Influence of Long-Distance Bicycle Riding on Serum/Urinary Biomarkers of Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Comparison of Pre- and Post-Ride Anthropometric Characteristics
2.2. Comparison of Pre- and Post-Ride Serum and Urinary Biochemical Markers
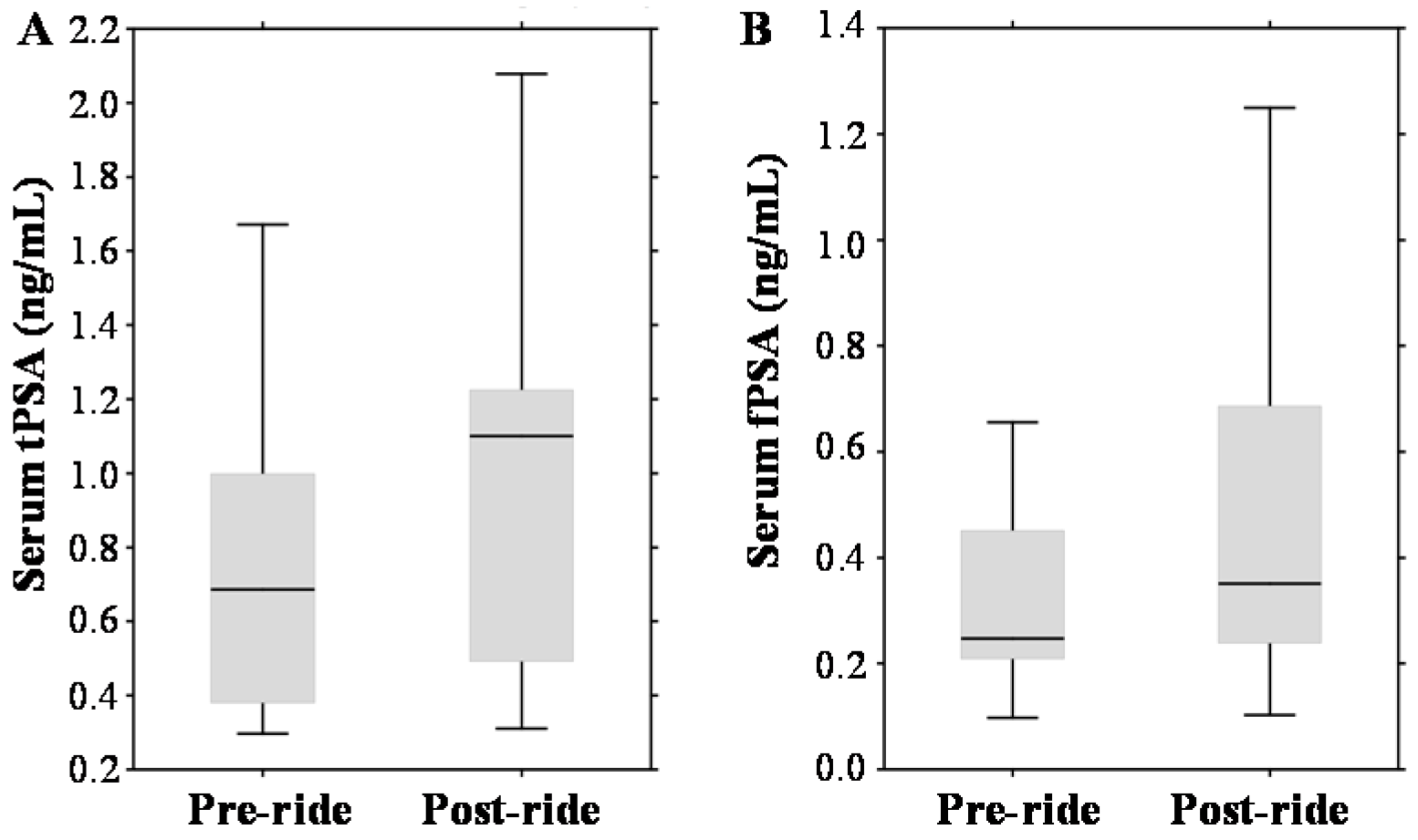
2.3. Evaluation of Pre- and Post-Ride Levels of Prostate Cancer (PCa) Biomarkers
3. Discussion
3.1. Evaluation of Pre- and Post-Ride Anthropometric and Serum Biochemical Parameters
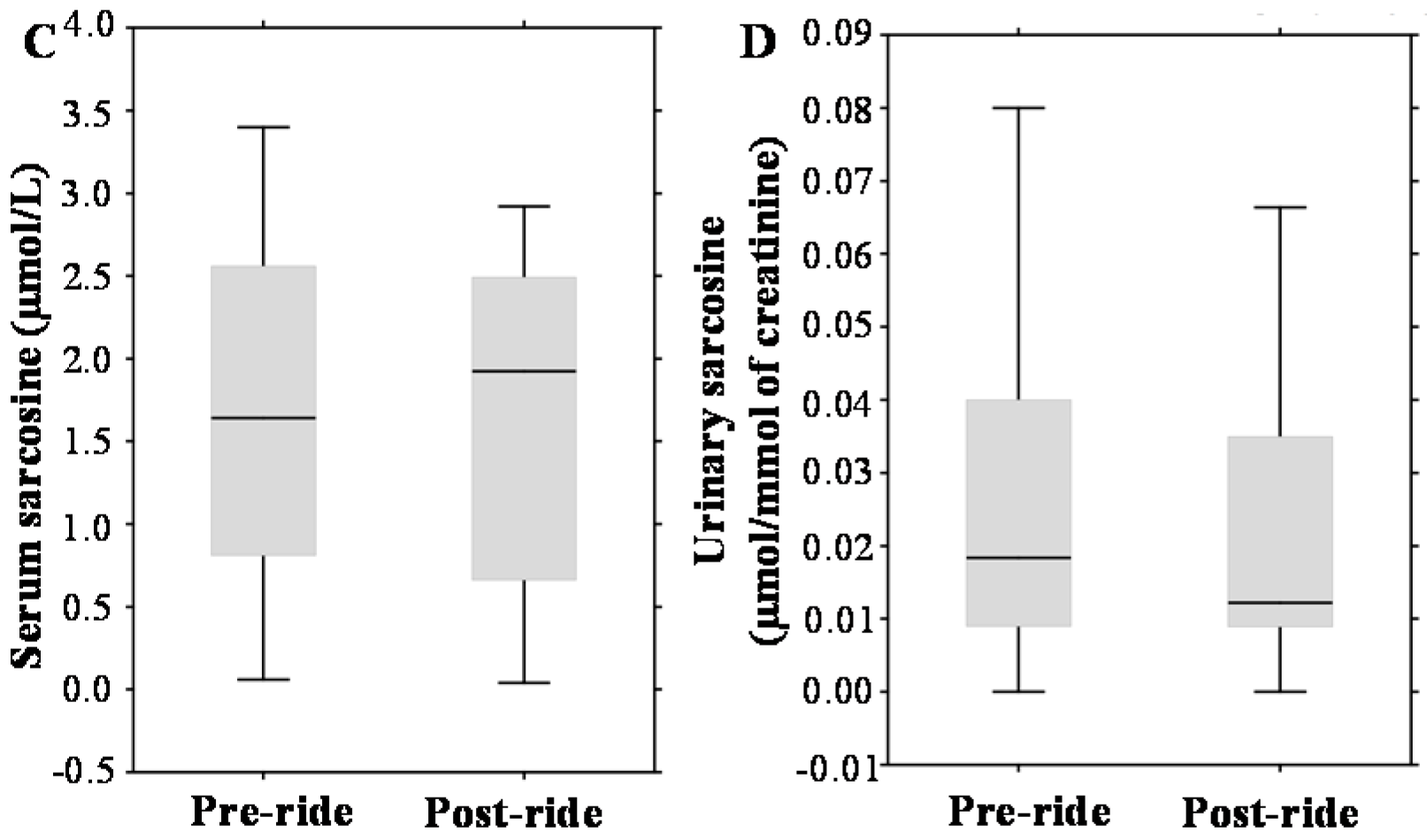
3.2. Evaluation of Pre- and Post-Ride Urinary Biochemical Parameters
3.3. The Effect of Physical Exercise on PCa Biomarkers
4. Materials and Methods
4.1. Chemical Compounds
4.2. Volunteers, Physical Exercise and Sample Collection
4.3. Anthropometric Measurements
4.4. Biochemical Analyses of Serum Specimens
4.5. Biochemical Analyses of Urinary Specimens
4.6. Preparation of Serum and Urinary Specimens for Sarcosine Determination
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| tPSA | Total prostate specific antigen |
| fPSA | Free prostate specific antigen |
| nPSA | Nicked PSA |
| PSA | Prostate specific antigen |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| GMT | Gamma glutamyltransferase |
| CRP | C-reactive protein |
| ATP | Adenosine triphosphate |
| AMP | Adenosine monophosphate |
| IMP | Inosine-5′-monophosphate |
| IEC-VIS | Ion-exchange chromatography with detector in visible range |
| hK2 | Kallikrein-related peptidase 2 |
| ACT | 1-antichymotrypsin |
| BR | Bicycle riding |
References
- Oberpenning, F.; Schmid, H.P.; Fuchs-Surdel, W.; Hertle, L.; Semjonow, A. The impact of intraoperative manipulation of the prostate on total and free prostate-specific antigen. Int. J. Biol. Markers 2002, 17, 154–160. [Google Scholar] [PubMed]
- Herrmann, M.; Scharhag, J.; Sand-Hill, M.; Kindermann, W.; Herrmann, W. Long-distance mountain biking does not disturb the measurement of total, free or complexed prostatespecific antigen in healthy men. Clin. Chem. Lab. Med. 2004, 42, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Mejak, S.L.; Bayliss, J.; Hanks, S.D. Long distance bicycle riding causes prostate-specific antigen to increase in men aged 50 years and over. PLoS ONE 2013, 8, e56030. [Google Scholar] [CrossRef] [PubMed]
- Oremek, G.M.; Seiffert, U.B. Physical activity releases prostate-specific antigen (PSA) from the prostate gland into blood and increases serum PSA concentrations. Clin. Chem. 1996, 42, 691–695. [Google Scholar] [PubMed]
- Kindermann, W.; Lehmann, V.; Herrmann, M.; Loch, T. Influencing of the PSA concentration in serum by physical exercise (especially bicycle riding). Urologe 2011, 50, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Safford, H.R.; Crawford, E.D.; Mackenzie, S.H.; Capriola, M. The effect of bicycle riding on serum prostate specific antigen levels. J. Urol. 1996, 156, 103–105. [Google Scholar] [CrossRef]
- Frymann, R.J.; Nuttall, M.C.; Carter, P.G. Case report: Endurance cycle ride associated with a significant rise in PSA. Int. Urol. Nephrol. 2006, 38, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Luboldt, H.J.; Peck, K.D.; Oberpenning, F.; Schmid, H.P.; Luboldt, W.; Semjonow, A. Bicycle riding has no important impact on total and free prostate-specific antigen serum levels in older men. Urology 2003, 61, 1177–1180. [Google Scholar] [CrossRef]
- Saka, T.; Sofikerim, M.; Demirtas, A.; Kulaksizoglu, S.; Caniklioglu, M.; Karacagil, M. Rigorous bicycling does not increase serum levels of total and free prostate-specific antigen (PSA), the free/total PSA ratio, gonadotropin levels, or uroflowmetric parameters. Urology 2009, 74, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Swain, R.A.; Montalto, N.; Ross, D. The effect of long-distance cycling on the prostate-specific antigen level. Arch. Fam. Med. 1997, 6, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; Oesterling, J.E. The clinical usefulness of prostate-specific antigen—Update-1994. J. Urol. 1994, 152, 1358–1368. [Google Scholar] [CrossRef]
- Holmstrom, B.; Johansson, M.; Bergh, A.; Stenman, U.H.; Hallmans, G.; Stattin, P. Prostate specific antigen for early detection of prostate cancer: Longitudinal study. Br. Med. J. 2009, 339, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; van der Maas, P.; Beemsterboer, P.; Kruger, A.B.; Hoedemaeker, R.; Rietbergen, J.; Kranse, R. Evaluation of the digital rectal examination as a screening test for prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Eisenberger, M.A.; Halabi, S.; Oudard, S.; Nanus, D.M.; Petrylak, D.P.; Sartor, A.O.; Scher, H.I. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur. Urol. 2012, 61, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Fanelli, M.; Larocca, A.M.V.; Germinario, C.A.; Rutigliano, M.; Vavallo, A.; Selvaggi, F.P.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Serum sarcosine increases the accuracy of prostate cancer detection in patients with total serum PSA less than 4.0 ng/mL. Prostate 2012, 72, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a potential prostate cancer biomarker—A review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.D.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, R.A.; Bartok, C. Hydration testing of athletes. Sports Med. 2002, 32, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L.; Poule, K.A.; Bland, E.G. Estimation of prepractice hydration status of national collegiate athletic association division i athletes. J. Athl. Train. 2009, 44, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P. Blood-pressure behavior during physical-activity. Sports Med. 1988, 5, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R. Scientific contributions of A. V. Hill: Exercise physiology pioneer. J. Appl. Physiol. 2002, 93, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Messonnier, L.; Denis, C.; Feasson, L.; Lacour, J.R. The following letters are in response to point: Counterpoint series “lactic acid accumulation is an advantage/disadvangage during muscle activity”. J. Appl. Physiol. 2006, 101, 1269–1269. [Google Scholar] [PubMed]
- Daly, W.; Seegers, C.A.; Rubin, D.A.; Dobridge, J.D.; Hackney, A.C. Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur. J. Appl. Physiol. 2005, 93, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Pusch, H.H.; Wolf, W.; Pilger, E.; Pessenhofer, H.; Schwaberger, G.; Pristautz, H.; Purstner, P. Serum FSH, LH, and testosterone in humans after physical exercise. Int. J. Sports Med. 1982, 3, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Arce, J.C.; Desouza, M.J. Exercise and male factor infertility. Sports Med. 1993, 15, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Zmuda, J.M.; Thompson, P.D.; Winters, S.J. Exercise increases serum testosterone and sex hormone-binding globulin levels in older men. Metab. Clin. Exp. 1996, 45, 935–939. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Targher, G.; Lippi, G. Serum bilirubin levels and cardiovascular disease risk: A Janus Bifrons? In Advances in Clinical Chemistry; Gregory, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Chapter 3; Volume 50, pp. 47–63. [Google Scholar]
- Swift, D.L.; Johannsen, N.M.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effect of different doses of aerobic exercise training on total bilirubin levels. Med. Sci. Sports Exerc. 2012, 44, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Trape, A.A.; Jacomini, A.M.; Muniz, J.J.; Sertorio, J.T.C.; Tanus-Santos, J.E.; do Amaral, S.L.; Zago, A.S. The relationship between training status, blood pressure and uric acid in adults and elderly. BMC Cardiovasc. Disord. 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.O.; Pepys, M.B.; Gudnason, V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.; Senden, J.; Saris, W.H.M.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Malm, C.; Sjodin, B.; Sjoberg, B.; Lenkei, R.; Renstrom, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, N.; Fujimura, R.; Tokuyama, K.; Suzuki, M. A bout of resistance exercise increases urinary calcium independently of osteoclastic activation in men. J. Appl. Physiol. 1997, 83, 1159–1163. [Google Scholar] [PubMed]
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Jinzu, H.; Nagao, K.; Noguchi, Y.; Shimba, N.; Miyano, H.; Watanabe, T.; Iseki, K. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J. Effect of exercise on amino-acid-concentrations in skeletal-muscle and plasma. J. Exp. Biol. 1991, 160, 149–165. [Google Scholar] [PubMed]
- Song, Y.H.; Shiota, M.; Kuroiwa, K.; Naito, S.; Oda, Y. The important role of glycine n-methyltransferase in the carcinogenesis and progression of prostate cancer. Mod. Pathol. 2011, 24, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Cernei, N.; Gumulec, J.; Masarik, M.; Eckschlager, T.; Hrabec, R.; Zitka, O.; Adam, V.; Kizek, R. Determination of common urine substances as an assay for improving prostate carcinoma diagnostics. Oncol. Rep. 2014, 31, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Cernei, N.; Heger, Z.; Matousek, M.; Kopel, P.; Kynicky, J.; Masarik, M.; Kizek, R.; Adam, V. Microfluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urine. Electrophoresis 2013, 34, 2639–2647. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Before (Median, Interquartile Range) | After (Median, Interquartile Range) | p-Value |
|---|---|---|---|
| Muscles (%) | 35.55 (33.1–38.4) | 34.6 (32.5–37.6) | 0.01 |
| Fat (%) | 22.5 (16.4–25.5) | 22.75 (18.4–27.3) | 0.01 |
| Temperature (°C) | 36.4 (36.2–36.6) | 36.1 (36–36.3) | 0.08 |
| Body mass index (BMI) (kg/m2) | 25.9 (23.6–27.7) | 25.9 (23.8–27.4) | 0.10 |
| Systolic pressure (mmHg) | 143 (136–149) | 130 (123–137) | 0.01 |
| Diastolic pressure (mmHg) | 93 (79–99) | 87 (78–96) | 0.10 |
| Parameter | Before (Median, Interquartile Range) | After (Median, Interquartile Range) | p-Value |
|---|---|---|---|
| Testosterone (nmol/L) | 6.9 (5.1–11.7) | 8.5 (5.9–9.4) | 0.727 |
| Triglycerides (mmol/L) | 1.7 (1.35–2.14) | 1.6 (1.32–1.97) | 0.81 |
| ALP (µkat/L) | 0.53 (0.5–1.29) | 0.87 (0.46–1.06) | 0.75 |
| ALT (µkat/L) | 0.54 (0.47–0.64) | 0.52 (0.49–0.56) | 0.31 |
| AST (µkat/L) | 0.43 (0.41–0.5) | 0.48 (0.43–0.51) | 0.13 |
| GMT (µkat/L) | 0.46 (0.38–0.69) | 0.45 (0.34–0.75) | 0.55 |
| Cholesterol (mmol/L) | 5.75 (5.34–6.21) | 5.9 (5.28–6.34) | 0.14 |
| Bilirubin (µmol/L) | 7.35 (6.38–10.33) | 9.8 (7.92–10.93) | 0.60 |
| Glucose (mmol/L) | 4.93 (4.56–5.17) | 4.48 (4.38–4.99) | 0.20 |
| Total protein (g/L) | 65.48 (62.26–66.36) | 65.71 (64.04–67.2) | 0.31 |
| Creatinine (µmol/L) | 100.74 (96.9–103.9) | 103.21 (95.02–109.31) | 0.42 |
| Parameter | Before (Median, Interquartile Range) | After (Median, Interquartile Range) | p-Value |
|---|---|---|---|
| pH | 5.7 (5.1–6) | 5.1 (4.8–5.5) | 0.04 |
| Potassium (mmol/mmol) * | 32 (23–38) | 28.5 (17–43) | 0.75 |
| Chlorides (mmol/mmol) * | 244.5 (187–294) | 204.5 (137–247) | 0.08 |
| Sodium (mmol/mmol) * | 118 (79–178) | 113 (56–164) | 0.51 |
| Total protein (g/L) | 16.65 (12–32.1) | 17.8 (11.5–25) | 0.27 |
| Creatinine (mmol/L) | 6.96 (5.2–8.29) | 6.59 (3.64–8.7) | 0.78 |
| Valine (µmol/mmol) * | 13.35 (7.9–24.4) | 13.2 (3.8–25.3) | 0.62 |
| Tyrosine (µmol/mmol) * | 9.25 (3.8–17.3) | 6.55 (4.1–14.6) | 0.40 |
| Threonine (µmol/mmol) * | 10.4 (1.8–19.4) | 9.55 (5.8–19.5) | 0.59 |
| Serine (µmol/mmol) * | 6.95 (3.5–11.6) | 6.45 (3.6–9.5) | 0.30 |
| Proline (µmol/mmol) * | 4.6 (3.3–9.7) | 4.75 (2.8–9.8) | 0.83 |
| Phenylalanine (µmol/mmol) * | 7.95 (6.2–15.2) | 8.35 (5.1–16.6) | 1.00 |
| Methionine (µmol/mmol) * | 5.15 (2.4–10.4) | 5.25 (2.6–11.3) | 0.64 |
| Lysine (µmol/mmol) * | 49.6 (38.6–85.6) | 51.5 (34.1–130.1) | 0.20 |
| Leucine (µmol/mmol) * | 4.05 (0.9–9.8) | 2 (0.8–3.5) | 0.11 |
| Isoleucine (µmol/mmol) * | 4.6 (1–9.5) | 5.35 (2.6–7.7) | 0.97 |
| Histidine (µmol/mmol) * | 14.75 (3.6–50.6) | 11.5 (7.2–44.8) | 0.36 |
| Glycine (µmol/mmol) * | 6.3 (3–13) | 7.35 (3.9–11.1) | 0.36 |
| Glutamic acid (µmol/mmol) * | 3.45 (1.1–6) | 3.5 (1.3–7.3) | 0.81 |
| Cysteine (µmol/mmol) * | 4.2 (2.8–10.4) | 4.45 (1.8–5.3) | 0.64 |
| Aspartic acid (µmol/mmol) * | 27.5 (15.6–64.2) | 33 (20.1–66.7) | 0.55 |
| Arginine (µmol/mmol) * | 39.55 (16.3–61.7) | 30.3 (18.6–38.1) | 0.06 |
| Alanine (µmol/mmol) * | 3.55 (1.9–6.3) | 4.25 (1.7–7.2) | 0.25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heger, Z.; Gumulec, J.; Ondrak, A.; Skoda, J.; Zitka, Z.; Cernei, N.; Masarik, M.; Zitka, O.; Adam, V. Influence of Long-Distance Bicycle Riding on Serum/Urinary Biomarkers of Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 377. https://doi.org/10.3390/ijms17030377
Heger Z, Gumulec J, Ondrak A, Skoda J, Zitka Z, Cernei N, Masarik M, Zitka O, Adam V. Influence of Long-Distance Bicycle Riding on Serum/Urinary Biomarkers of Prostate Cancer. International Journal of Molecular Sciences. 2016; 17(3):377. https://doi.org/10.3390/ijms17030377
Chicago/Turabian StyleHeger, Zbynek, Jaromir Gumulec, Ales Ondrak, Jan Skoda, Zdenek Zitka, Natalia Cernei, Michal Masarik, Ondrej Zitka, and Vojtech Adam. 2016. "Influence of Long-Distance Bicycle Riding on Serum/Urinary Biomarkers of Prostate Cancer" International Journal of Molecular Sciences 17, no. 3: 377. https://doi.org/10.3390/ijms17030377
APA StyleHeger, Z., Gumulec, J., Ondrak, A., Skoda, J., Zitka, Z., Cernei, N., Masarik, M., Zitka, O., & Adam, V. (2016). Influence of Long-Distance Bicycle Riding on Serum/Urinary Biomarkers of Prostate Cancer. International Journal of Molecular Sciences, 17(3), 377. https://doi.org/10.3390/ijms17030377









