Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats
Abstract
1. Introduction
2. Results
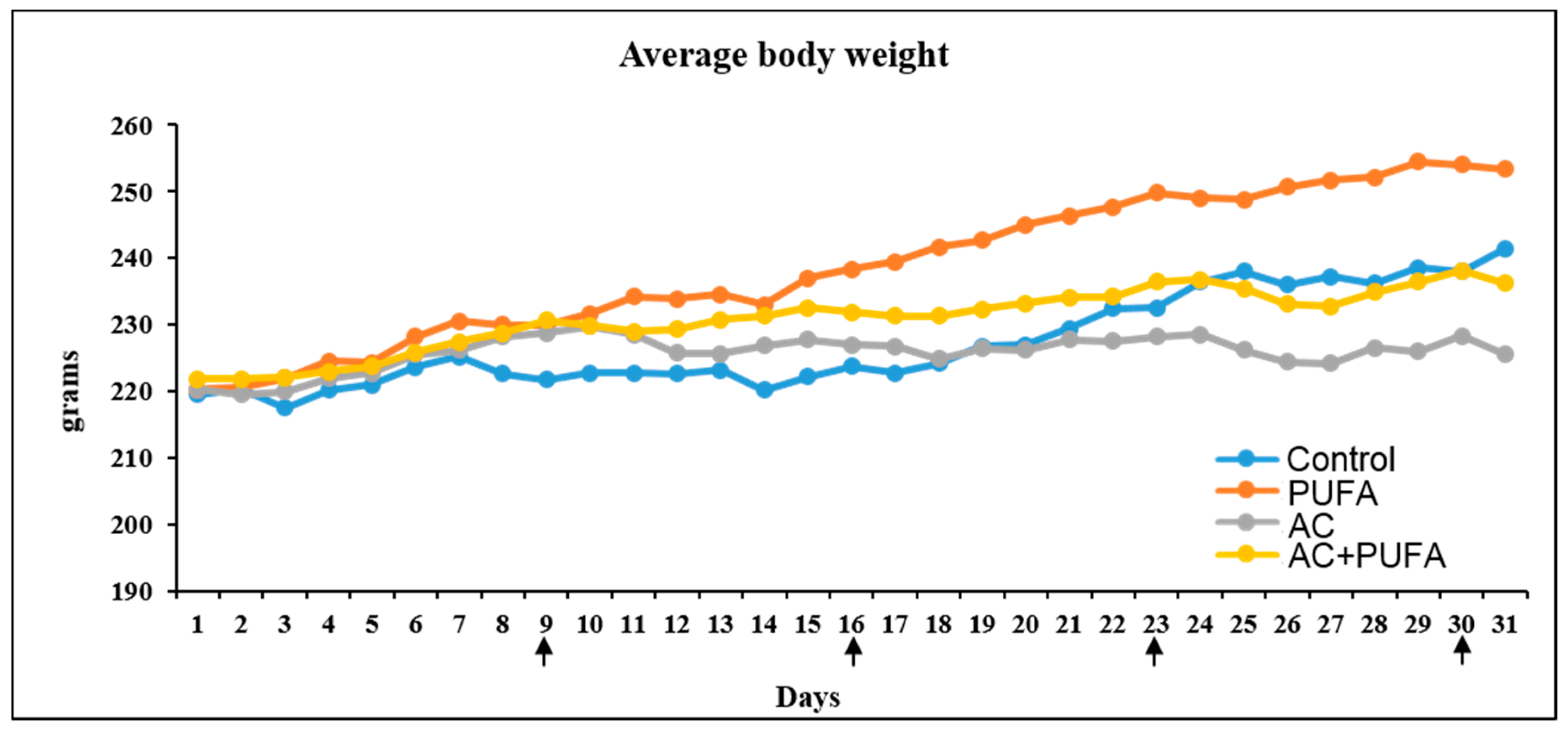
2.1. Treatment Effects on Body Weight Changes
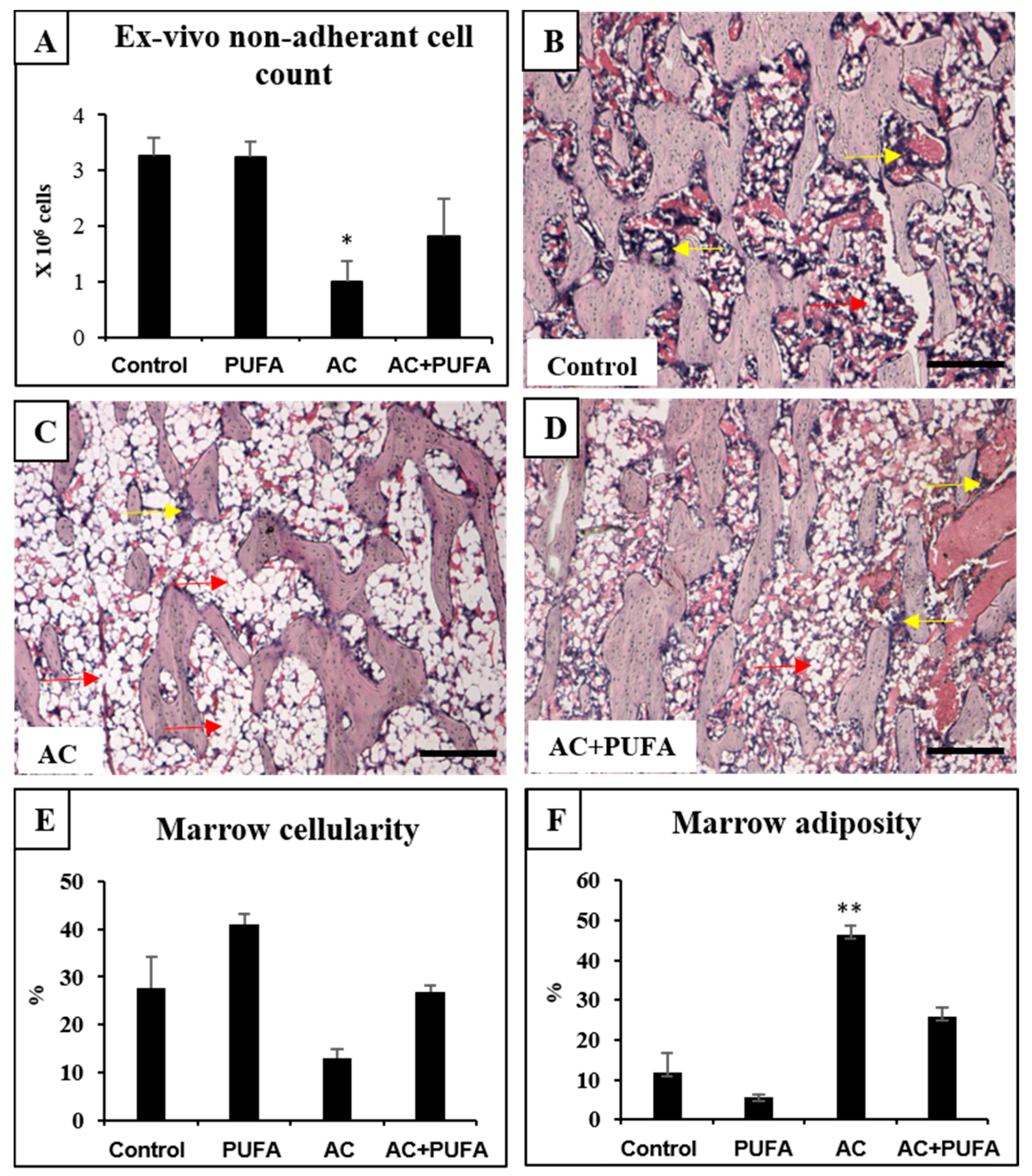
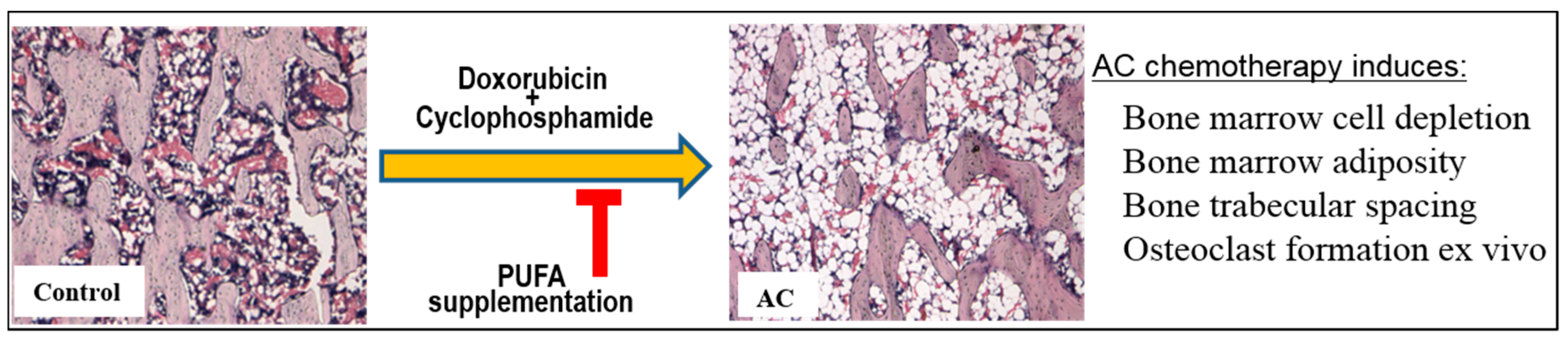
2.2. Treatment Effects on Bone Marrow Microenvironment
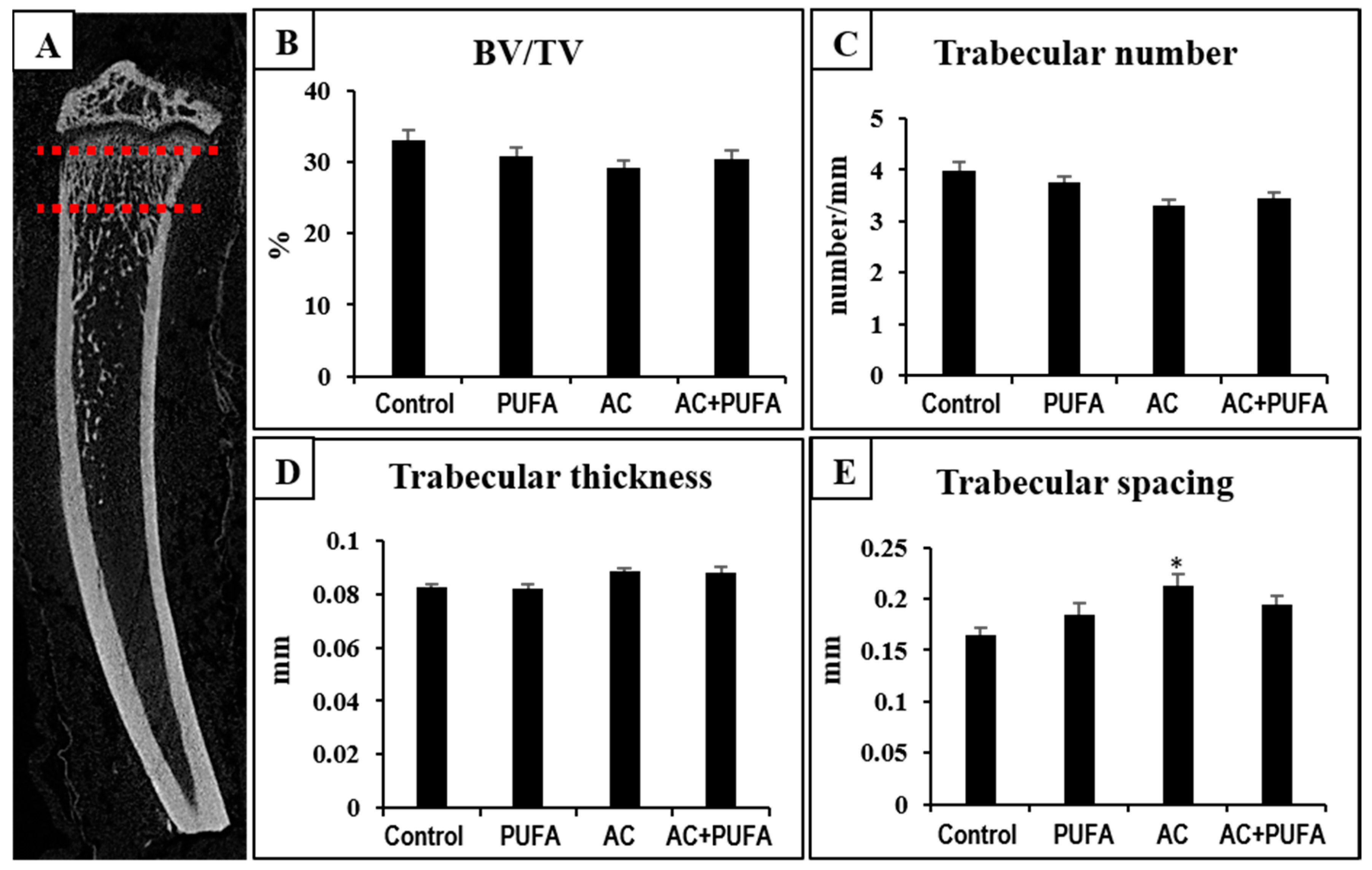
2.3. Treatment Effects on Overall Trabecular Bone Volume and Structures in the Metaphysis
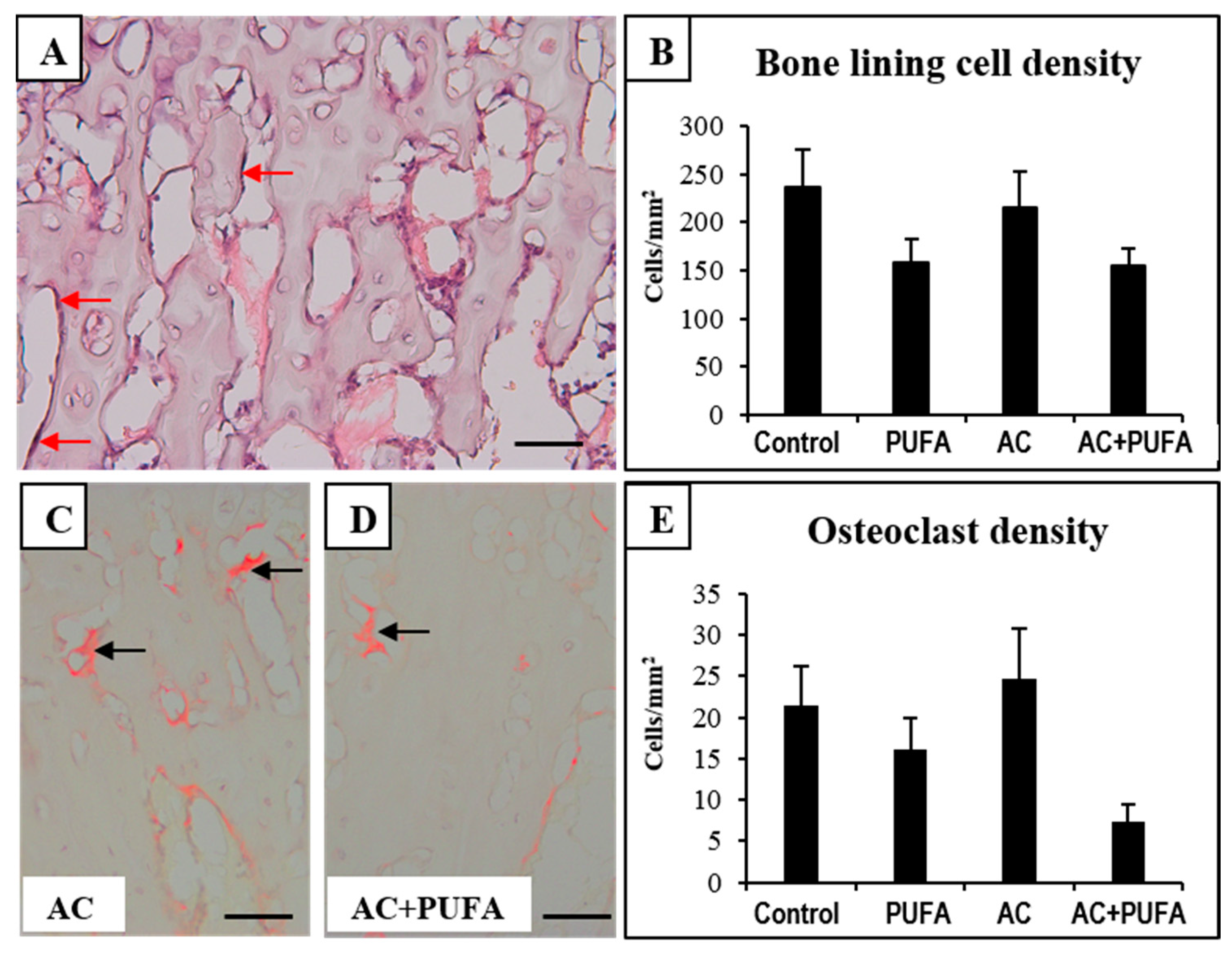
2.4. Treatment Effects on Bone Cells at the Metaphysis
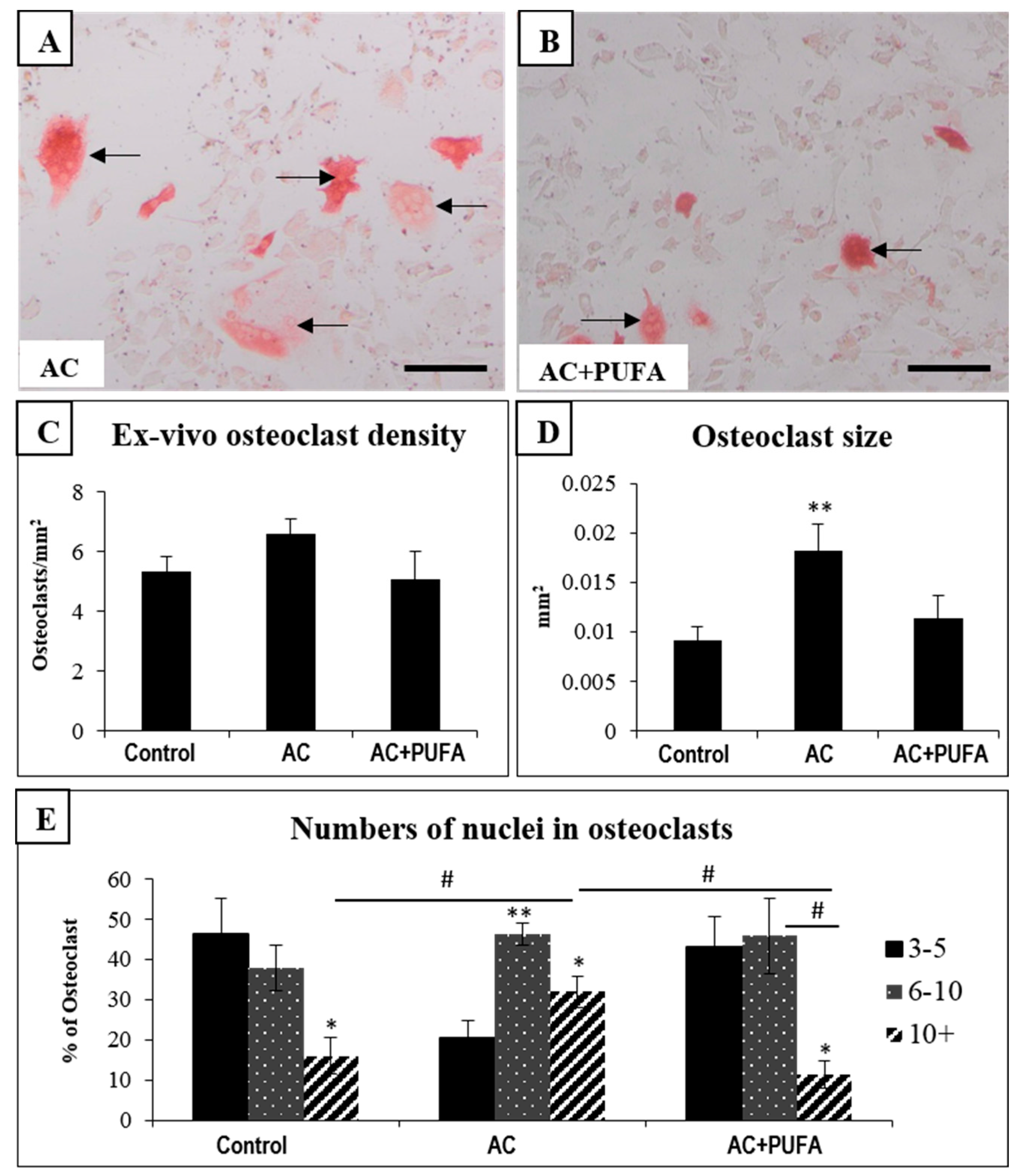
2.5. Treatment Effects on Ex Vivo Osteoclast Formation Potential
3. Discussion
4. Materials and Methods
4.1. Animal Trial
4.2. Specimen Collection
4.3. Ex Vivo µ-CT
4.4. Histological Analysis
4.5. Ex Vivo Osteoclast Formation Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nurgalieva, Z.; Liu, C.C.; Du, X.L.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med. Oncol. 2011, 28, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.M.; Georgiou, K.R.; Morris, H.A.; McKinnon, R.A.; Keefe, D.M.K.; Howe, P.R.; Xian, C.J. Combination breast cancer chemotherapy with doxorubicin and cyclophosphamide damages bone and bone marrow in a female rat model. Breast Cancer Res. Treat. 2017, 165, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Georgiou, K.R.; McKinnon, R.A.; Keefe, D.M.; Howe, P.R.; Xian, C.J. Combination chemotherapy with cyclophosphamide, epirubicin and 5-fluorouracil causes trabecular bone loss, bone marrow cell depletion and marrow adiposity in female rats. J. Bone Miner. Metab. 2016, 34, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L.; Manola, J.; Leboff, M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J. Clin. Oncol. 2001, 19, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Pan, X.; Cheek, F.; Ing, S.W.; Jackson, R.D. A systematic review of omega-3 fatty acids and osteoporosis. Br. J. Nutr. 2012, 107 (Suppl. 2), S253–S260. [Google Scholar] [CrossRef] [PubMed]
- Hogstrom, M.; Nordstrom, P.; Nordstrom, A. n-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 Study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [PubMed]
- Bullon, P.; Battino, M.; Varela-Lopez, A.; Perez-Lopez, P.; Granados-Principal, S.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Gonzalez-Alonso, A.; Ramirez-Tortosa, C.L.; et al. Diets Based on Virgin Olive Oil or Fish Oil but Not on Sunflower Oil Prevent Age-Related Alveolar Bone Resorption by Mitochondrial-Related Mechanisms. PLoS ONE 2013, 8, e74234. [Google Scholar] [CrossRef] [PubMed]
- Vanek, C.; Connor, W.E. Do n-3 fatty acids prevent osteoporosis? Am. J. Clin. Nutr. 2007, 85, 647–648. [Google Scholar] [PubMed][Green Version]
- Matsushita, H.; Barrios, J.A.; Shea, J.E.; Miller, S.C. Dietary fish oil results in a greater bone mass and bone formation indices in aged ovariectomized rats. J. Bone Miner. Metab. 2008, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Raghu Nadhanan, R.R.; Skinner, J.; Chung, R.; Su, Y.W.; Howe, P.R.; Xian, C.J. Supplementation with Fish Oil and Genistein, Individually or in Combination, Protects Bone against the Adverse Effects of Methotrexate Chemotherapy in Rats. PLoS ONE 2013, 8, e71592. [Google Scholar] [CrossRef] [PubMed]
- Raghu Nadhanan, R.R.; Fan, C.M.; Su, Y.W.; Howe, P.R.C.; Xian, C.J. Fish Oil in Comparison to Folinic Acid for Protection Against Adverse Effects of Methotrexate Chemotherapy on Bone. J. Orthop. Res. 2014, 32, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Foster, B.K.; Wallace, W.H.; Xian, C.J. Pathobiology and Prevention of Cancer Chemotherapy-Induced Bone Growth Arrest, Bone Loss, and Osteonecrosis. Curr. Mol. Med. 2011, 11, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.R.; Hui, S.K.; Xian, C.J. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am. J. Stem Cells 2012, 1, 205–224. [Google Scholar] [PubMed]
- Mohamed, A.M. An Overview of Bone Cells and Their Regulating Factors of Differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar] [PubMed]
- Kalder, M.; Hadji, P. Breast Cancer and Osteoporosis-Management of Cancer Treatment-Induced Bone Loss in Postmenopausal Women with Breast Cancer. Breast Care 2014, 9, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Ziller, M.; Maskow, C.; Albert, U.; Kalder, M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur. J. Cancer 2009, 45, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Glackin, C.A.; Murray, E.J.; Murray, S.S. Doxorubicin inhibits differentiation and enhances expression of the helix-loop-helix genes Id and mTwi in mouse osteoblastic cells. Biochem. Int. 1992, 28, 67–75. [Google Scholar] [PubMed]
- Fonseca, H.; Carvalho, A.; Esteves, J.; Esteves, V.I.; Moreira-Goncalves, D.; Duarte, J. Effects of doxorubicin administration on bone strength and quality in sedentary and physically active Wistar rats. Osteoporos. Int. 2016, 27, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chen, K.M.; Hassanshahi, M.; Tang, Q.; Howe, P.R.; Xian, C.J. Childhood cancer chemotherapy-induced bone damage: Pathobiology and protective effects of resveratrol and other nutraceuticals. Ann. N. Y. Acad. Sci. 2017, 1403, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Rucker-Martin, C.; Gressette, M.; Lefebvre, F.; Gresikova, M.; Solgadi, A.; Veksler, V.; et al. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: Potential role of energy metabolism remodeling. Circ. Heart Fail. 2015, 8, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Shiozawa, K.; Uesugi, T.; Hiromatsu, R.; Fukuda, M.; Kitaura, K.; Minami, T.; Matsumoto, S. Collaborative work on evaluation of ovarian toxicity. 7) Effects of 2- or 4-week repeated dose studies and fertility study of cyclophosphamide in female rats. J. Toxicol. Sci. 2009, 34 (Suppl. 1), SP83–SP89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todorova, V.K.; Kaufmann, Y.; Klimberg, V.S. Increased efficacy and reduced cardiotoxicity of metronomic treatment with cyclophosphamide in rat breast cancer. Anticancer Res. 2011, 31, 215–220. [Google Scholar] [PubMed]
- Wang, Y.; Probin, V.; Zhou, D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr. Cancer Ther. Rev. 2006, 2, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Gerber, S.; Vanel, D.; Brisse, H.; Leclere, J. Improving the interpretation of bone marrow imaging in cancer patients. Cancer Imaging 2006, 6, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, Y.; Chang, J.; Luo, Y.; Meng, A.; Zhou, D. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl. Cancer Res. 2013, 2, 397–411. [Google Scholar] [PubMed]
- Georgiou, K.R.; Scherer, M.A.; Fan, C.M.; Cool, J.C.; King, T.J.; Foster, B.K.; Xian, C.J. Methotrexate chemotherapy reduces osteogenesis but increases adipogenic potential in the bone marrow. J. Cell. Physiol. 2012, 227, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.R.; King, T.J.; Scherer, M.A.; Zhou, H.; Foster, B.K.; Xian, C.J. Attenuated Wnt/b-catenin signalling mediates methotrexate chemotherapy-induced bone loss and marrow adiposity in rats. Bone 2012, 50, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Błogowski, W.; Ratajczak, M.Z.; Zyżniewska-Banaszak, E.; Dołęgowska, B.; Starzyńska, T. Adipose tissue as a potential source of hematopoietic stem/progenitor cells. Obesity 2012, 20, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259. [Google Scholar] [CrossRef] [PubMed]
- Pronk, C.J.; Veiby, O.P.; Bryder, D.; Jacobsen, S.E. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: Involvement of two distinct receptors. J. Exp. Med. 2011, 208, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.J.; Wu, M.Q.; Li, Z.J.; Zhang, Y.; Liu, K.Y. Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int. J. Hematol. 2013, 97, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Li, X.P.; Cheng, L.; Han, M.T.; Zhang, M.M.; Shao, Q.X.; Xu, H.X.; Qi, L. Fish Oil-Rich Diet Promotes Hematopoiesis and Alters Hematopoietic Niche. Endocrinology 2015, 156, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Halade, G.; Williams, P. Omega-3 fatty acid-rich fish oil supplementation prevents rosiglitazone-induced osteopenia in insulin resistant C57BL/6 mice. FASEB J. 2016, 30, 692–693. [Google Scholar]
- Miller, K.K.; Klibanski, A. Clinical review 106: Amenorrheic bone loss. J. Clin. Endocrinol. Metab. 1999, 84, 1775–1783. [Google Scholar] [PubMed][Green Version]
- Berliere, M.; Dalenc, F.; Malingret, N.; Vindevogel, A.; Piette, P.; Roche, H.; Donnez, J.; Symann, M.; Kerger, J.; Machiels, J.P. Incidence of reversible amenorrhea in women with breast cancer undergoing adjuvant anthracycline-based chemotherapy with or without docetaxel. BMC Cancer 2008, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A. Management of cancer-treatment-induced bone loss in postmenopausal women undergoing adjuvant breast cancer therapy: A Z-FAST update. Semin. Oncol. 2006, 33 (Suppl. 7), S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Vehmanen, L.; Saarto, T.; Elomaa, I.; Makela, P.; Valimaki, M.; Blomqvist, C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur. J. Cancer 2001, 37, 2373–2378. [Google Scholar] [CrossRef]
- Fan, C.M.; Foster, B.K.; Hui, S.K.; Xian, C.J. Prevention of bone growth defects, increased bone resorption and marrow adiposity with folinic acid in rats receiving long-term methotrexate. PLoS ONE 2012, 7, e46915. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; Paragius, T.; Foster, B.K. Damage and recovery of the bone growth mechanism in young rats following 5-fluorouracil acute chemotherapy. J. Cell. Biochem. 2006, 99, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; Scherer, M.A.; Macsai, C.E.; Fan, C.M.; Covino, M.; Foster, B.K. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone 2007, 41, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; van Gangelen, J.; Foster, B.K.; Howarth, G.S. Effects of Etoposide and cyclophosphamide acute chemotherapy on growth plate and metaphyseal bone in rats. Cancer Biol. Ther. 2007, 6, 170–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-Mediated Bone Loss in Breast Cancer Bone Metastases Is Driven by an Interplay between Oxidative Stress and Induction of TGF beta. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ek-Rylander, B.; Karlstrom, E.; Wendel, M.; Andersson, G. Osteoclast size heterogeneity in rat long bones is associated with differences in adhesive ligand specificity. Exp. Cell Res. 2008, 314, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.S.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Iitsuka, N.; Tsukamoto, I. Fish oil suppresses bone resorption by inhibiting osteoclastogenesis through decreased expression of M-CSF, PU.1, MITF and RANK in ovariectomized rats. Mol. Med. Rep. 2013, 7, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Raghu Nadhanan, R.; Abimosleh, S.M.; Su, Y.W.; Scherer, M.A.; Howarth, G.S.; Xian, C.J. Dietary emu oil supplementation suppresses 5-fluorouracil chemotherapy-induced inflammation, osteoclast formation, and bone loss. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1440–E1449. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.M.; Cool, J.C.; Scherer, M.A.; Foster, B.K.; Shandala, T.; Tapp, H.; Xian, C.J. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone 2009, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
| Groups | Injection | Gavage |
|---|---|---|
| Control (n = 6) | Sterile water | Sterile water |
| LCn3PUFA (n = 6) | Sterile water | LCn3PUFA |
| AC (n = 8) | AC | Sterile water |
| AC + LCn3PUFA (n = 8) | AC | LCn3PUFA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.-M.; Su, Y.-W.; Howe, P.R.; Xian, C.J. Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats. Int. J. Mol. Sci. 2018, 19, 484. https://doi.org/10.3390/ijms19020484
Fan C-M, Su Y-W, Howe PR, Xian CJ. Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats. International Journal of Molecular Sciences. 2018; 19(2):484. https://doi.org/10.3390/ijms19020484
Chicago/Turabian StyleFan, Chia-Ming, Yu-Wen Su, Peter R. Howe, and Cory J. Xian. 2018. "Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats" International Journal of Molecular Sciences 19, no. 2: 484. https://doi.org/10.3390/ijms19020484
APA StyleFan, C.-M., Su, Y.-W., Howe, P. R., & Xian, C. J. (2018). Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats. International Journal of Molecular Sciences, 19(2), 484. https://doi.org/10.3390/ijms19020484







