Development of a Fluorophore-Bound l-Tryptophan Derivative for Evaluating Indoleamine 2,3-Dioxygenase Activity by HPLC with Fluorescence Detection: An In Vivo Microdialysis Study Using Rat Kidney
Abstract
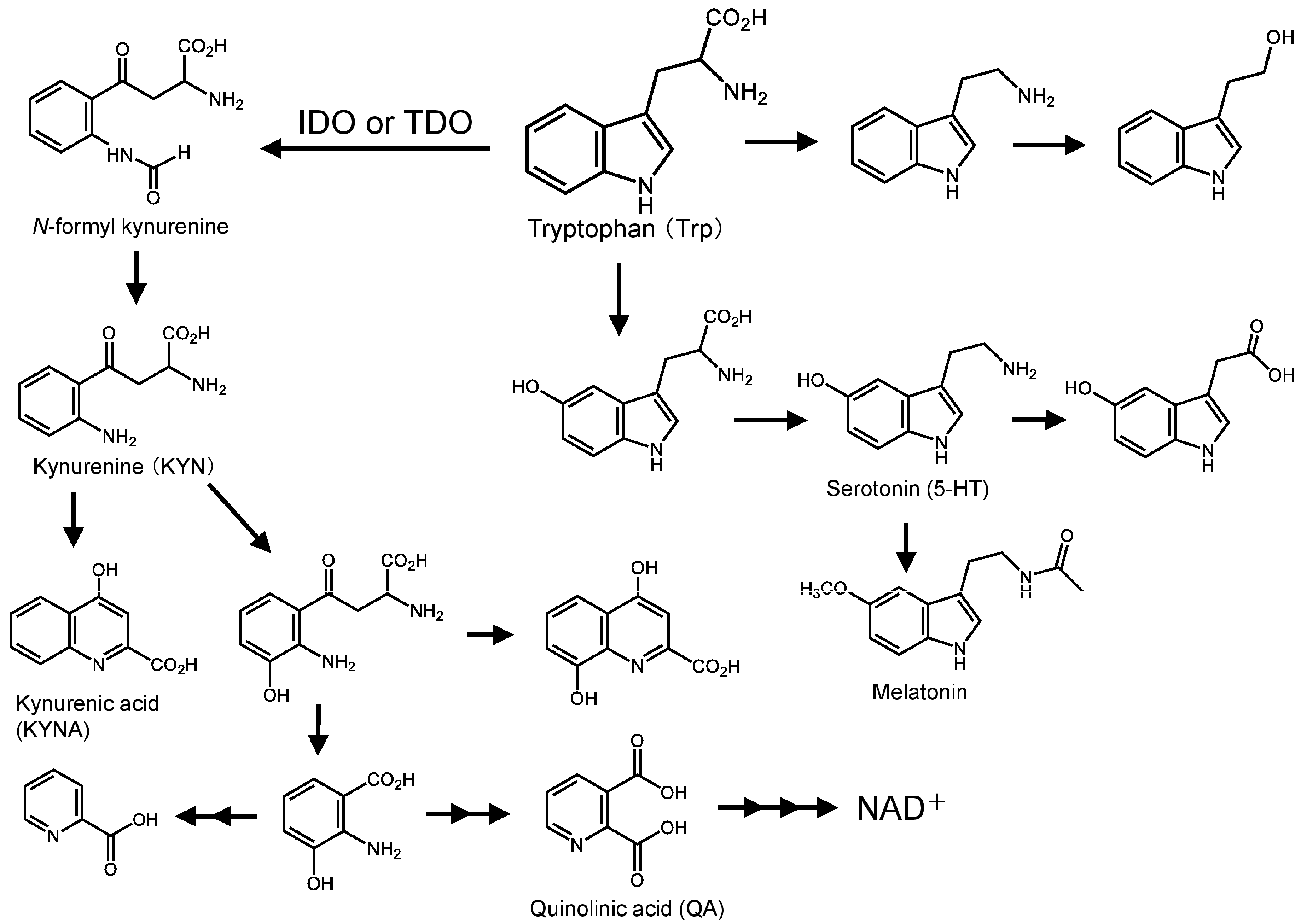
1. Introduction
2. Results
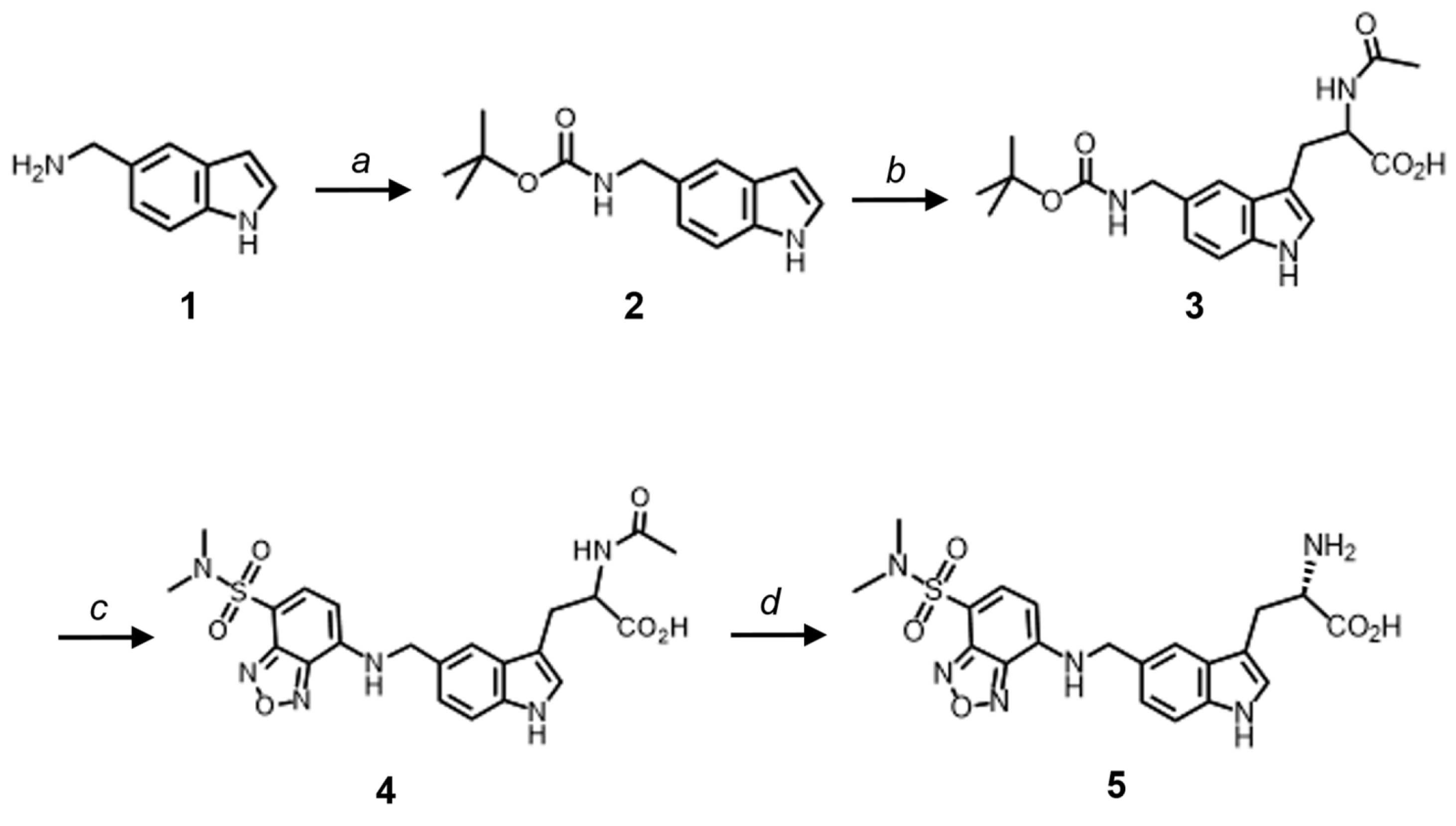
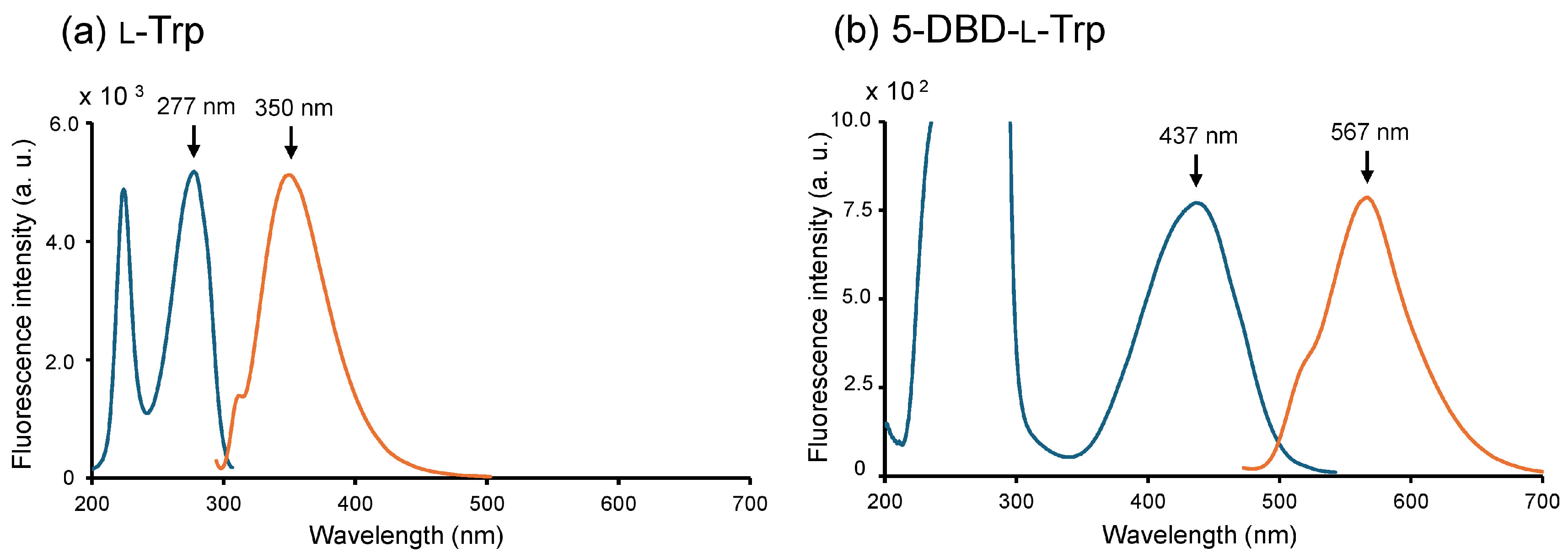
2.1. Preparation of 5-DBD-l-Trp
2.2. Results of In Vivo MD Study in Rats
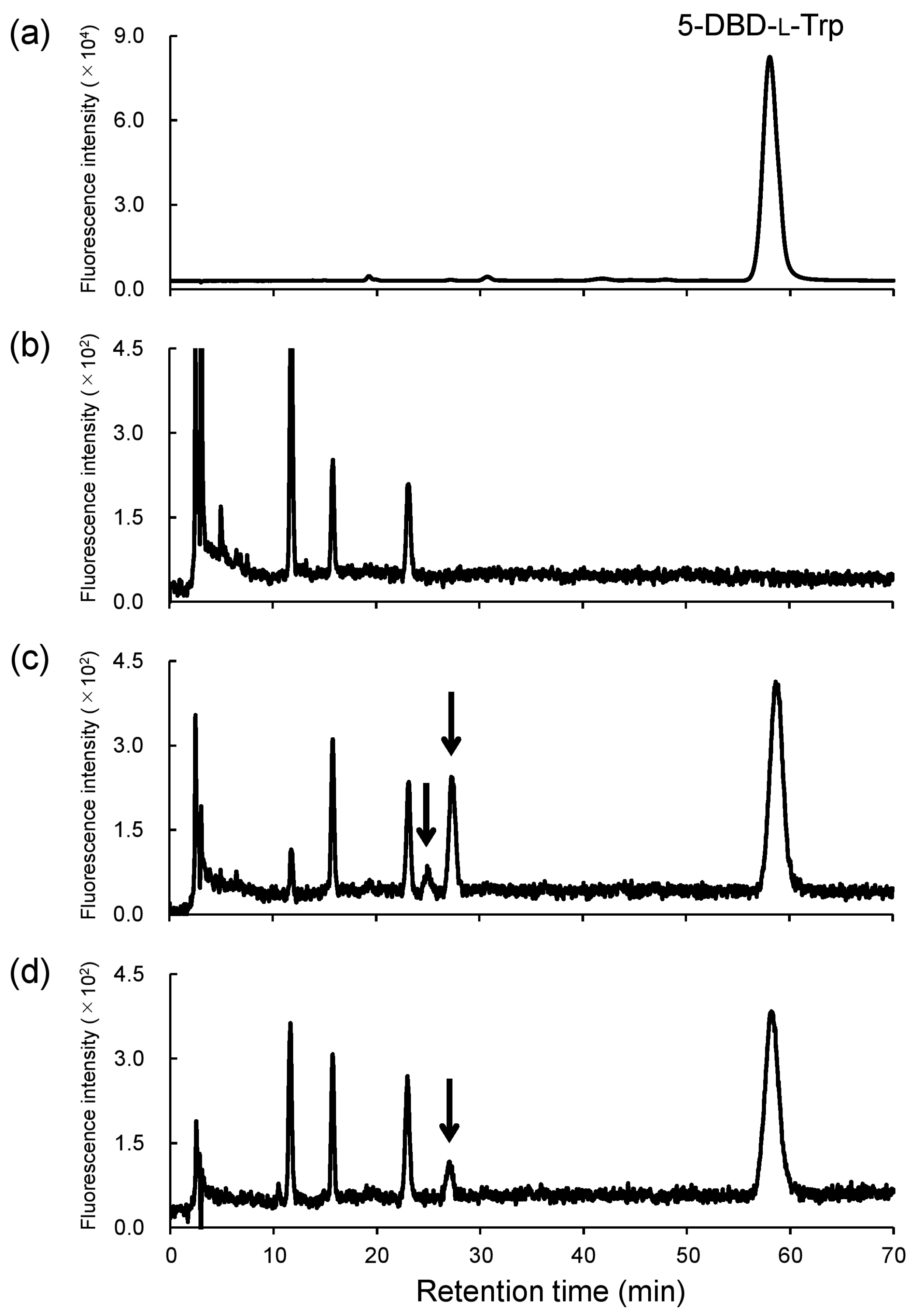
2.2.1. MD Study in Rat Kidneys
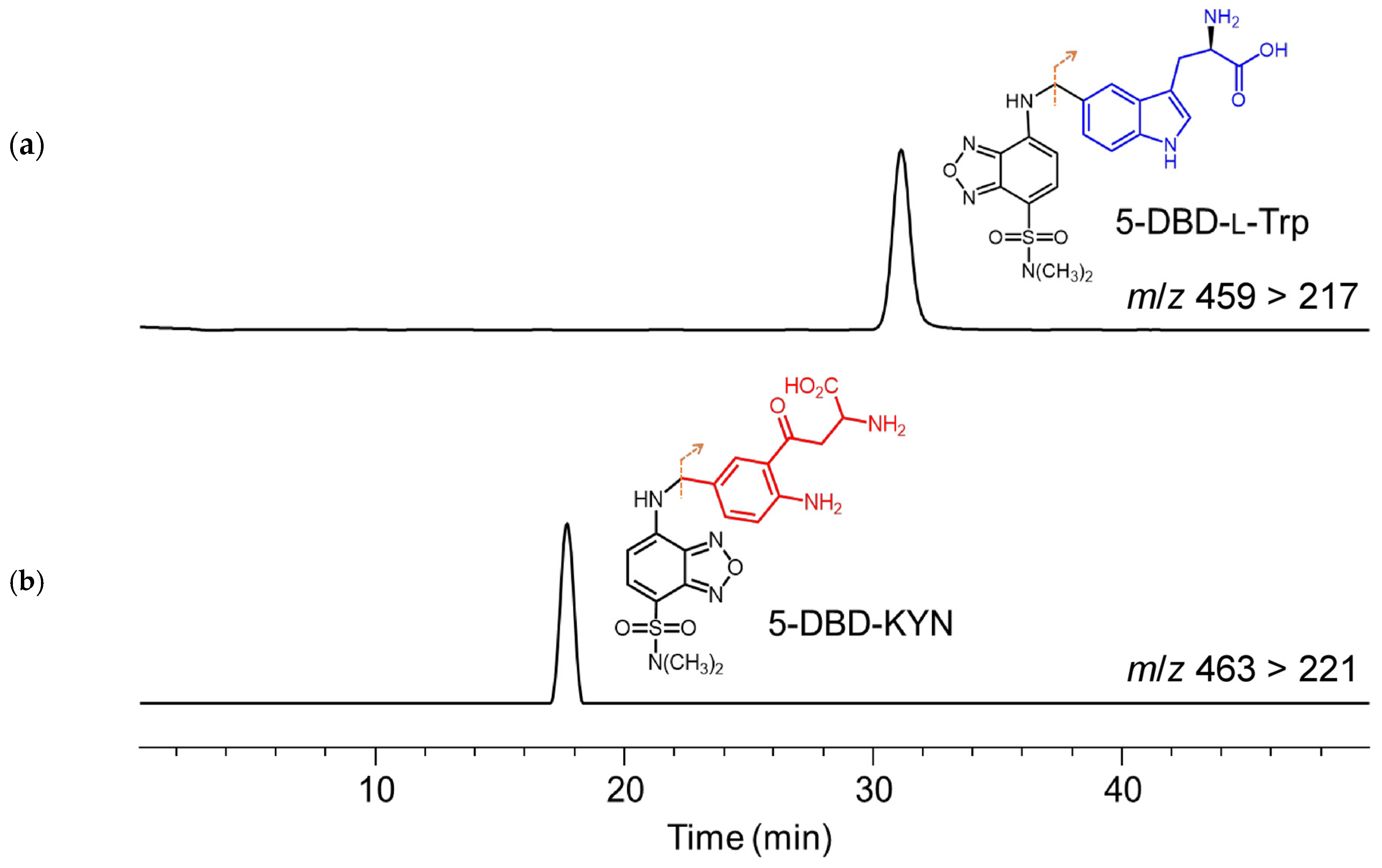
2.2.2. LC–MS Analysis
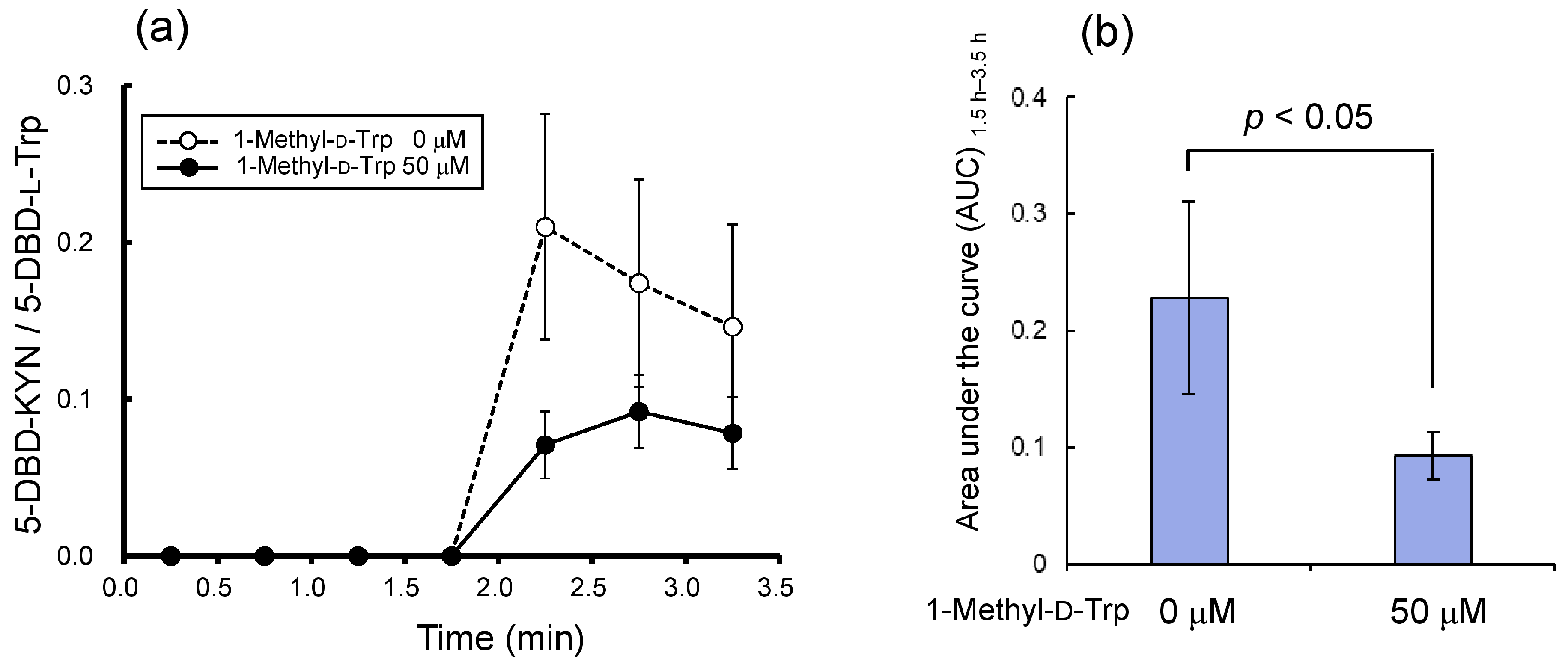
2.2.3. Co-Infusion with 1-Methyl-d-Trp
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of 5-DBD-l-Trp Derivative
4.2.1. Tert-butyl ((1H-indol-5-yl)methyl)carbamate (2)
4.2.2. 2-Acetamido-3-(5-(((tert-butoxycarbonyl)amino)methyl)-1H-indol-3-yl)propanoic acid (3)
4.2.3. 2-Acetamido-3-(5-(((7-(N,N-dimethylsulfamoyl)benzo[c][1,2,5]oxadiazol-4-yl)amino)methyl)-1H-indol-3-yl)propanoic acid (4)
4.2.4. 2-Amino-3-(5-(((7-(N,N-dimethylsulfamoyl)benzo[c][1,2,5]oxadiazol-4-yl)amino)methyl)-1H-indol-3-yl)propanoic acid (5-DBD-l-Trp) (5)
4.3. Measurement of the Fluorescence Spectrum
4.4. MD Experiments
4.5. HPLC with Fluorescence Detection
4.6. LC-MS
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Trp | tryptophan |
| HPLC | high-performance liquid chromatography |
| DBD | 4-N,N-dimethylamino-2,1,3-benzoxadiazole |
| LC-MS | liquid chromatography–mass spectrometry |
References
- Leklem, J.E. Quantitative aspects of tryptophan metabolism in humans and other species: A review. Am. J. Clin. Nutr. 1971, 24, 659−672. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379−401. [Google Scholar] [CrossRef]
- Macchiarulo, A.; Camaioni, E.; Nuti, R.; Pellicciari, R. Highlights at the gate of tryptophan catabolism: A review on the mechanisms of activation and regulation of indoleamine 2,3-dioxygenase (IDO), a novel target in cancer disease. Amino Acids 2009, 37, 219−229. [Google Scholar] [CrossRef]
- Zhu, M.M.T.; Dancsok, A.R.; Nielsen, T.O. Indoleamine dioxygenase inhibitors: Clinical rationale and current development. Curr. Oncol. Rep. 2019, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Umino, M.; Sakamoto, T.; Onozato, M. A review of chromatographic methods for bioactive tryptophan metabolites, kynurenine, kynurenic acid, quinolinic acid, and others, in biological fluids. Biomed. Chromatogr. 2022, 36, e5308. [Google Scholar] [CrossRef]
- Rangel, M.V.S.; Alam, M.A.; Islamuddin, M.; Chen, Z.; Qin, X.; Borges, J.P. Exercise-induced effects on atherogenesis and tryptophan catabolism via the kynurenine pathway in an HIV-associated atherosclerosis mouse model. Exp. Physiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Schröcksnadel, K.; Widner, B.; Bergant, A.; Neurauter, G.; Schennach, H.; Schröcksnadel, H.; Fuchs, D. Longitudinal study of tryptophan degradation during and after pregnancy. Life Sci. 2003, 72, 785−793. [Google Scholar] [CrossRef]
- Thomas, J.; Khanam, R.; Vohora, D. Activation of indoleamine 2, 3- dioxygenase pathway by olanzapine augments antidepressant effects of venlafaxine in mice. Psychiatry Res. 2017, 258, 444−448. [Google Scholar] [CrossRef]
- Naseem, W.; Bano, S. Chronic administration of St. John’s Wort attenuates alcohol intake and brain indoleamine 2, 3-dioxygenase activity in mice. Pak. J. Pharm. Sci. 2018, 31, 1203−1207. [Google Scholar]
- Stenken, J.A.; Holunga, D.M.; Decker, S.A.; Sun, L. Experimental and theoretical microdialysis studies of in situ metabolism. Anal. Biochem. 2001, 290, 314−323. [Google Scholar] [CrossRef]
- Chumakov, I.; Blumenfeld, M.; Guerassimenko, O.; Cavarec, L.; Palicio, M.; Abderrahim, H.; Bougueleret, L.; Barry, C.; Tanaka, H.; La Rosa, P.; et al. Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. USA 2002, 99, 13675−13680. [Google Scholar] [CrossRef]
- Fukushima, T.; Kansaku, A.; Umino, M.; Sakamoto, T.; Onozato, M. Evaluation of d-amino acid oxidase activity in rat kidney using a d-kynurenine derivative, 6-methylthio-d-kynurenine: An in vivo microdialysis study. Drug Discov. Ther. 2023, 17, 434−439. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Uzu, S.; Toyo’oka, T. Fluorogenic reagents, having benzofurazan structure, in liquid-chromatography. J. Pharm. Biomed. Anal. 1989, 7, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Toyo’oka, T.; Suzuki, T.; Saito, Y.; Uzu, S.; Imai, K. Evaluation of benzofurazan derivatives as fluorogenic reagents for thiols and amines using high-performance liquid-chromatography. Analyst 1989, 114, 1233–1240. [Google Scholar] [CrossRef]
- Ohta, Y.; Kubo, H.; Yashiro, K.; Ohashi, K.; Tsuzuki, Y.; Wada, N.; Yamamoto, Y.; Saito, K. Effect of water-immersion restraint stress on tryptophan catabolism through the kynurenine pathway in rat tissues. J. Physiol. Sci. 2017, 67, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Blaser, G.; Sanderson, J.; Batsanov, A.; Howard, J. The facile synthesis of a series of tryptophan derivatives. Tetrahedron Lett. 2008, 49, 2795−2798. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nakakoshi, M.; Okuno, H.; Sakamoto, Y.; Sakurai, S. Mechanism for the direct synthesis of tryptophan from indole and serine: A useful NMR technique for the detection of a reactive intermediate in the reaction mixture. Magn. Reson. Chem. 2010, 48, 811−817. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Sugiura, A.; Furuta, I.; Iwasa, S.; Iizuka, H.; Ichiba, H.; Onozato, M.; Hikawa, H.; Yokoyama, Y. Enantiomeric separation of monosubstituted tryptophan derivatives and metabolites by HPLC with a cinchona alkaloid-based zwitterionic chiral stationary phase and its application to the evaluation of the optical purity of synthesized 6-chloro-l-tryptophan. Int. J. Tryptophan Res. 2015, 8, 1−5. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Hikawa, H.; Mitsuhashi, M.; Uyama, A.; Murakami, Y. Syntheses without protection: A three-step synthesis of optically active clavicipitic acid by utilizing biomimetic synthesis of 4-bromotryptophan. Tetrahedron Lett. 1999, 40, 7803−7806. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int. Rev. Cell Mol. Biol. 2018, 336, 175−203. [Google Scholar]
- Watanabe, N.; Toyo’oka, T.; Imai, K. HPLC electrochemical fluorometric detection of amino acids including tryptophan using 4-fluoro-7-nitrobenzo-2-oxa-1,3-diazole. Biomed. Chromatogr. 1987, 2, 99−103. [Google Scholar] [CrossRef]
- Imai, K.; Ueda, E.; Toyo’oka, T. High-performance liquid-chromatography with photochemical fluorimetric detection of tryptophan based on 4-fluoro-7-nitrobenzo-2-oxa-1,3-diazole—Total protein amino-acid analysis. Anal. Chim. Acta 1988, 205, 7−14. [Google Scholar] [CrossRef]
- Zakharia, Y.; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, V.; Tang, S.; Dillon, P.; Montero, A.; Poklepovic, A.; Melin, S.; Nuhad, I.; Nikolinakos, P.; Kennedy, E.; Han, H.; et al. Results of a phase II double-blinded, randomized, placebo-controlled clinical trial of Indoximod, an Indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor, in combination with Taxane chemotherapy in metastatic breast cancer (MBC). Cancer Res. 2020, 80, PD1-04. [Google Scholar] [CrossRef]
- Hou, D.Y.; Muller, A.J.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D.H. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007, 67, 792−801. [Google Scholar] [CrossRef]
- Onozato, M.; Fu, S.; Takaura, T.; Sakamoto, T.; Fukushima, T. Enantiomeric separation of 1-methyl-tryptophan on a mixed-mode stationary phase via pre-column derivatization and in vivo assessment of chiral inversion in rats. J. Chromatogr. Open 2025, 8, 100257. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Hunt, N.H.; Ball, H.J. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: Characteristics and potential roles in health and disease. Amino Acids 2013, 45, 1319−1329. [Google Scholar] [CrossRef]
- Toyo’oka, T.; Liu, Y.M.; Jinno, H.; Hanioka, N.; Ando, M.; Imai, K. Chiral separation of amines by high-performance liquid chromatography after tagging with 4-(N,N-dimethylaminosulphonyl)-7-(2-chloroformylpyrrolidin-1-yl)- 2,1,3-benzoxadiazole. Biomed. Chromatogr. 1994, 8, 85−89. [Google Scholar] [CrossRef]
- Ota, T.; Yasuda, M.; Iijima, R.; Yui, S.; Fukuuchi, T.; Yamaoka, N.; Mawatari, K.; Kaneko, K.; Nakagomi, K. Development of a fluorescence analysis method for N-acetylneuraminic acid and its oxidized product ADOA. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2013, 932, 152−157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Onozato, M.; Aoki, R.; Yamaguchi, M.; Fujimoto, H.; Sakamoto, T.; Fukushima, T. Development of a Fluorophore-Bound l-Tryptophan Derivative for Evaluating Indoleamine 2,3-Dioxygenase Activity by HPLC with Fluorescence Detection: An In Vivo Microdialysis Study Using Rat Kidney. Molecules 2026, 31, 283. https://doi.org/10.3390/molecules31020283
Onozato M, Aoki R, Yamaguchi M, Fujimoto H, Sakamoto T, Fukushima T. Development of a Fluorophore-Bound l-Tryptophan Derivative for Evaluating Indoleamine 2,3-Dioxygenase Activity by HPLC with Fluorescence Detection: An In Vivo Microdialysis Study Using Rat Kidney. Molecules. 2026; 31(2):283. https://doi.org/10.3390/molecules31020283
Chicago/Turabian StyleOnozato, Mayu, Reika Aoki, Mai Yamaguchi, Honoka Fujimoto, Tatsuya Sakamoto, and Takeshi Fukushima. 2026. "Development of a Fluorophore-Bound l-Tryptophan Derivative for Evaluating Indoleamine 2,3-Dioxygenase Activity by HPLC with Fluorescence Detection: An In Vivo Microdialysis Study Using Rat Kidney" Molecules 31, no. 2: 283. https://doi.org/10.3390/molecules31020283
APA StyleOnozato, M., Aoki, R., Yamaguchi, M., Fujimoto, H., Sakamoto, T., & Fukushima, T. (2026). Development of a Fluorophore-Bound l-Tryptophan Derivative for Evaluating Indoleamine 2,3-Dioxygenase Activity by HPLC with Fluorescence Detection: An In Vivo Microdialysis Study Using Rat Kidney. Molecules, 31(2), 283. https://doi.org/10.3390/molecules31020283





