Asymmetric Mannich Reaction of α-(2-Nitrophenylsulfenyl)imino Acetamide: A Cyclization-Driven Process
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
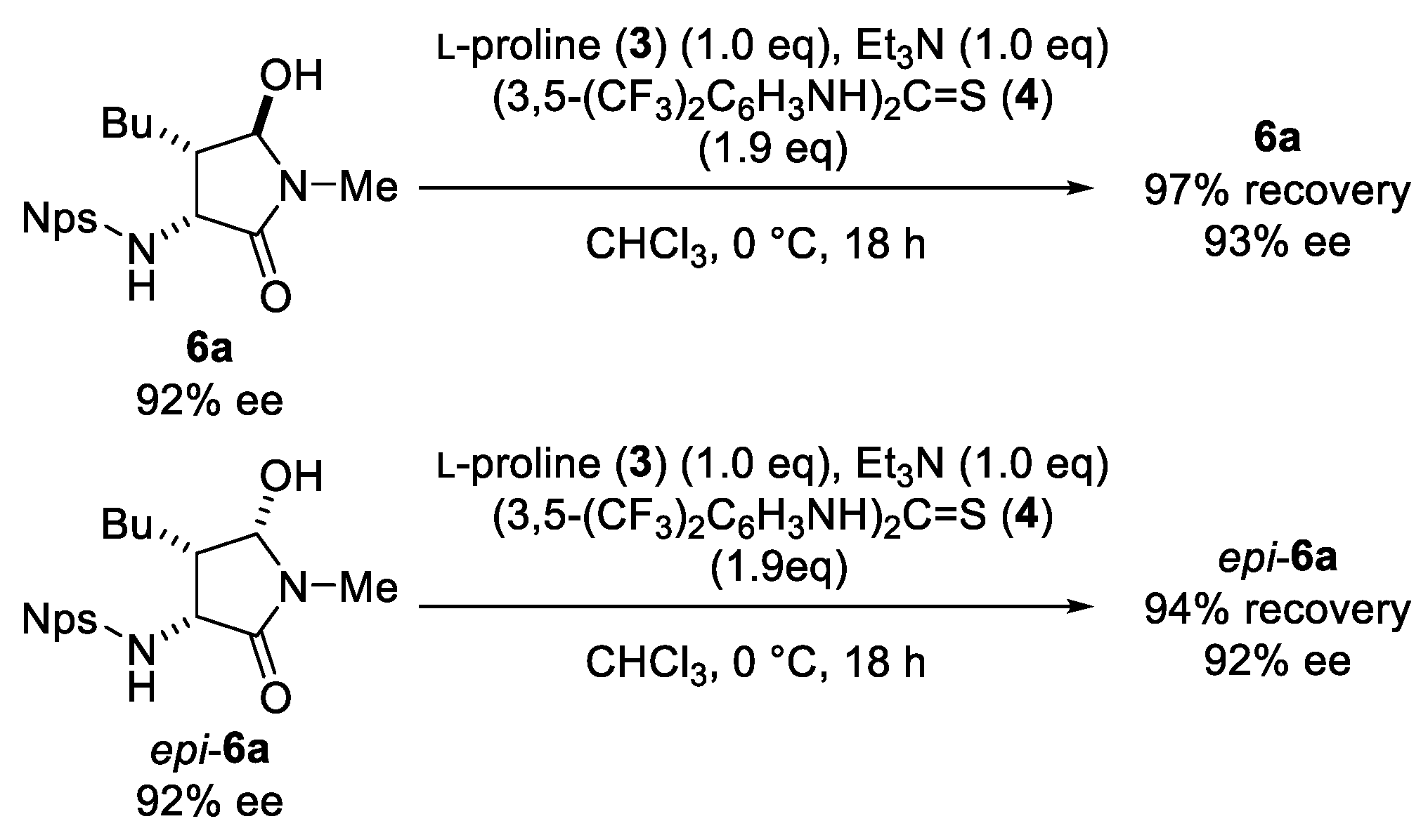
3.2. General Procedure for Mannich Reaction of N-Nps Imino Acetamide 1: (3R,4S,5R)-4-Butyl-5-hydroxy-1-methyl-3-(2-nitrophenylthioamino)pyrrolidin-2-one (6a) and (3R,4S,5S)-4-Butyl-5-hydroxy-1-methyl-3-(2-nitrophenylthioamino)pyrrolidin-2-one (epi-6a)
3.3. Mannich Reaction of Imino Ester 9 (Scheme 1)
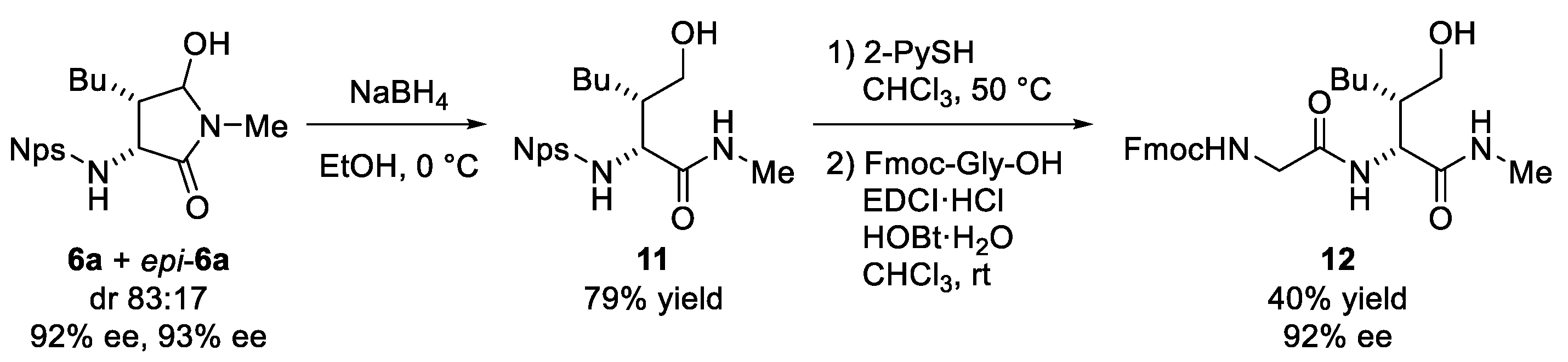
3.4. Synthesis of the Dipeptide 12 Bearing Homoserine Derivative (Scheme 3)
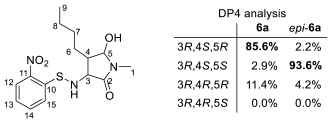
3.5. Determination of Relative Configuration of 6a (Scheme 4)
3.6. Calculations of the 13C NMR Chemical Shifts (Table 3)
3.7. Calculations of the ECD Spectra (Figure 1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, K.K.; Sharma, A.; Jain, R. Peptide-based Drug Discovery: Current Status and Recent Advances. Drug Discov. Today 2023, 28, 103464. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic Therapeutic Peptides: Science and Market. Drug Discov. Today 2010, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.G.; Melle-Franco, M.; Sousa, C.E.A.; Cavaco-Paulo, A.; Marcos, J.C. Non-Canonical Amino Acids as Building Blocks for Peptidomimetics: Structure, Function, and Applications. Biomolecules 2023, 13, 981. [Google Scholar] [CrossRef]
- Ding, Y.; Ting, J.P.; Liu, J.; Al-Azzam, S.; Pandya, P.; Afshar, S. Impact of non-proteinogenic amino acids in the discovery and development of peptide therapeutics. Amino Acids 2020, 52, 1207. [Google Scholar] [CrossRef]
- Götze, S.; Vij, R.; Burow, K.; Thome, N.; Urbat, L.; Schlosser, N.; Pflanze, S.; Müller, R.; Hänsch, V.G.; Schlabach, K.; et al. Ecological Niche-Inspired Genome Mining Leads to the Discovery of Crop-Protecting Nonribosomal Lipopeptides Featuring a Transient Amino Acid Building Block. J. Am. Chem. Soc. 2023, 145, 2342. [Google Scholar] [CrossRef]
- Coraiola, M.; Lo Cantore, P.; Lazzaroni, S.; Evidente, A.; Iacobellis, N.S.; Dalla Serra, M. WLIP and Tolaasin I, Lipodepsipeptides from Pseudomonas Reactans and Pseudomonas Tolaasii, Permeabilise Model Membranes. Biochim. Biophys. Acta 2006, 1758, 1713. [Google Scholar] [CrossRef]
- Song, B.; Kibler, P.; Malde, A.; Kodukula, K.; Galande, A.K. Design of Short Linear Peptides That Show Hydrogen Bonding Constraints in Water. J. Am. Chem. Soc. 2010, 132, 4508. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Baldwin, R.L. Straight-Chain Non-Polar Amino Acids are Good Helix-formers in Water. J. Mol. Biol. 1991, 219, 135. [Google Scholar] [CrossRef]
- Cummings, A.E.; Miao, J.; Slough, D.P.; McHugh, S.M.; Kritzer, J.A.; Lin, Y.-S. β-Branched Amino Acids Stabilize Specific Conformations of Cyclic Hexapeptides. Biophys. J. 2019, 116, 433. [Google Scholar] [CrossRef]
- Wolf, J.P.; Rapoport, H. Conformationally Constrained Peptides. Chirospecific Synthesis of 4-Alkyl-Substituted γ-Lactam-Bridged Dipeptides from L-Aspartic Acid. J. Org. Chem. 1989, 54, 3164. [Google Scholar] [CrossRef]
- Córdova, A.; Watanabe, S.; Tanaka, F.; Notz, W.; Barbas, C.F., III. A Highly Enantioselective Route to Either Enantiomer of Both α-and β-Amino Acid Derivatives. J. Am. Chem. Soc. 2002, 124, 1866. [Google Scholar] [CrossRef] [PubMed]
- Notz, W.; Tanaka, F.; Barbas, C.F., III. Enamine-based Organocatalysis with Proline and Diamines: The Development of Direct Catalytic Asymmetric Aldol, Mannich, Michael, and Diels-Alder Reactions. Acc. Chem. Res. 2004, 37, 580. [Google Scholar] [CrossRef] [PubMed]
- Veverková, E.; Štrasserová, J.; Šebesta, R.; Toma, Š. Asymmetric Mannich Reaction Catalyzed by N-Arylsulfonyl-L-proline Amides. Tetrahedron Asymmetry 2010, 21, 58. [Google Scholar] [CrossRef]
- Šramel, P.; Šebesta, R. Organocatalytic Mannich Type Reactions of Glyoxylate Imines and Related Compounds. Tetrahedron Lett. 2024, 143, 155129. [Google Scholar] [CrossRef]
- Inokuma, T.; Nishida, K.; Shigenaga, A.; Yamada, K.; Otaka, A. Direct Enantioselective Indolylation of Peptidyl Imine for the Synthesis of Indolyl Glycine-containing Peptides. Heterocycles 2018, 97, 1269. [Google Scholar]
- Inokuma, T.; Sakakibara, T.; Someno, T.; Masui, K.; Shigenaga, A.; Otaka, A.; Yamada, K. Asymmetric Synthesis of α-Amino Phosphonic Acids using Stable Imino Phosphonate as a Universal Precursor. Chem. Eur. J. 2019, 25, 13829. [Google Scholar] [CrossRef]
- Inokuma, T. Synthesis of Non-canonical Amino Acids and Peptide Containing Them for Establishment of the Template for Drug Discovery. Chem. Pharm. Bull. 2021, 69, 303. [Google Scholar] [CrossRef]
- Inokuma, T.; Masui, K.; Fukuhara, K.; Yamada, K. Preparation of N-2-Nitrophenylsulfenyl Imino Peptides and Their Catalyst-Controlled Diastereoselective Indolylation. Chem. Eur. J. 2023, 29, e202203120. [Google Scholar] [CrossRef]
- Inokuma, T.; Fukuhara, K.; Sugano, M.; Miyamoto, M.; Someno, T.; Yamada, K. Palladium-Catalyzed Asymmetric Arylation of α-2-Nitrophenylsulfenylimino Acetamide. Tetrahedron 2025, in press. [Google Scholar] [CrossRef]
- Wang, J.-F.; Lei, M.; Li, Q.; Ge, Z.-M.; Wang, X.; Li, R.-T. A Novel and Efficient Direct Aldol Condensation from Ketones and Aromatic Aldehydes Catalyzed by Proline–TEA through a New Pathway. Tetrahedron 2009, 65, 4826. [Google Scholar] [CrossRef]
- Lu, H.; Lv, J.; Zhou, C.; Zhou, M.; Fang, Y.; Dong, J.; Kato, T.; Liu, Y.; Maruoka, K. Remarkable Effect of tert-Amine Additives in the Asymmetric Direct Michael Reaction of Ketones with β-Arylnitroethenes Catalyzed by an L-Hydroxyproline-Based Amino Tf-Amide Organocatalyst. Eur. J. Org. Chem. 2021, 12, 1909. [Google Scholar] [CrossRef]
- Demir, A.S.; Baseken, S. Study of Asymmetric Aldol and Mannich Reactions Catalyzed by Proline–Thiourea Host–Guest Complexes in Nonpolar Solvents. Tetrahedron Asymmetry 2013, 24, 515. [Google Scholar] [CrossRef]
- Companyó, X.; Valero, G.; Crovetto, L.; Moyano, A.; Rios, R. Highly Enantio- and Diastereoselective Organocatalytic Desymmetrization of Prochiral Cyclohexanones by Simple Direct Aldol Reaction Catalyzed by Proline. Chem. Eur. J. 2009, 15, 6564. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Sinha, D.; Rana, N.K.; Trieu-Do, V.; Zhao, J.C.-G. List−Barbas−Mannich Reaction Catalyzed by Modularly Designed Organocatalysts. J. Org. Chem. 2013, 78, 10947. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.-N.; Zhan, Z.-X.; Li, C.; Tian, M.-Y.; Yang, M.-Q.; Fan, W.-Q.; Shu, X.-R.; Yu, J.; Wang, Y.; Wei, W.-T. Ligand-Promoted Copper-Catalyzed Radical Relay for Alkyl-Alkynylation of Alkenes. Org. Lett. 2025, 27, 12396. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J. Am. Chem. Soc. 2010, 132, 12946. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Morita, H. Circular Dichroism Calculation for Natural Products. J. Nat. Med. 2014, 68, 1–10. [Google Scholar] [CrossRef]
- Yamasaki, K.; Yamauchi, A.; Inokuma, T.; Miyakawa, Y.; Wang, Y.; Oriez, R.; Yamaoka, Y.; Takasu, K.; Tanaka, N.; Kashiwada, Y.; et al. Mechanistic Support for Intramolecular Migrative Cyclization of Propargyl Sulfones Provided by Catalytic Asymmetric Induction with a Chiral Counter Cation Strategy. Asian J. Org. Chem. 2021, 10, 1828. [Google Scholar] [CrossRef]
- Wanner, M.J.; Hauwert, P.; Schoemaker, H.E.; de Gelder, R.; van Maarseveen, J.H.; Hiemstra, H. Synthesis of Enantiopure (S)-Indolylglycine by Organocatalyzed Friedel–Crafts Alkylation of Indole. Eur. J. Org. Chem. 2008, 2008, 180. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Mukherjee, S. Lewis Base Catalysis by Thiourea: N-Bromosuccinimide-Mediated Oxidation of Alcohols. J. Org. Chem. 2012, 77, 1592. [Google Scholar] [CrossRef]
- Grünenfelder, C.E.; Kisunzu, J.K.; Wennemers, H. Peptide-Catalyzed Stereoselective Conjugate Addition Reactions of Aldehydes to Maleimide. Angew. Chem. Int. Ed. 2016, 55, 8571. [Google Scholar] [CrossRef]
- Spartan’18, Version 1.4.5; Wavefunction Inc.: Irvine, CA, USA, 2020.
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]

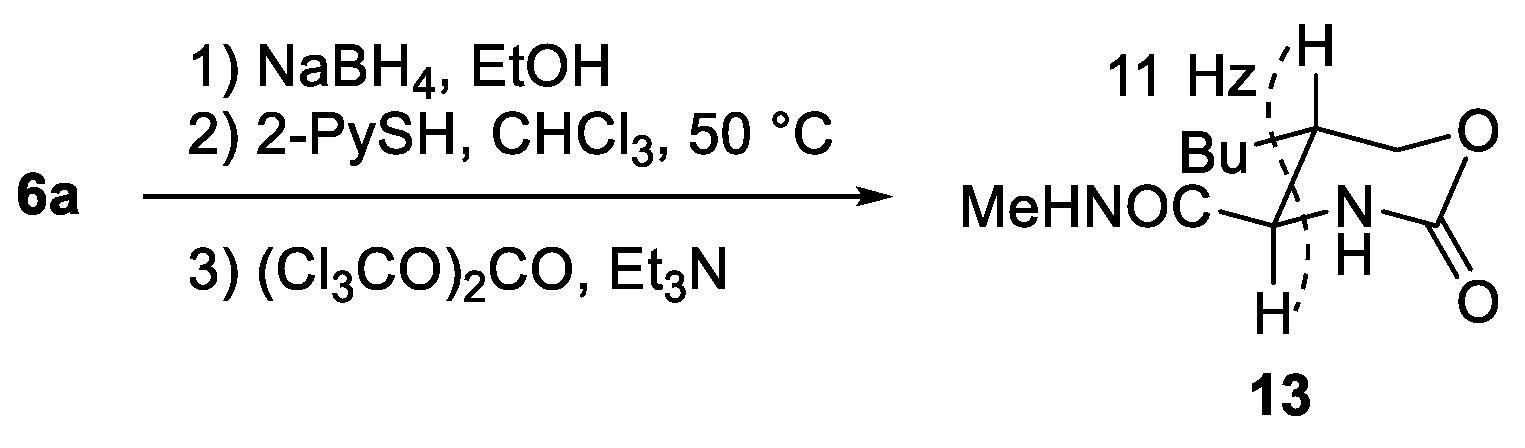

 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Base (equiv) | 3 (equiv) | 4 (equiv) | Solvent | Yield (%)[b] | dr[c] | ee of 6a (%)[d] |
| 1 | --- | 0.25 | --- | CH2Cl2 | --- | --- | --- |
| 2 | Et3N (0.25) | 0.25 | --- | CH2Cl2 | Trace | --- | --- |
| 3 | Et3N (0.25) | 0.25 | 0.50 | CH2Cl2 | 24 | 81:19 | 92 |
| 4 | DBU (0.25) | 0.25 | 0.50 | CH2Cl2 | Trace | --- | --- |
| 5 | TBD (0.25) | 0.25 | 0.50 | CH2Cl2 | 18 | 80:20 | 92 |
| 6 | TBD (0.25) | 0.25 | 0.50 | DMSO | --- | --- | --- |
| 7 | TBD (0.25) | 0.25 | 0.50 | DMF | --- | --- | --- |
| 8 | Cs2CO3 (0.25) | 0.25 | 0.50 | CH2Cl2 | 10 | 86:14 | 89 |
| 9 | Et3N (1.0) | 1.0 | 1.9 | CH2Cl2 | 23 | 83:17 | 94 |
| 10[e] | Et3N (1.0) | 1.0 | 1.9 | CH2Cl2 | 56 | 82:18 | 95 |
| 11[e] | Et3N (0.25) | 0.25 | 0.50 | CH2Cl2 | 25 | 80:20 | 94 |
| 12[e] | Et3N (1.5) | 1.5 | 3.0 | CH2Cl2 | 27 | 79:21 | 91 |
| 13[e] | Et3N (1.0) | 1.0 | 1.9 | CHCl3 | 58 | 83:17 | 92 |
| 14[e] | Et3N (1.0) | 1.0 | 1.9 | Toluene | 38 | 81:19 | 92 |
| 15[e] | Et3N (1.0) | 1.0 | 1.9 | THF | 43 | 76:24 | 96 |
 | ||||
|---|---|---|---|---|
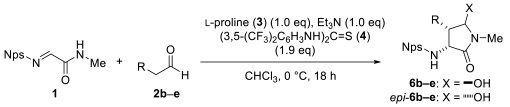
| Entry | R (2) | Yield | dr[c] | ee of 6 |
| (%)[b] | (%)[d] | |||
| 1[e] | Bu (2a) | 58 | 83:17 | 92 |
| 2 | i-Pr (2b) | 22 | 53:47 | 97 |
| 3 | PhCH2 (2c) | 21 | 82:18 | 89 |
| 4 | CH3(CH2)4CH=CHCH2 (2d) | 22 | 81:19 | 81 |
| 5 | BocNH(CH2)4 (2e) | 28 | 76:24 | 89 |
 | ||||||
|---|---|---|---|---|---|---|
| Observed (ppm) | Calculated (ppm) | |||||
| 6a | epi-6a | 3R,4S,5R | 3R,4S,5S | 3R,4R,5R | 3R,4R,5S | |
| C-1 | 27.7 | 27.7 | 26.9 | 26.2 | 27.2 | 26.2 |
| C-2 | 173.1 | 173.4 | 174.0 | 172.1 | 175.5 | 173.3 |
| C-3 | 60.9 | 63.2 | 59.2 | 61.4 | 61.5 | 57.8 |
| C-4 | 45.8 | 42.9 | 47.8 | 47.2 | 47.0 | 44.0 |
| C-5 | 87.1 | 85.6 | 88.5 | 85.1 | 84.7 | 91.0 |
| C-6 | 26.3 | 23.9 | 25.6 | 25.3 | 25.7 | 29.3 |
| C-7 | 29.2 | 29.6 | 28.9 | 28.7 | 28.3 | 27.4 |
| C-8 | 22.8 | 22.9 | 22.5 | 22.5 | 21.5 | 20.4 |
| C-9 | 14.0 | 14.0 | 13.9 | 13.6 | 13.4 | 14.6 |
| C-10 | 144.6 | 144.5 | 144.8 | 143.1 | 147.1 | 140.6 |
| C-11 | 142.8 | 142.4 | 144.9 | 144.1 | 144.6 | 147.2 |
| C-12 | 126.0 | 125.6 | 127.4 | 126.6 | 127.4 | 128.2 |
| C-13 | 124.3 | 125.1 | 124.0 | 125.2 | 123.9 | 124.8 |
| C-14 | 134.2 | 134.1 | 133.1 | 133.3 | 133.1 | 132.8 |
| C-15 | 125.0 | 125.0 | 126.0 | 128.2 | 125.7 | 126.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Inokuma, T.; Miyamoto, M.; Okada, K.; Nagai, G.; Yamada, K.-i. Asymmetric Mannich Reaction of α-(2-Nitrophenylsulfenyl)imino Acetamide: A Cyclization-Driven Process. Molecules 2026, 31, 449. https://doi.org/10.3390/molecules31030449
Inokuma T, Miyamoto M, Okada K, Nagai G, Yamada K-i. Asymmetric Mannich Reaction of α-(2-Nitrophenylsulfenyl)imino Acetamide: A Cyclization-Driven Process. Molecules. 2026; 31(3):449. https://doi.org/10.3390/molecules31030449
Chicago/Turabian StyleInokuma, Tsubasa, Maki Miyamoto, Kazuki Okada, Genki Nagai, and Ken-ichi Yamada. 2026. "Asymmetric Mannich Reaction of α-(2-Nitrophenylsulfenyl)imino Acetamide: A Cyclization-Driven Process" Molecules 31, no. 3: 449. https://doi.org/10.3390/molecules31030449
APA StyleInokuma, T., Miyamoto, M., Okada, K., Nagai, G., & Yamada, K.-i. (2026). Asymmetric Mannich Reaction of α-(2-Nitrophenylsulfenyl)imino Acetamide: A Cyclization-Driven Process. Molecules, 31(3), 449. https://doi.org/10.3390/molecules31030449





