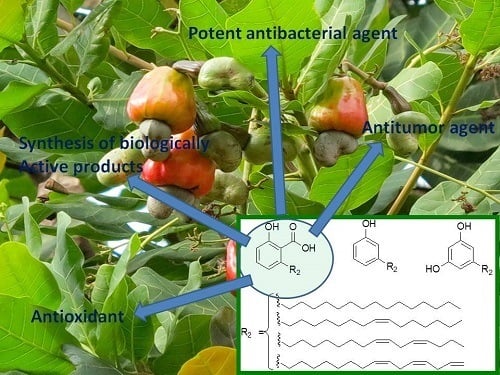
Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives
Abstract
:1. Introduction
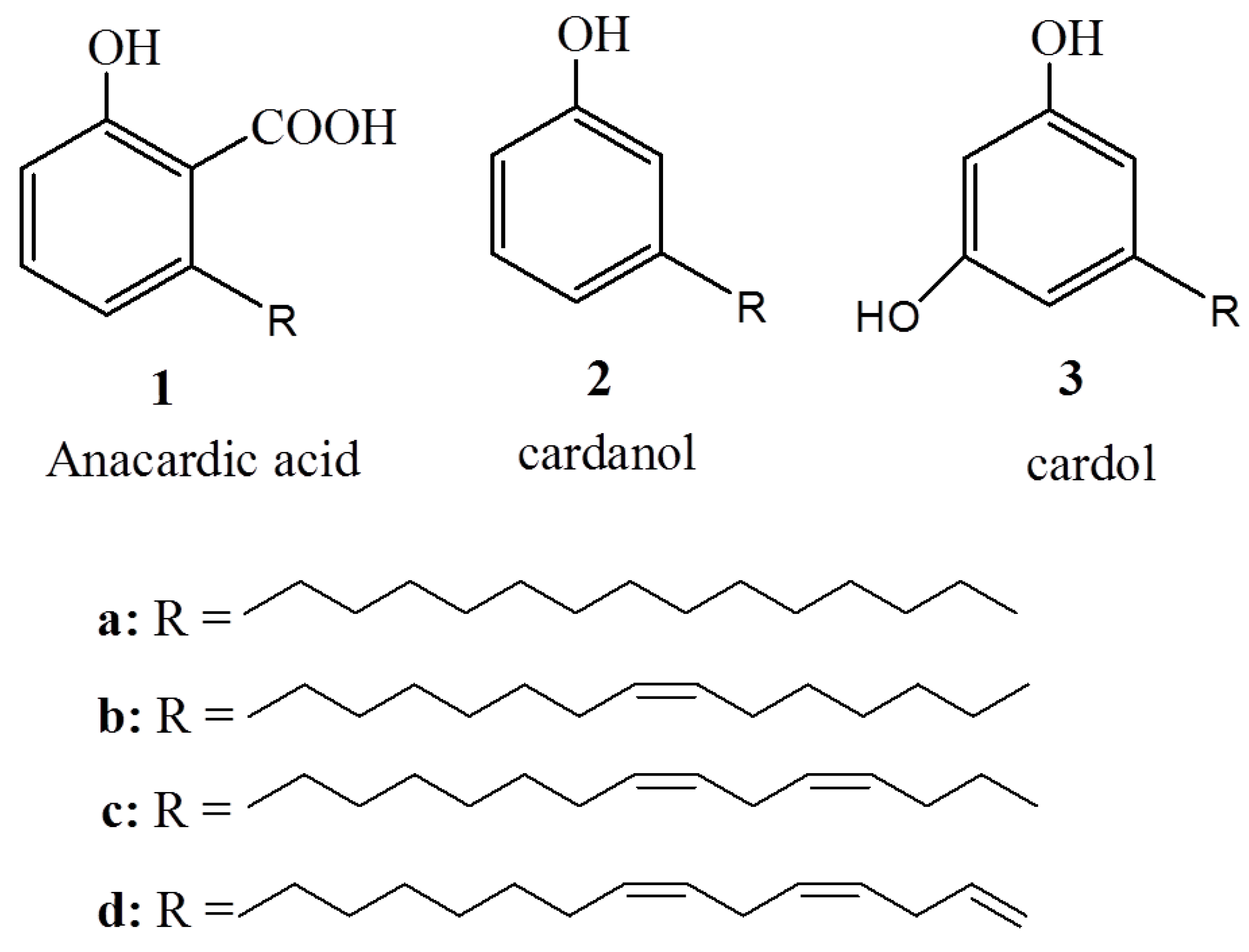
2. Biological Activity of Anacardic Acids
2.1. Antibacterial Activity of Anacardic Acids
| Bacterial | Antibacterial | MIC (µg/mL) | Reference |
|---|---|---|---|
| S. mutans (ATCC 25175) | 1a | ˃800 | [20] |
| 1b | 6.25 | [20] | |
| 1c | 3.13 | [20] | |
| 1d | 1.56 | [20] | |
| Vancomycin | 1 | [22] | |
| Ampicillin | 0.15 | [22] | |
| S. aureus (ATCC 12598) | 1a | ˃800 | [20] |
| 1b | 100 | [20] | |
| 1c | 25 | [20] | |
| 1d | 6.25 | [20] | |
| Methicillin | 1.56 | [23] | |
| Penicillin G | 0.049 | [23] | |
| P. acnes (ATCC 11827) | 1a | 0.78 | [20] |
| 1b | 0.78 | [20] | |
| 1c | 0.78 | [20] | |
| 1d | 0.78 | [20] | |
| Amoxicillin | 0.117 (MIC90) | [24] | |
| Penicillin G | 0.125 (MIC90) | [24] |
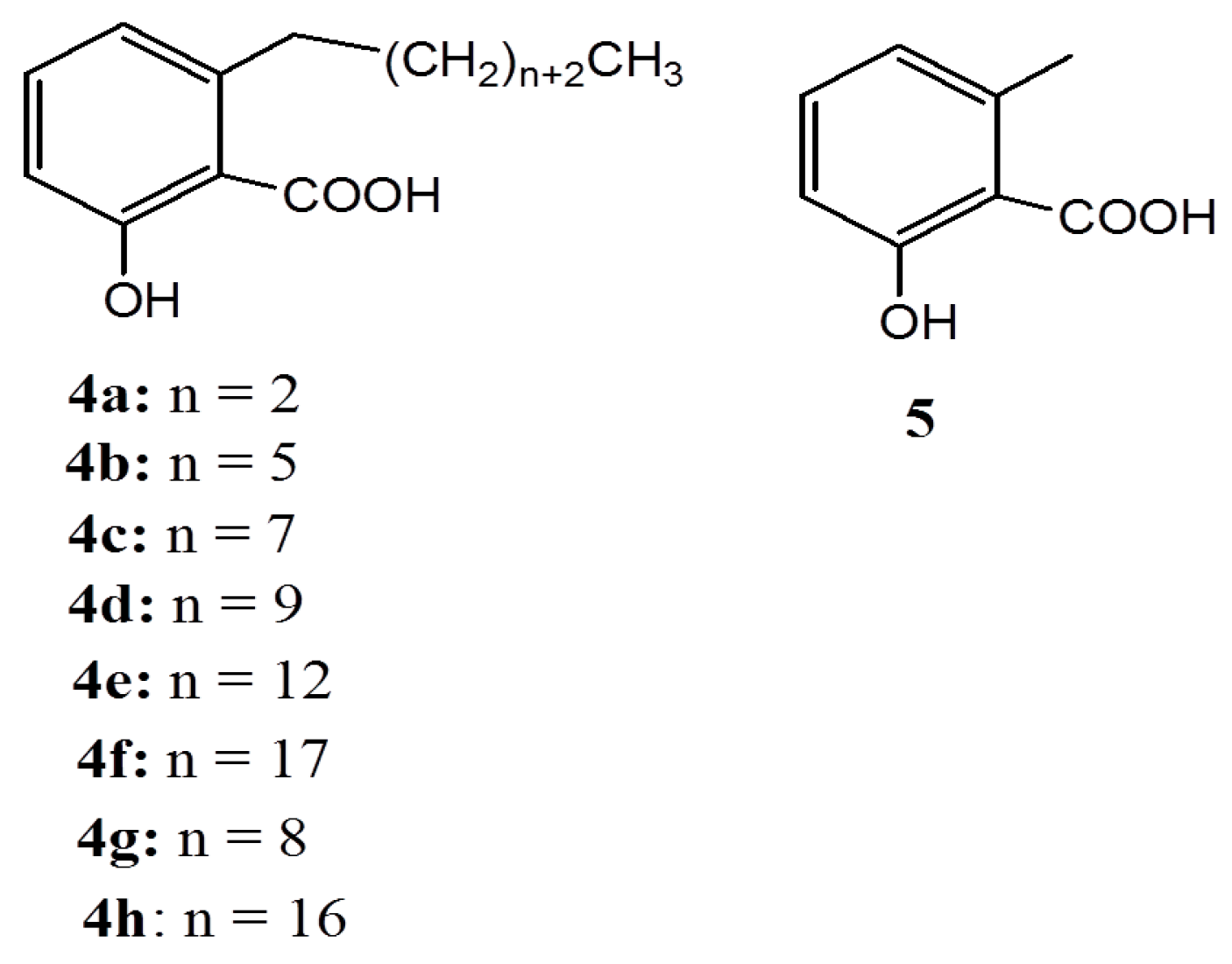
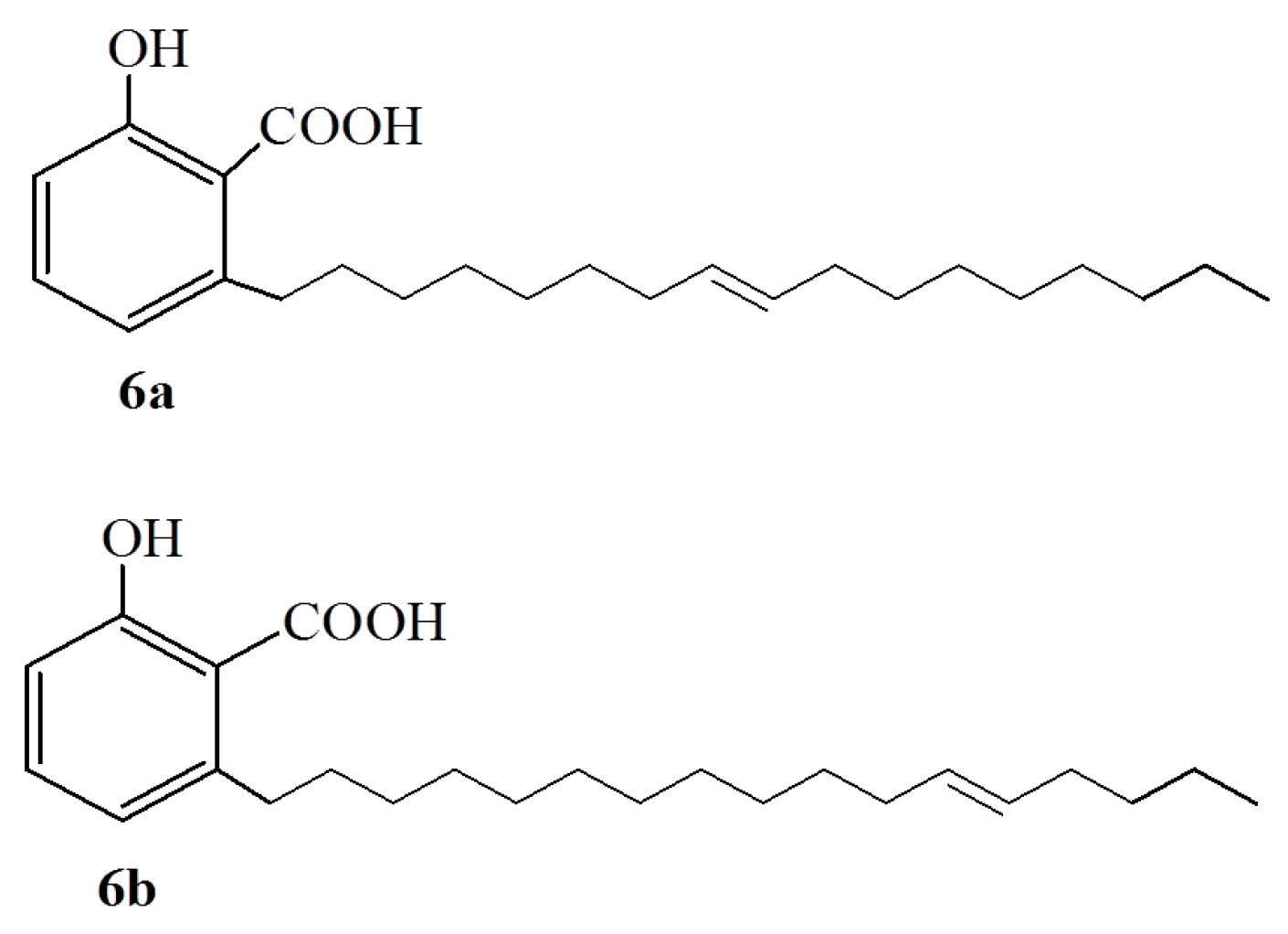
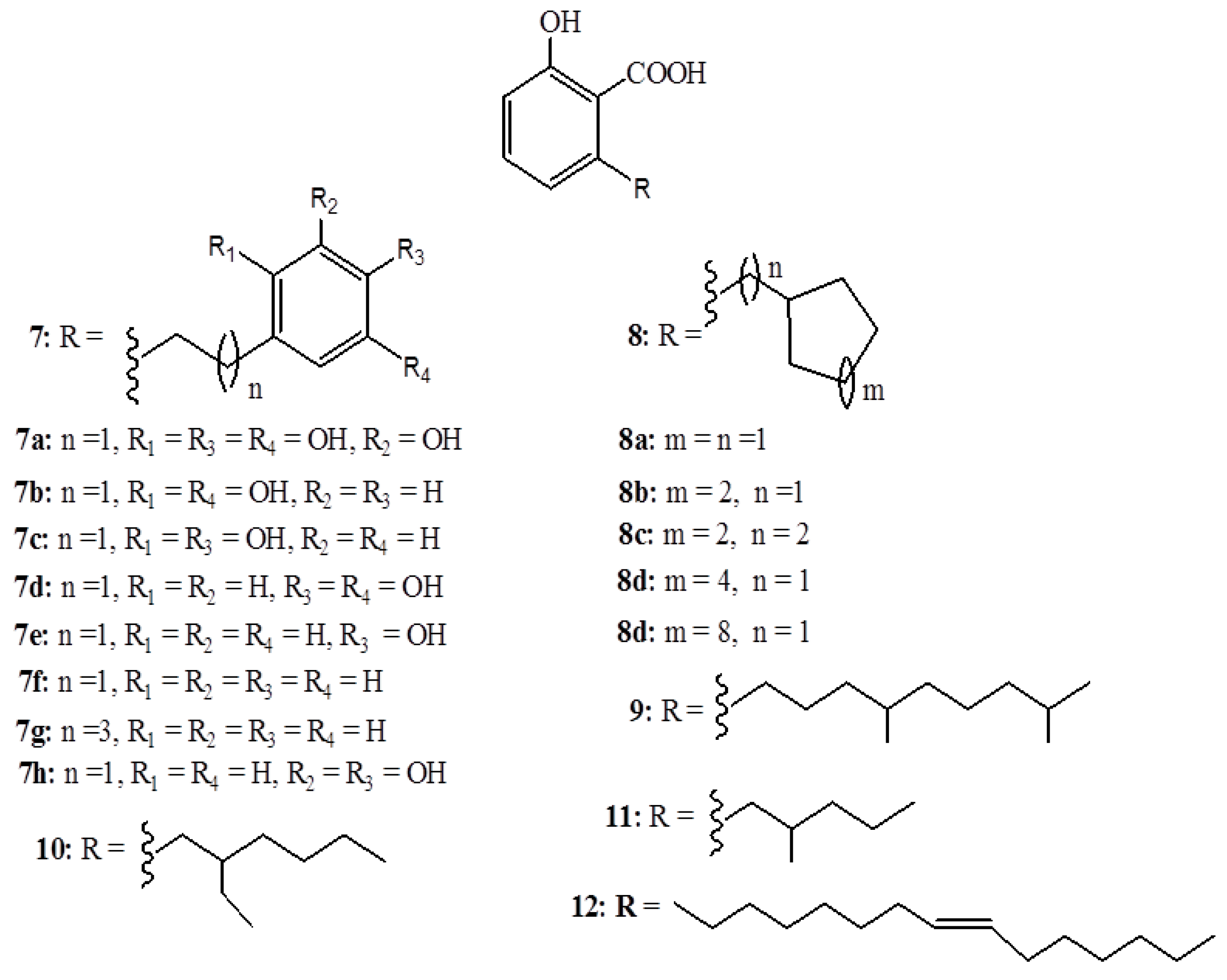
2.2. Anacardic Acids as Antitumor Agents
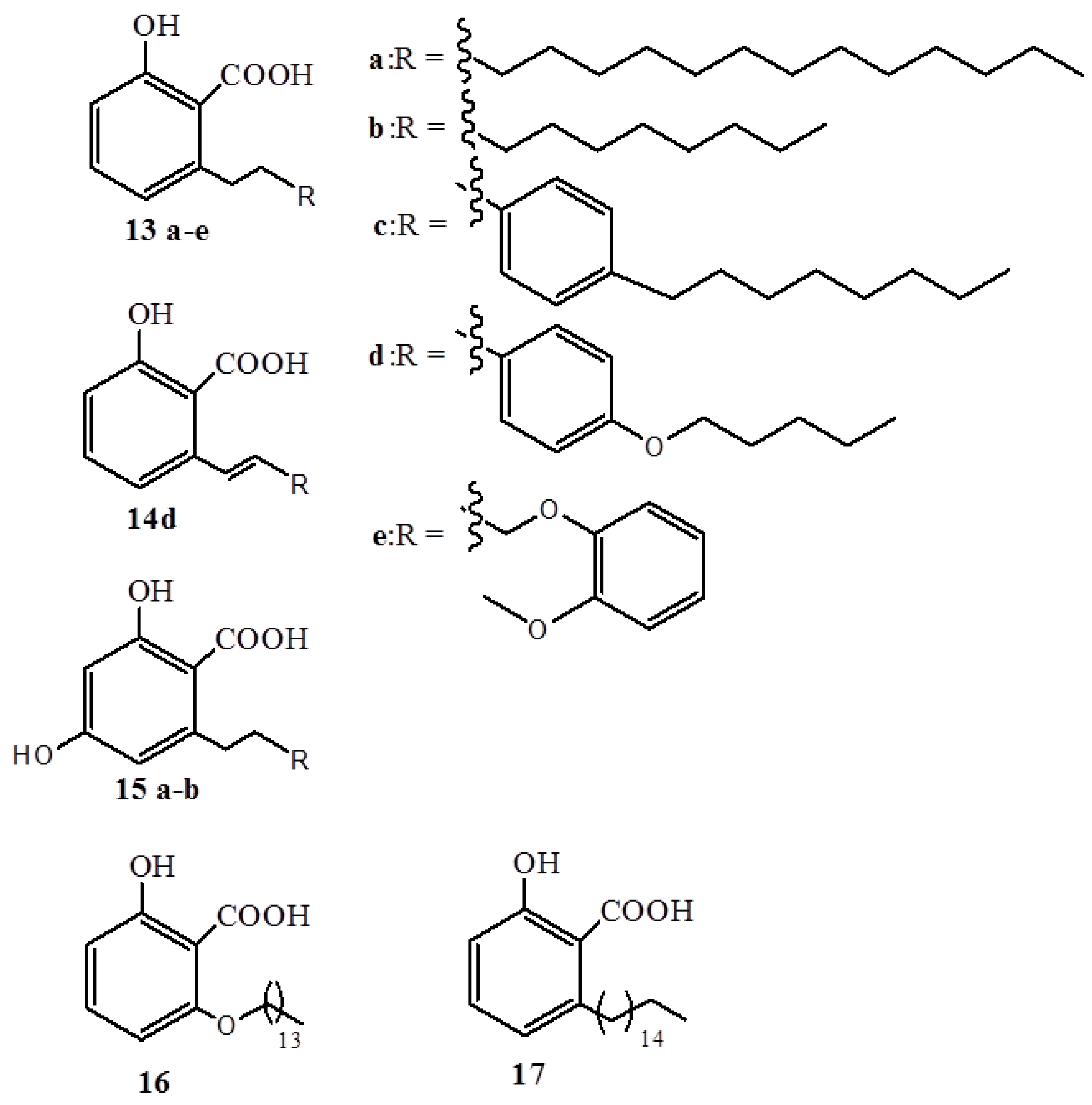
2.3. Anacardic Acidsas Antioxidants
3. Anacardic Acids as Synthons for Synthesis of Biologically Active Products
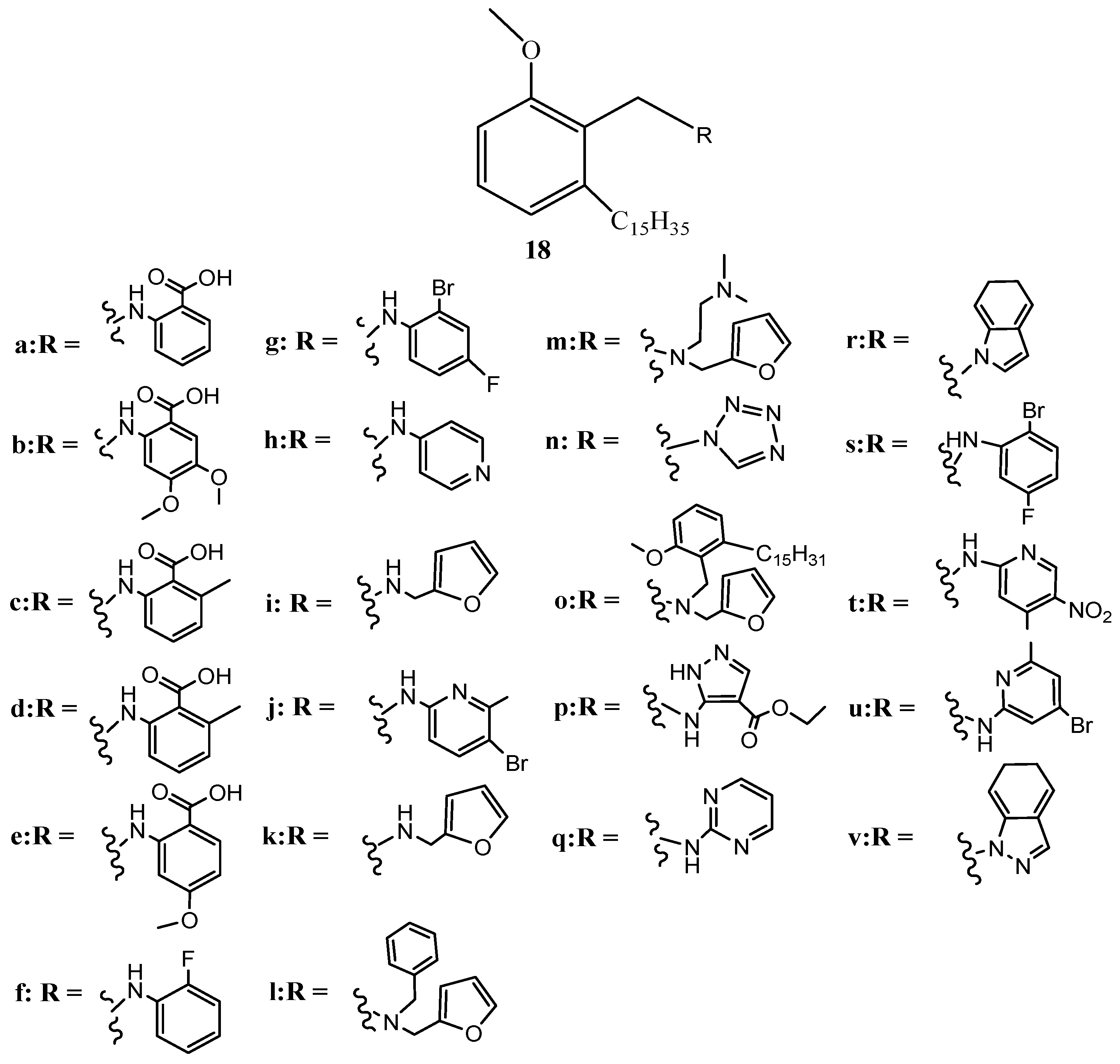

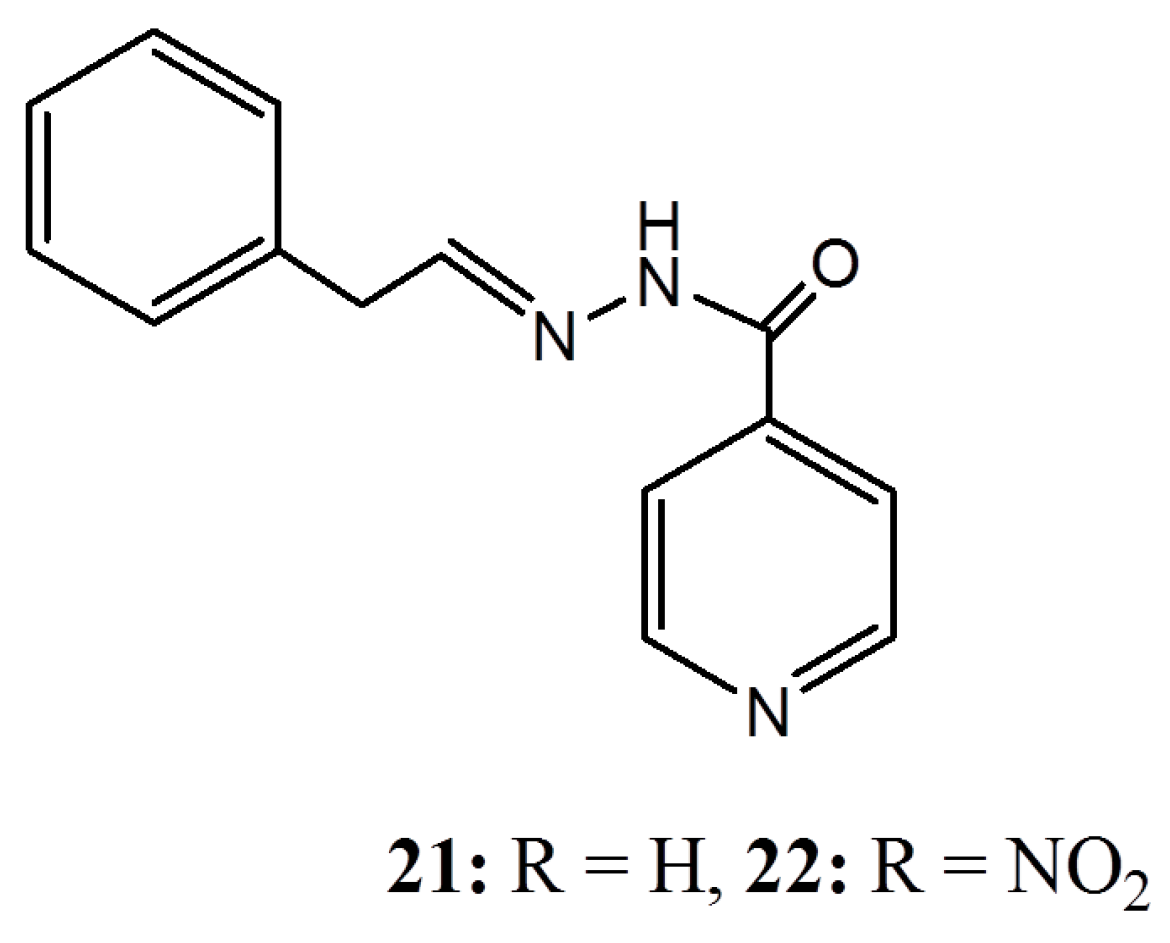
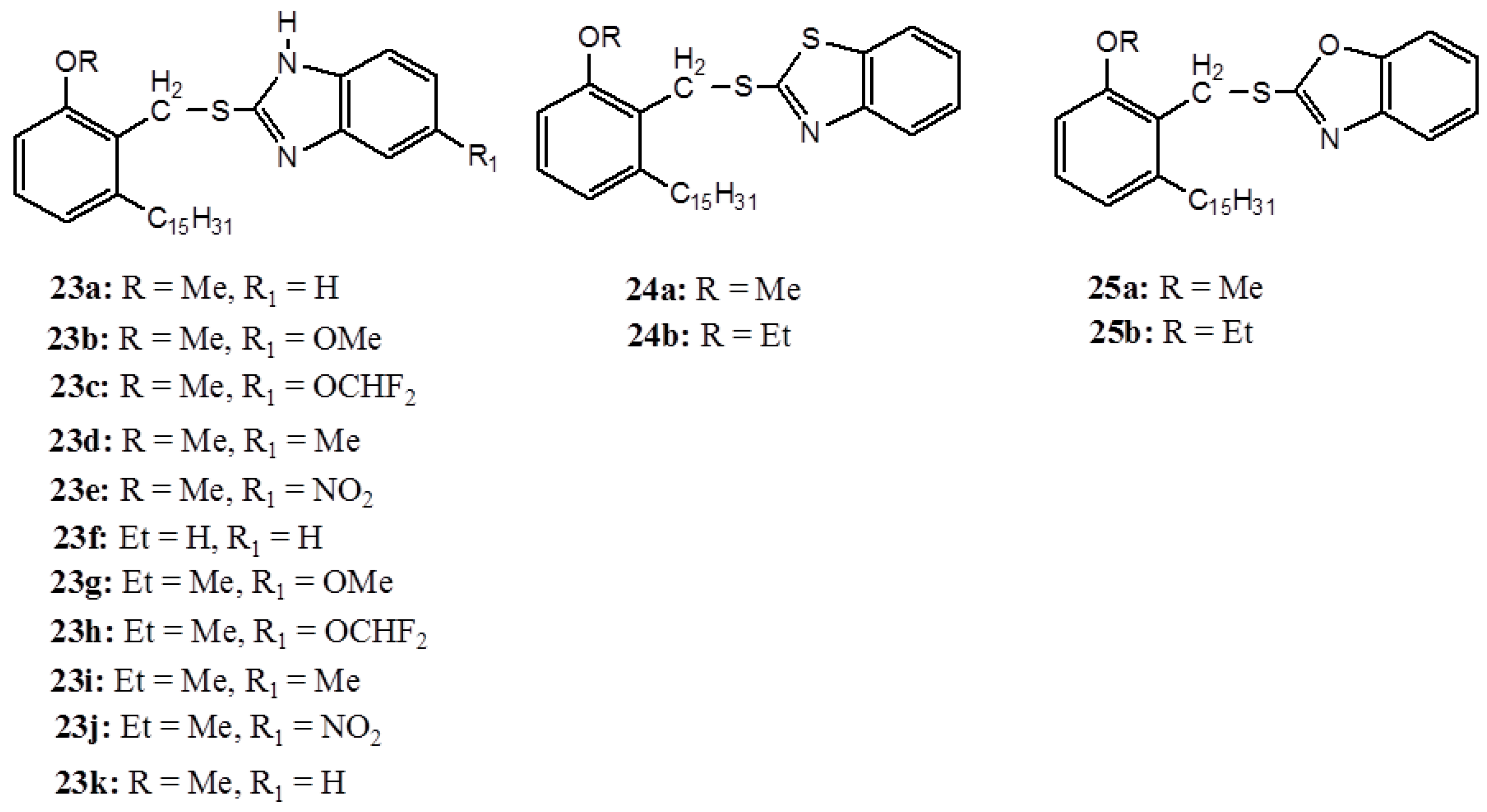
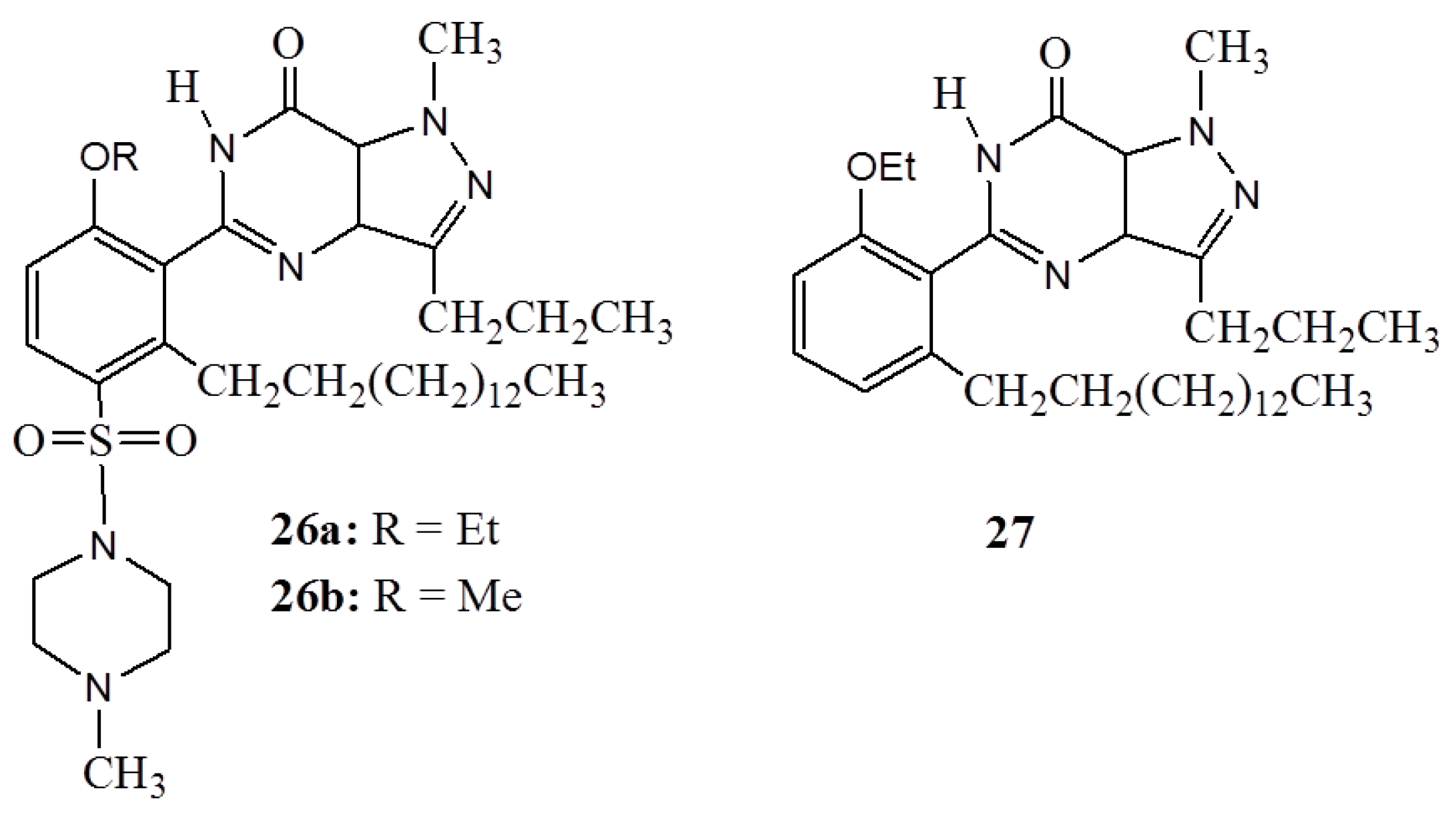
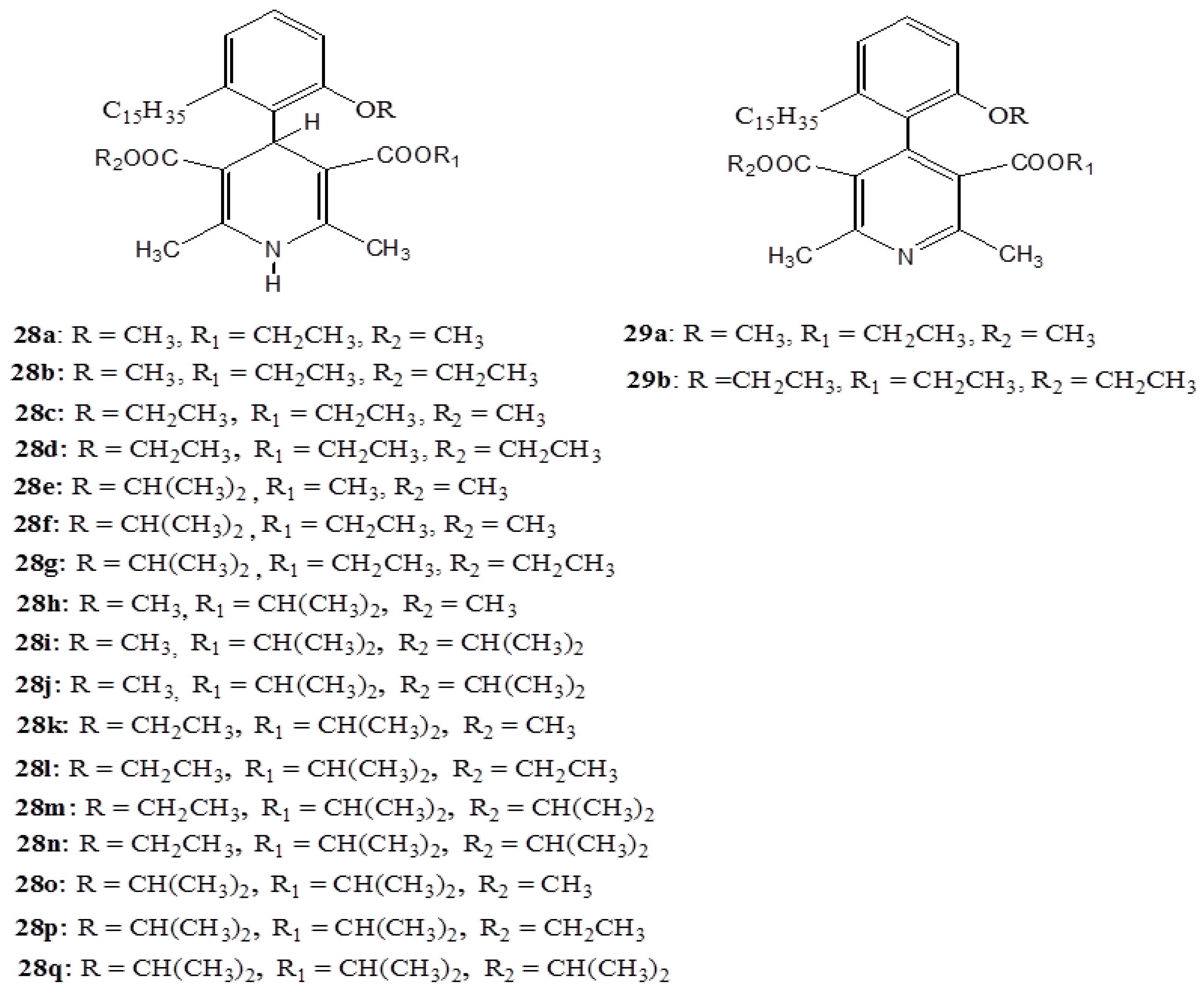
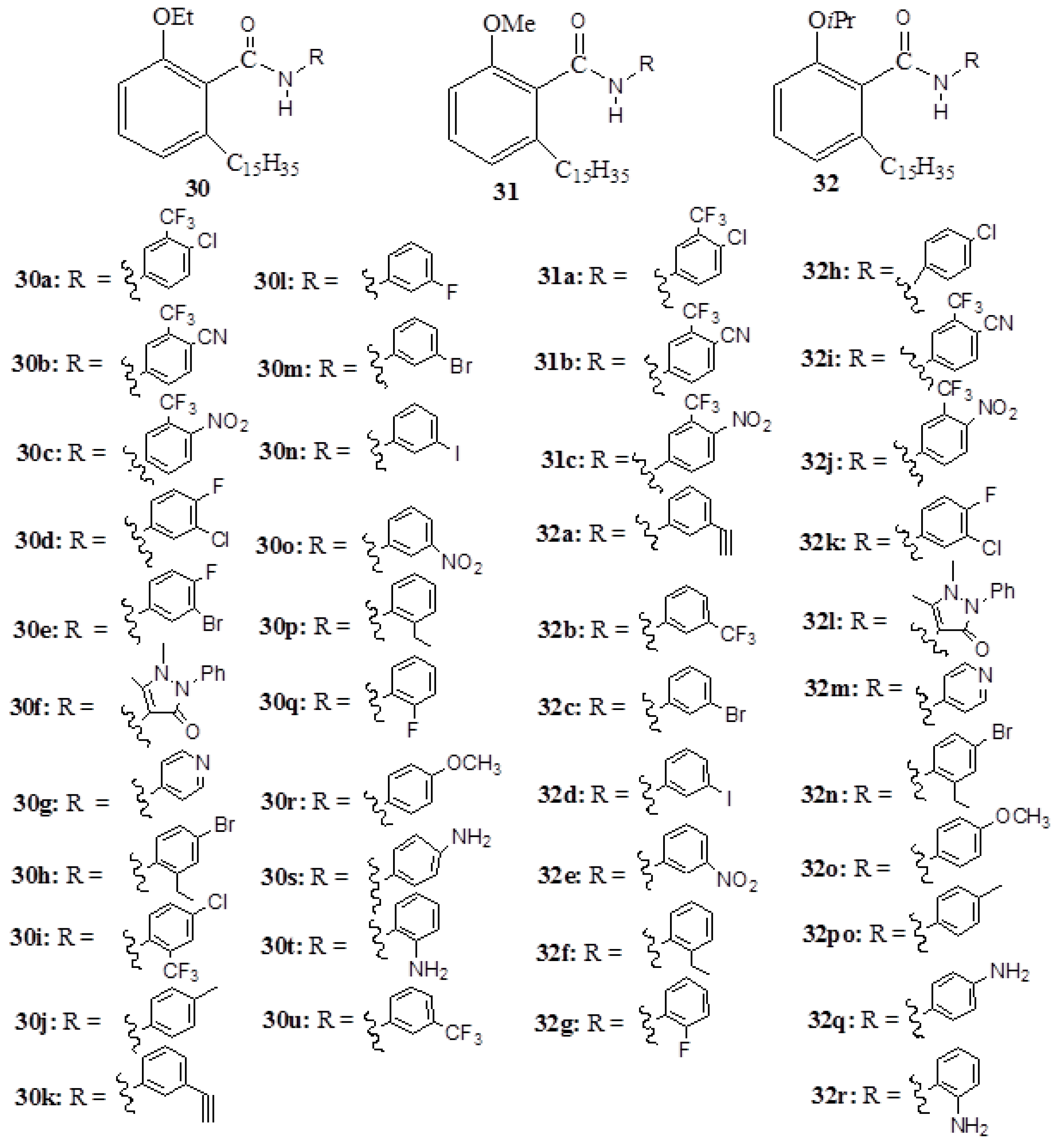
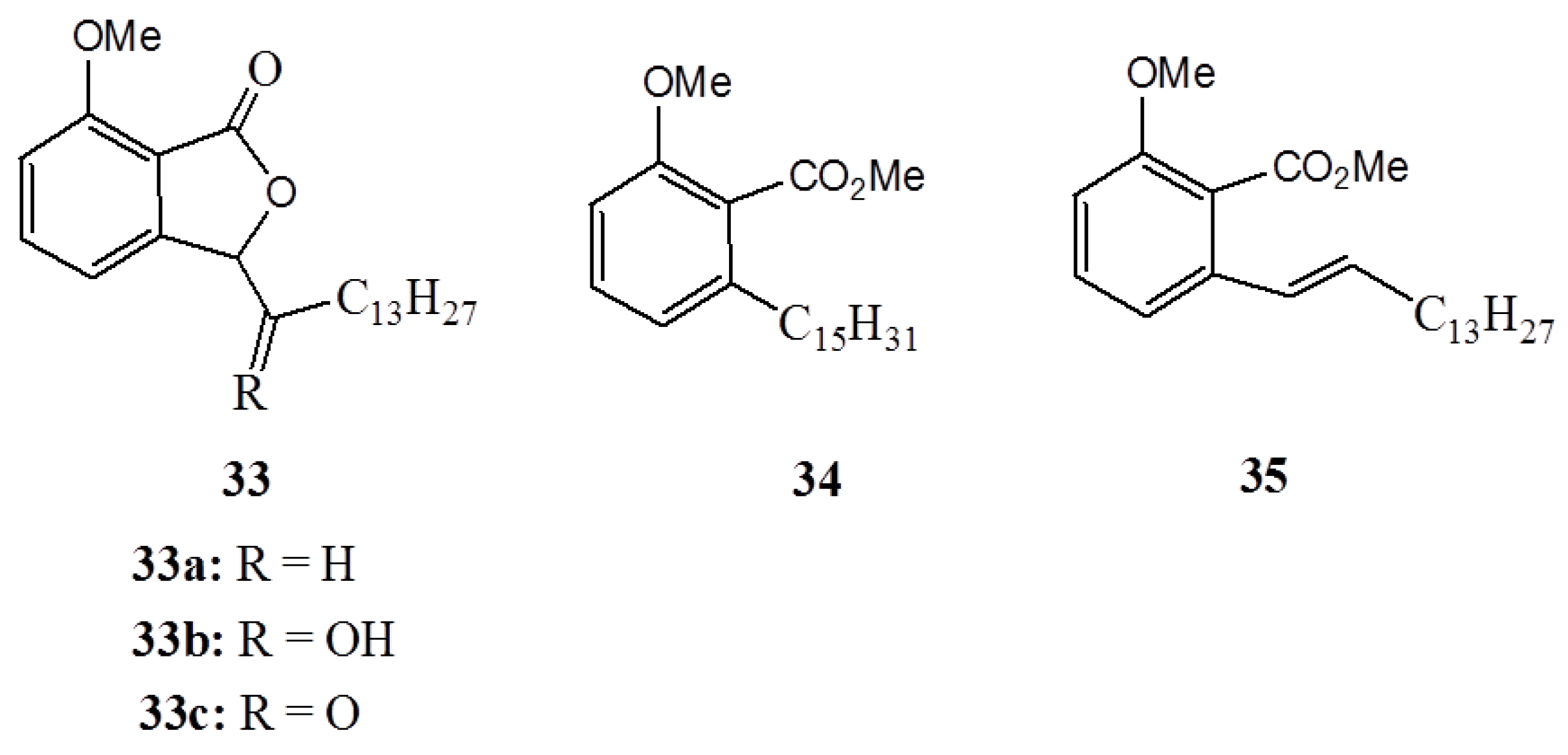
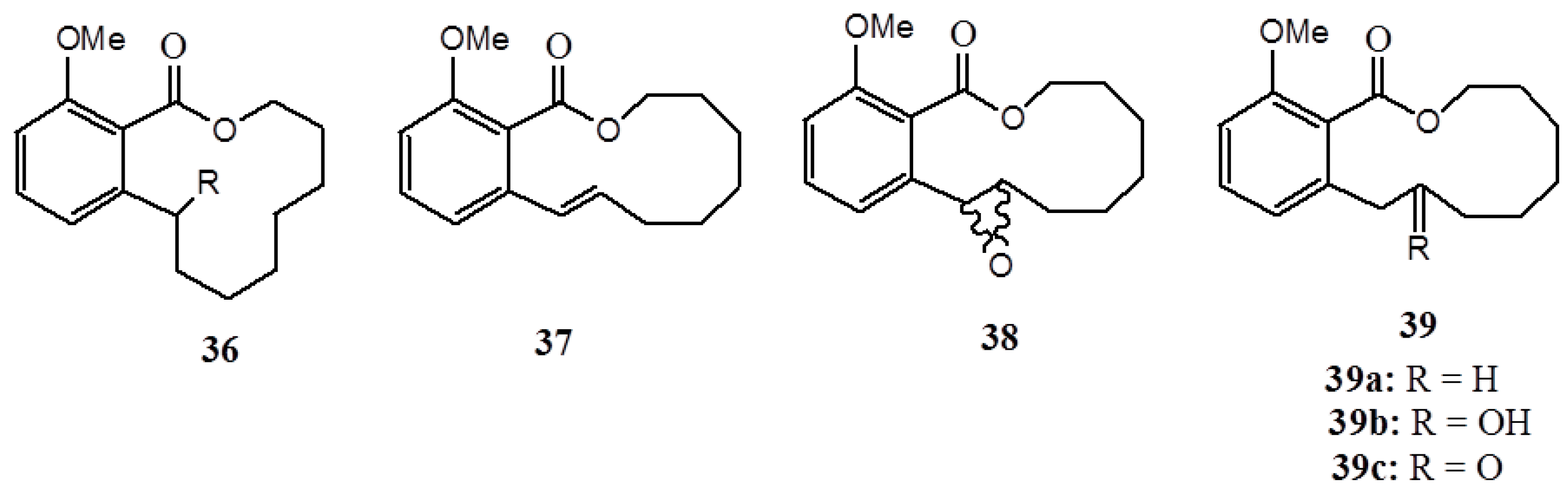
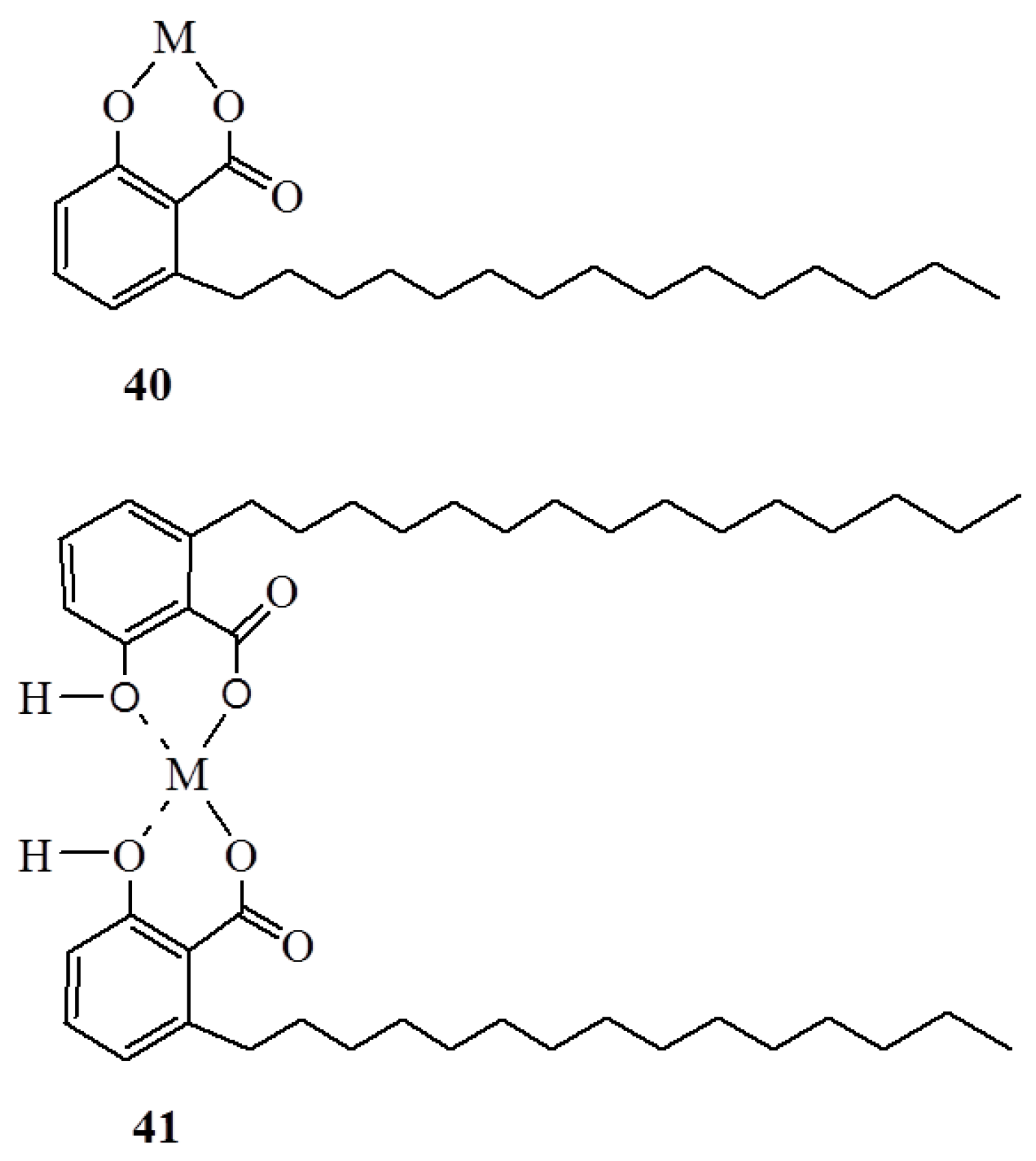
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mazzetto, S.E.; Lemonaco, D.; Mele, G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Quim. Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Tyman, J.H.P. Synthetic and Natural Phenols; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Manjula, S.; Pillai, C.K.S. Thermal characterization of cardanol-formaldehyde resins and cardanol-formaldehyde/poly(methyl methacrylate) semi-interpenetrating polymer networks. Thermochim. Acta 1990, 159, 255–266. [Google Scholar] [CrossRef]
- Tyman, J.H.P.; Johnson, R.A.; Muir, M.; Rokhgar, R. The extraction of natural cashewnut-shell liquid from the cashew nut (Anacardium occidentale). J. Am. Oil Chem. Soc. 1989, 66, 553–537. [Google Scholar] [CrossRef]
- Jain, P.K.; Sivala, K.J. Development of a cashew nut sheller. Food Eng. 1997, 32, 339–345. [Google Scholar] [CrossRef]
- Setianto, W.B.; Yoshikawa, S.; Smith, R.L., Jr.; Inomata, H.; Florusse, L.J.; Peters, C.J. Pressure profile separation of phenolic liquid compounds from cashew (Anacardium occidentale) shell with supercritical carbon dioxide and aspects of its phase equilibria. J. Supercrit. Fluids 2009, 48, 203–210. [Google Scholar] [CrossRef]
- Kumar, P.P.; Paramashivappa, R.; Vithayathil, P.J.; Rao, P.V.S.; Rao, A.S. Process for isolation of cardanol from technical cashew (Anacardium occidentale) nut shell liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef] [PubMed]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Trevisan, M.T.; Pfundstein, S.B.; Haubner, R.; Wurtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006, 44, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Mlowe, S.S.; Pullabhotla, R.R.; Mubofu, E.B.; Ngassapa, F.N.; Revaprasadu, N.N. Low temperature synthesis of anacardic-acid-capped cadmium chalcogenide nanoparticles. Int. Nano Lett. 2014, 4, 106–111. [Google Scholar] [CrossRef]
- Mlowe, S.; Nejo, A.A.; Rajasekhar Pullabhotla, V.S.R.; Mubofu, E.B.; Ngassapa, F.N.; O’Brien, P.; Revaprasadu, N. Lead chalcogenides stabilized by anacardic acid. Mater. Sci. Semicond. Process. 2013, 16, 263–268. [Google Scholar] [CrossRef]
- Pimentel, M.F.; de Lima, D.P.; Martins, L.R.; Beatriz, A.; Santaella, S.T.; Lotufo, L.V.C. Ecotoxicological analysis of cashew nut industry effluents, specifically two of its major phenolic components, cardol and cardanol. Pan Am. J. Aquat. Sci. 2009, 4, 363–368. [Google Scholar]
- Nagabhushana, K.S.; Ravindranath, B. Efficient medium-scale chromatographic group separation of anacardic acids from solvent-extracted cashew nut (Anacardium occidentale) shell liquid. Agric. Food Chem. 1995, 43, 2381–2383. [Google Scholar] [CrossRef]
- Tsunetaro, K.; Mitsuo, K. Separation and purification of anacardic acids using anion-exchange resin. Japanese Patent JP1995000062290, 22 March 1995. [Google Scholar]
- Kubo, I.; Komatsu, S.; Ochi, M. Molluscicides from the Cashew (Anacardium occidentale) and their large-scale isolation. J. Agric. Food Chem. 1986, 34, 970–973. [Google Scholar] [CrossRef]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.Y.N.; Francisco, J.D.C.; Dey, E.S.; Buchweishaija, J.; Mkayula, L.L.; Ye, L. Isolation of anacardic acid from natural cashew nut shell liquid (CNSL) using supercritical carbon dioxide. J. Agric. Food Chem. 2008, 56, 9350–9354. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; O’Brien, P.; Tuna, F.; Pritchard, R.; Buchweishaija, J.; Elianaso, K.; Mubofu, E.B. The synthesis, spectroscopy and X-ray single crystal structure of catena-[(µ-anacardato)-copper(II)bipyridine][Cu2{(µ-O2CC6H3(o-OH)(o-C15H31(NC5H5)2]. Dalton Trans. 2013, 42, 14438–14444. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M.; Yamagiwa, Y.; Mera, H.; Tokushima, K.; Ohta, S.; Kamikawa, T. Structure and antimicrobial activity relationships of anacardic acids. J. Agric. Food Chem. 1993, 41, 1016–1019. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Naturally occurring antiacne agents. J. Nat. Prod. 1994, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, X.; Shi, W.; Lu, Q.; Go, V.L.; Heber, D. Inhibition of growth of Streptococcus mutans, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci by kurarinone, a bioactive flavonoid isolated from Sophora flavescens. J. Clin. Microbiol. 2005, 43, 3574–3575. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resist ant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Hohman, D.W.; Nodzo, S.R.; Duquin, T.R. Antimicrobial Susceptibility of Propionibacterium acnes Isolates from Shoulder Surgery. Antimicrob. Agents Chemother. 2013, 57, 3424–3426. [Google Scholar] [CrossRef] [PubMed]
- Green, I.R.; Tocoli, F.E.; Lee, S.H.; Nihei, K.; Kubo, I. Design and evaluation of anacardic acid derivatives as anticavity agents. Eur. J. Med. Chem. 2008, 43, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Bactericidal activity of anacardic acids against streptococcus mutans and their potentiation. J. Agric. Food Chem. 1993, 41, 1780–1783. [Google Scholar] [CrossRef]
- Kubo, I.; Nihel, K.; Tsujimoto, K. Antibacterial action of anacardic acids against methicillin resistant Staphylococcus aureus (MRSA). J. Agric. Food Chem. 2003, 51, 7624–7628. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.J.; Haisma, H.J. Histone acetyl transferases as emerging drug targets. Drug Discov. Today 2009, 14, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, K.; Swaminathan, V.; Ranganathan, A.; Kundu, T.K. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 2003, 278, 19134–19140. [Google Scholar] [CrossRef]
- Ghizzoni, M.; Boltjes, A.; de Graaf, C.; Haisma, H.J.; Dekker, F. Improved inhibition of the histone acetyltransferase PCAF by an anacardic acid derivative. J. Bioorg. Med. Chem. 2010, 18, 5826–5834. [Google Scholar] [CrossRef]
- Kusio-Kobialka, M.; Dudka-Ruszkowska, W.; Ghizzoni, M.; Dekker, F.J.; Piwocka, K. Inhibition of PCAF by anacardic acid derivative leads to apoptosis and breaks resistance to DNA damage in BCR-ABL-expressing Cells. Anti-Cancer Agents Med. Chem. 2013, 13, 762–767. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Chen, S.; Price, B.D. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006, 580, 4353–4356. [Google Scholar] [CrossRef] [PubMed]
- Cate, R.T.; Krawczyk, P.; Aten, J.S.A.; Franken, N.A.P. Radiosensitizing effect of the histone acetyltransferase inhibitor anacardic acid on various mammalian cell lines. Oncol. Lett. 2010, 1, 765–769. [Google Scholar] [PubMed]
- Cui, L.; Miao, J.; Furuya, T.; Fan, Q.; Li, X.; Rathod, P.K.; Su, X.; Cui, L. Development plasmodium falciparum gene expression during in vitroanacardic acid causes changes in global histone acetyltransferase inhibitor. Eukaryot. Cell 2008, 7, 1200–1210. [Google Scholar]
- Sung, B.; Pandey, M.K.; Ahn, K.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-κB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-κBα kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; de Bosscher, K.; Boone, E.; Plaisance, S.; Haegeman, G. The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J. Biol. Chem. 1999, 274, 32091–32098. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, P.S.; Rahman, I.; Donaldson, K.; MacNee, W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L533–L540. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, B.; He, L; Tang, Y.; Jiang, Z.; Yin, G.; Wang, J.; Jiang, X. Anacardic acid (6-pentadecylsalicylic acid) induces apoptosis of prostate cancer cells through inhibition of androgen receptor and activation of p53 signaling. Chin. J. Cancer Res. 2012, 24, 275–283. [Google Scholar] [CrossRef]
- Wu, Y.; He, L.; Li, Z.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.H.; Vedamurthy, B.M.; Mantelingu, K.; Agrawal, S.; Reddy, B.A.A.; Rangappa, K.S.; Roy, S.; Kundu, T.K. Specific small-molecule activator of aurora kinase a induces autophosphorylation in a cell-free system. J. Med. Chem. 2008, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Ochi, M.; Vieira, P.C.; Komatsu, S. Antitumor agents from the cashew (Anacardium occidentale) apple juice. J. Agric. Food Chem. 1993, 41, 1012–1015. [Google Scholar] [CrossRef]
- Schultz, D.J.; Wickramasinghe, N.S.; Ivanova, M.M.; Isaacs, S.M.; Dougherty, S.M.; Imbert-Fernandez, Y.; Cunningham, A.R.; Chen, C.; Klinge, C.M. Anacardic acid inhibits estrogen receptor α-DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol. Cancer Ther. 2010, 9, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.; Shin, P.; Kim, G. Anacardic acid induces mitochondrial-mediated apoptosis in the A549 human lung adenocarcinoma cells. Int. J. Oncol. 2013, 42, 1045–1051. [Google Scholar] [PubMed]
- Legut, M.; Lipka, D.; Filipczak, N.; Piwoni, A.; Kozubek, A; Gubernator, J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines—In vitro studies. Int. J. Nanomed. 2014, 9, 653–668. [Google Scholar]
- Grazzini, R.; Hesk, D.; Heininger, E.; Hildenbrandt, G.; Reddy, C.C.; Cox-Foster, D.; Medford, J.; Craig, R.; Mumma, R.O. Inhibition of lipoxygenase and prostaglandin endoperoxide synthase by anacardic acids. Biochem. Biophys. Res. Commun. 1991, 176, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Kubo, I. Lipoxygenase inhibitory activity of anacardic acids. J. Agric. Food Chem. 2005, 53, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Tsujimotoa, K.; Hayashia, A.; Hab, T.J.; Kubo, I. Anacardic acids and ferric ion chelation. Z. Naturforsch. 2007, 62c, 710–716. [Google Scholar]
- Shobha, S.V.; Ramadoss, C.S.; Ravindranath, B. Regiospecific hydroperoxidation of anacardic acid (15:2) by soybean lipoxygenase 1. J. Nat. Prod. 1992, 55, 818–821. [Google Scholar] [CrossRef]
- Shobm, S.V.; Ramados, C.S.; Ravindranath, B. Inhibition of soybean lipoxygenase-1 by anacardic acids, cardols, and cardanols. J. Nat. Prod. 1994, 57, 1755–1757. [Google Scholar] [CrossRef]
- Prigge, S.T.; Boyington, J.C.; Faig, M.; Doctor, K.S.; Gaffney, B.J.; Amzel, L.M. Structure and mechanism of lipoxygenases. Biochimie 1997, 79, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Ha, T.J.; Tsujimoto, K.; Tocoli, F.E.; Green, I.R. Evaluation of lipoxygenase inhibitory activity of anacardic acids. Z. Naturforsch. 2008, 63c, 539–546. [Google Scholar]
- Kubo, I.; Masuoka, N.; Ha, T.J.; Tsujimoto, K. Antioxidant activity of anacardic acids. Food Chem. 2006, 99, 555–562. [Google Scholar] [CrossRef]
- Masuoka, N.; Kubo, I. Characterization of xanthine oxidase inhibition by anacardic acids. Biochim. Biophys. Acta 2004, 1688, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Morii, H.; Tamura, M.; Hayaishi, O.; Watanabe, Y. A possible mechanism of mitochondrial dysfunction during cerebral ischemia; Inhibition of mitochondrial respiration activity of arochidonic acid. Arch. Biohem. Biophys. 1991, 289, 33–38. [Google Scholar] [CrossRef]
- Toyomizu, M.; Okamoto, K.; Ishibashi, T.; Chent, Z.; Nakatsu, T. Uncoupling effect of anacardic acids from cashew nut shell oil on oxidative phosphorylation of rat liver mitochondria. Life Sci. 2000, 66, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.N.; Durão, A.C.C.S.; Almeida, F.M.; Annoni, R.; Torres, L.H.L.; Shimada, A.L.B.; Hebeda, C.B.; Lopes, F.D.T.Q.S.; Martins, M.A.; Silva, L.F.F.; et al. Anacardic acids from cashew nuts ameliorate lung damageinduced by exposure to diesel exhaust particles in mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Morais, T.C.; Pinto, N.B.; Carvalho, K.M.M.B.; Ricardo, N.M.P.S.; Trevisan, M.T.S.; Rao, V.S.; Rios, J.B.; Santos, F.A. Protective effect of anacardic acids from cashew (Anacardium occidentale) on ethanol-induced gastric damage in mice. Chem. Biol. Interact. 2010, 183, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Toyomizu, M.; Sugiyama, S.; Jin, R.L.; Nakatsu, T. α-Glucosidase and aldose reductase inhibitors: Constituents of cashew, anacardium occidentale, nut shell liquids. Phytochem. Res. 1993, 7, 252–254. [Google Scholar]
- Vempati, R.K.; Reddy, N.S.; Alapati, S.R.; Dubey, P.K. Synthesis of novel benzylamine analogues of anacardic acid as potent antibacterial agents. Der Pharma Chem. 2011, 3, 500–512. [Google Scholar]
- Vempati, R.K.; Alapati, S.R.; Reddy, N.S.; Naresh, V.V.; Dubey, P.K. Synthesis of novel quinoline carboxylic acids from anacardic acid. Der Pharma Chem. 2012, 4, 248–254. [Google Scholar]
- Swamy, B.N.; Suma, T.K.; Rao, G.V.; Reddy, G.C. Synthesis of isonicotinoylhydrazones from anacardic acid and their in vitro activity against Mycobacterium smegmatis. Eur. J. Med. Chem. 2007, 42, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Paramashivappa, R.; Kumar, P.P.; Rao, P.V.S.; Rao, A.S. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Wagner, G. Physiology of penile erection. Physiol. Rev. 1995, 75, 191–236. [Google Scholar] [PubMed]
- Paramashivappa, R.; Kumar, P.P.; Rao, P.V.S.; Rao, A.S. Synthesis of sildenafil analogues from anacardic acid and their phosphodiesterase-5 inhibition. J. Agric. Food Chem. 2002, 50, 7709–7713. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Stotz, S.C.; Paramashivappa, R.; Beedle, A.M.; Zamponi, G.W.; Rao, A.S. Synthesis and evaluation of a new class of nifedipine analogs with T-type calcium channel blocking activity. Mol. Pharmacol. 2002, 61, 649–658. [Google Scholar] [CrossRef]
- Mantelingu, K.; Kishore, A.H.; Balasubramanyam, K.; Kumar, G.V.P.; Altaf, M.; Swamy, S.N.; Selvi, R.; Das, C.; Narayana, C.; Rangappa, K.S.; et al. Activation of p300 histone acetyltransferase by small molecules altering enzyme structure: Probed by surface-enhanced raman spectroscopy. J. Phys. Chem. B 2007, 111, 4527–4534. [Google Scholar] [CrossRef]
- Chandregowda, V.; Kush, A.; Reddy, G.C. Synthesis of benzamide derivatives of anacardic acid and their cytotoxic activity. Eur. J. Med. Chem. 2009, 44, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Logrado, L.P.L.; Santos, C.O.; Romeiro, L.A.S.; Costa, A.M.; Ferreira, J.R.O.; Cavalcanti, B.C.; de Moraes, O.M.; Costa-Lotufo, L.V.; Pessoa, C.; dos Santos, M.L. Synthesis and cytotoxicity screening of substituted isobenzofuranones designedfrom anacardic acids. Eur. J. Med. Chem. 2010, 45, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Logrado, L.P.L.; Silveira, D.; Romeiro, L.A.S.; de Moraes, M.O.; Cavalcanti, B.C.; Costa-Lotufo, L.V.; do Ó Pessoa, C.; dos Santos, M.L. Synthesis and biological evaluation of new salicylate macrolactones from anacardic acids. J. Braz. Chem. Soc. 2005, 16, 1217–1225. [Google Scholar] [CrossRef]
- Nagabhushana, K.S.; Shobha, S.V.; Ravindran, B. Selective ionophoric properties of anacardic acid. J. Nat. Prod. 1995, 58, 807. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, F.B.; Mubofu, E.B. Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives. Int. J. Mol. Sci. 2015, 16, 8569-8590. https://doi.org/10.3390/ijms16048569
Hamad FB, Mubofu EB. Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives. International Journal of Molecular Sciences. 2015; 16(4):8569-8590. https://doi.org/10.3390/ijms16048569
Chicago/Turabian StyleHamad, Fatma B., and Egid B. Mubofu. 2015. "Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives" International Journal of Molecular Sciences 16, no. 4: 8569-8590. https://doi.org/10.3390/ijms16048569
APA StyleHamad, F. B., & Mubofu, E. B. (2015). Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives. International Journal of Molecular Sciences, 16(4), 8569-8590. https://doi.org/10.3390/ijms16048569