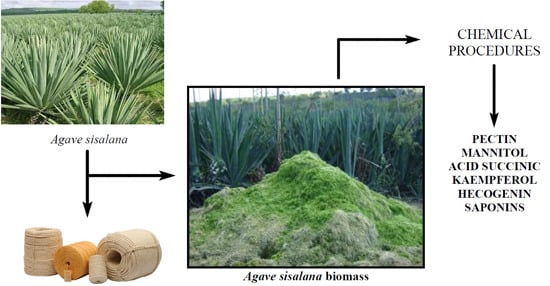
Chemicals from Agave sisalana Biomass: Isolation and Identification
Abstract
:1. Introduction
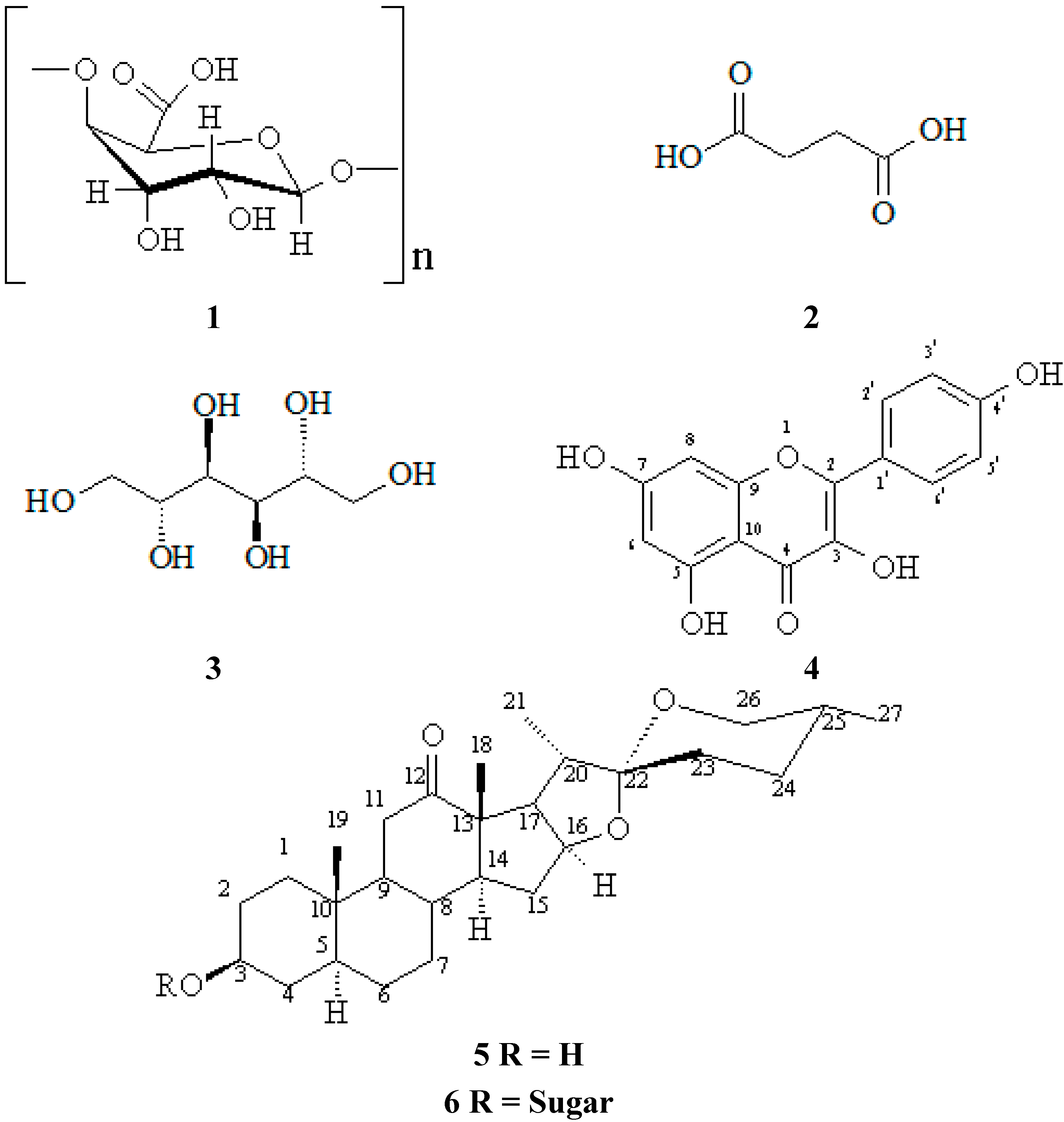
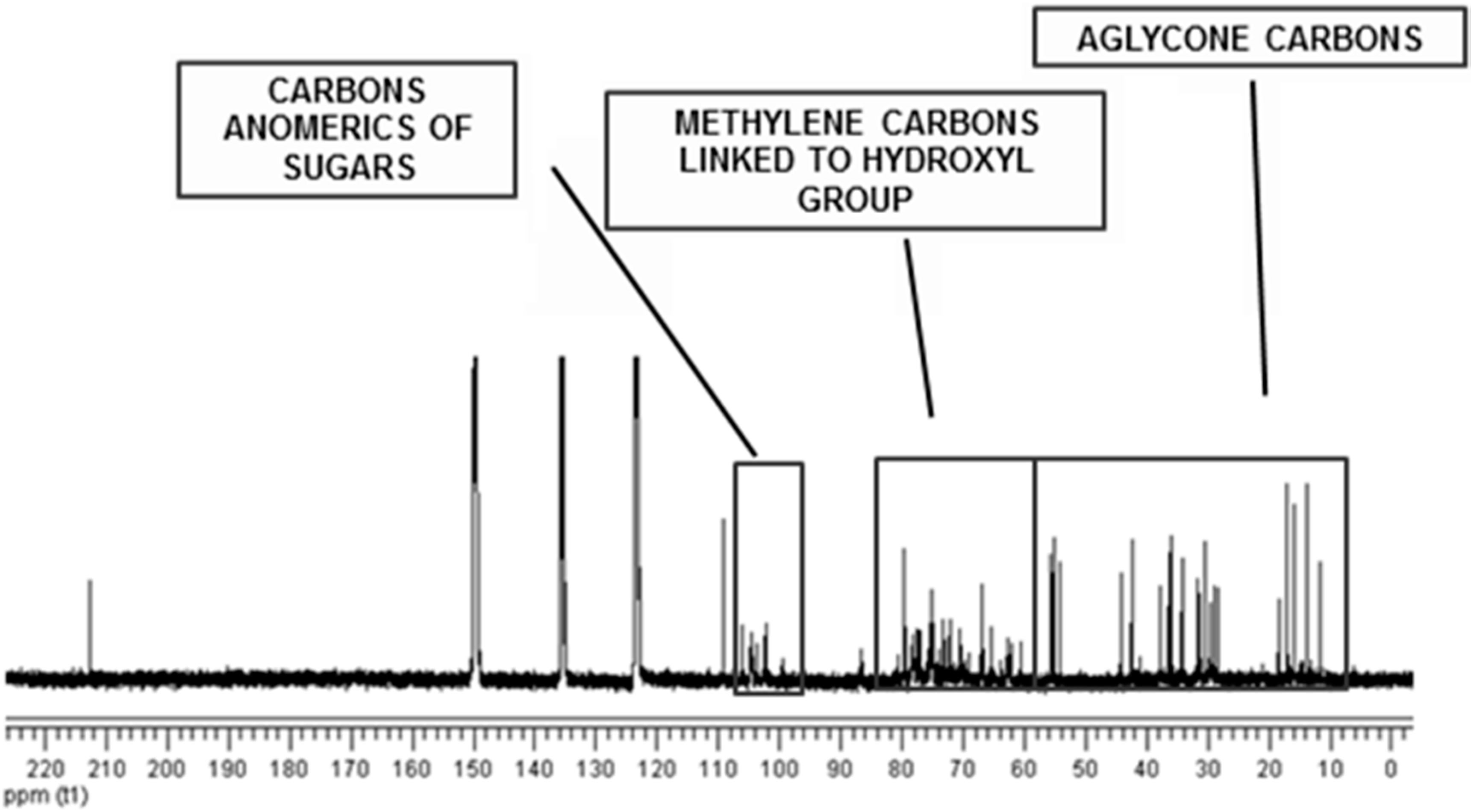
2. Results and Discussion
Isolation and Characterization of Chemicals from Sisal Biomass
| Position | HSQC | HMBC | Model | |||
|---|---|---|---|---|---|---|
| C | δC | δH | 2JHC | 3JHC | δC | δH |
| 10 | 36.0 | - | 3H-19 | - | 36.3 | - |
| 12 | 212.6 | - | - | - | 212.8 | - |
| 13 | 55.1 | - | 3H-18 | - | 55.5 | - |
| 22 | 109.2 | - | - | 3H-21 | 109.2 | - |
| CH | δC | δH | 2JHC | 3JHC | δC | δH |
| 3 | 76.9 | 3.83 | - | H-1' | 76.7 | 3.88 (m) |
| 5 | 44.2 | 0.84 | - | 3H-19 | 44.3 | 0.86 (m) |
| 8 | 34.1 | 1.73 | - | - | 34.4 | 1.65 |
| 9 | 55.2 | 0.89 | - | 3H-19 | 55.3 | 0.92 |
| 14 | 55.6 | 1.35 | - | 3H-18 | 55.8 | 1.38 |
| 16 | 79.5 | 4.58 | - | - | 79.6 | 4.99 (m) |
| 17 | 54.2 | 2.75 (t, 8.5) | 3H-18; 3H-21 | 54.2 | 2.76 (dd) | |
| 20 | 42.4 | 1.90 | 3H-21 | - | 42.5 | 1.96 |
| 25 | 30.3 | 1.54 | - | - | 30.4 | 1.57 |
| CH2 | δC | δH | 2JHC | 3JHC | δC | δH |
| 1 | 36.1 | 1.35, 0.70 | - | 3H-19 | 36.6 | 1.36, 0.76 |
| 2 | 29.4 | 1.55, 1.40 | - | - | 29.6 | 2.00, 1.76 |
| 4 | 34.4 | 1.76, 1.32 | - | - | 34.1 | 1.90, 1.78 |
| 6 | 28.3 | 1.10 | - | - | 28.5 | 1.16 |
| 7 | 31.5 | 1.69, 1.64 | - | - | 31.7 | 1.63, 0.78 |
| 11 | 37.7 | 2.35 (t, 14.5), 2.22 (dd, 14.5, 5.0) | - | - | 37.9 | 2.41 (dd, 13.8, 13.7) 2.25 (dd, 13.8, 5.0) |
| 15 | 31.2 | 2.10, 1.58 | - | - | 31.4 | 2.10, 1.61 |
| 23 | 31.4 | 1.55 | - | - | 31.6 | 1.69 (m), 2H |
| 24 | 29.0 | 1.98 | - | 3H-27 | 29.1 | 1.56 (m), 2H |
| 26 | 66.7 | 3.58 (dd, 10.5), 3.48 (t, 105) | 3H-27 | 66.9 | 3.59 (br d, 11.8) 3.48 (dd, 11.8, 10.6) | |
| CH3 | δC | δH | 2JHC | 3JHC | δC | δH |
| 18 | 15.9 | 1.07 (s) | - | - | 16.0 | 1.07 (s) |
| 19 | 11.5 | 0.64 (s) | - | - | 11.8 | 0.88 (s) |
| 21 | 13.7 | 1.34 (d, 7.0) | - | - | 13.8 | 1.35 (d, 6.9) |
| 27 | 17.1 | 0.68 (d, 6.0) | - | - | 17.2 | 0.69 (d, 5.7) |
3. Experimental Section
3.1. General Procedures
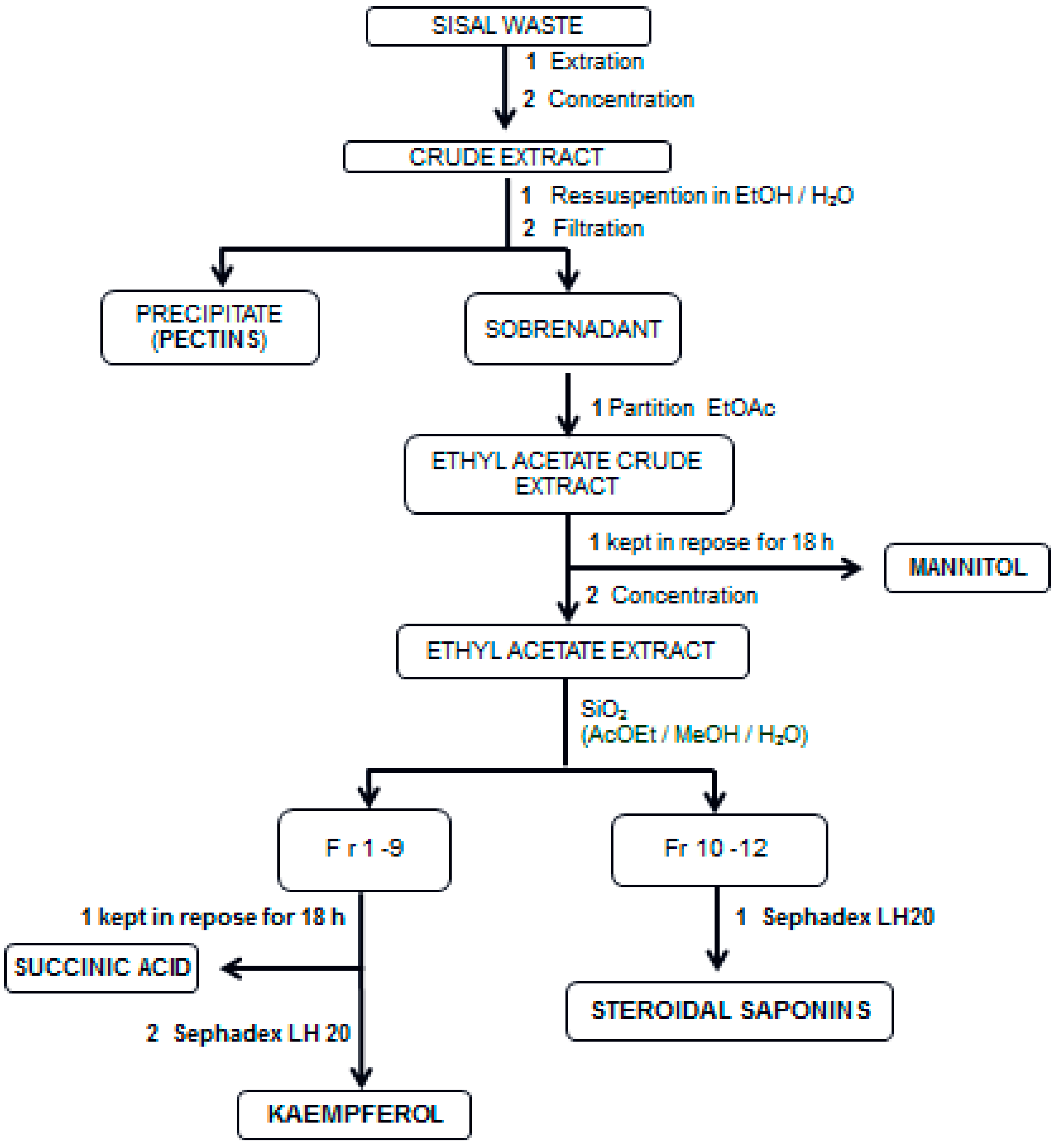
3.2. Obtaining Crude Extract from Sisal Waste
3.3. Systematic Chemical Procedures on Crude Extract
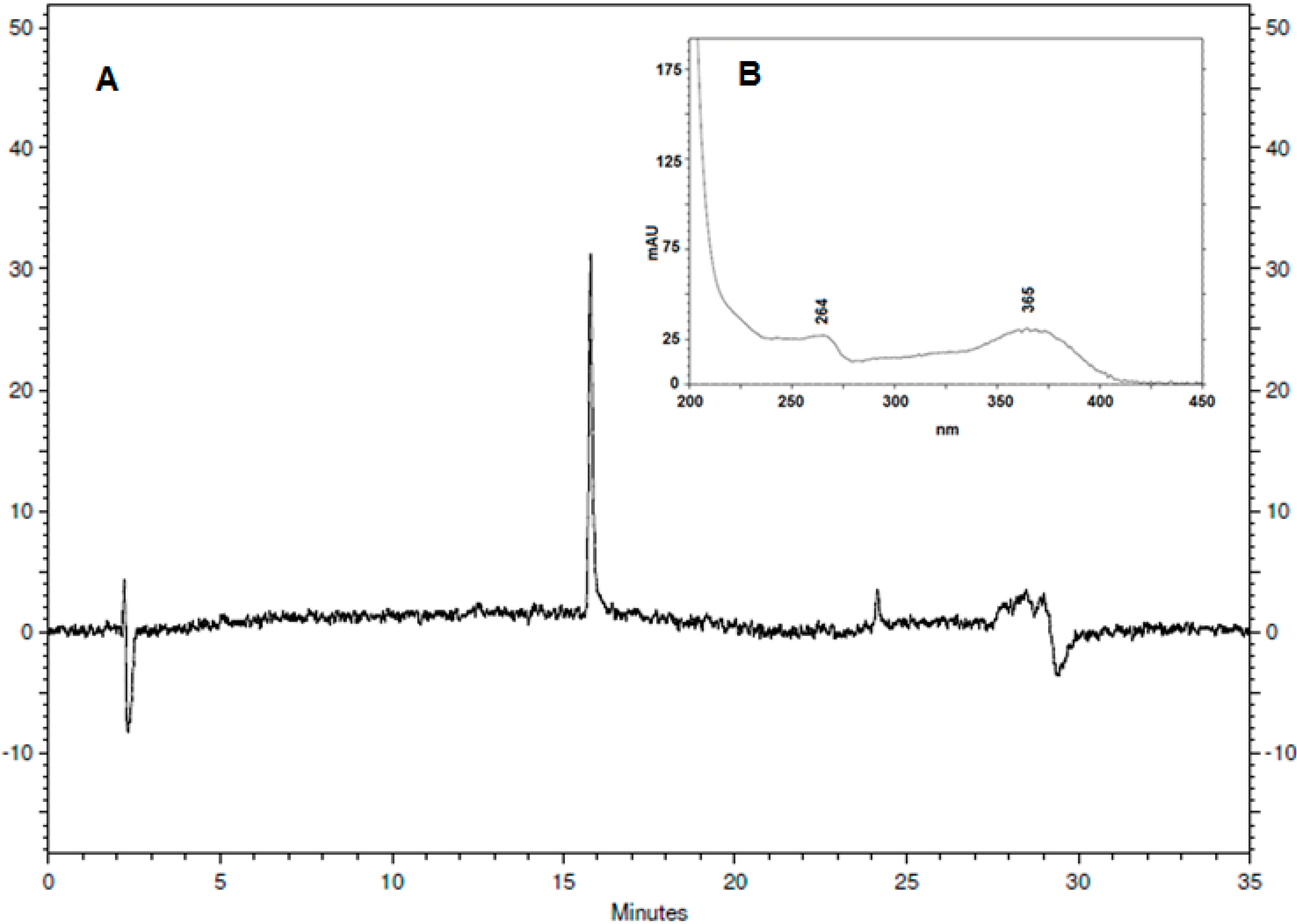
3.4. HPLC-DAD Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mishra, S.; Mohanty, A.K.; Drzal, L.T.; Misra, M.; Hinrichsen, G. A review on pineapple leaf fibers, sisal fibers and their biocomposites. Macromol. Mater. Eng. 2004, 289, 955–974. [Google Scholar] [CrossRef]
- Ramzy, A.; Beermann, D.; Steuernagel, L.; Meiners, D.; Ziegmann, G. Developing a new generation of sisal composite fibres for use in industrial applications. Comp. Part. B Eng. 2014, 66, 287–298. [Google Scholar] [CrossRef]
- FAO STAT Database. Available online: http://faostat.fao.org (accessed on 28 June 2014).
- Sharma, S.; Varshney, V.K. Chemical analysis of Agave sisalana juice for its possible utilization. Acta Chim. Pharm. Indica 2012, 2, 60–66. [Google Scholar]
- Zhang, X.; Liu, L.; Lin, C. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from Sisal waste. Food Hydrocoll. 2014, 39, 10–18. [Google Scholar] [CrossRef]
- Ding, Y.; Tian, R.H.; Yang, C.R.; Chen, Y.Y.; Nohara, T. Two new steroidal saponins from dried fermented residues of leaf-juices of Agave sisalana forma Dong No.1. Chem. Pharm. Bull. 1993, 41, 557–560. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds e recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Savastano Junior, H.; Pimentel, L.L. Viabilidade do aproveitamento de resíduos de fibras vegetais para fins de obtenção de material de construção (in Portuguese). R. Bras. Eng. Agric. Ambiental. 2000, 4, 103–110. [Google Scholar]
- Muchovej, R.M.C.; Pacovsky, R.S. Future directions of by-products and wastes in agriculture. In Agricultural Uses of by-Products and Wastes; Rechcigl, J., MacKinnon, H.C, Eds.; ACS Symposium Series, American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Arvanitoyannis, I.S.; Tserkezou, P. Corn and rice waste: A comparative and critical presentation of methods and current and potential uses of treated waste. Int. J. Food Sci. Technol. 2008, 43, 958–988. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Amico, V.; Chillemi, R.; Mangiafico, S.; Spatafora, C.; Tringali, C. Polyphenol-enriched fractions from Sicilian grape pomace: HPLC-DAD analysis and antioxidant activity. Bioresour. Technol. 2008, 99, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Figueiredo, E.A.T.; Ricardo, N.M.S.; Vieira, I.G.P.; Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Preparation of bioactive extracts from asparagus by-product. Food Bioprod. Process. 2013, 91, 74–82. [Google Scholar] [CrossRef]
- Cetkovic, G.; Savatovic, S.; Canadanovic-Brunet, J.; Djilas, S.; Vulic, J.; Mandic, A.; Cetojevic-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Debnath, M.; Pandey, M.; Sharma, R.; Thakur, G.S.; Lal, P. Biotechnological intervention of Agave sisalana: A unique fiber yielding plant with medicinal property. J. Med. Plants Res. 2010, 4, 177–187. [Google Scholar]
- Santos, J.D.G.; Branco, A. GC-MS Characterisation of sapogenins from sisal waste and a method to isolate pure hecogenin. Bioresources 2014, 9, 1325–1333. [Google Scholar]
- Botura, M.B.; Silva, G.D.; Lima, H.G.; Oliveira, J.V.A.; Souza, T.S.; Santos, J.D.G.; Branco, A.; Moreira, E.L.T.; Almeida, M.A.O.; Batatinha, M.J.M. In vivo ovicidal and larvicidal activity of Agave sisalana Perr.(sisal) on gastrointestinal nematodes of goats. Vet. Parasitol. 2011, 177, 204–210. [Google Scholar]
- Santos, J.D.G.; Branco, A.; Silva, A.F.; Pinheiro, C.S.R.; Góes Neto, A.; Uetanabaro, A.P.T.; Queiroz, S.R.O.D.; Osuna, J.T.A. Antimicrobial activity of Agave sisalana. Afr. J. Biotechnol. 2009, 8, 6181–6184. [Google Scholar]
- Botura, M.B.; dos Santos, J.D.G.; da Silva, G.D.; de Lima, H.G.; de Oliveira, J.V.; de Almeida, M.A.; Batatinha, M.J.; Branco, A. In vitro ovicidal and larvicidal activity of Agave sisalana Perr.(sisal) on gastrointestinal nematodes of goats. Vet. Parasitol. 2013, 192, 211–217. [Google Scholar] [CrossRef]
- Silveira, R.X.; Chagas, A.C.; Botura, M.B.; Batatinha, M.J.; Katiki, L.M.; Carvalho, C.O.; Bevilaqua, C.M.; Branco, A.; Machado, E.A.; Borges, S.L.; et al. Action of sisal (Agave sisalana Perrine) extract in the in vitro development of sheep and goat gastrointestinal nematodes. Exp. Parasitol. 2012, 131, 162–168. [Google Scholar]
- Yan, X.; Tan, D.K.Y.; Inderwildi, O.R.; Smith, J.A.C.; King, D.A. Life cycle energy and greenhouse gas analysis for agave-derived bioethanol. Energy Environ. Sci. 2011, 4, 3110–3121. [Google Scholar] [CrossRef]
- Garcia-Moya, E.; Romero-Manzanares, A.; Nobel, P.S. Highlights for agave productivity. GCB Bioenergy 2011, 3, 4–14. [Google Scholar] [CrossRef]
- Izydorczyk, M. Understanding the chemistry of food carbohydrates. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; Cui, S.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Santos, J.D.G.; Espeleta, A.F.; Branco, A.; Assis, S.A. Aqueous extraction of pectin from sisal waste. Carbohydr. Polym. 2013, 92, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Santos, J.D.G.; Pimentel, M.A.M.; Osuna, J.T.A.; Lima, L.S.; David, J.M. d-Mannitol from Agave sisalana biomass waste. Ind. Crop. Prod. 2010, 32, 507–510. [Google Scholar] [CrossRef]
- Markham, K.R.; Ternai, B.; Stanley, R.; Geiger, H.; Mabry, T.J. Carbon-13 NMR studies of flavonoids—III: Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 1978, 34, 1389–1397. [Google Scholar] [CrossRef]
- Ujikawa, K.; Purchio, A. Substâncias Antifúngicas, inibidoras de Aspergillus flavus e de outras espécies fúngicas, isoladas de Agave sisalana (sisal) (in Portuguese). Cien Cult. 1989, 41, 1218–1224. [Google Scholar]
- Yu, H.-S.; Zou, P.; Song, X.-B.; Kang, L.-P.; Liu, Y.-X.; Pang, X.; Zhang, J.; Fu, J.; Zhao, Y.; Xiong, C.-Q.; et al. Two new steroidal saponins from the fresh leaves of Agave sisalana. Helv. Chim. Acta 2011, 94, 1351–1358. [Google Scholar]
- Chen, P.Y.; Chen, C.H.; Kuo, C.C.; Lee, T.H.; Kuo, Y.H.; Lee, C.K. Cytotoxic steroidal saponins from Agave sisalana. Planta Med. 2011, 77, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan-Yong, C.; de-Zua, W.; Chong-Ren, Y. Steroidal saponins from a cultivated of Agave sisalana. Phytochemistry 1989, 28, 2787–2791. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.D.G.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Chemicals from Agave sisalana Biomass: Isolation and Identification. Int. J. Mol. Sci. 2015, 16, 8761-8771. https://doi.org/10.3390/ijms16048761
Santos JDG, Vieira IJC, Braz-Filho R, Branco A. Chemicals from Agave sisalana Biomass: Isolation and Identification. International Journal of Molecular Sciences. 2015; 16(4):8761-8771. https://doi.org/10.3390/ijms16048761
Chicago/Turabian StyleSantos, Jener David Gonçalves, Ivo Jose Curcino Vieira, Raimundo Braz-Filho, and Alexsandro Branco. 2015. "Chemicals from Agave sisalana Biomass: Isolation and Identification" International Journal of Molecular Sciences 16, no. 4: 8761-8771. https://doi.org/10.3390/ijms16048761
APA StyleSantos, J. D. G., Vieira, I. J. C., Braz-Filho, R., & Branco, A. (2015). Chemicals from Agave sisalana Biomass: Isolation and Identification. International Journal of Molecular Sciences, 16(4), 8761-8771. https://doi.org/10.3390/ijms16048761