Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction
Abstract
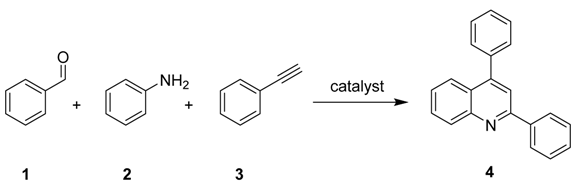
:1. Introduction
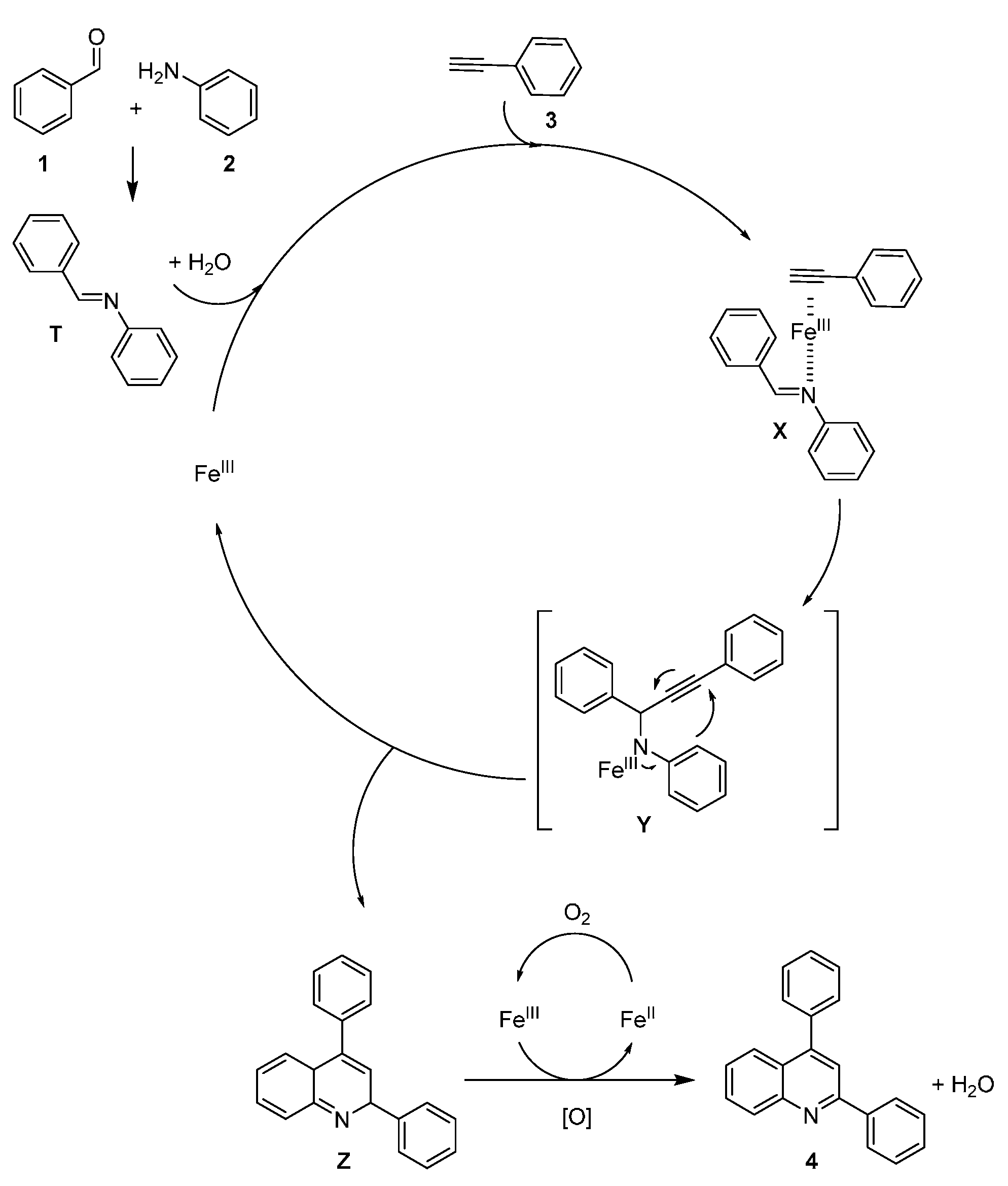
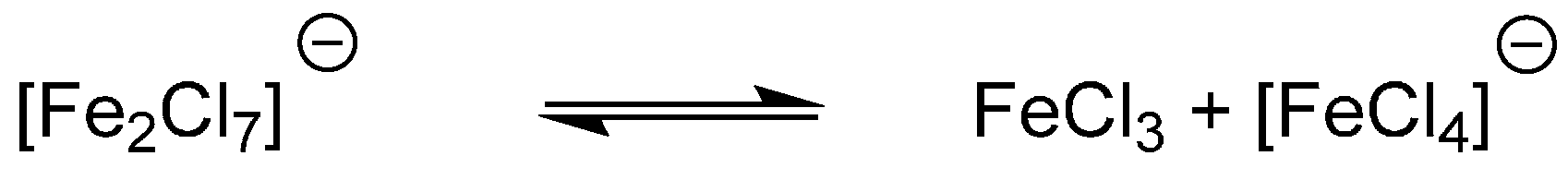
2. Results
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. General Experimental for the Preparation of 2,4-Diphenylquinoline 4
4.3. Preparation of Metallic Ionic Liquids
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; Volumes 1–2. [Google Scholar]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids as Green Solvents: Progress and Prospects; American Chemical Society: Washington, DC, USA, 2003; pp. 2–12. [Google Scholar]
- Chowdhury, S.; Mohan, R.S.; Scott, J.L. Reactivity of ionic liquids. Tetrahedron 2007, 63, 2363–2389. [Google Scholar] [CrossRef]
- Jurčík, V.; Wilhelm, R. An imidazolinium salt as ionic liquid for medium and strong bases. Green Chem. 2005, 7, 844–848. [Google Scholar] [CrossRef]
- Dupont, J.; Spencer, J. On the Noninnocent Nature of 1,3-Dialkylimidazolium Ionic Liquids. Angew. Chem. Int. Ed. 2004, 43, 5296–5297. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Singh, S.; Kirichenko, K.; Holbrey, J.D.; Smiglak, M.; Reichert, W.M.; Rogers, R.D. 1-Butyl-3-methylimidazolium 3,5-dinitro-1,2,4-triazolate: A novel ionic liquid containing a rigid, planar energetic anion. Chem. Commun. 2005, 21, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.B.; Culver, S.L.; Fox, P.A.; Goode, R.D.; Ntai, I.; Tickell, M.D.; Traylor, R.K.; Hoffman, N.W.; Davis, J.H. Sweet success: Ionic liquids derived from non-nutritive sweeteners. Chem. Commun. 2004, 10, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Baan, Z.; Hermecz, I.; Novak, T.; Odinets, I.L. The Phosphorus Aspects of Green Chemistry: The Use of Quaternary Phosphonium Salts and 1,3-Dialkylimidazolium Hexafluorophosphates in Organic Synthesis. Curr. Org. Chem. 2007, 11, 107–126. [Google Scholar] [CrossRef]
- Steinrück, H.P.; Wasserscheid, P. Ionic Liquids in Catalysis. Catal. Lett. 2015, 145, 380–397. [Google Scholar] [CrossRef]
- Petkovic, M.; Kenneth, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Pereira, J.F.B.; Debbeti, V.; Wang, H.; Rogers, R.D. Mixing ionic liquids—“simple mixtures” or “double salts”? Green Chem. 2014, 16, 2051–2083. [Google Scholar] [CrossRef]
- Niedermeyer, H.; Hallett, J.P.; Villar-Garcia, I.J.; Hunt, P.A.; Welton, T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Baudequin, C.; Baudoux, J.; Levillain, J.; Cahard, D.; Gaumont, A.C.; Plaquevent, J.C. Ionic liquids and chirality: Opportunities and challenges. Tetrahedron: Asymmetry 2003, 14, 3081–3093. [Google Scholar] [CrossRef]
- Jain, N.; Kumar, A.; Chauhan, S.; Chauhan, S.M.S. Chemical and biochemical transformations in ionic liquids. Tetrahedron 2005, 61, 1015–1060. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Hardacre, C. Catalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Teuma, E.; Goez, M. Ionic liquids as a medium for enantioselective catalysis. C. R. Chim. 2007, 10, 152–177. [Google Scholar] [CrossRef]
- Sereda, O.; Tabassum, S.; Wilhelm, R. Lewis acid organocatalysts. Top. Curr. Chem. 2010, 291, 349–394. [Google Scholar] [PubMed]
- Estager, J.; Holbrey, J.D.; Swadzba-Kwasny, M. Halometallate ionic liquids—Revisited. Chem. Soc. Rev. 2014, 43, 847–886. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, W.; Wang, Y.; Zhang, J.; Lub, J.; Yana, Y. Deep oxidative desulfurization of fuels in redox ionic liquids based on iron chloride. Green Chem. 2009, 11, 810–815. [Google Scholar] [CrossRef]
- Ko., N.H.; Lee, J.S.; Huh, E.S.; Lee, H.; Jung, K.D.; Kim, H.S.; Cheong, M. Extractive Desulfurization Using Fe-Containing Ionic Liquids. Energy Fuels 2008, 22, 1687–1690. [Google Scholar] [CrossRef]
- Hu, Y.L.; Liu, Q.F.; Lu, T.T.; Lu, M. Highly efficient oxidation of organic halides to aldehydes and ketones with H5IO6 in ionic liquid [C12MIM][FeCl4]. Catal. Commun. 2010, 11, 923–927. [Google Scholar] [CrossRef]
- Bica, K.; Leder, S.; Gaertner, P. From solvent to sustainable catalysis. Chloroferrate ionic liquids in synthesis. Curr. Organ. Synth. 2011, 8, 824–839. [Google Scholar] [CrossRef]
- Jurčík, V.; Wilhelm, R. The preparation of new enantiopure imidazolinium salts and their evaluation as catalysts and shift reagents. Tetrahedron: Asymmetry 2006, 17, 801–810. [Google Scholar] [CrossRef]
- Jurčík, V.; Gilani, M.; Wilhelm, R. Easily accessible chiral imidazolinium salts bearing two hydroxy-containing substituents as shift reagents and carbene precursors. Eur. J. Org. Chem. 2006, 2, 5103–5109. [Google Scholar] [CrossRef]
- Winkel, A.; Wilhelm, R. New Chiral Ionic Liquids Based on Imidazolinium Salts. Tetrahedron: Asymmetry 2009, 20, 2344–2350. [Google Scholar] [CrossRef]
- Winkel, A.; Wilhelm, R. New Chiral Ionic Liquids Based on Enantiopure Sulphate and Sulfonate Anions for Chiral Recognition. Eur. J. Org. Chem. 2010, 30, 5817–5824. [Google Scholar] [CrossRef]
- Blanrue, A.; Wilhelm, R. Methylated Imidazolinium-Dithiocarboxylates: Two Representatives of a New Class of Ionic Liquids. Synthesis 2009, 4, 583–586. [Google Scholar]
- Jurčík, V.; Wilhelm, R. Imidazolinium salts as catalysts for the aza-Diels-Alder reaction. Org. Biomol. Chem. 2005, 3, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sereda, O.; Clemens, N.; Heckel, T.; Wilhelm, R. Imidazolinium and amidinium salts as Lewis acid organocatalysts. Beilstein J. Org. Chem. 2012, 8, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Heckel, T.; Konieczna, D.D.; Wilhelm, R. An Ionic Liquid Solution of Chitosan as Organocatalyst. Catalysts 2013, 3, 914–921. [Google Scholar] [CrossRef]
- Heckel, T.; Winkel, A.; Wilhelm, R. Chiral ionic liquids based on nicotine for the chiral recognition of carboxylic acids. Tetrahedron: Asymmetry 2013, 24, 1127–1133. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1997, 14, 605–618. [Google Scholar] [CrossRef]
- Solomonov, I.; Osipova, M.; Feldman, Y.; Baehtz, C.; Kjaer, K.; Robinson, I.K.; Webster, G.T.; McNaughton, D.; Wood, B.R.; Weissbuch, I.; et al. Crystal nucleation, growth, andmorphology of the synthetic malaria pigment beta-hematin and the effect thereon by quinoline additives: The malaria pigment as a target of various antimalarial drugs. J. Am. Chem. Soc. 2007, 129, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Dormer, P.G.; Eng, K.K.; Farr, R.N.; Humphrey, G.R.; McWilliams, J.C.; Reider, P.J.; Sager, J.W.; Volante, R.P. Highly regioselective Friedlander annulations with unmodified ketones employing novel amine catalysts: Syntheses of 2-substituted quinolines, 1,8-naphthyridines, and related heterocycles. J. Org. Chem. 2003, 68, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.; Rivera, N.R.; Yasuda, N.; Hughes, D.L.; Reider, P.J. Highly regioselective Friedlander reaction. Org. Lett. 2001, 3, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Z.; Zhang, D.; Thummel, R.P. Friedlander approach for the incorporation of 6-bromoquinoline into novel chelating ligands. Org. Lett. 2003, 5, 2251–2253. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, B.R.; Miller, B.L. A mild and efficient one-step synthesis of quinolines. Org. Lett. 2003, 5, 4257–4259. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Torok, F.; Torok, B. Energy efficiency of heterogeneous catalytic microwave-assisted organic reactions. Green Chem. 2014, 16, 3623–3634. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, V.K. Microwave-Assisted and Yb(OTf)3-Promoted One-Pot Multicomponent Synthesis of Substituted Quinolines in Ionic Liquid. Synlett 2011, 15, 2157–2162. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Inoue, Y.; Takai, K. Copper(I)- and gold(I)-catalyzed synthesis of 2,4-disubstituted quinoline derivatives from N-aryl-2-propynylamines. Chem. Lett. 2007, 36, 1422–1423. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.M.; Mao, D.; Wang, W.B.; Zhang, L.; Wu, S.Y.; Xie, Y.S. Ytterbium pentafluorobenzoate as a novel fluorous Lewis acid catalyst in the synthesis of 2,4-disubstituted quinolines. Tetrahedron 2011, 67, 8465–8469. [Google Scholar] [CrossRef]
- Xiao, F.P.; Chen, Y.L.; Liu, Y.; Wang, J.B. Sequential catalytic process: Synthesis of quinoline derivatives by AuCl3/CuBr-catalyzed three-component reaction of aldehydes, amines, and alkynes. Tetrahedron 2008, 64, 2755–2761. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.Q.; Shu, X.; Gao, Y.; Lv, H.P.; Zhu, J. Silver-Mediated C-H Activation: Oxidative Coupling/Cyclization of N-Arylimines and Alkynes for the Synthesis of Quinolines. J. Org. Chem. 2012, 77, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhang, F.M.; Tu, Y.Q.; Zhuo, X.T.; Fan, C.A. Iron(III)-Catalyzed and Air-MediatedTandem Reaction of Aldehydes, Alkynes and Amines: An Efficient Approach to Substituted Quinolines. Chem. Eur. J. 2009, 15, 6332–6334. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Patil, S.V.; Bobade, V.D. Synthesis of Aminoindolizine and Quinoline Derivatives via Fe(acac)3/TBAOH-Catalyzed Sequential Cross-Coupling-Cycloisomerization Reactions. Synlett 2011, 16, 2379–2383. [Google Scholar] [CrossRef]
- Yao, C.S.; Qin, B.B.; Zhang, H.H.; Lu, J.; Wang, D.L.; Tu, S.J. One-pot solvent-free synthesis of quinolines by C-H activation/C-C Bond formation catalyzed by recyclable iron(III) triflate. RSC Adv. 2012, 2, 3759–3764. [Google Scholar] [CrossRef]
- Bauer, I.; Knolker, H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.E.; Hatter, J.E.; Nieuwenhuyzen, M.; Pitner, W.R.; Seddon, K.R.; Thied, R.C. Precipitation of a dioxouranium(VI) species from a room temperature ionic liquid medium. Inorg. Chem. 2002, 41, 1692–1694. [Google Scholar] [CrossRef] [PubMed]
- Oxley, J.D.; Prozorov, T.; Suslick, K.S. Sonochemistry and sonoluminescence of room-temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 11138–11139. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Gaertner, P. An iron-containing ionic liquid as recyclable catalyst for aryl grignard cross-coupling of alkyl halides. Org. Lett. 2006, 8, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Nguyen, L.V.; Jeon, E.H.; Kim, J.H.; Cheong, M.; Kim, H.S.; Lee, J.S. Fe-containing ionic liquids as catalysts for the dimerization of bicyclo[2.2.1]hepta-2,5-diene. J. Catal. 2008, 258, 5–13. [Google Scholar] [CrossRef]
- Vincze, L.; Papp, S. Calculation of Association Constants and Distribution of Contact and Dipolar Interactions for [R3P+R'][FeCl4]− Ion-Pairs. Acta Chim. Hung. 1982, 110, 163–173. [Google Scholar]
- Santos, E.; Albo, J.; Rosatella, A.; Afonso, C.A.M.; Irabien, A. Synthesis and characterization of Magnetic Ionic Liquids (MILs) for CO2 separation. J. Chem. Technol. Biotechnol. 2014, 89, 866–871. [Google Scholar] [CrossRef]
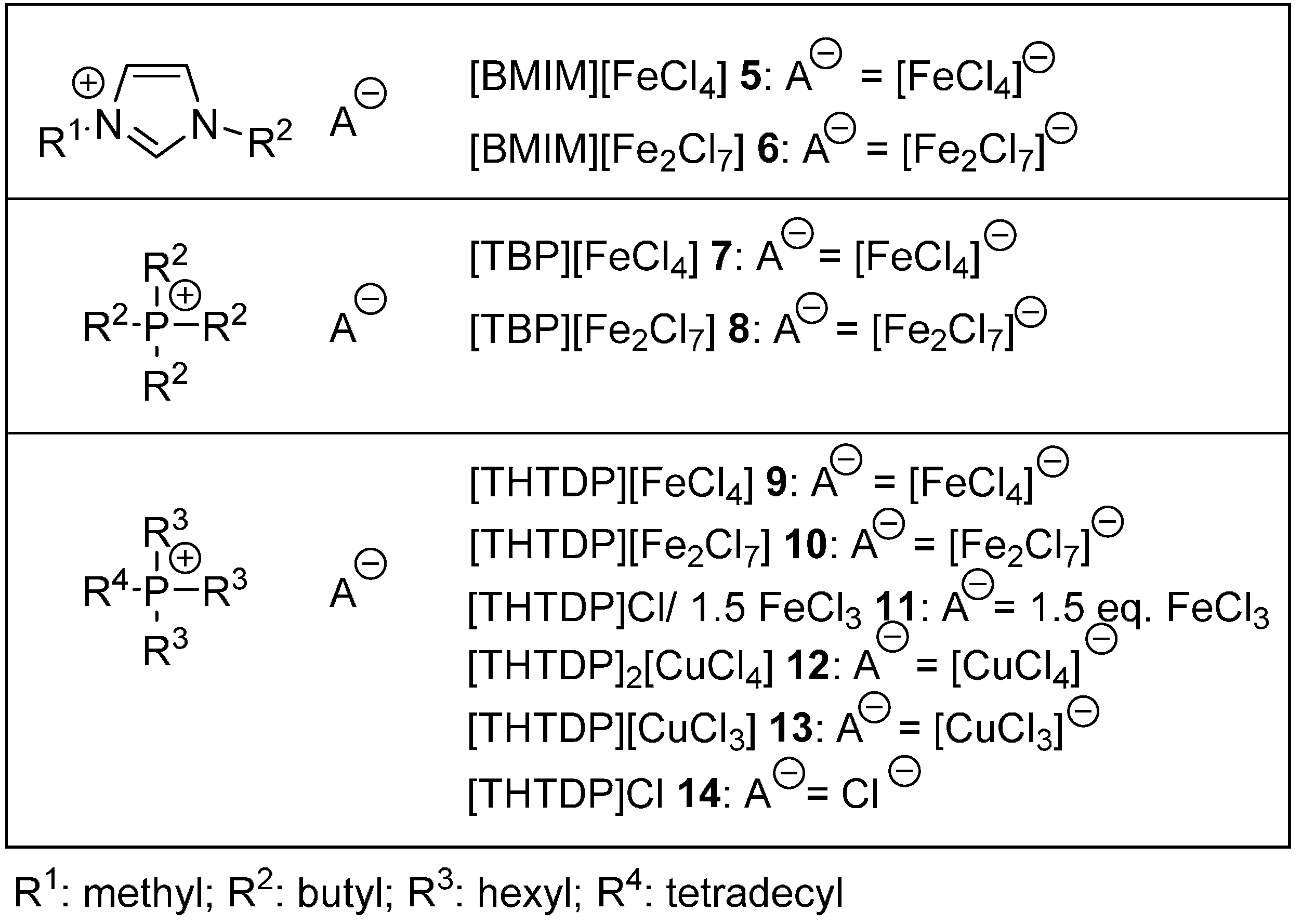
| Entry a | Catalyst | Solvents | Time | T [°C] | Yield [%] b |
|---|---|---|---|---|---|
| 1 | FeCl3·6H2O | toluene | 24 h | 110 | 53 |
| 2 | [BMIM][FeCl4] 5 | toluene | 24 h | 110 | - |
| 3 | [TBP][FeCl4] 7 | toluene | 24 h | 110 | - |
| 4 | [THTDP][FeCl4] 9 | toluene | 24 h | 110 | - |
| 5 | [BMIM][Fe2Cl7] 6 | toluene | 24 h | 110 | 44 |
| 6 | [BMIM][Fe2Cl7] 6 | toluene | 18 h | 110 | 40 |
| 7 | [BMIM][Fe2Cl7] 6 | - | 24 h | 130 | 16 |
| 8 | [TBP][Fe2Cl7] 8 | toluene | 24 h | 110 | 62 |
| 9 | [THTDP][Fe2Cl7] 10 | toluene | 24 h | 110 | 54 |
| 10 | [THTDP][Fe2Cl7] 10 | 1,2-dichloroethane | 24 h | 85 | 50 |
| 11 | [THTDP][Fe2Cl7] 10 | - | 24 h | 110 | 30 |
| 12 | [THTDP][Fe2Cl7] 10 | - | 24 h | 130 | 38 |
| 13 c | [THTDP][Fe2Cl7] 10 | - | 10 min | 110 | 41 |
| 14 | [THTDP]Cl/1.5 FeCl3 11 | toluene | 24 h | 110 | 48 |
| 15 | [THTDP]Cl/1.5 FeCl3 11 | 1,2-dichloroethane | 24 h | 85 | 38 |
| 16 | [THTDP]Cl/1.5 FeCl3 11 | toluene | 14 h | 110 | 38 |
| 17 | [THTDP]Cl/1.5 FeCl3 11 | - | 24 h | 110 | 36 |
| 18 | [THTDP]Cl/1.5 FeCl3 11 | - | 24 h | 130 | 31 |
| 19 c | [THTDP]Cl/1.5 FeCl3 11 | - | 20 min | 110 | 43 |
| 20 c | [THTDP]Cl/1.5 FeCl3 11 | - | 10 min | 110 | 10 |
| 21 c | [THTDP]Cl 14 | - | 20 min | 110 | - |
| 22 | CuCl2 | toluene | 24 h | 110 | - |
| 23 | [THTDP]2[CuCl4] 12 | toluene | 24 h | 110 | - |
| 24 | [THTDP][CuCl3] 13 | toluene | 24 h | 110 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntzeck, M.; Wilhelm, R. Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction. Int. J. Mol. Sci. 2016, 17, 860. https://doi.org/10.3390/ijms17060860
Muntzeck M, Wilhelm R. Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction. International Journal of Molecular Sciences. 2016; 17(6):860. https://doi.org/10.3390/ijms17060860
Chicago/Turabian StyleMuntzeck, Maren, and René Wilhelm. 2016. "Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction" International Journal of Molecular Sciences 17, no. 6: 860. https://doi.org/10.3390/ijms17060860
APA StyleMuntzeck, M., & Wilhelm, R. (2016). Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction. International Journal of Molecular Sciences, 17(6), 860. https://doi.org/10.3390/ijms17060860