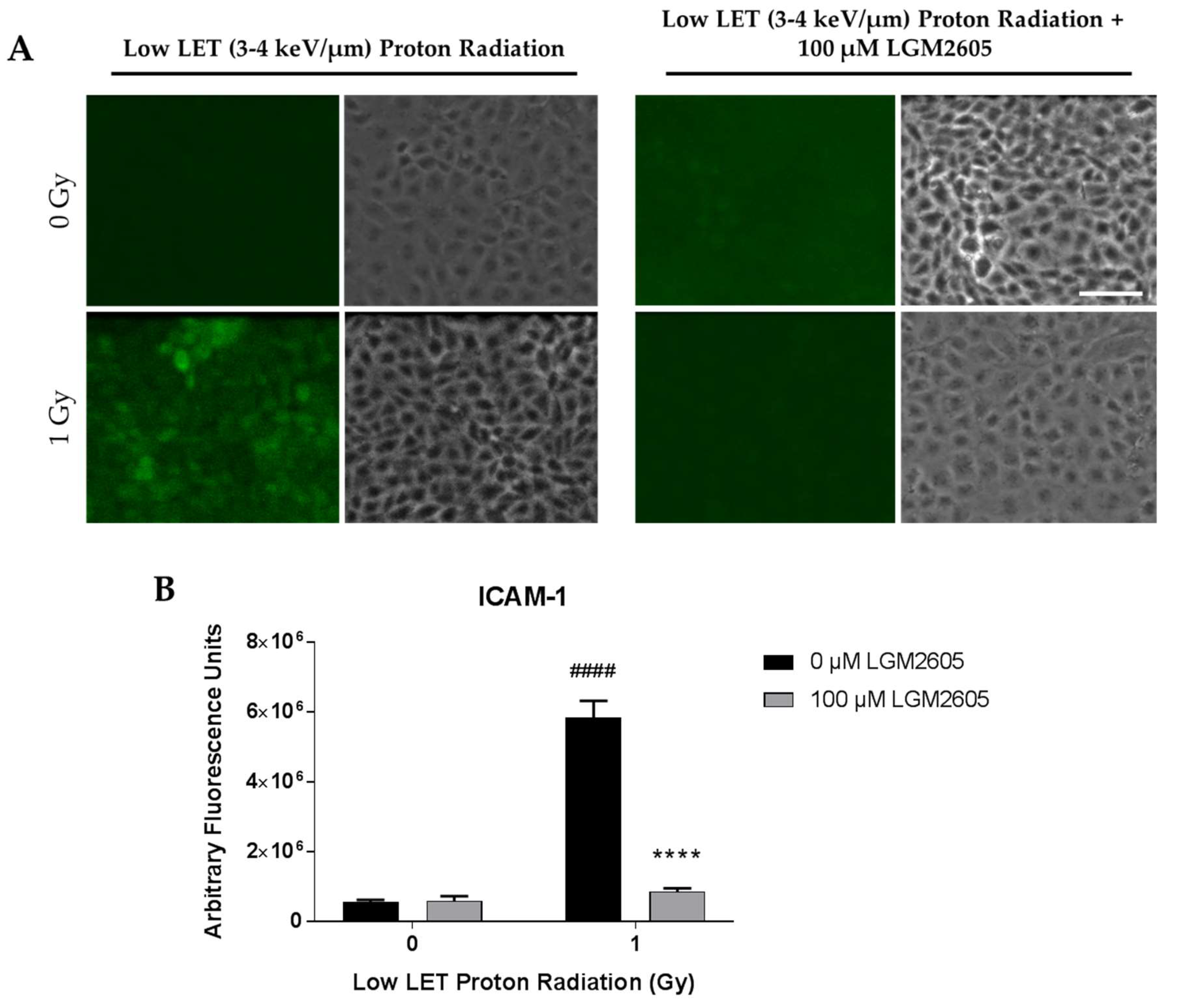
2.2. LGM2605 Blunts Low LET Proton Radiation-Induced Inflammatory Phenotype in In Vitro Lung Vascular Networks
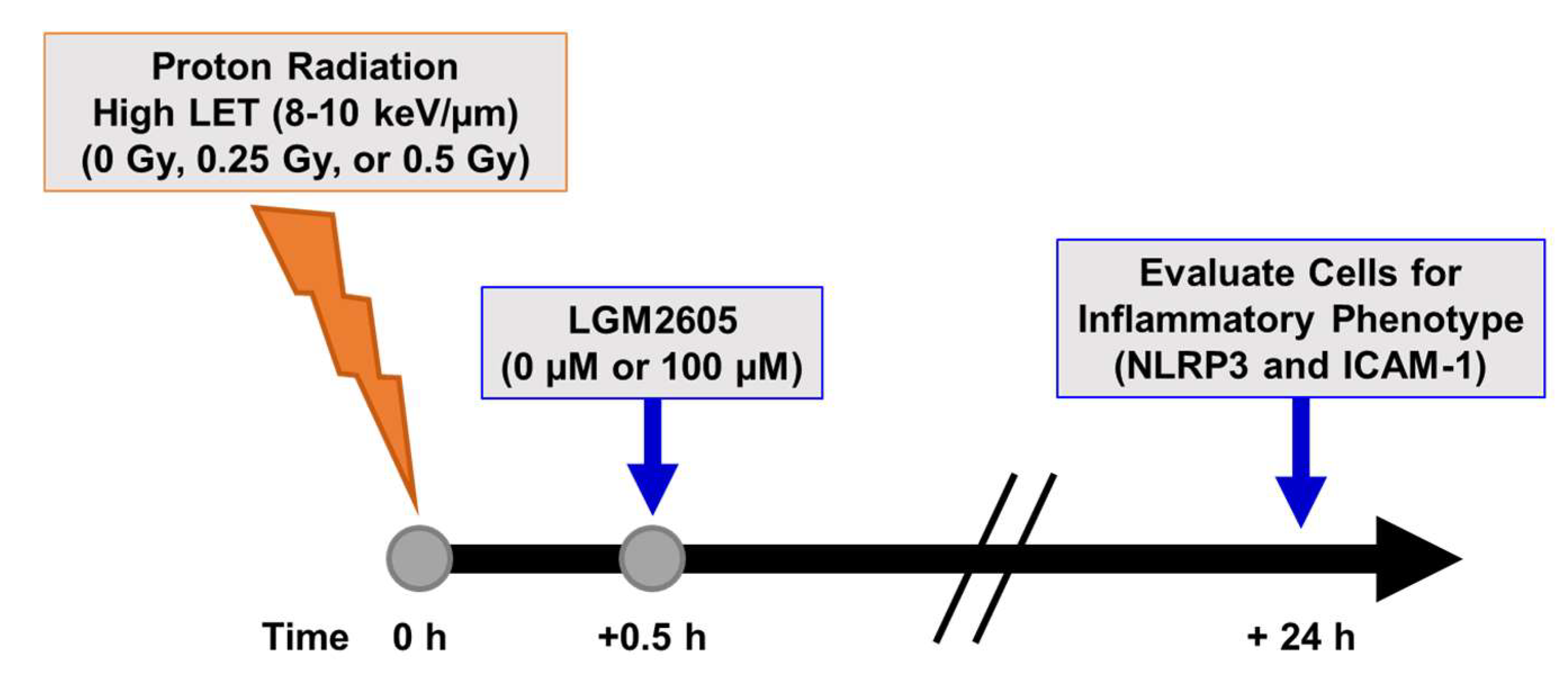
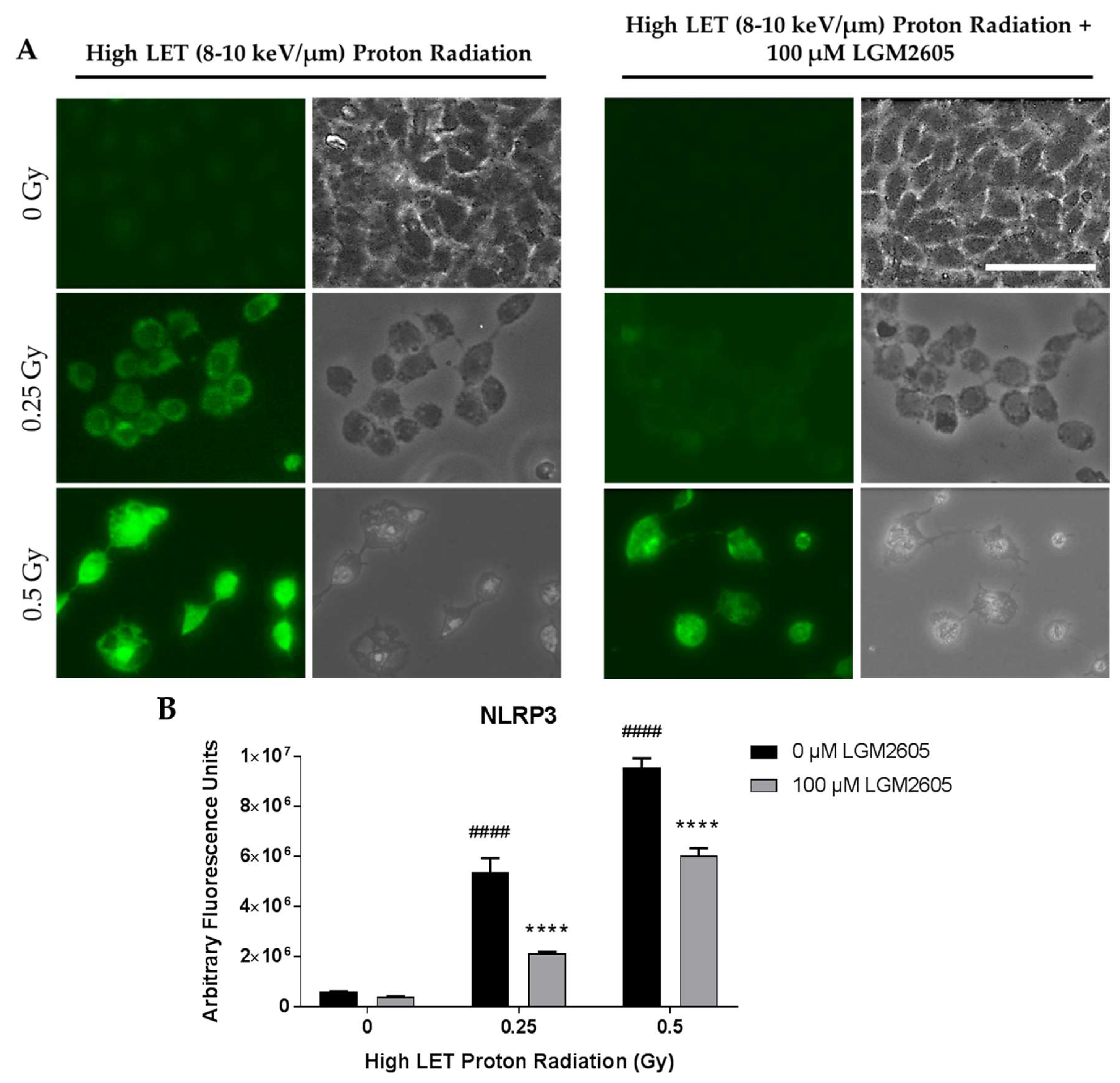
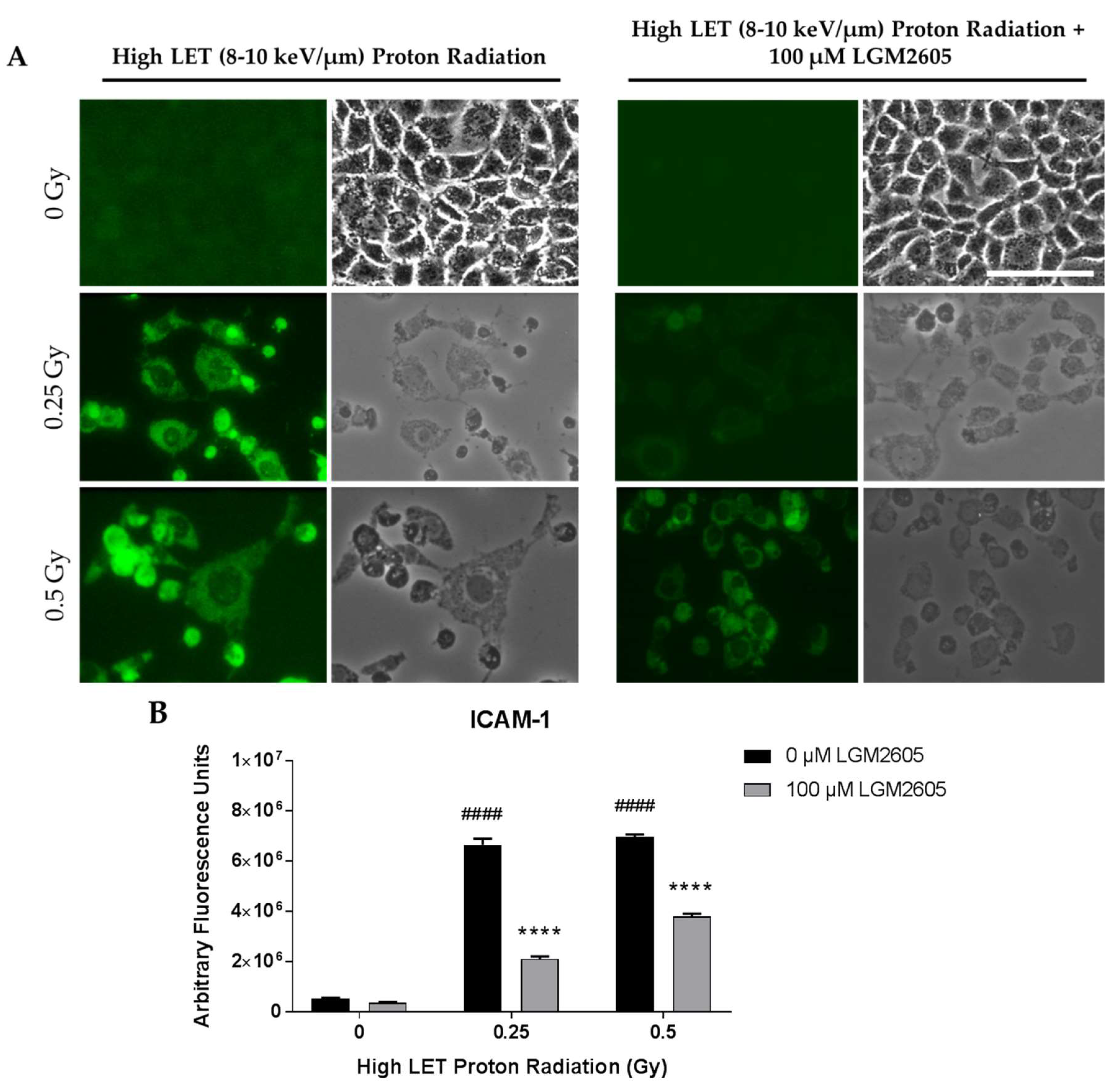
2.3. High LET Proton Radiation-Induced Increases in ICAM-1 and NLRP3 Are Mitigated by LGM2605 Treatment in In Vitro Lung Vascular Networks
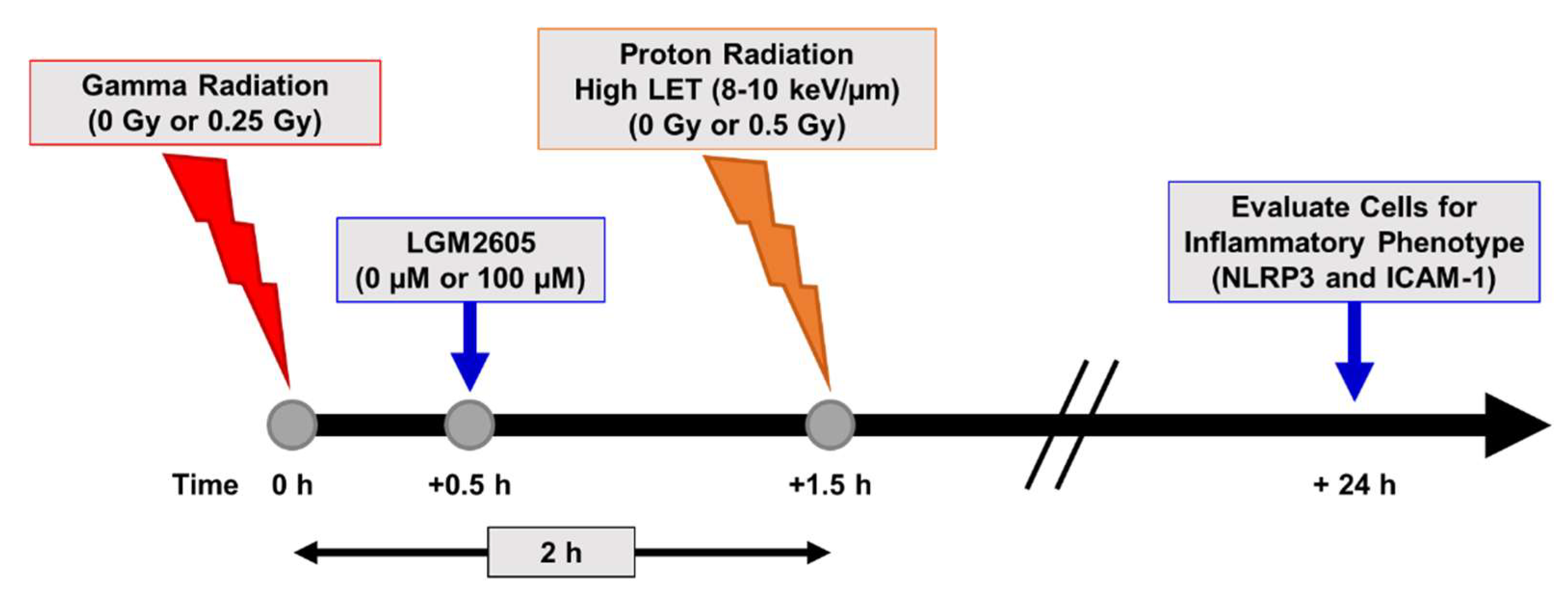
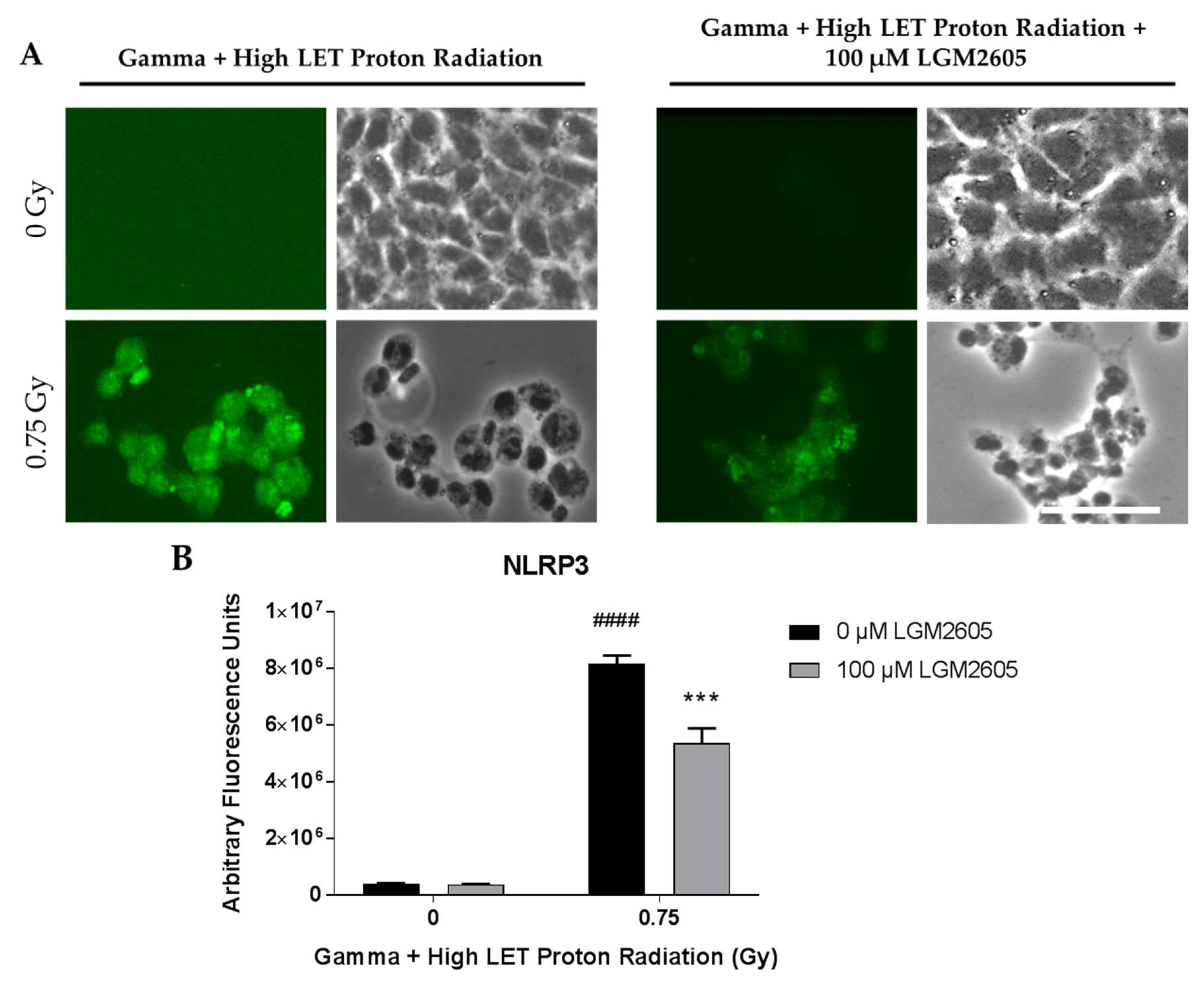
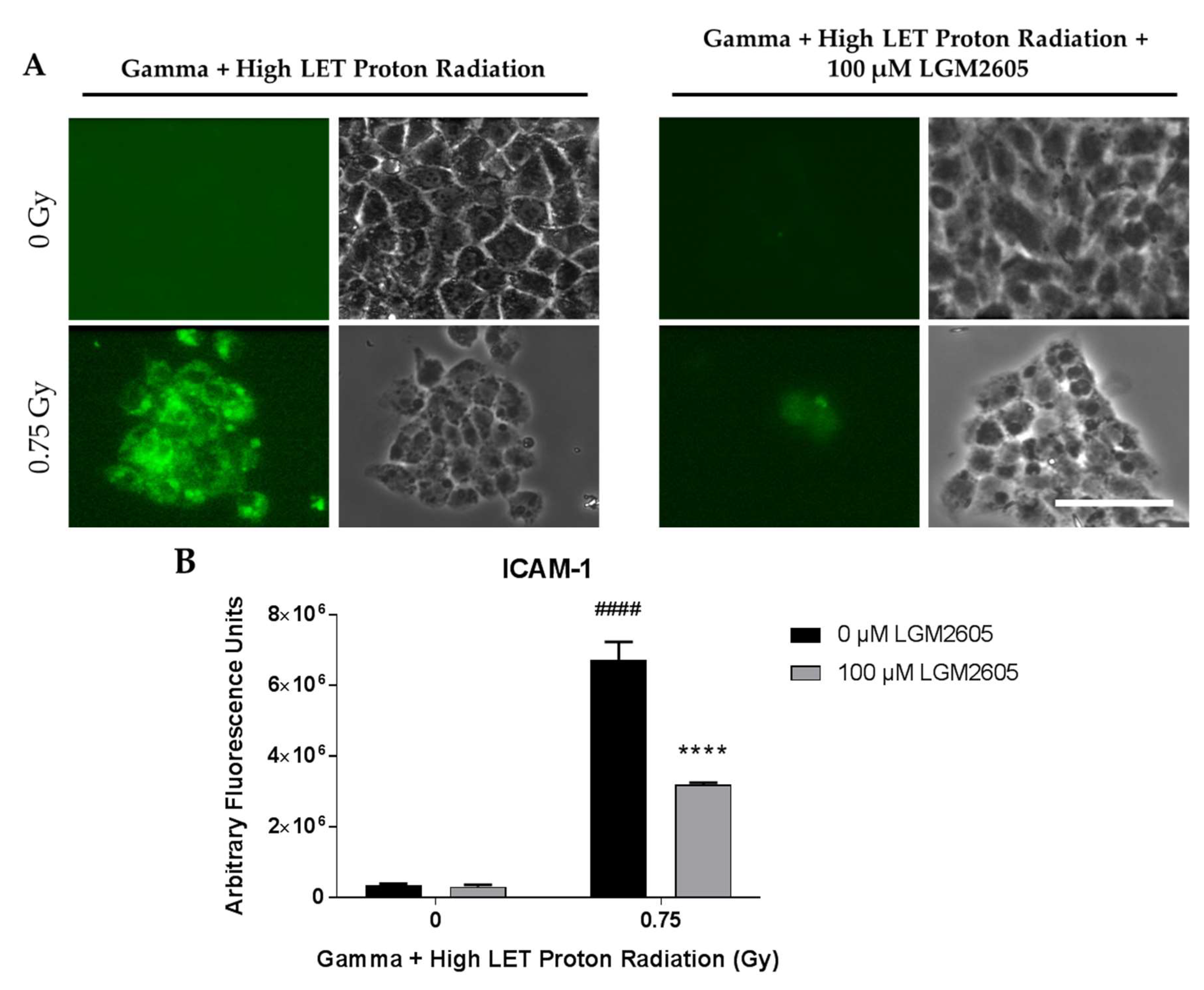
2.4. LGM2605 Ameliorates the Inflammatory Phenotype Induced by Mixed Field Gamma and Proton Radiation Exposure in In Vitro Lung Vascular Networks
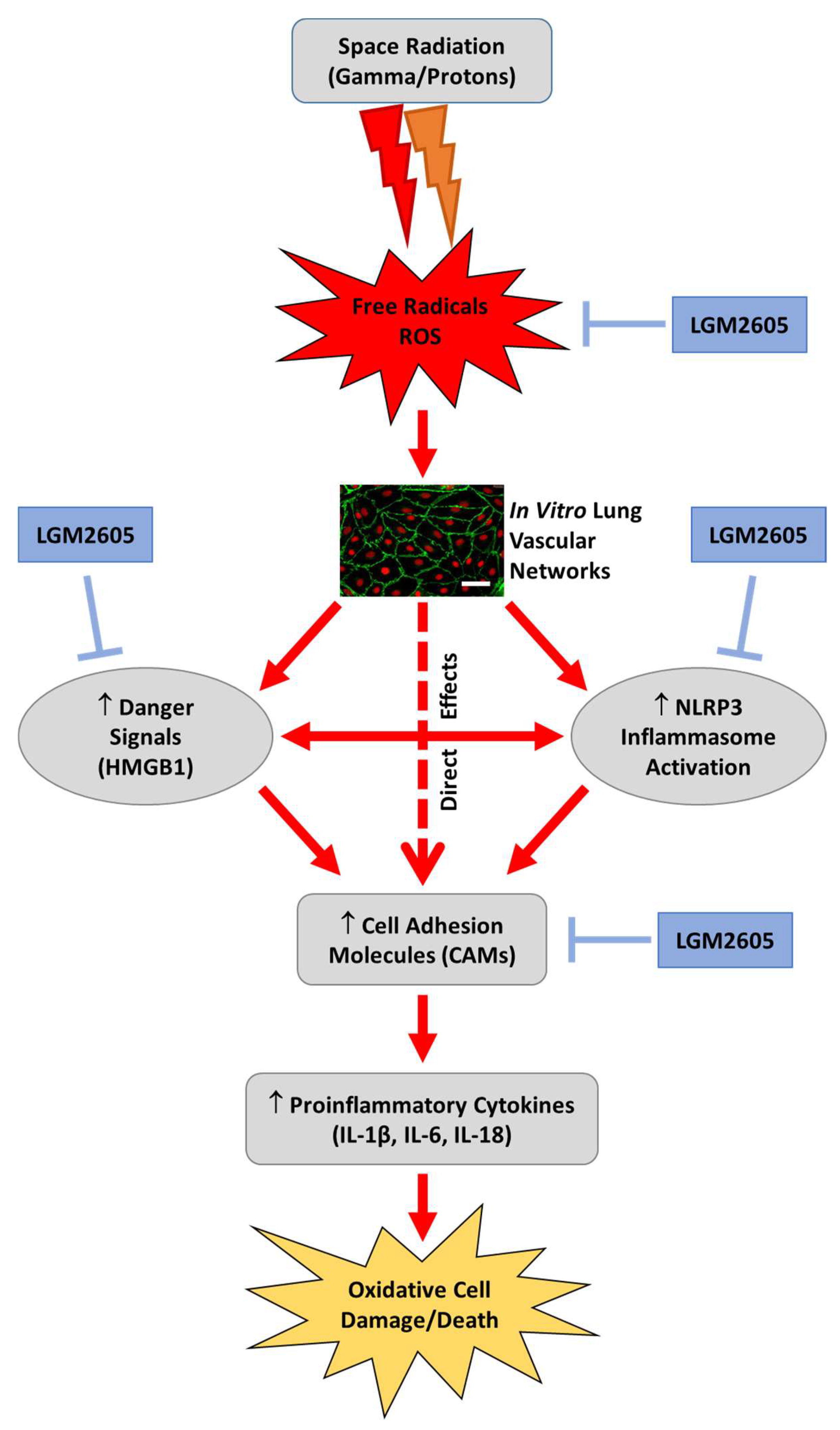
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Media
4.2. Exposure of Cells to Shear Stress
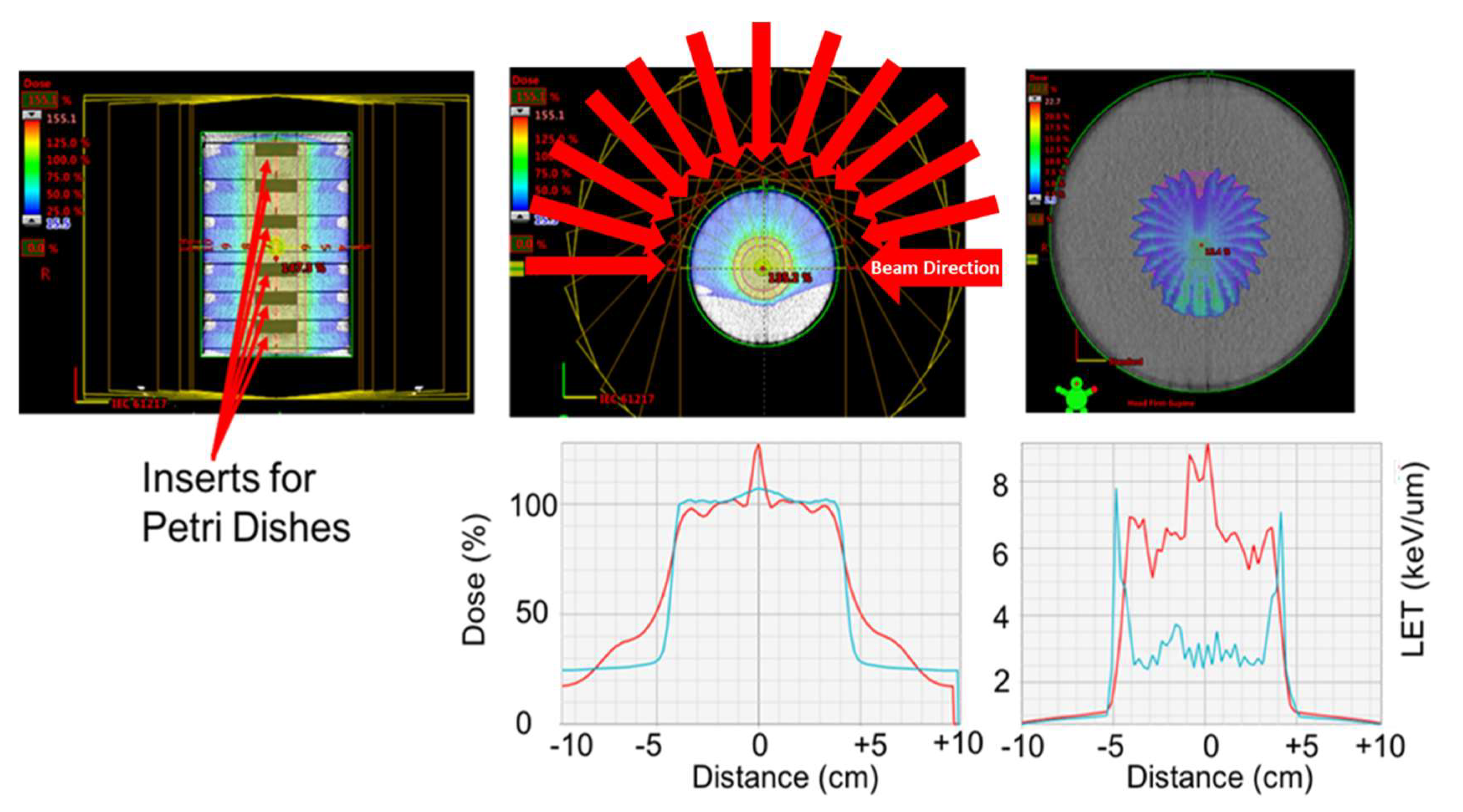
4.3. Radiation Exposure
4.4. LGM2605 Treatment
4.5. Determination of ICAM-1 and NLRP3 Expression
4.6. RNA Isolation and Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACS | active chlorine species |
| ARE | antioxidant response element |
| CTL | Control |
| DAMP | danger-associated molecular pattern |
| EC | endothelial cell |
| ELISA | enzyme-linked immunosorbent assay |
| FAEC FLC | flow-adapted endothelial cell flaxseed lignan component |
| FS | Flaxseed |
| GCR | galactic cosmic ray |
| GSTM1 | glutathione S-transferase mu 1 |
| HO-1 | heme oxygenase-1 |
| huPCLS | human precision-cut lung sections |
| HZE | high-energy nuclei |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| LGM2605 | synthetic secoisolariciresinol diglucoside |
| MFO | multi-field dose optimization |
| NLR | nucleotide-binding oligomerization domain-like receptor |
| NLRP3 | NOD-like receptor protein 3 |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| Nqo1 | NADPH: quinone oxidoreductase-1 |
| PAMP | pathogen-associated molecular pattern |
| PBS | phosphate-buffered saline |
| qPCR | quantitative polymerase chain reaction |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SDG | secoisolariciresinol diglucoside |
| SEP | solar energetic particle |
| SOBP | spread of Bragg peak |
| SPE | solar particle event |
| TNFα | tumor necrosis factor alpha |
| TRR | total radiation risk |
References
- Ehresmann, B.; Hassler, D.M.; Zeitlin, C.; Guo, J.; Kohler, J.; Wimmer-Schweingruber, R.F.; Appel, J.K.; Brinza, D.E.; Rafkin, S.C.; Bottcher, S.I.; et al. Charged particle spectra measured during the transit to Mars with the Mars Science Laboratory Radiation Assessment Detector (MSL/RAD). Life Sci. Space Res. (Amst) 2016, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Ehresmann, B.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Hassler, D.M.; Reitz, G.; Brinza, D.E.; Appel, J.; Bottcher, S.; Bohm, E.; et al. Measurements of the neutron spectrum in transit to Mars on the Mars Science Laboratory. Life Sci. Space Res. (Amst.) 2015, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef] [PubMed]
- Gauger, G.E.; Tobias, C.A.; Yang, T.; Whitney, M. The effect of space radiation of the nervous system. Adv. Space Res. 1986, 6, 243–249. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Peterson, L.E.; Feiveson, A.H.; Wear, M.L.; Manuel, F.K.; Tung, W.H.; Hardy, D.S.; Marak, L.J.; Cucinotta, F.A. NASA study of cataract in astronauts (NASCA). Report 1: Cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat. Res. 2009, 172, 10–20. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Plante, I.; Ponomarev, A.L.; Kim, M.H. Nuclear interactions in heavy ion transport and event-based risk models. Radiat. Prot. Dosim. 2011, 143, 384–390. [Google Scholar] [CrossRef]
- Turner, N.D.; Braby, L.A.; Ford, J.; Lupton, J.R. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition 2002, 18, 904–912. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Chappell, L.J. Updates to astronaut radiation limits: Radiation risks for never-smokers. Radiat. Res. 2011, 176, 102–114. [Google Scholar] [CrossRef]
- Shafirkin, A.V.; Petrov, V.M.; Kolomensky, A.V.; Shurshakov, V.A. Lifetime total radiation risk of cosmonauts for orbital and interplanetary flights. Adv. Space Res. 2002, 30, 999–1003. [Google Scholar] [CrossRef]
- Little, M.P. Cancer and non-cancer effects in Japanese atomic bomb survivors. J. Radiol. Prot. 2009, 29, A43–A59. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Jaenke, R.S.; Robbins, M.E.; Bywaters, T.; Whitehouse, E.; Rezvani, M.; Hopewell, J.W. Capillary endothelium. Target site of renal radiation injury. Lab. Investig. J. Tech. Methods Pathol. 1993, 68, 396–405. [Google Scholar]
- Rubin, D.; Griem, M.L. The histopathology of the irradiated endothelium. In The Radiation Biology of the Vascular Endothelium; Rubin, D., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 13–38. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Report to the General Assembly. Annex G. Early Effects in Man of High Doses of Radiation. 1988, pp. 545–565. Available online: http://www.unscear.org/unscear/en/publications/1988.html (accessed on 11 November 2018).
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef] [PubMed]
- Tungjai, M.; Whorton, E.B.; Rithidech, K.N. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to 28Silicon (28Si) ions. Radiat. Environ. Biophys. 2013, 52, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Takahashi, A.; Ohnishi, T. Radiation-induced adaptive responses and bystander effects. Biol. Sci. Space 2004, 18, 247–254. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: A novel mechanism of tissue radioprotection by flaxseed. Cancer Boil. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Chatterjee, S.; Pietrofesa, R.A.; Koziol-White, C.; Panettieri, R.A.; Lin, L.; Tuttle, S.; Berman, A.; Koumenis, C.; Christofidou-Solomidou, M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Velalopoulou, A.; Lehman, S.L.; Arguiri, E.; Solomides, P.; Koch, C.J.; Mishra, O.P.; Koumenis, C.; Goodwin, T.J.; Christofidou-Solomidou, M. Novel Double-Hit Model of Radiation and Hyperoxia-Induced Oxidative Cell Damage Relevant to Space Travel. Int. J. Mol. Sci. 2016, 17, 953. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2016, 37, 177–187. [Google Scholar] [CrossRef]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.S.; Latif, M.J.; Li, X.; Afthinos, J.N.; Ippagunta, N.; Schwartz, G.; Sagalovich, D.; Belsley, S.J.; Connery, C.P.; Jour, G.; et al. Dietary flaxseed protects against lung ischemia reperfusion injury via inhibition of apoptosis and inflammation in a murine model. J. Surg. Res. 2011, 171, e113–e121. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int. J. Mol. Sci. 2015, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Al-Mehdi, A.B.; Levitan, I.; Stevens, T.; Fisher, A.B. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am. J. Physiol. Cell. Physiol. 2003, 285, C959–C967. [Google Scholar] [CrossRef] [PubMed]
- Browning, E.; Wang, H.; Hong, N.; Yu, K.; Buerk, D.G.; DeBolt, K.; Gonder, D.; Sorokina, E.M.; Patel, P.; De Leon, D.D.; et al. Mechanotransduction drives post ischemic revascularization through K(ATP) channel closure and production of reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 872–886. [Google Scholar] [CrossRef]
- Chatterjee, C.; Browning, E.; Hong, N.; Debolt, K.M.; Sorokina, E.M.; Liu, W.; Birmbaum, M.J.; Fisher, A.B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H105–H114. [Google Scholar] [CrossRef]
- Tao, J.Q.; Sorokina, E.M.; Vazquez Medina, J.P.; Mishra, M.K.; Yamada, Y.; Satalin, J.; Nieman, G.F.; Nellen, J.R.; Beduhn, B.; Cantu, E.; et al. Onset of Inflammation With Ischemia: Implications for Donor Lung Preservation and Transplant Survival. Am. J. Transplant. 2016, 16, 2598–2611. [Google Scholar] [CrossRef]
- Brinley, A.A.; Theriot, C.A.; Nelman-Gonzalez, M.; Crucian, B.; Stowe, R.P.; Barrett, A.D.; Pierson, D.L. Characterization of Epstein-Barr virus reactivation in a modeled spaceflight system. J. Cell. Biochem. 2013, 114, 616–624. [Google Scholar] [CrossRef]
- Canova, S.; Fiorasi, F.; Mognato, M.; Grifalconi, M.; Reddi, E.; Russo, A.; Celotti, L. “Modeled microgravity” affects cell response to ionizing radiation and increases genomic damage. Radiat. Res. 2005, 163, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Fisher, A.B. Mechanotransduction: Forces, sensors, and redox signaling. Antioxid. Redox Signal. 2014, 20, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Fisher, A.B. Mechanotransduction in the endothelium: Role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid. Redox Signal. 2014, 20, 899–913. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hong, N.K.; Yu, K.; Fisher, A.B. Endothelial mechanotransduction with loss of shear is a signal for angiogenesis. FASEB J. 2010, 24, 602–603. [Google Scholar]
- Chatterjee, S.; Levitan, I.; Wei, Z.; Fisher, A.B. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 2006, 13, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nieman, G.F.; Christie, J.D.; Fisher, A.B. Shear stress-related mechanosignaling with lung ischemia: Lessons from basic research can inform lung transplantation. Am. J. Physiology. Lung Cell. Mol. Physiol. 2014, 307, L668–L680. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Tzima, E.; Del Pozo, M.A.; Kiosses, W.B.; Mohamed, S.A.; Li, S.; Chien, S.; Schwartz, M.A. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002, 21, 6791–6800. [Google Scholar] [CrossRef]
- Sasi, S.P.; Yan, X.; Lee, J.; Sisakyan, H.; Carrozza, J.; Goukassian, D.A. Radiation-associated degenerative cardiovascular risks during normal aging and after adverse CV event 10 months post-initial exposure. J. Radiat. Res. 2014, 55, i111–i112. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Song, J.; Carrozza, J.; Goukassian, D.A. Radiation-associated cardiovascular risks for future deep-space missions. J. Radiat. Res. 2014, 55, i37–i39. [Google Scholar] [CrossRef]
- Rodman, C.; Almeida-Porada, G.; George, S.K.; Moon, J.; Soker, S.; Pardee, T.; Beaty, M.; Guida, P.; Sajuthi, S.P.; Langefeld, C.D.; et al. In vitro and in vivo assessment of direct effects of simulated solar and galactic cosmic radiation on human hematopoietic stem/progenitor cells. Leukemia 2017, 31, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.R.; Li, M.; Chen, W.; Liu, T.; de Toledo, S.M.; Pandey, B.N.; Li, H.; Rabin, B.M.; Azzam, E.I. In vivo space radiation-induced non-targeted responses: Late effects on molecular signaling in mitochondria. Curr. Mol. Pharmacol. 2011, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Porada, G.; Rodman, C.; Kuhlman, B.; Brudvik, E.; Moon, J.; George, S.; Guida, P.; Sajuthi, S.P.; Langefeld, C.D.; Walker, S.J.; et al. Exposure of the Bone Marrow Microenvironment to Simulated Solar and Galactic Cosmic Radiation Induces Biological Bystander Effects on Human Hematopoiesis. Stem Cells Dev. 2018, 27, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Woodruff, P.; Hwang, W.T.; Patel, P.; Chatterjee, S.; Albelda, S.M.; Christofidou-Solomidou, M. The Synthetic Lignan Secoisolariciresinol Diglucoside Prevents Asbestos-Induced NLRP3 Inflammasome Activation in Murine Macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 7395238. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Chatterjee, S.; Park, K.; Arguiri, E.; Albelda, S.M.; Christofidou-Solomidou, M. Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605) Reduces Asbestos-Induced Cytotoxicity in an Nrf2-Dependent and -Independent Manner. Antioxidants (Basel) 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Boil. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M. The Role of Proinflammatory Cytokine Interleukin-18 in Radiation Injury. Health Phys. 2016, 111, 212–217. [Google Scholar] [CrossRef]
- Shan, Y.X.; Jin, S.Z.; Liu, X.D.; Liu, Y.; Liu, S.Z. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: Dose-response relationship, mechanisms and implications. Radiat. Environ. Biophys. 2007, 46, 21–29. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Q.; Chu, Y.; Xu, B.; Song, Q. The Influence of Radiotherapy on AIM2 Inflammasome in Radiation Pneumonitis. Inflammation 2016, 39, 1827–1834. [Google Scholar] [CrossRef]
- Sohn, S.H.; Lee, J.M.; Park, S.; Yoo, H.; Kang, J.W.; Shin, D.; Jung, K.H.; Lee, Y.S.; Cho, J.; Bae, H. The inflammasome accelerates radiation-induced lung inflammation and fibrosis in mice. Environ. Toxicol. Pharmacol. 2015, 39, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Inflammasomes, the cardinal pathology mediators are activated by pathogens, allergens and mutagens: A critical review with focus on NLRP3. Biomed. Pharmacother. 2017, 92, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, M.; Aravindan, N.; Sprague, E.A.; Mohan, S. Hemodynamic Flow-Induced Mechanotransduction Signaling Influences the Radiation Response of the Vascular Endothelium. Radiat. Res. 2016, 186, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chapman, K.E.; Fisher, A.B. Lung ischemia: A model for endothelial mechanotransduction. Cell. Biochem. Biophys 2008, 52, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M. Pulmonary Complications of Anticancer Treatment, 3rd ed.; Churchill Livingston: London, UK, 2003. [Google Scholar]
- Marks, L.B.; Fan, M.; Clough, R.; Munley, M.; Bentel, G.; Coleman, R.E.; Jaszczak, R.; Hollis, D.; Anscher, M. Radiation-induced pulmonary injury: Symptomatic versus subclinical endpoints. Int. J. Radiat. Biol. 2000, 76, 469–475. [Google Scholar] [PubMed]
- Franko, A.J.; Sharplin, J. Development of fibrosis after lung irradiation in relation to inflammation and lung function in a mouse strain prone to fibrosis. Radiat. Res. 1994, 140, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Scherpereel, A.; Santiago, J.; Lee, J.; McDonough, J.; Kinniry, P.; Arguiri, E.; Shuvaev, V.V.; Sun, J.; Cengel, K.; et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2006, 81, 196–205. [Google Scholar] [CrossRef]
- Lee, J.C.; Bhora, F.; Sun, J.; Cheng, G.; Arguiri, E.; Solomides, C.C.; Chatterjee, S.; Christofidou-Solomidou, M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am. J. Physiology. Lung Cell. Mol. Physiol. 2008, 294, L255–L265. [Google Scholar] [CrossRef]
- Gorshkova, I.; Zhou, T.; Mathew, B.; Jacobson, J.R.; Takekoshi, D.; Bhattacharya, P.; Smith, B.; Aydogan, B.; Weichselbaum, R.R.; Natarajan, V.; et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J. Lipid Res. 2012, 53, 1553–1568. [Google Scholar] [CrossRef]
- Jones, J.A.; Riggs, P.K.; Yang, T.C.; Pedemonte, C.H.; Clarke, M.S.; Feeback, D.L.; Au, W.W. Ionizing radiation-induced bioeffects in space and strategies to reduce cellular injury and carcinogenesis. Aviat. Space Environ. Med. 2007, 78, A67–A78. [Google Scholar]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Lindsey, K.; Borowicz, P.; Christofidou-Solomidou, M.; Reindl, K.M. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Lindsey, K.; Dhillon, H.; Mamidi, S.; Kittilson, J.; Christofidou-Solomidou, M.; Reindl, K.M. Enterolactone Induces G1-phase Cell Cycle Arrest in Nonsmall Cell Lung Cancer Cells by Downregulating Cyclins and Cyclin-dependent Kinases. Nutr. Cancer 2017, 69, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Mamidi, S.; Sreedasyam, A.; Chittem, K.; Pietrofesa, R.; Zuppa, A.; Moorthy, G.; Dyer, N.; Christofidou-Solomidou, M.; Reindl, K.M. Flaxseed Consumption Inhibits Chemically Induced Lung Tumorigenesis and Modulates Expression of Phase II Enzymes and Inflammatory Cytokines in A/J Mice. Cancer Prev. Res. (Phila) 2018, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Christofidou-Solomidou, M. Gamma-irradiation produces active chlorine species (ACS) in physiological solutions: Secoisolariciresinol diglucoside (SDG) scavenges ACS—A novel mechanism of DNA radioprotection. Biochim. Biophys. Acta 2016, 1860, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Nakamaru-Ogiso, E.; Andrake, M.; Christofidou-Solomidou, M. Synthetic secoisolariciresinol diglucoside (LGM2605) inhibits myeloperoxidase activity in inflammatory cells. Biochim. Biophys. Acta 2018, 1862, 1364–1375. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef]
- Dorr, W.; Alheit, H.; Appold, S.; Enghardt, W.; Haase, M.; Haberer, T.; Hinz, R.; Jakel, O.; Kellerer, A.M.; Kramer, M.; et al. Response of pig lung to irradiation with accelerated 12C-ions. Radiat. Environ. Biophys 1999, 38, 185–194. [Google Scholar]
- Nishimura, H.; Miyamoto, T.; Yamamoto, N.; Koto, M.; Sugimura, K.; Tsujii, H. Radiographic pulmonary and pleural changes after carbon ion irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 861–866. [Google Scholar] [CrossRef]
- Saito-Fujita, T.; Iwakawa, M.; Nakamura, E.; Nakawatari, M.; Fujita, H.; Moritake, T.; Imai, T. Attenuated lung fibrosis in interleukin 6 knock-out mice after C-ion irradiation to lung. J. Radiat. Res. 2011, 52, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, K.H.; Leith, J.T.; Lyman, J.T.; Tobias, C.A. Morphologic and morphometric analysis of the early effects of X-ray and heavy-ion irradiation of hamster lung. Am. J. Pathol. 1976, 82, 287–298. [Google Scholar] [PubMed]
- Wu, Z.; Wang, X.; Yang, R.; Liu, Y.; Zhao, W.; Si, J.; Ma, X.; Sun, C.; Liu, Y.; Tan, Y.; et al. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Exp. Ther. Med. 2013, 5, 771–776. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Pietrofesa, R.A.; Park, K.; Tao, J.-Q.; Carabe-Fernandez, A.; Berman, A.T.; Koumenis, C.; Sielecki, T.; Christofidou-Solomidou, M. LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. Int. J. Mol. Sci. 2019, 20, 176. https://doi.org/10.3390/ijms20010176
Chatterjee S, Pietrofesa RA, Park K, Tao J-Q, Carabe-Fernandez A, Berman AT, Koumenis C, Sielecki T, Christofidou-Solomidou M. LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. International Journal of Molecular Sciences. 2019; 20(1):176. https://doi.org/10.3390/ijms20010176
Chicago/Turabian StyleChatterjee, Shampa, Ralph A. Pietrofesa, Kyewon Park, Jian-Qin Tao, Alejandro Carabe-Fernandez, Abigail T. Berman, Constantinos Koumenis, Thais Sielecki, and Melpo Christofidou-Solomidou. 2019. "LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks" International Journal of Molecular Sciences 20, no. 1: 176. https://doi.org/10.3390/ijms20010176
APA StyleChatterjee, S., Pietrofesa, R. A., Park, K., Tao, J.-Q., Carabe-Fernandez, A., Berman, A. T., Koumenis, C., Sielecki, T., & Christofidou-Solomidou, M. (2019). LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. International Journal of Molecular Sciences, 20(1), 176. https://doi.org/10.3390/ijms20010176






