Abstract
The complement system plays an important role in inflammation, innate and acquired immunity, as well as homeostasis. Despite these functions, the effects of spaceflight conditions on the complement system have not yet been intensively studied. Consequently, we investigated the effects of five types of chronic stressors, similar to those encountered during a stay onboard the International Space Station, on C3 expression in larvae of the urodele amphibian Pleurodeles waltl. We focused on C3 because it is a critical component of this system. These studies were completed by the analysis of adult mice exposed to two models of inflight stressors. Our data show that simulating space radiation, or combining a modification of the circadian rhythm with simulated microgravity, affects the amount of C3 proteins. These results suggest that C3 expression could be modified under real spaceflight conditions, potentially increasing the risk of inflammation and associated tissue damage.
1. Introduction
Since Yuri Gagarin became the first human to leave the confines of Earth in 1961, an increasing number of humans have traveled to space and permanently inhabited space stations (Mir and then the International Space Station (ISS)) have been constructed. Studies performed on humans or animals sent to these stations, or subjected to ground-based models used to simulate space conditions, have revealed that these missions induce physiological dysregulations such as muscle atrophy, bone demineralization, cardiovascular and metabolic dysfunctions, impaired cognitive processes, and reduced immunological competence.
Immune system dysregulation occurs during flight and persists during 6-month orbital spaceflight [1,2]. A recent study revealed that about 50% of the astronauts who spent six months onboard the ISS faced immunological problems [3], thereby confirming in-flight dysregulation distinct from the influences of landing and readaptation following deconditioning [1,3,4]. All compartments of the immune system are affected by spaceflight. Concerning innate immunity, it was shown that astronauts’ monocytes exhibit phenotypic and cytokine-production deregulations and a reduced ability to engulf E. coli, to elicit an oxidative burst, and to degranulate [5,6,7]. Reactive oxygen species production by macrophages [8] as well as neutrophils phagocytosis and oxidative burst capacities are also significantly reduced [9]. Regarding acquired immunity, several studies reported a reduction of T-cell activation under low gravity conditions [10,11,12]. This phenomenon results from changes in gene expression, cellular interactions, cytoskeleton structure, signal transduction, and disturbed expression of cell cycle regulatory proteins (reviewed in [13,14]). As to humoral immunity, it was shown using the urodele amphibian Pleurodeles waltl as animal model [15] that spaceflight affects antibody production in response to an antigenic stimulation [16,17,18]. This was recently confirmed in mice subjected to hindlimb unloading, a model frequently used to simulate the effects of microgravity exposure [19]. Hypergravity and simulated microgravity also impaired the proliferative responses of murine B-lymphocytes [20,21]. Finally, the maturation of immune cells belonging to the myeloid [22,23,24,25,26], B- [27,28,29], and T- [30,31,32] lineages were shown to be reduced under low gravity conditions.
It therefore appears that immunological changes induced by space, an adverse environment in which human and animals face a combination of stressors (e.g., radiation, microgravity, confinement, isolation, and disrupted circadian rhythm), must be seriously investigated and understood to be able to preserve astronauts’ health during future deep-space exploration missions such as the deployment of a Lunar station followed by multiple Mars flyby missions.
In this context, the effects of spaceflight conditions on the complement system, which is key for immune surveillance and homeostasis, need to be studied. Complement constitutes a first line of defense against microbial intruders and orchestrates immunological and inflammatory processes [33]. Indeed, opsonization by complement fragments and pro-inflammatory signaling by anaphylatoxins (resulting from complement activation) recruits macrophages and enables phagocytosis and the formation of a lytic membrane attack complex on cells such as gram-negative bacteria. Complement coordinates innate immunity in cooperation with Toll-like receptors (TLR) [34], links innate response to both humoral and cellular adaptive immunity [35], and regulates the mobilization of hematopoietic stem-progenitor cells from bone marrow to replenish the immune system [36]. It also contributes to the resolution of inflammation by promoting the safe clearance of apoptotic cells and immune complexes [37,38]. Moreover, it participates in homeostasis by promoting tissue repair [39,40], potentiating coagulation to limit the spread of infection [41]; it is also implicated in synaptogenesis (it eliminates weak or immature synapses) [42] and in the differentiation and migration of neural progenitor cells [43]. Finally, it is established that any trigger that tips the delicate balance between complement activation and regulation can induce self-attack and lead to various immune, inflammatory, neurodegenerative, ischemic, and age-related diseases [33].
Given this functional repertoire, and a recent study having shown that hypergravity (an acute stressor faced during takeoff and landing) increases in a g-dependent manner C3 complement component expression [29], we investigated whether chronic stressors encountered during a space mission could affect the expression of this molecule conserved from amphibians to mammals [44,45]. We focused on C3 because it is the most abundant complement protein and all complement activation pathways (extracellularly through the classical, lectins, or alternative pathways or intracellularly via a cathepsin-dependent mechanism) converge at the level of this component [46].
2. Results
Different groups of P. waltl larvae at the same developmental stage were subjected, in parallel, to various environmental modifications similar to those encountered during a mission onboard the ISS or some combination thereof. These stressors were recreated in the laboratory to the best of our technical abilities and were applied for a maximum of 10 days. Experiments were performed on larvae, and not adults, because many of them can be accommodated in small aquaria (miniaquaria—Figure 1) conceived to allow amphibian embryo development onboard the ISS.
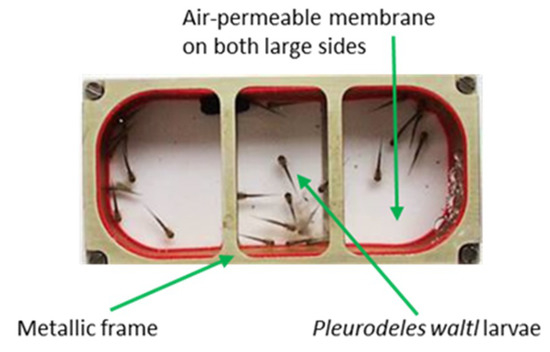
Figure 1.
Miniaquarium developed by EADS (European aeronautic defense and space company)-Astrium and the German Space Agency to allow amphibian embryo development onboard the international space station (ISS). These miniaquaria measure 80 mm in length by 40 mm in width and 20 mm in height, and have two transparent sides that are permeable to O2 and CO2. Once in the ISS, these miniaquaria are placed in the Kubik incubator to maintain temperature at 20 °C.
2.1. Effects of Simulated Microgravity, Circadian Rhythm Modification, Confinement, Vibration, and Radiation on P. waltl C3 Transcription
To determine how these five individual stressors affect C3 transcription, P. waltl larvae were exposed to simulated microgravity or darkness during 10 days, confinement, vibration, or a simulation of space radiation. The total dose of radiation mimicked the one received during a 10-day-stay in the Columbus Laboratory of the ISS [47,48], because miniaquaria were developed to fit within the Kubik incubator located in that part of the ISS. Then, C3 transcripts were quantified by RT-qPCR.
Our results show that the amount of C3 mRNAs is 1.5 times higher in larvae exposed to simulated microgravity (10−2 to 10−3 g) using a random positioning machine (RPM) (Figure 2A), 5 times higher in larvae subjected to the ISS radiation environment (Figure 2B), 1.7 times higher in larvae subjected to confinement (Figure 2C), and 0.5 times lower in larvae subjected to vibration (Figure 2D). The perturbation of the circadian rhythm (darkness as miniaquaria are in the dark within the Kubik incubator) did not affect the amount of C3 mRNAs (Figure 2E). Taken together, these results indicate that C3 expression is impacted at the transcriptional level by numerous spaceflight-associated stressors.
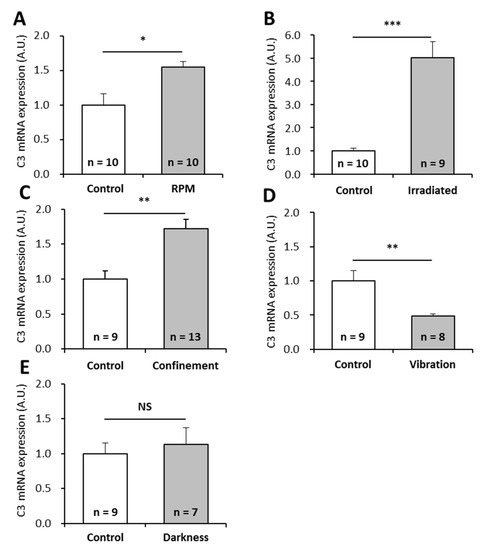
Figure 2.
Effects of individual stressors, similar to those encountered during a stay in the ISS, on P. waltl complement C3 mRNA levels. Complement C3 mRNAs were quantified by RT-qPCR in larvae subjected to (A) simulated microgravity, (B) simulated ISS radiation environment, (C) confinement, (D) vibration, or (E) obscurity. Relative values obtained with unstressed controls were set to 1. Results are expressed as means ± SEM. “A.U.” stands for “arbitrary units”. Differences were found using Student’s t-tests or Mann—Whitney tests. * p < 0.05, ** p < 0.01, *** p < 0.001, NS., no statistical difference.
2.2. Effects of Two Combinations of Stressors on P. waltl C3 Expression
Since astronauts are submitted to a combination of stressors during spaceflight, and not to single stressors as tested above, we wondered how combinations of individual stressors would affect C3 expression. Because it is technically impossible to test all possible combinations, we focused on two combinations of stressors that could be simulated in the laboratory: the combination of simulated microgravity with darkness (Figure 3) and the combination of space radiation with darkness (Figure 4). Interestingly, exposure of larvae to simulated microgravity and darkness did not affect C3 mRNA level (Figure 3A). Thus, it appears that combining simulated microgravity with darkness annuls the effect of microgravity on C3 mRNA expression. However, the amount of C3 proteins was decreased by a factor of two by comparison to controls (Figure 3B,C). Exposure of larvae to simulated space radiation and darkness led to a 3.5-fold increase of C3 mRNA level (Figure 4A) and a 1.5-fold increase of C3 proteins (Figure 4B,C) in comparison to controls. Combining darkness with space radiation did not statistically change the amount of C3 mRNAs and proteins when compared to space radiation alone.
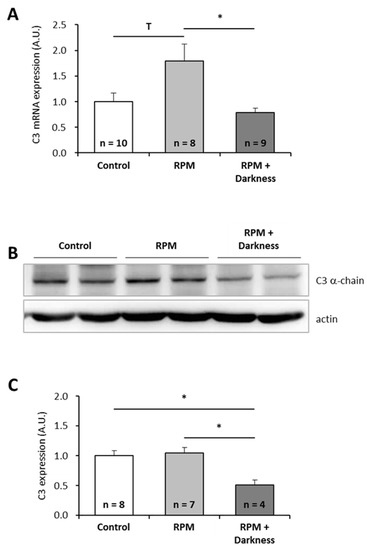
Figure 3.
Effects of the combination of simulated microgravity and darkness on P. waltl complement C3 expression. (A) C3 mRNA were quantified by RT-qPCR. (B, C) C3 proteins were quantified by western blotting. Western blot results were visualized by chemiluminescence, analyzed by densitometry, and normalized to α-actin. Relative values obtained with control larvae placed in a miniaquarium positioned on the base of the random positioning machine (RPM) and subjected to normal light conditions were set to one in (A) and (C). Results are expressed as means ± SEM. “A.U.” stands for “arbitrary units”. Differences were found using Kruskal–Wallis followed by a Dunn test (A) or ANOVA followed by a Tukey–Kramer test (C). * p < 0.05, T indicates a tendency (statistically significant difference with a 10% alpha risk but not with a 5% alpha risk), NS., no statistical difference.
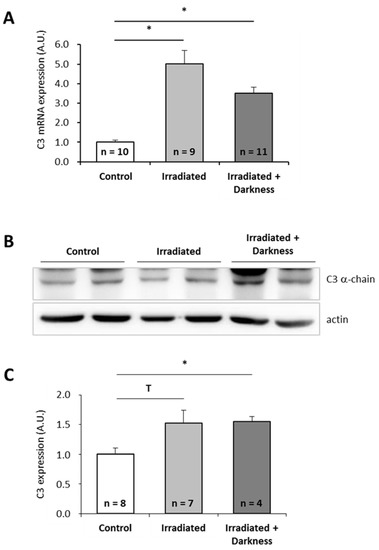
Figure 4.
Effects of the combination of radiation and darkness on P. waltl complement C3 expression. (A) C3 mRNA were quantified by RT-qPCR. (B, C) C3 proteins were quantified by western blotting. Western blot results were visualized by chemiluminescence, analyzed by densitometry and normalized to α-actin. Relative values obtained with control larvae reared in a nonirratiated miniaquarium subjected to normal light conditions were set to one in (A) and (C). Results are expressed as means ± SEM. “A.U.” stands for “arbitrary units”. Differences were found using Kruskal–Wallis followed by a Dunn test. * p < 0.05, T indicates a tendency (statistically significant difference with a 10% alpha risk but not with a 5% alpha risk), NS., no statistical difference.
2.3. Effects of Spaceflight Stressors on Murine C3 Expression
To further investigate the effects of chronic spaceflight-associated stressors, we exposed mice to hindlimb unloading (HU) used to simulate microgravity [49] or to the CUMS model used to simulate socio-environmental stressors encountered during a stay in a space station [50]. CUMS implies submitting mice to confinement, isolation, disrupted circadian rhythm, interpersonal issues, perturbation of spatial references, lower dietary intake, and uncomfortable living conditions in a chronic and unpredictable manner. This model replicates some spaceflight-induced immunological changes [50]. Then, we quantified C3 mRNAs and proteins in the liver of these animals because this tissue is the major source of complement. Our data show that HU and CUMS exposures did not affect the expression of C3 at the mRNA and protein levels (Figure 5 and Figure 6), indicating that simulations of chronic socio-environmental stressors or of microgravity did not affect C3 expression. This last observation is in agreement with the fact that RPM exposure did not modify the amount of P. waltl C3 proteins (Figure 3C).
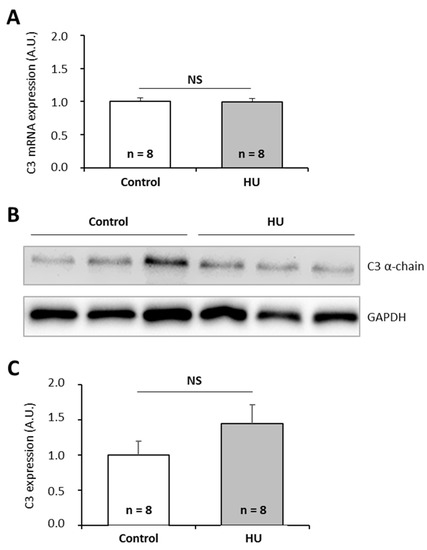
Figure 5.
Effects of the hindlimb unloading model, used to simulate microgravity, on murine complement C3 expression. (A) C3 mRNAs were quantified by RT-qPCR. (B, C) C3 proteins were quantified by western blotting. Western blot results were visualized by chemiluminescence, analyzed by densitometry, and normalized to GAPDH. Relative values obtained with control mice reared under normal housing conditions were set to one in (A) and (C). Results are expressed as means ± SEM. “A.U.” stands for “arbitrary units”. No differences were found using student’s t-tests. NS., no statistical difference.
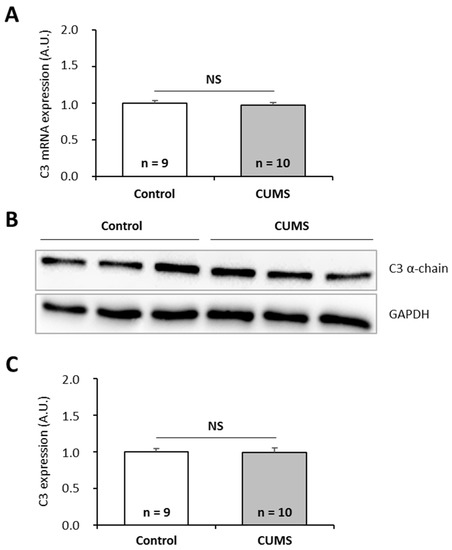
Figure 6.
Effects of the CUMS (Chroni Unpredictable Mild psychosocial and environmental Stressors) model, used to simulate socio-environmental stressors encountered during a stay in a space station, on murine complement C3 expression. (A) C3 mRNAs were quantified by RT-qPCR. (B, C) C3 proteins were quantified by western blotting. Western blot results were visualized by chemiluminescence, analyzed by densitometry, and normalized to GAPDH. Relative values obtained with control mice reared under normal housing conditions were set to one in (A) and (C). Results are expressed as means ± SEM. “A.U.” stands for “arbitrary units”. No differences were found using student’s t-tests. NS., no statistical difference.
3. Discussion
During spaceflight, humans and animals are subjected to various environmental modifications. In this study, P. waltl larvae were exposed to chronic stressors similar to those encountered during a stay in the ISS (microgravity, perturbed circadian rhythm, radiation, confinement, and vibration) to determine how these individual stressors, but also two combinations of them, affect the expression of a critical component of the complement system. P. waltl larvae were used as model organisms because (i) they fulfills many technical requirements associated with spaceflight experimentation; (ii) a hardware has been developed to allow amphibian development onboard the ISS; (iii) this model was shown to be useful for improving our understanding of the immunosuppressive effects of spaceflight [15] and (iv) the cardinal elements of its immune system are conserved [51,52,53], including C3. These studies were completed by the analysis of mice exposed to two models of inflight stressors.
We noted that, in complement of a previous study having shown that hypergravity exposure increases murine C3 protein expression [29], simulating space radiation or combining simulated microgravity with darkness affects the amount of P. waltl C3 proteins, thereby suggesting that C3 expression could be modified under real spaceflight conditions. Indeed, it is known that the complement system is sensitive to disturbances of homeostasis or assault [54]. Furthermore, as reported here, previous studies showed (i) that radiotherapy upregulates human and murine C3 at mRNA and protein levels [55], (ii) that head-down tilt bed, used to simulate weightlessness, does not change human plasma complement factor C3 concentration [56], and (iii) that sleep deprivation does not affect circadian fluctuation of human C3 and C4 plasma concentrations [57].
Changes of C3 mRNA and protein levels could be mounted by P. waltl larvae to neutralize harmful free radicals induced by stressors. Indeed, previous studies have shown that spaceflight can cause oxidative damage and the generation of reactive oxygen species (ROS) in humans, rodents, and amphibians [58,59,60,61], and that complement plays an important role in the inflammatory process after oxidative stress [62]. Furthermore, there is evidence that ROS activate the complement system and that C3 provides protection against oxidative stress [62,63,64]. These data might explain why Baqai and colleagues noted that exposure to the spaceflight environment increases murine anti-inflammatory mechanisms [65]. Another possibility is that danger signals, such as heat shock proteins (a hallmark of stressed cells and organisms is the increased synthesis of HSPs), whose expression is affected by spaceflight [66], regulate C3 expression, as shown, for example, in photodynamic therapy-treated tumors [67]. This hypothesis is supported by the presence of heat shock binding sites in human, mouse, and rat C3 promotors [45]. Given the conservation of C3 across vertebrates, it is very likely that the promotor of the P. waltl C3 gene also contains such binding sites.
Changes in C3 expression could be involved in numerous spaceflight-associated physiological changes because, besides its obvious involvement in eliminating microbes, complement participates in such diverse processes as synapse maturation, clearance of immune complexes, angiogenesis, mobilization of hematopoietic stem-progenitor cells, tissue regeneration, and lipid metabolism [33]. Furthermore, this system is required at early stage of animal development, for example, at P. waltl [45] and X. laevis [68] neurulation stages but also for the proper migration of neural crest cells during early vertebrate development [69].
In the future, it will be interesting to quantify complement activation products, because numerous factors can induce the production of these molecules. Indeed, in addition to pathway-dependent activations, coagulation factors such as thrombin, plasmin, FXa (factor Xa), and FXIa (factor XIa) have been shown to induce the cleavage of C3 and C5 proteins promoting the generation of C3a and C5a fragments [70]. Furthermore, radiation upregulate C3a and C5a production [55] and modified membranes of late apoptotic and necrotic cells are potent activators of complement [33]. Thus, changes in coagulation cascade protein expression noted during and after spaceflight [29,71,72], space radiation, and apoptosis, which is frequently increased in microgravity [73,74,75,76], could increase the production of C3a and C5a. Since these molecules have pro-inflammatory properties and increase the expression of MHC (major histocompatibility complex) class II and costimulatory molecules on antigen presenting cells such as dendritic cells, they could influence T cell activation and polarization towards a tolerogenic or inflammatory profile [77,78]. In support of this hypothesis, a recent in vivo study in which OT-II cells (CD4+ T cells expressing a transgenic TCR specifically recognizing chicken ovalbumin) were transferred into mice, which were stimulated with ovalbumin inflight, showed an alteration of tolerance [79] and an increase of pro-inflammatory cytokines produced by murine splenocytes. This last observation could be mediated via extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) pathways [80] that are sensitive to microgravity [81,82,83]. Finally, as C3a and C5a anaphylatoxins are powerful chemoattractants for neutrophils, monocytes, and macrophages, the modification of their production could help explain changes in leukocyte distribution noted after space missions [13,14].
In conclusion, the analysis of P. waltl larvae submitted to recreated-in-the-lab ISS-associated stressors show that some of them affect C3 expression, potentially increasing the risk of inflammation and associated tissue damages that could affect various organs and physiological systems during future deep-space missions. Future investigations performed on mammals embarked in a space station will be required to confirm this study and to determine if spaceflight affects the production of complement activation products that have numerous essential biological functions. Given the sensitivity of the complement system to a vast array of stimuli, strategies to mitigate the potential increase of complement activation products will likely need to target several factors including the control of microbial load inside the spacecraft and strategies to moderate stress responses such as β-blockers as suggested by Crucian and colleagues [84].
4. Materials and Methods
4.1. Animals
P. waltl embryos at stage 19–20 (3 days after laying) and larvae at stage 34 (11 days after laying) or 36 (13 days after laying) of development, as defined by Shi and Boucaut [85], were used in this study and reared in Evian spring water to avoid potential contamination/infection during treatments.
Three-month-old C57BL/6J male mice were purchased from Janvier Laboratories (Le Genest-Saint-Isle, France). Mice were housed in vented animal cabinets (Noroit, Bouaye, France) under controlled temperature (22 °C) and 12 h light/dark cycles. Prior to the start of the experiments, mice were allowed to rest for one week in groups of four in standard cages.
Animals were treated in accordance with the French Legislation and the Council Directive of the European Communities on the Protection of Animals Used for Experimental and Other Scientific Purposes (2010/63/UE). Local ethic committee approved the protocols (“Comité Ethique Lorrain en Matière d’Expérimentation Animale”, authorizations 04112004, and 20120008).
4.2. Simulation of Microgravity Using the RPM
Stage 19–20 embryos were placed in a miniaquarium (Figure 1) developed by EADS-Astrium and the German Space Agency to allow amphibian embryo development onboard the ISS. This miniaquarium was mounted on a desktop random positioning machine (RPM) (Dutch Space, Leiden, Netherlands) placed at 20 ± 2 °C. RPM is a 3D-clinostat classically used to simulate microgravity. Herranz et al. [86] indicated that RPM might be used with very early larval stages (before they can swim freely). This is the case here because, due to its slow development, P. waltl hatching occurs 13 days after laying [85], just at the end of RPM exposure. Random speed, direction, and interval, with an angular velocity of rotation between 55 and 65 degrees per second, were applied on the RPM for 10 days. The continuous three-dimensional movement of the samples provided by the RPM randomized the direction of the gravity force, resulting in an average net force approaching zero, therefore simulating microgravity. As negative control, another miniaquarium was placed on the base of the RPM.
4.3. Circadian Rhythm Perturbation
Because miniaquaria are kept in the dark in the ISS (they are stored inside the Kubik incubator (Comat Aerospace, Toulouse, France) located inside the Columbus Laboratory), we investigated the effects of continuous darkness. For that purpose, a miniaquarium containing embryos at stage 19–20 of development was kept in the dark 24h/24h during 10 days at 20 °C. A miniaquarium containing embryos at the same developmental stage was subjected for the same duration to natural light/dark cycles for comparison.
4.4. Simulation of Space Radiation
The ISS radiation environment was simulated using 137Cs γ (low-linear energy transfer (LET)) and 252Cf neutron (high-LET particles) sources. 137Cs is a mono-energetic source of 0.662 MeV γ rays (LET up to 10 keV μm−1). The neutron source 252Cf is expelling a neutron spectrum with an average energy of 2.1 MeV (LET up to 250 keV μm−1) [87]. Stage 34 P. waltl larvae were housed in a miniaquarium and exposed to ionizing radiation under rotation at 2 r.p.m for 65 h within the calibration room facility of SCK•CEN, as previously described [27]. The total dose received during 65 h (30 µSv/h totaling 1.950 mSv, energy of 660 keV of γ rays and 31.2 µ Sv/h totaling 2.028mSv, mean energy of 2.1 MeV of neutrons) imitated the one received during a stay of 10 days in the ISS as determined by Berger et al. [47,48]. One control miniaquarium was kept outside the irradiation bunker at 20 °C for the same duration.
4.5. Confinement
P. waltl larvae at stage 36 of development were placed in 0.5 mL Eppendorf tubes (one larvae/tube) in 400 µL of water at 20 °C during 7 h to induce confinement. Note that the volume of water could not be lowered and the duration of this experiment could not be extended due to oxygen consumption. Larvae reared under classical conditions were used as controls.
4.6. Vibration
To evaluate the impact of vibration, P. waltl larvae at stage 36 of development were placed in a Falcon tube containing 50 mL of water at 20 °C and subjected to vibration at 15 Hz for 5 h. Another group of P. waltl larvae at the same developmental stage was placed in a Falcon tube and kept at 20 °C on the bench for comparison.
4.7. Combinations of Stressors
During a stay in the ISS, animals are simultaneously subjected to the stressors presented above. However, in a laboratory, it is technically impossible to combine all these stressors. Consequently, we focused on two combinations of stressors: (i) the combination of simulated microgravity with darkness and (ii) the combination of simulated radiation with darkness. In the first case, a miniaquarium containing P. waltl embryos at stage 19–20 of development was kept in the dark and subjected to simulated microgravity (RPM) during 10 days at 20 °C. Control embryos were placed in a miniaquarium located on the base of the RPM and subjected to normal light conditions. In the second case, a miniaquarium containing P. waltl embryos at stage 19–20 of development was kept in the dark during 10 days at 20 °C and subjected to simulated space radiation during the last 65 h. Embryos of the same developmental stage placed in a miniaquarium and reared under standard conditions were used as controls.
4.8. Simulation of Microgravity using the Hindlimb Unloading Model
Mice were isolated five days before the beginning of the procedure in standard or hindlimb unloading (HU) cages (312 mm in length, 197 mm in width, and 260 mm in height). Mice were suspended by using a dressing retention sheet wrapped around the tail and a wire hooked on a swivel pulley system as previously described [20,28]. During suspension, mice were provided with food and water ad libitum. Unsuspended control mice were kept individually in same size cages.
4.9. Simulation of Socio-Environmental Stressors Encountered During a Space Mission Using the CUMS Model
Mice were isolated and subjected to six mild environmental or psychosocial stressors: 30° cage tilt for 1 h, 2 h, or 15 h; confinement in a small cage (110 × 80 × 80 mm) for 1 h or 2 h; paired housing for 2 h; one 15 h overnight period with difficult access to food without a reduction in the daily food ration; one 15 h overnight period with permanent light; and one 15 h overnight period in a soiled cage (50 mL of water in 1000 mL of bedding) as previously described [50,88]. Control mice were housed by five in standard cages placed in another room of the animal facility.
4.10. RT-qPCR
At the end of treatments, P. waltl larvae and mice liver were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) for RNA extraction according to the instructions of the manufacturer. RNA was reverse transcribed using random primers, RNAout, and MML-V (Moloney murine leukemia virus) reverse transcriptase (Invitrogen, Cergy Pontoise, France). qPCR were then performed. Primers specific for P. waltl and murine C3 as well as housekeeping transcripts (Table 1) were designed using the Genscript software (http://genscript.com/ssl-bin/app/primer) in different exons to ensure that they could not hybridize to potential traces of genomic DNA. The specificity of each primer pair was checked with BLAST (basic local alignment search tool, NCBI, Bethesda, MD, USA). The Mesa Green qPCR Master Mix (Eurogentec, Seraing, Belgium) and a Mastercycler® realplex2 real-time PCR machine (Eppendorf, Hamburg, Germany) were used to performed real-time PCR. We used the following cycling protocol: 3 min at 95 °C, 40 cycles of 15 s at 94 °C, and 30 s at the annealing temperature indicated in Table 1. Amplification efficiencies were controlled with standard curves and validated in the range of 90%–110%. Each qPCR was performed in triplicate. Data were analyzed using the Pfaffl model [89]. Relative expressions, expressed in arbitrary units (A.U.), were calculated by comparison to housekeeping transcripts using GeNorm software and Vandesompele’s methodology [90].

Table 1.
Primers used in this study. F, forward. R, reverse.
4.11. Western Blotting
Proteins were prepared by lysing P. waltl larvae in lysis buffer containing 50 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS, 10% glycerol, 50 mM NaF, and 1 mM Na3VO4 or 20–25 mg of mice liver in lysis buffer containing 15 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100. Both buffers were supplemented with proteases inhibitor. Forty µg of proteins from P. waltl larvae and 30 µg of proteins from mice liver were heated at 95 °C for 5 min, run on 8% SDS-polyacrylamide gels, and electrotransferred to PVDF (polyvinylidene difluoride) membranes (Amersham, Buckinghamshire, UK). Membranes were incubated with antibodies detecting human and murine complement C3 alpha chain (GTX101316, Tebubio, Le Perray-en-Yvelines, France). As analysis revealed several bands, the specific one was identified based on its molecular weight and quantified. Then, membranes were stripped (stripping buffer from Thermo Fisher Scientific, Waltham, Massachusetts, USA) and incubated with an antibody against α-actin for P. waltl samples or an antibody against GAPDH for murine samples. Immunodetection was performed using the Pierce ECL western blotting substrate (Thermo Fisher Scientific, Illkrich, France). Signals were visualized by chemiluminescence (FX7, Vilbert-Lourmat, Marne la Vallée, France), analyzed by densitometry (ImageJ®, NIH, USA), and normalized to α-actin or GAPDH used as housekeeping proteins.
4.12. Statistics
Statistical analyses were performed using the StatView software (SAS Campus Drive Cary, NC, USA) and the Anastats website (http://www.anastats.fr/outils.php). Outlier values were determined by a boxplot of each studied group. When normality and homogeneity of variances were ascertained, as determined by Kolmogorov-Smirnov and Fisher tests, respectively, student’s t-tests were used to perform two groups’ comparisons. When data were not normally distributed or if there was a heterogeneity of variances, Mann—Whitney tests were used. For three groups’ comparisons, homogeneity of variances was checked using the Levene test. If there was a heterogeneity of variances, Kruskal–Wallis tests were performed followed by a Dunn test for two by two comparisons. In the other cases, ANOVA tests were done followed by a Tukey–Kramer test. Stars indicate statistically significant differences. T indicates trends determined using a 10% alpha risk. All results are shown as means ± SEM.
Author Contributions
Conceptualization, J.-P.F.; Formal analysis, J.J. and S.G.; Funding acquisition, S. B. and J.-P.F.; Investigation, N.G., J.J., S.B., and S.G.; Supervision, J.-P.F.; Validation, N.G., J.J., S.K., and S.G.; Visualization, J.J. and S.G.; Writing—original draft, J.-P.F.; Writing—review and editing, N.G., J.J., S.K., S.B., S.G., and J.-P.F.
Funding
This research was funded by the French National Space Agency (“Centre National d’Etudes Spatiales”, grants DAR 4800000786, DAR 4800000841, and DAR 4800000894), the French Ministry of Higher Education and Research, the Université de Lorraine, the Région Lorraine, and the Belgian Science Policy (grant 42-000-90-380, C4000109861 ESA/PRODEX IMPULSE contract).
Acknowledgments
We thank C. Tankosic for her efficient technical support and SCK•CEN staff for support during irradiation experiments.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Abbreviations
| A.U. | Arbitrary unit |
| BLAST | Basic local alignment search tool |
| EADS | European aeronautic defense and space company |
| ERK | Extracellular signal-regulated kinase |
| FXa | Factor Xa |
| FXIa | Factor XIa |
| HU | Hindlimb unloading |
| ISS | International space station |
| MHC | Major histocompatibility complex |
| MML-V | Moloney murine leukemia virus |
| PI3K | Phosphatidylinositol 3-kinase |
| PVDF | Polyvinylidene difluoride |
| RT-qPCR | Reverse transcription-quantitative PCR |
| RPM | Random positioning machine |
| ROS | Reactive oxygen species |
| TLR | Toll-like receptors |
References
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. Npj Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef]
- Crucian, B.; Babiak-Vazquez, A.; Johnston, S.; Pierson, D.L.; Ott, C.M.; Sams, C. Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 2016, 9, 383–391. [Google Scholar] [CrossRef]
- Crucian, B.; Johnston, S.; Mehta, S.; Stowe, R.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Laudenslager, M.L.; Sams, C. A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J. Allergy Clin. Immunol. Pract. 2016, 4, 759–762.e8. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Quiriarte, H.; Pierson, D.; Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat. Space Environ. Med. 2011, 82, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Simons, E.R.; Kapadia, A.S.; Ott, C.M.; Pierson, D.L. Effect of spaceflight on ability of monocytes to respond to endotoxins of gram-negative bacteria. Clin. Vaccine Immunol. CVI 2008, 15, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Rykova, M.P.; Antropova, E.N.; Larina, I.M.; Morukov, B.V. Humoral and cellular immunity in cosmonauts after the ISS missions. Acta Astronaut. 2008, 63, 697–705. [Google Scholar] [CrossRef]
- Brungs, S.; Kolanus, W.; Hemmersbach, R. Syk phosphorylation—a gravisensitive step in macrophage signalling. Cell Commun. Signal. CCS 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Mark Ott, C.; Pierson, D.L. Changes in neutrophil functions in astronauts. Brain. Behav. Immun. 2004, 18, 443–450. [Google Scholar] [CrossRef]
- Cogoli, A.; Tschopp, A.; Fuchs-Bislin, P. Cell sensitivity to gravity. Science 1984, 225, 228–230. [Google Scholar] [CrossRef]
- Cogoli, A. The effect of space flight on human cellular immunity. Environ. Med. Annu. Rep. Res. Inst. Environ. Med. Nagoya Univ. 1993, 37, 107–116. [Google Scholar]
- Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Rizvi, A.; Chapes, S.K.; Stodieck, L.S.; Ferguson, V.L.; Pecaut, M.J. Spaceflight effects on T lymphocyte distribution, function and gene expression. J. Appl. Physiol. Bethesda Md 1985 2009, 106, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, J.-P.; Crucian, B.E.; de Quervain, D.J.-F.; Grimm, D.; Montano, N.; Praun, S.; Roozendaal, B.; Schelling, G.; Thiel, M.; Ullrich, O.; et al. Towards human exploration of space: The THESEUS review series on immunology research priorities. NPJ Microgravity 2016, 2, 16040. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.-P. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, J.-P. Contribution of the urodele amphibian Pleurodeles waltl to the analysis of spaceflight-associated immune system deregulation. Mol. Immunol. 2013, 56, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Bascove, M.; Huin-Schohn, C.; Guéguinou, N.; Tschirhart, E.; Frippiat, J.-P. Spaceflight-associated changes in immunoglobulin VH gene expression in the amphibian Pleurodeles waltl. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Boxio, R.; Dournon, C.; Frippiat, J.-P. Effects of a long-term spaceflight on immunoglobulin heavy chains of the urodele amphibian Pleurodeles waltl. J. Appl. Physiol. Bethesda Md 1985 2005, 98, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Bascove, M.; Guéguinou, N.; Schaerlinger, B.; Gauquelin-Koch, G.; Frippiat, J.-P. Decrease in antibody somatic hypermutation frequency under extreme, extended spaceflight conditions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Rettig, T.A.; Bye, B.A.; Nishiyama, N.C.; Hlavacek, S.; Ward, C.; Pecaut, M.J.; Chapes, S.K. Effects of skeletal unloading on the antibody repertoire of tetanus toxoid and/or CpG treated C57BL/6J mice. PLoS ONE 2019, 14, e0210284. [Google Scholar] [CrossRef] [PubMed]
- Gaignier, F.; Schenten, V.; De Carvalho Bittencourt, M.; Gauquelin-Koch, G.; Frippiat, J.-P.; Legrand-Frossi, C. Three weeks of murine hindlimb unloading induces shifts from B to T and from th to tc splenic lymphocytes in absence of stress and differentially reduces cell-specific mitogenic responses. PLoS ONE 2014, 9, e92664. [Google Scholar] [CrossRef]
- Guéguinou, N.; Bojados, M.; Jamon, M.; Derradji, H.; Baatout, S.; Tschirhart, E.; Frippiat, J.-P.; Legrand-Frossi, C. Stress response and humoral immune system alterations related to chronic hypergravity in mice. Psychoneuroendocrinology 2012, 37, 137–147. [Google Scholar] [CrossRef]
- Vacek, A.; Michurina, T.V.; Serova, L.V.; Rotkovská, D.; Bartonícková, A. Decrease in the number of progenitors of erythrocytes (BFUe, CFUe), granulocytes and macrophages (GM-CFC) in bone marrow of rats after a 14-day flight onboard the Cosmos-2044 Biosatellite. Folia Biol. (Praha) 1991, 37, 35–41. [Google Scholar]
- Ichiki, A.T.; Gibson, L.A.; Jago, T.L.; Strickland, K.M.; Johnson, D.L.; Lange, R.D.; Allebban, Z. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J. Leukoc. Biol. 1996, 60, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Wiesmann, W.; Kidwell, W.; Cannon, T.; Kerns, L.; Serke, C.; Delaplaine, T.; Pranger, A.; Lee, K.P. Effect of spaceflight on human stem cell hematopoiesis: Suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 1996, 60, 69–76. [Google Scholar] [CrossRef]
- Ortega, M.T.; Pecaut, M.J.; Gridley, D.S.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K. Shifts in bone marrow cell phenotypes caused by spaceflight. J. Appl. Physiol. Bethesda Md 1985 2009, 106, 548–555. [Google Scholar] [CrossRef]
- Sotnezova, E.V.; Markina, E.A.; Andreeva, E.R.; Buravkova, L.B. Myeloid Precursors in the Bone Marrow of Mice after a 30-Day Space Mission on a Bion-M1 Biosatellite. Bull. Exp. Biol. Med. 2017, 162, 496–500. [Google Scholar] [CrossRef]
- Huin-Schohn, C.; Guéguinou, N.; Schenten, V.; Bascove, M.; Gauquelin-Koch, G.; Baatout, S.; Tschirhart, E.; Frippiat, J.-P. Gravity changes during animal development affect IgM heavy-chain transcription and probably lymphopoiesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lescale, C.; Schenten, V.; Djeghloul, D.; Bennabi, M.; Gaignier, F.; Vandamme, K.; Strazielle, C.; Kuzniak, I.; Petite, H.; Dosquet, C.; et al. Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Tascher, G.; Gerbaix, M.; Maes, P.; Chazarin, B.; Ghislin, S.; Antropova, E.; Vassilieva, G.; Ouzren-Zarhloul, N.; Gauquelin-Koch, G.; Vico, L.; et al. Analysis of femurs from mice embarked on board BION-M1 biosatellite reveals a decrease in immune cell development, including B cells, after 1 wk of recovery on Earth. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, fj201801463R. [Google Scholar] [CrossRef]
- Woods, C.C.; Banks, K.E.; Gruener, R.; DeLuca, D. Loss of T cell precursors after spaceflight and exposure to vector-averaged gravity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1526–1528. [Google Scholar] [CrossRef]
- Woods, C.C.; Banks, K.E.; Lebsack, T.W.; White, T.C.; Anderson, G.A.; Maccallum, T.; Gruener, R.; DeLuca, D. Use of a microgravity organ culture dish system to demonstrate the signal dampening effects of modeled microgravity during T cell development. Dev. Comp. Immunol. 2005, 29, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Ghislin, S.; Ouzren-Zarhloul, N.; Kaminski, S.; Frippiat, J.-P. Hypergravity exposure during gestation modifies the TCRβ repertoire of newborn mice. Sci. Rep. 2015, 5, 9318. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lambris, J.D. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010, 31, 154–163. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Jalili, A.; Shirvaikar, N.; Marquez-Curtis, L.; Qiu, Y.; Korol, C.; Lee, H.; Turner, A.R.; Ratajczak, M.Z.; Janowska-Wieczorek, A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp. Hematol. 2010, 38, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Flierman, R.; Daha, M.R. The clearance of apoptotic cells by complement. Immunobiology 2007, 212, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Blom, A.M.; Gasque, P. Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 2008, 45, 1199–1207. [Google Scholar] [CrossRef]
- Markiewski, M.M.; DeAngelis, R.A.; Lambris, J.D. Liver inflammation and regeneration: Two distinct biological phenomena or parallel pathophysiologic processes? Mol. Immunol. 2006, 43, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mastellos, D.C.; Deangelis, R.A.; Lambris, J.D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013, 25, 29–38. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. Complement and coagulation: Strangers or partners in crime? Trends Immunol. 2007, 28, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Anderson, A.J.; Barnum, S.R.; Stevens, B.; Tenner, A.J. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J. Neurochem. 2008, 107, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Ståhlberg, A.; Dragunow, M.; Pekny, M.; Pekna, M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells Dayt. Ohio 2009, 27, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Yoshizaki, F. Evolution of the complement system. Mol. Immunol. 2004, 40, 897–902. [Google Scholar] [CrossRef]
- Guéguinou, N.; Huin-Schohn, C.; Ouzren-Zarhloul, N.; Ghislin, S.; Frippiat, J.-P. Molecular cloning and expression analysis of Pleurodeles waltl complement component C3 under normal physiological conditions and environmental stresses. Dev. Comp. Immunol. 2014, 46, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Kolev, M.; Le Friec, G.; Leung, M.; Bertram, P.G.; Fara, A.F.; Subias, M.; Pickering, M.C.; Drouet, C.; Meri, S.; et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 2013, 39, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Przybyla, B.; Matthiä, D.; Reitz, G.; Burmeister, S.; Labrenz, J.; Bilski, P.; Horwacik, T.; Twardak, A.; Hajek, M.; et al. DOSIS & DOSIS 3D: Long-term dose monitoring onboard the Columbus Laboratory of the International Space Station (ISS). J. Space Weather Space Clim. 2016, 6, A39. [Google Scholar]
- Berger, T.; Burmeister, S.; Matthiä, D.; Przybyla, B.; Reitz, G.; Bilski, P.; Hajek, M.; Sihver, L.; Szabo, J.; Ambrozova, I.; et al. DOSIS & DOSIS 3D: Radiation measurements with the DOSTEL instruments onboard the Columbus Laboratory of the ISS in the years 2009–2016. J. Space Weather Space Clim. 2017, 7, A8. [Google Scholar]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. Bethesda Md 1985 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Gaignier, F.; Legrand-Frossi, C.; Stragier, E.; Mathiot, J.; Merlin, J.-L.; Cohen-Salmon, C.; Lanfumey, L.; Frippiat, J.-P. A Model of Chronic Exposure to Unpredictable Mild Socio-Environmental Stressors Replicates Some Spaceflight-Induced Immunological Changes. Front. Physiol. 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Schaerlinger, B.; Frippiat, J.-P. IgX antibodies in the urodele amphibian Ambystoma mexicanum. Dev. Comp. Immunol. 2008, 32, 908–915. [Google Scholar] [CrossRef]
- Bascove, M.; Frippiat, J.-P. Molecular characterization of Pleurodeles waltl activation-induced cytidine deaminase. Mol. Immunol. 2010, 47, 1640–1649. [Google Scholar] [CrossRef]
- Fonte, C.; Gruez, A.; Ghislin, S.; Frippiat, J.-P. The urodele amphibian Pleurodeles waltl has a diverse repertoire of immunoglobulin heavy chains with polyreactive and species-specific features. Dev. Comp. Immunol. 2015, 53, 371–384. [Google Scholar] [CrossRef]
- Kolev, M.; Le Friec, G.; Kemper, C. Complement--tapping into new sites and effector systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef]
- Surace, L.; Lysenko, V.; Fontana, A.O.; Cecconi, V.; Janssen, H.; Bicvic, A.; Okoniewski, M.; Pruschy, M.; Dummer, R.; Neefjes, J.; et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity 2015, 42, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.A.; Schwarzenberg, M.; Tkaczuk, J.; Hebrard, S.; Brandenberger, G.; Mauco, G.; Cogoli-Greuter, M.; Abbal, M. Head-down tilt bed rest and immune responses. Pflugers Arch. 2000, 441, R79–R84. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.S.; Lange, T.; Köhl, G.; Herrmann, A.; Tschulakow, A.V.; Naujoks, J.; Born, J.; Köhl, J. Sleep and circadian rhythm regulate circulating complement factors and immunoregulatory properties of C5a. Brain. Behav. Immun. 2011, 25, 1416–1426. [Google Scholar] [CrossRef]
- Hollander, J.; Gore, M.; Fiebig, R.; Mazzeo, R.; Ohishi, S.; Ohno, H.; Ji, L.L. Spaceflight downregulates antioxidant defense systems in rat liver. Free Radic. Biol. Med. 1998, 24, 385–390. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Rossi, F.; Zava, S.; Montorfano, G.; Adorni, L.; Cotronei, V.; Zanini, A.; Berra, B. Antioxidant metabolism in Xenopus laevis embryos is affected by stratospheric balloon flight. Cell Biol. Int. 2007, 31, 716–723. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Deeva, I.; Mariani, S.; Maiani, G.; Stancato, A.; Korkina, L. Monitoring antioxidant defenses and free radical production in space-flight, aviation and railway engine operators, for the prevention and treatment of oxidative stress, immunological impairment, and pre-mature cell aging. Toxicol. Ind. Health 2009, 25, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tahimic, C.G.T.; Globus, R.K. Redox Signaling and Its Impact on Skeletal and Vascular Responses to Spaceflight. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Lekowski, R.; Jordan, J.E.; Agah, A.; Stahl, G.L. Complement activation following oxidative stress. Mol. Immunol. 1999, 36, 941–948. [Google Scholar] [CrossRef]
- Collard, C.D.; Väkevä, A.; Morrissey, M.A.; Agah, A.; Rollins, S.A.; Reenstra, W.R.; Buras, J.A.; Meri, S.; Stahl, G.L. Complement activation after oxidative stress: Role of the lectin complement pathway. Am. J. Pathol. 2000, 156, 1549–1556. [Google Scholar] [CrossRef]
- Hart, M.L.; Walsh, M.C.; Stahl, G.L. Initiation of complement activation following oxidative stress. In vitro and in vivo observations. Mol. Immunol. 2004, 41, 165–171. [Google Scholar] [CrossRef]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. Bethesda Md 1985 2009, 106, 1935–1942. [Google Scholar] [CrossRef]
- Ishihara, A.; Fujino, H.; Nagatomo, F.; Takeda, I.; Ohira, Y. Gene expression levels of heat shock proteins in the soleus and plantaris muscles of rats after hindlimb suspension or spaceflight. J. Physiol. Sci. JPS 2008, 58, 413–417. [Google Scholar] [CrossRef]
- Stott, B.; Korbelik, M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol. Immunother. CII 2007, 56, 649–658. [Google Scholar] [CrossRef]
- McLin, V.A.; Hu, C.-H.; Shah, R.; Jamrich, M. Expression of complement components coincides with early patterning and organogenesis in Xenopus laevis. Int. J. Dev. Biol. 2008, 52, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Fontaine, C.; Theveneau, E.; Tzekou, A.; Tada, M.; Woods, M.; Page, K.M.; Parsons, M.; Lambris, J.D.; Mayor, R. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev. Cell 2011, 21, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. Baltim. Md 1950 2010, 185, 5628–5636. [Google Scholar] [CrossRef] [PubMed]
- Kimzey, S.L.; Ritzmann, S.E.; Mengel, C.E.; Fischer, C.L. Skylab experiment results: Hematology studies. Acta Astronaut. 1975, 2, 141–154. [Google Scholar] [CrossRef]
- Larina, I.M.; Percy, A.J.; Yang, J.; Borchers, C.H.; Nosovsky, A.M.; Grigoriev, A.I.; Nikolaev, E.N. Protein expression changes caused by spaceflight as measured for 18 Russian cosmonauts. Sci. Rep. 2017, 7, 8142. [Google Scholar] [CrossRef]
- Lewis, M.L.; Reynolds, J.L.; Cubano, L.A.; Hatton, J.P.; Lawless, B.D.; Piepmeier, E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 1007–1018. [Google Scholar] [CrossRef]
- Sharma, C.S.; Sarkar, S.; Periyakaruppan, A.; Ravichandran, P.; Sadanandan, B.; Ramesh, V.; Thomas, R.; Hall, J.C.; Wilson, B.L.; Ramesh, G.T. Simulated microgravity activates apoptosis and NF-kappaB in mice testis. Mol. Cell. Biochem. 2008, 313, 71–78. [Google Scholar] [CrossRef]
- Kang, C.-Y.; Zou, L.; Yuan, M.; Wang, Y.; Li, T.-Z.; Zhang, Y.; Wang, J.-F.; Li, Y.; Deng, X.-W.; Liu, C.-T. Impact of simulated microgravity on microvascular endothelial cell apoptosis. Eur. J. Appl. Physiol. 2011, 111, 2131–2138. [Google Scholar] [CrossRef]
- Lin, S.-C.; Gou, G.-H.; Hsia, C.-W.; Ho, C.-W.; Huang, K.-L.; Wu, Y.-F.; Lee, S.-Y.; Chen, Y.-H. Simulated Microgravity Disrupts Cytoskeleton Organization and Increases Apoptosis of Rat Neural Crest Stem Cells Via Upregulating CXCR4 Expression and RhoA-ROCK1-p38 MAPK-p53 Signaling. Stem Cells Dev. 2016, 25, 1172–1193. [Google Scholar] [CrossRef] [PubMed]
- Strainic, M.G.; Liu, J.; Huang, D.; An, F.; Lalli, P.N.; Muqim, N.; Shapiro, V.S.; Dubyak, G.R.; Heeger, P.S.; Medof, M.E. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008, 28, 425–435. [Google Scholar] [CrossRef]
- Weaver, D.J.; Reis, E.S.; Pandey, M.K.; Köhl, G.; Harris, N.; Gerard, C.; Köhl, J. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur. J. Immunol. 2010, 40, 710–721. [Google Scholar] [CrossRef]
- Chang, T.T.; Spurlock, S.M.; Candelario, T.L.T.; Grenon, S.M.; Hughes-Fulford, M. Spaceflight impairs antigen-specific tolerance induction in vivo and increases inflammatory cytokines. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 4122–4132. [Google Scholar] [CrossRef]
- Hawlisch, H.; Belkaid, Y.; Baelder, R.; Hildeman, D.; Gerard, C.; Köhl, J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity 2005, 22, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2015, 64, 303–311. [Google Scholar] [CrossRef]
- Tauber, S.; Hauschild, S.; Crescio, C.; Secchi, C.; Paulsen, K.; Pantaleo, A.; Saba, A.; Buttron, I.; Thiel, C.S.; Cogoli, A.; et al. Signal transduction in primary human T lymphocytes in altered gravity—results of the MASER-12 suborbital space flight mission. Cell Commun. Signal. CCS 2013, 11, 32. [Google Scholar] [CrossRef]
- Kim, J.; Montagne, K.; Nemoto, H.; Ushida, T.; Furukawa, K.S. Hypergravity down-regulates c-fos gene expression via ROCK/Rho-GTP and the PI3K signaling pathway in murine ATDC5 chondroprogenitor cells. PLoS ONE 2017, 12, e0185394. [Google Scholar] [CrossRef]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Shi, D.L.; Boucaut, J.C. The chronological development of the urodele amphibian Pleurodeles waltl (Michah). Int. J. Dev. Biol. 1995, 39, 427–441. [Google Scholar]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vanhavere, F.; Coeck, M. Comparison between thermoluminescence and electronic dosimetry results at the Belgian Nuclear Research Centre. Radiat. Prot. Dosimetry 2001, 96, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Fonte, C.; Kaminski, S.; Vanet, A.; Lanfumey, L.; Cohen-Salmon, C.; Ghislin, S.; Frippiat, J.-P. Socioenvironmental stressors encountered during spaceflight partially affect the murine TCR-β repertoire and increase its self-reactivity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).