In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth
Abstract
1. Introduction
2. Results and Discussion
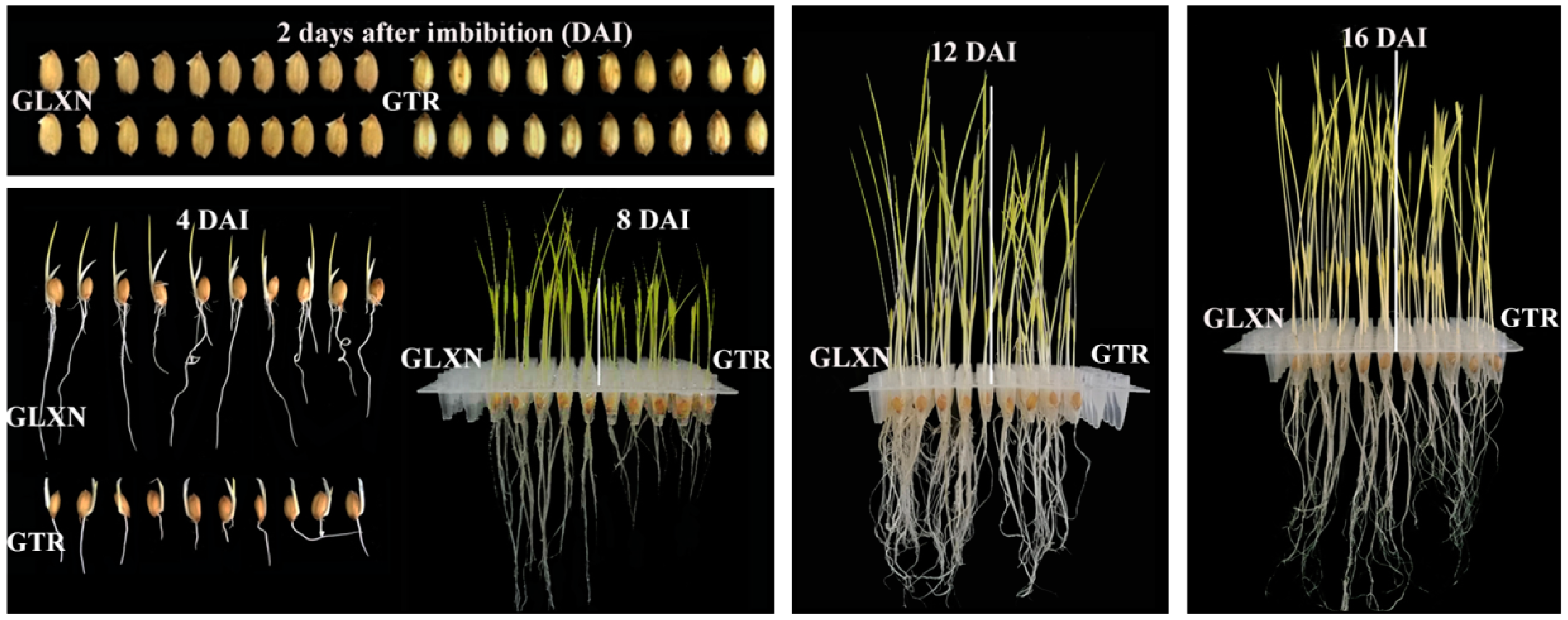
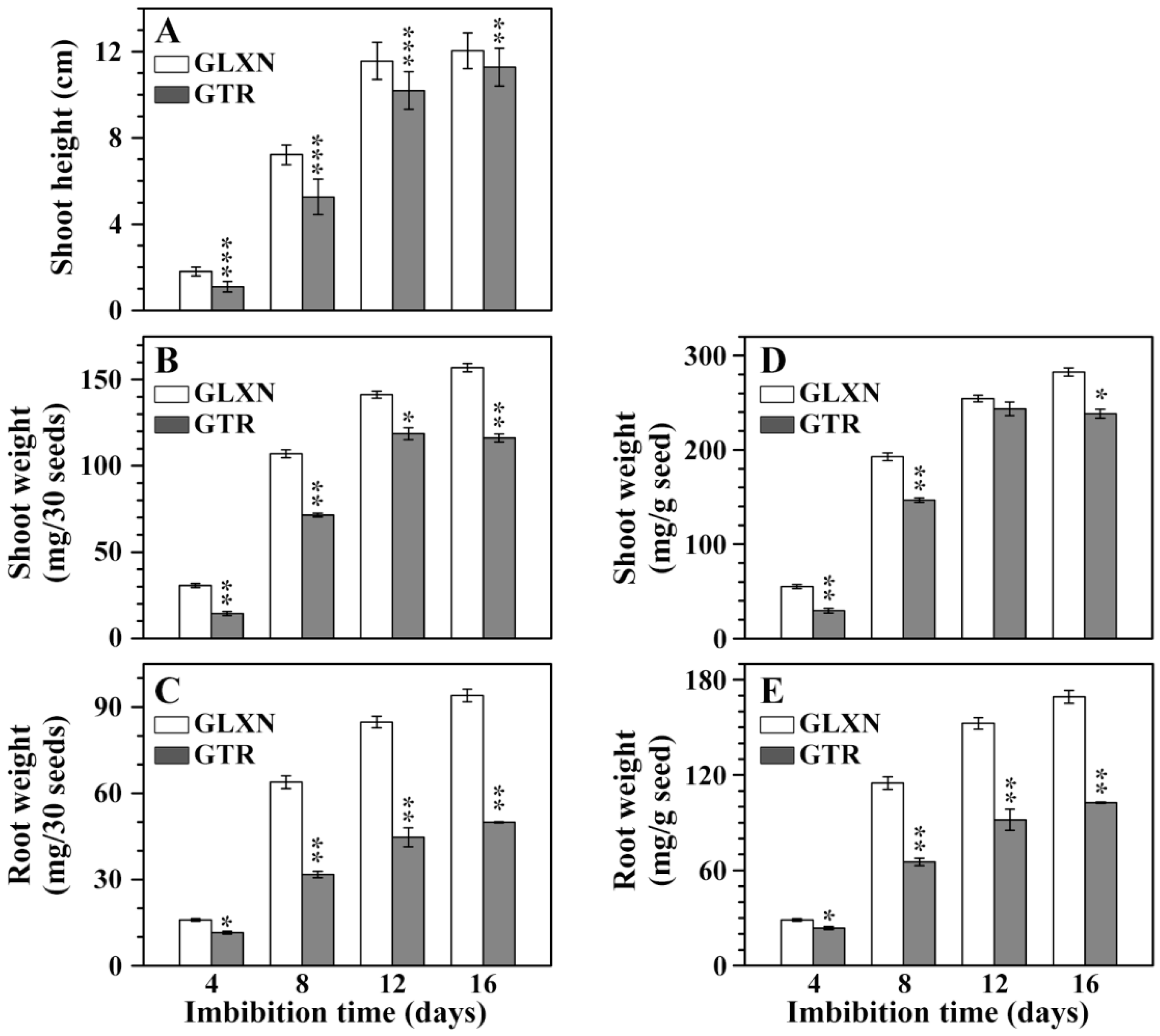
2.1. Growth Dynamics of Rice Seedlings in the Dark Only in Deionized Water
2.2. In Vitro Culture of Mature Embryo
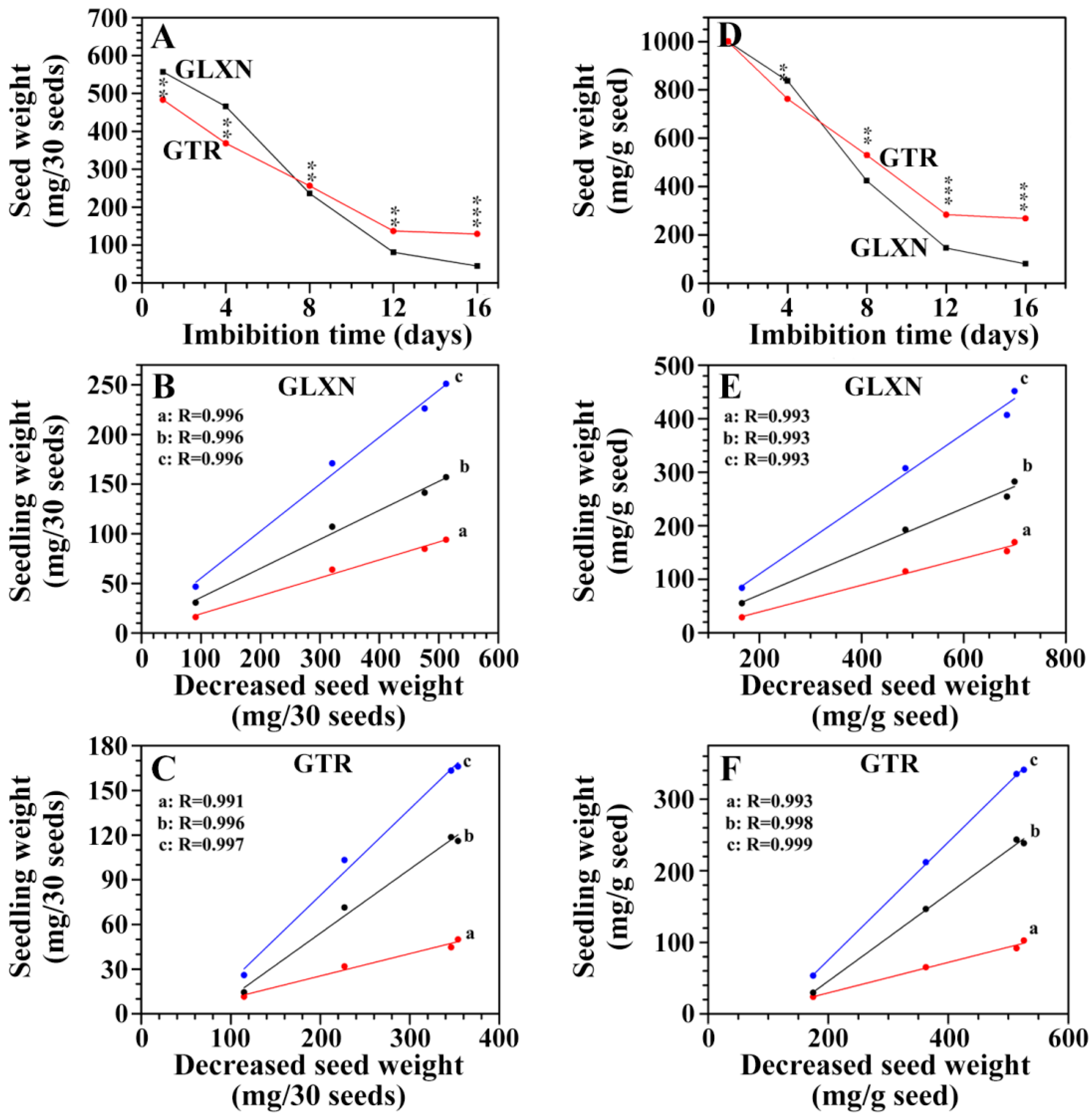
2.3. The Consumption of Seed Material and Its Relationship with Seedling Growth
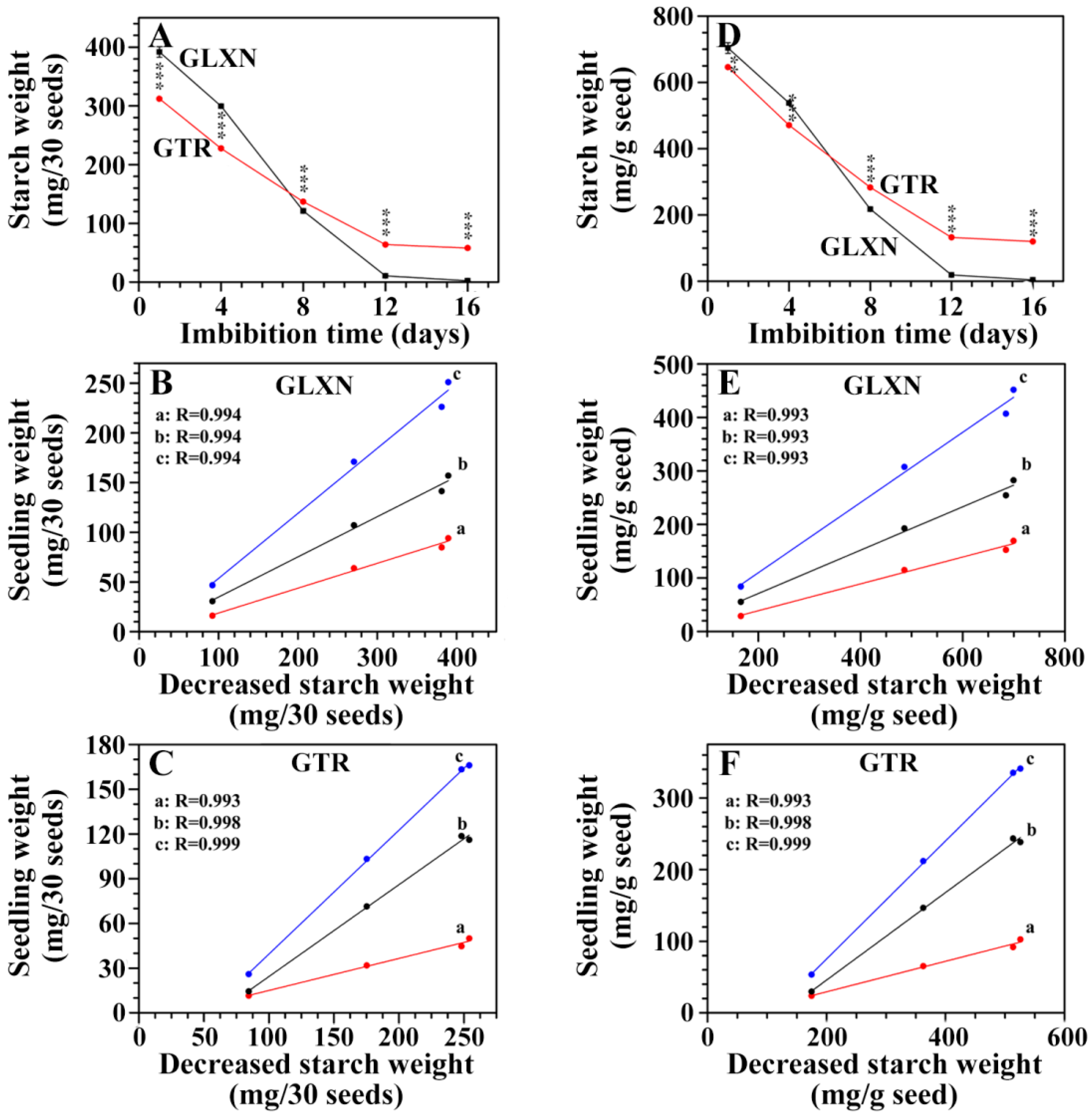
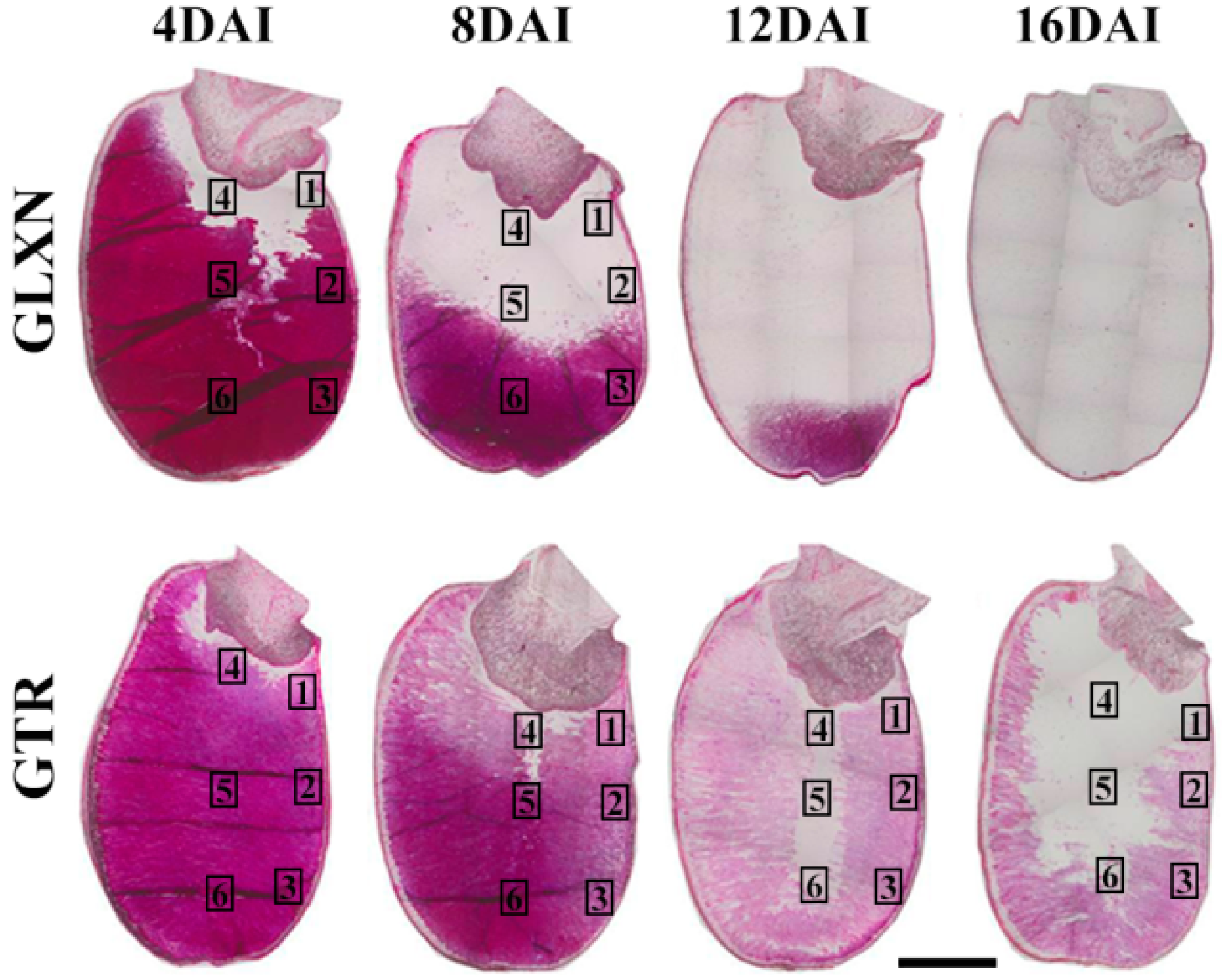
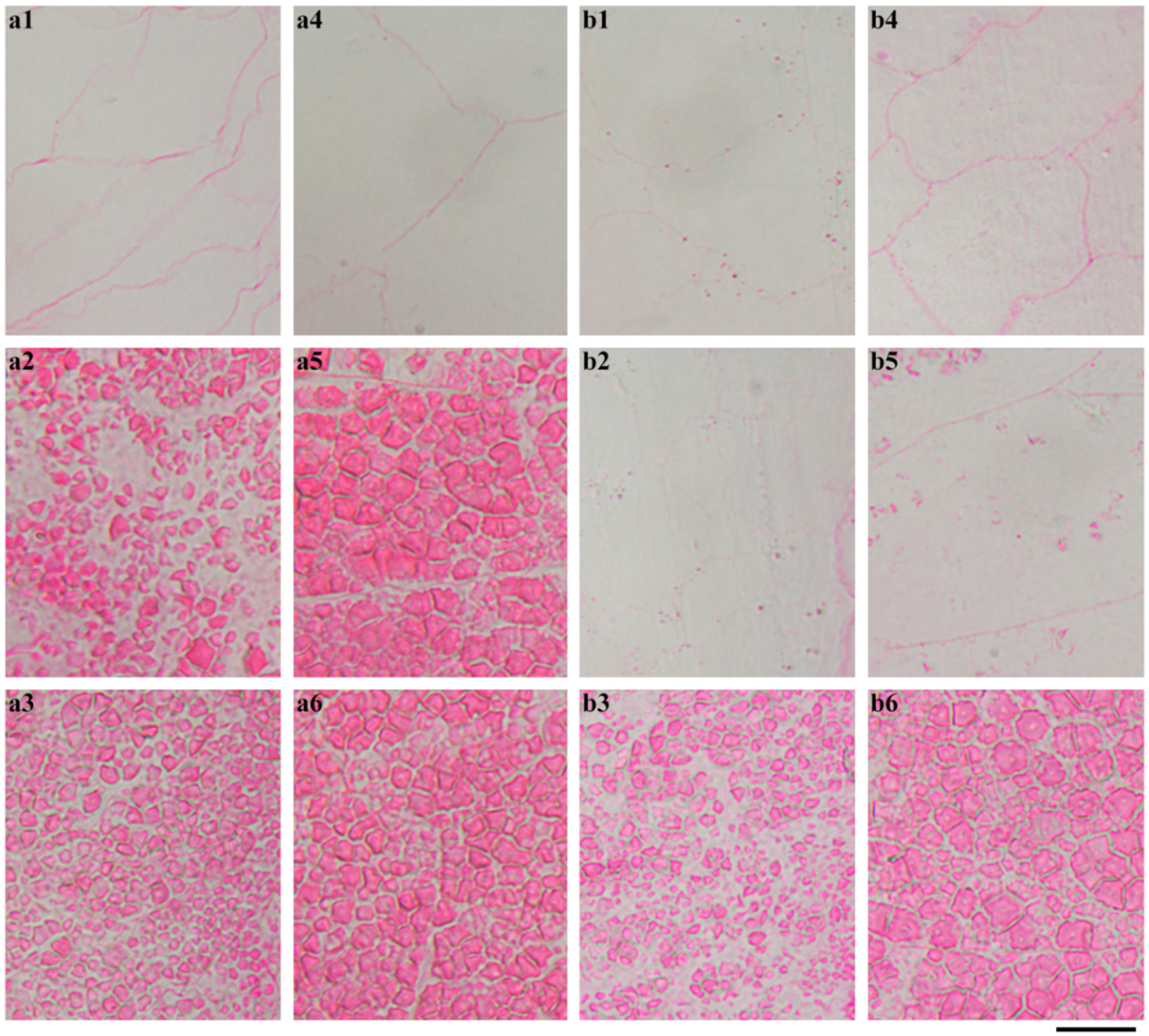
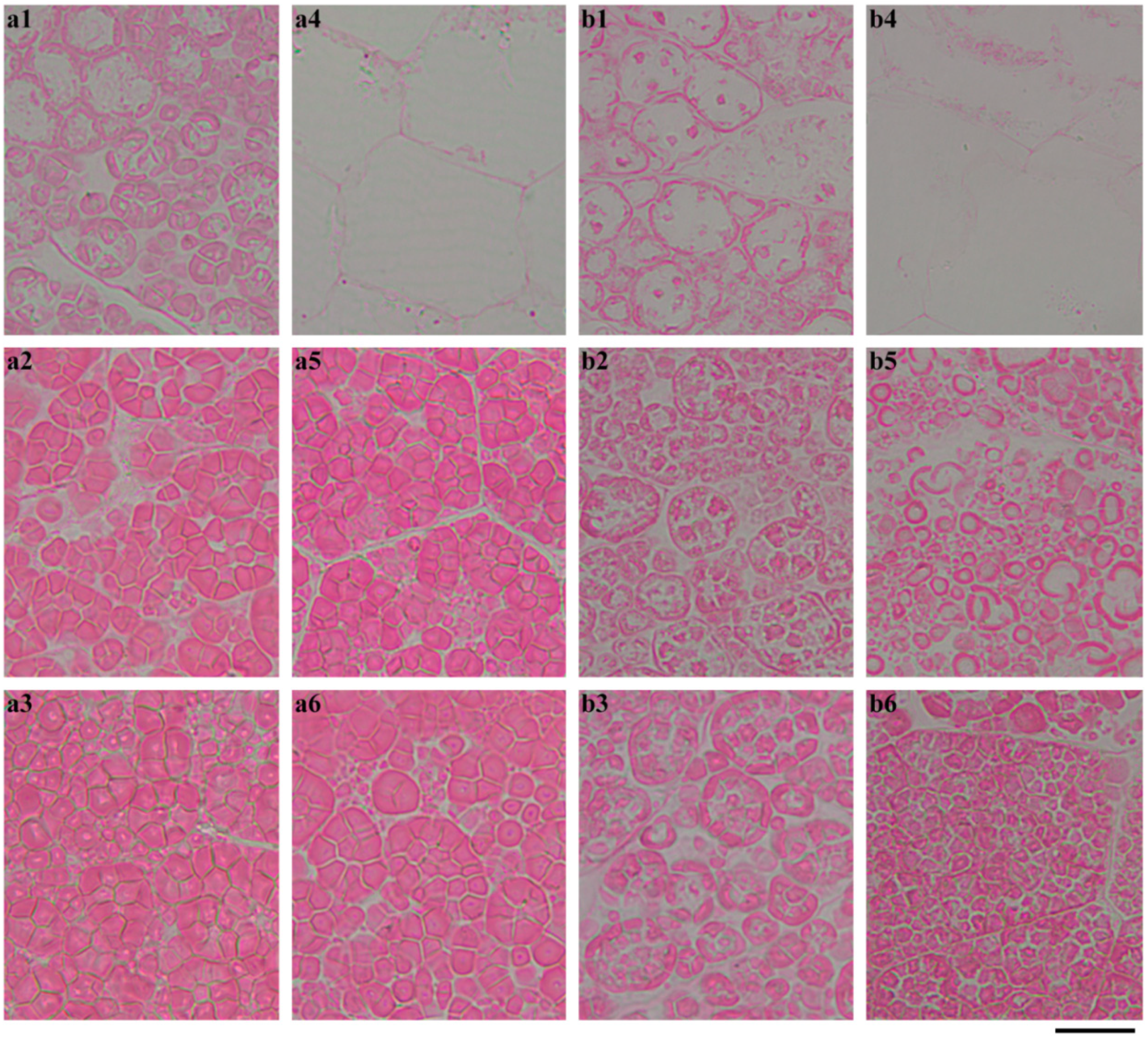
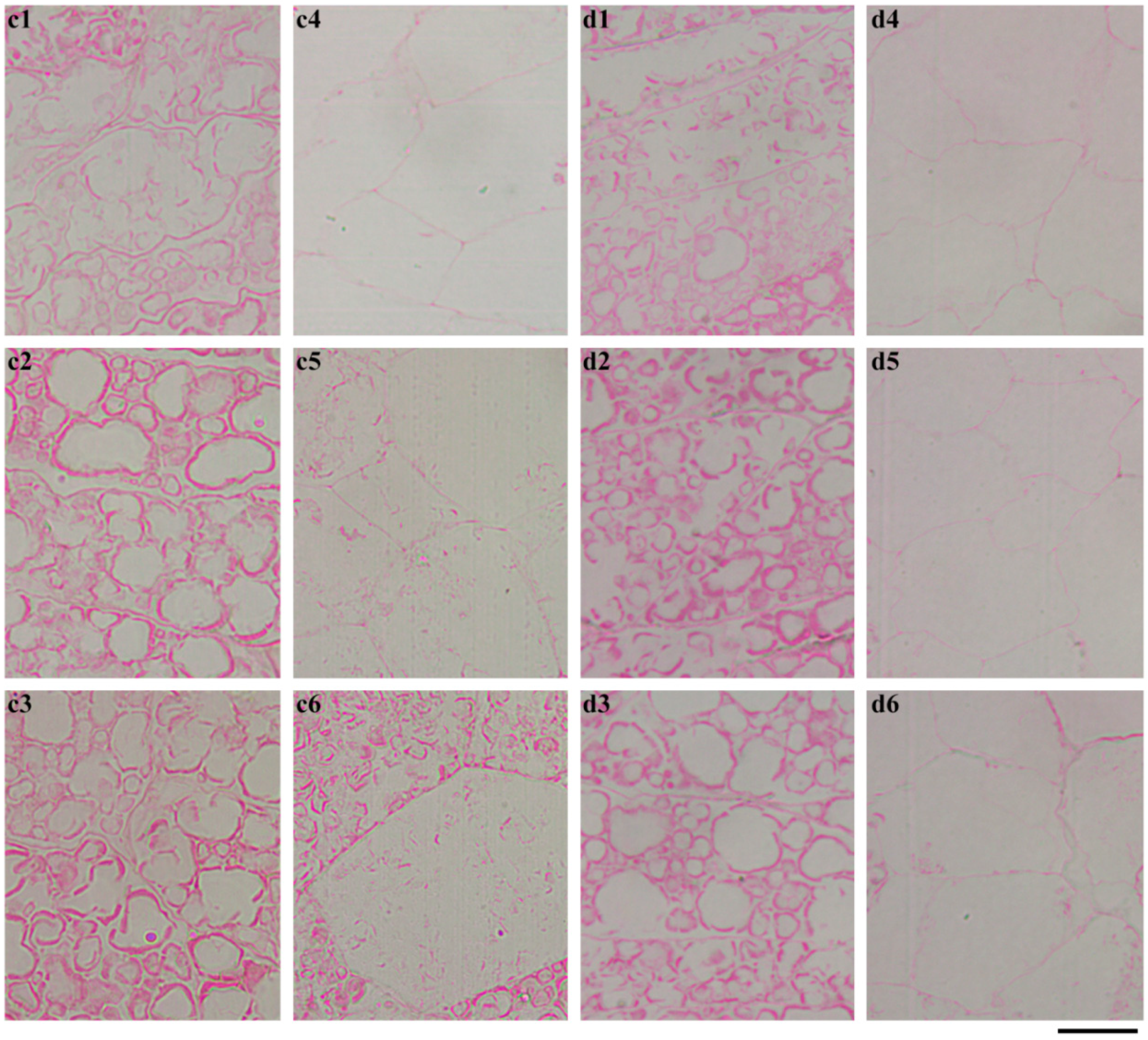
2.4. In Situ Degradation of Endosperm Starch during Seedling Growth
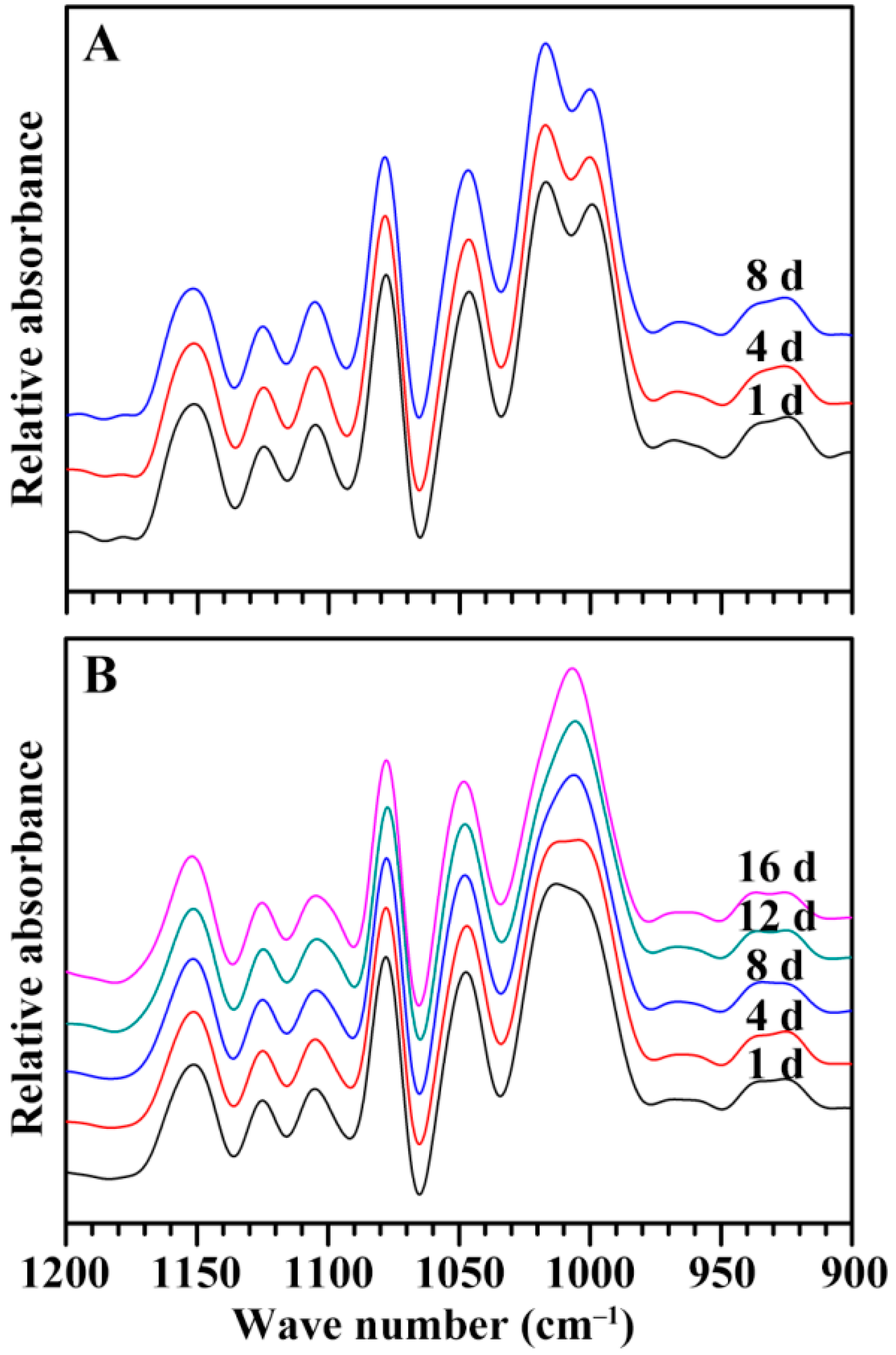
2.5. Characterization of Endosperm Starch during Seedling Growth
3. Materials and Methods
3.1. Plant Materials
3.2. Germination of Rice Grain and Seedling Growth
3.3. Determination of Shoot Height and the Dry Weight of Shoot, Root, Seed, and Endosperm Starch
3.4. In Vitro Culture of Mature Embryo
3.5. Preparation and Observation of Section of Whole Seed
3.6. Isolation of Endosperm Starch
3.7. Determination of Iodine Absorption Spectrum of Starch
3.8. Crystalline Structure Analysis of Starch
3.9. Ordered Structure Analysis of Starch
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Lin, L.; Cai, C.; Gilbert, R.G.; Li, E.; Wang, J.; Wei, C. Relationships between amylopectin molecular structures and functional properties of different-sized fractions of normal and high-amylose maize starches. Food Hydrocoll. 2016, 52, 359–368. [Google Scholar] [CrossRef]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sic. USA. 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Kosar-Hashemi, B.; Ling, S.; Li, Z.Y.; Rahman, S.; Morell, M. Control of starch branching in barley defined through differential RNAi suppression of starch branching enzyme IIa and IIb. J. Exp. Bot. 2010, 61, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, H.; Campbell, M.; Blanco, M.; Jane, J.L. Characterization of maize amylose-extender (ae) mutant starches. Part I: Relationship between resistant starch contents and molecular structures. Carbohydr. Polym. 2008, 74, 396–404. [Google Scholar] [CrossRef]
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buléon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol. 2012, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.J.; McGuire, C.; Loeffler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Holm, A.; Brandt, K.M.; Steine, M.N.; Goodstal, J.F.; et al. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, M.; Meng, X.; Cheung, S.C.K.; Yu, H.; Huang, J.; Sun, Y.; Shi, Y.C.; Liu, Q. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotech. J. 2012, 10, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.S.; Carciofi, M.; Martens, H.J.; Hebelstrup, K.H.; Blennow, A. Starch bioengineering affects cereal grain germination and seedling establishment. J. Exp. Bot. 2014, 65, 2257–2270. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 45, S33–S50. [Google Scholar]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Xia, H.; Yandeau-Nelson, M.; Thompson, D.B.; Guiltinan, M.J. Deficiency of maize starch-branching enzyme i results in altered starch fine structure, decreased digestibility and reduced coleoptile growth during germination. BMC Plant Biol. 2011, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Lin, L.S.; Wang, J.; Liu, Q.Q.; Wei, C.X. Long branch-chains of amylopectin with B-type crystallinity in rice seed with inhibition of starch branching enzyme I and IIb resist in situ degradation and inhibit plant growth during seedling development. BMC Plant Biol. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shang, Z.; Man, J.; Liu, Q.; Zhu, C.; Wei, C. Comparison of molecular structures and functional properties of high-amylose starches from rice transgenic line and commercial maize. Food Hydrocoll. 2015, 46, 172–179. [Google Scholar] [CrossRef]
- Wang, J.; Hu, P.; Chen, Z.; Liu, Q.; Wei, C. Progress in high-amylose cereal crops through inactivation of starch branching enzymes. Front. Plant Sci. 2017, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C. Effect of simultaneous inhibition of starch branching enzymes I and IIb on the crystalline structure of rice starches with different amylose contents. J. Agric. Food Chem. 2013, 61, 9930–9937. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lin, L.; Wang, J.; Zhang, L.; Liu, Q.; Wei, C. Inhibition of starch branching enzymes in waxy rice increases the proportion of long branch-chains of amylopectin resulting in the comb-like profiles of starch granules. Plant Sci. 2018. [Google Scholar] [CrossRef]
- Wang, J.; Hu, P.; Lin, L.; Chen, Z.; Liu, Q.; Wei, C. Gradually decreasing starch branching enzyme expression is responsible for the formation of heterogeneous starch granules. Plant Physiol. 2018, 176, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Huang, J.; Zhao, L.; Liu, Q.; Zhang, C.; Wei, C. Heterogeneous structure and spatial distribution in endosperm of high-amylose rice starch granules with different morphologies. J. Agric. Food Chem. 2014, 62, 10143–10152. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, Q.; Zhang, L.; Wei, C. Evaluation of molecular structural parameters of normal rice starch and their relationships with its thermal and digestion properties. Molecules 2017, 22, 1526. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohyd. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Buléon, A.; Gérard, C.; Riekel, C.; Vuong, R.; Chanzy, H. Details of the crystalline ultrastructure of C-starch granules revealed by synchrotron microfocus mapping. Macromolecules 1998, 31, 6605–6610. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Zhao, L.; Pan, T.; Cai, C.; Wang, J.; Wei, C. Application of whole sections of mature cereal seeds to visualize the morphology of endosperm cell and starch and the distribution of storage protein. J. Cereal Sci. 2016, 71, 19–27. [Google Scholar] [CrossRef]



| Time | GLXN | GTR | ||||
|---|---|---|---|---|---|---|
| λmax (nm) | OD 620/550 | BV (OD680) | λmax (nm) | OD 620/550 | BV (OD680) | |
| 1 DAI | 525.3 ± 1.8a | 0.572 ± 0.014a | 0.041 ± 0.002a | 548.3 ± 2.5a | 0.700 ± 0.015a | 0.105 ± 0.003a |
| 4 DAI | 526.8 ± 1.8a | 0.577 ± 0.012a | 0.044 ± 0.001a | 549.8 ± 1.1a | 0.708 ± 0.008a | 0.105 ± 0.001a |
| 8 DAI | 528.8 ± 2.5a | 0.586 ± 0.018a | 0.041 ± 0.003a | 553.8 ± 0.4a | 0.735 ± 0.001a | 0.141 ± 0.002b |
| 12 DAI | 527.5 ± 0.7a | 0.583 ± 0.024a | 0.039 ± 0.003a | 555.8 ± 2.5a | 0.740 ± 0.019a | 0.163 ± 0.005c |
| 16 DAI | – | – | – | 555.8 ± 3.2a | 0.741 ± 0.019a | 0.163 ± 0.006c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.; Lin, L.; Liu, Q.; Wei, C. In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth. Int. J. Mol. Sci. 2018, 19, 3397. https://doi.org/10.3390/ijms19113397
Pan T, Lin L, Liu Q, Wei C. In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth. International Journal of Molecular Sciences. 2018; 19(11):3397. https://doi.org/10.3390/ijms19113397
Chicago/Turabian StylePan, Ting, Lingshang Lin, Qiaoquan Liu, and Cunxu Wei. 2018. "In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth" International Journal of Molecular Sciences 19, no. 11: 3397. https://doi.org/10.3390/ijms19113397
APA StylePan, T., Lin, L., Liu, Q., & Wei, C. (2018). In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth. International Journal of Molecular Sciences, 19(11), 3397. https://doi.org/10.3390/ijms19113397





