Gas Chromatography/Mass Spectrometry Chemical Profiling of Volatile Compounds from Cranberry Plant Byproducts as Potential Antibacterials, Antifungals, and Antioxidants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profiling
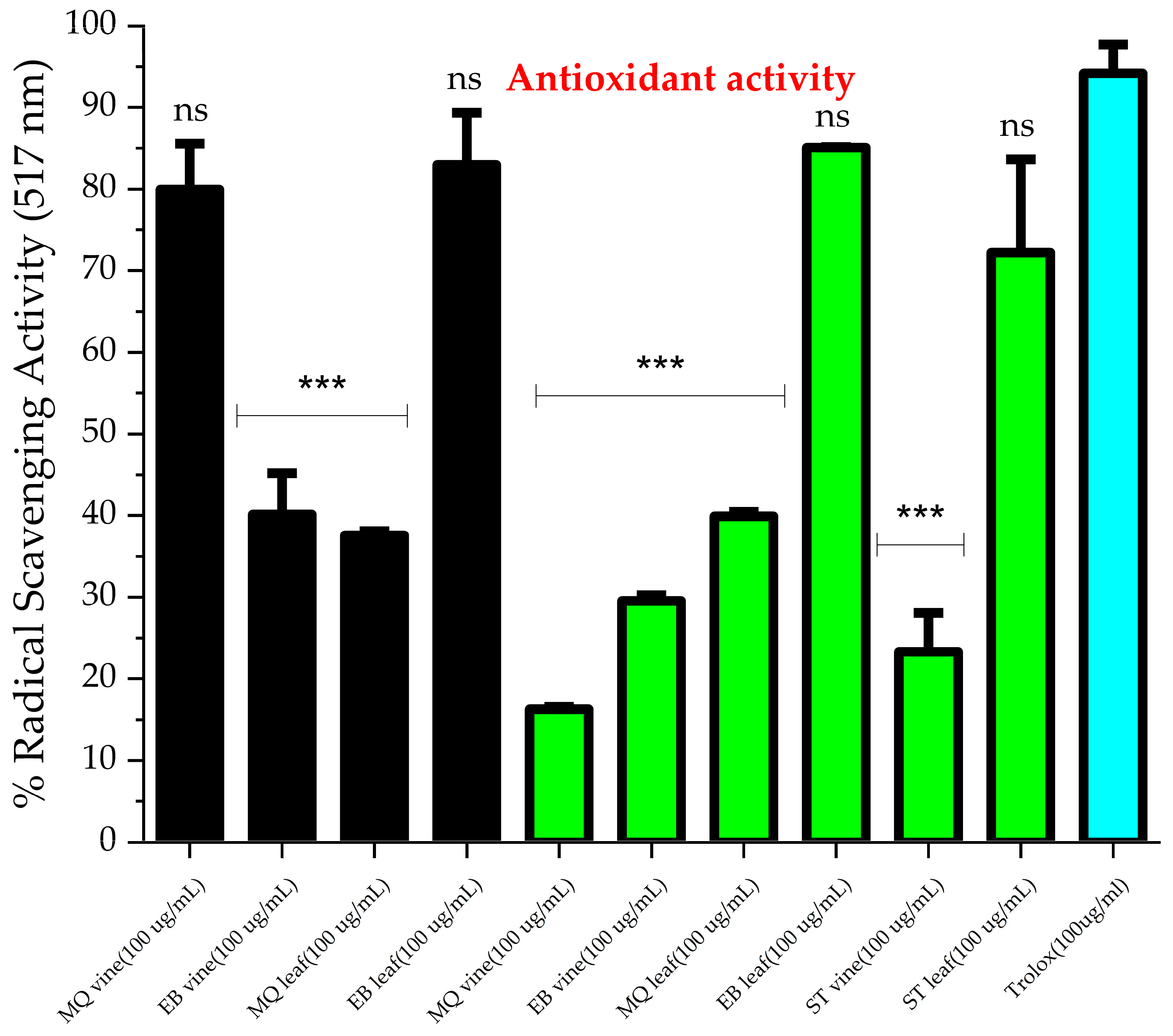
2.2. Antioxidant Activity
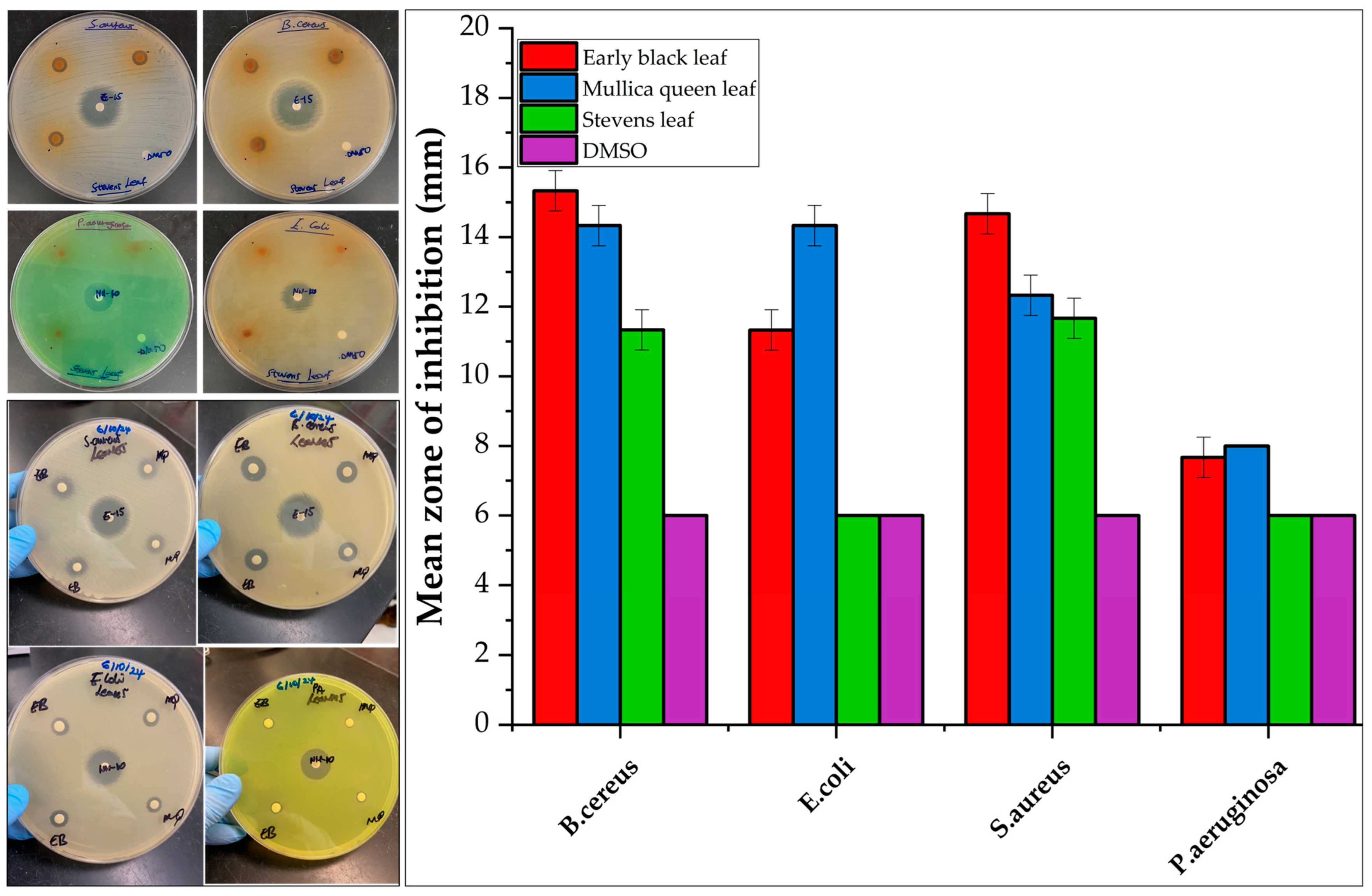
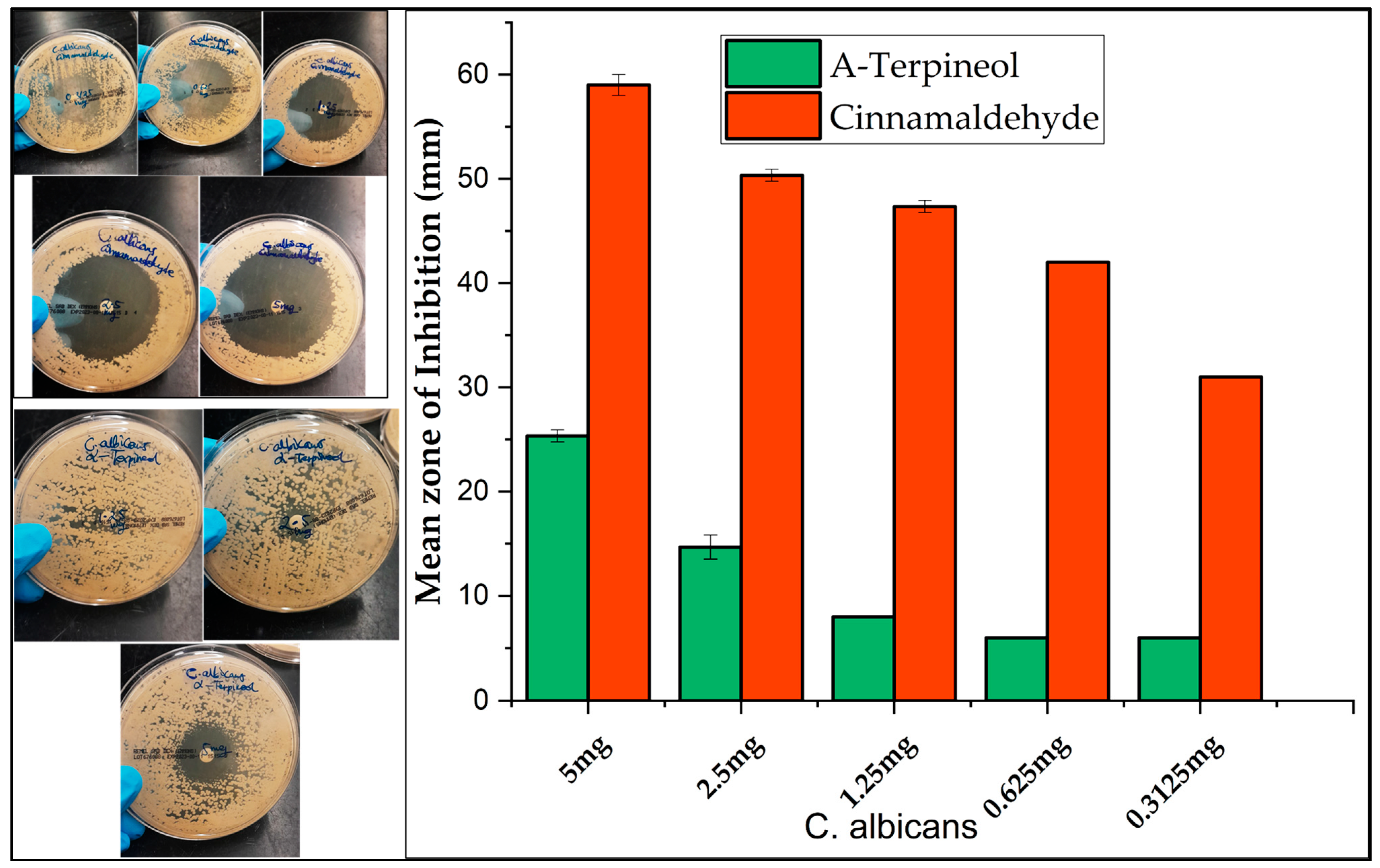
2.3. Antimicrobial Screening
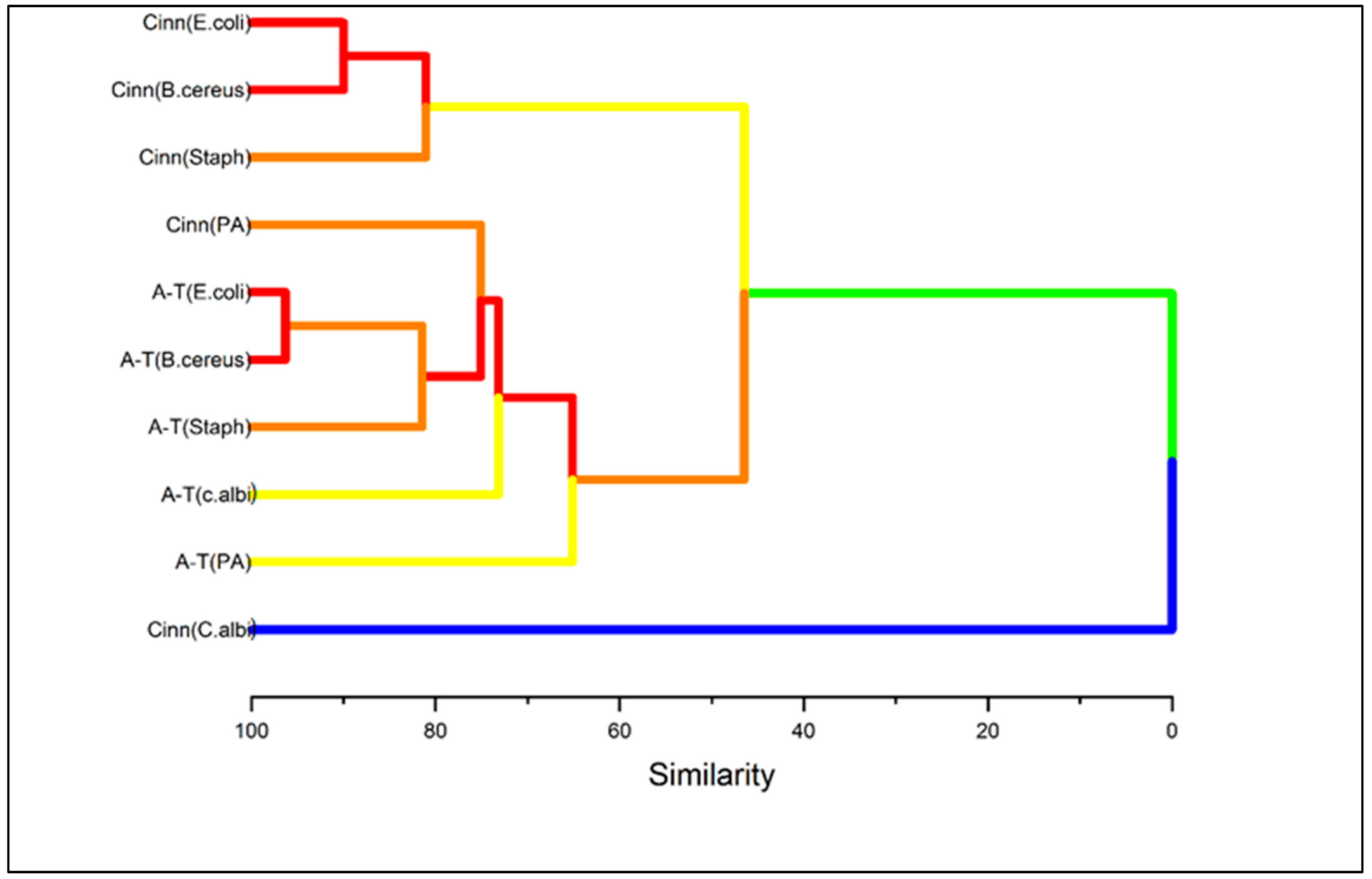
2.4. Multivariate Analysis
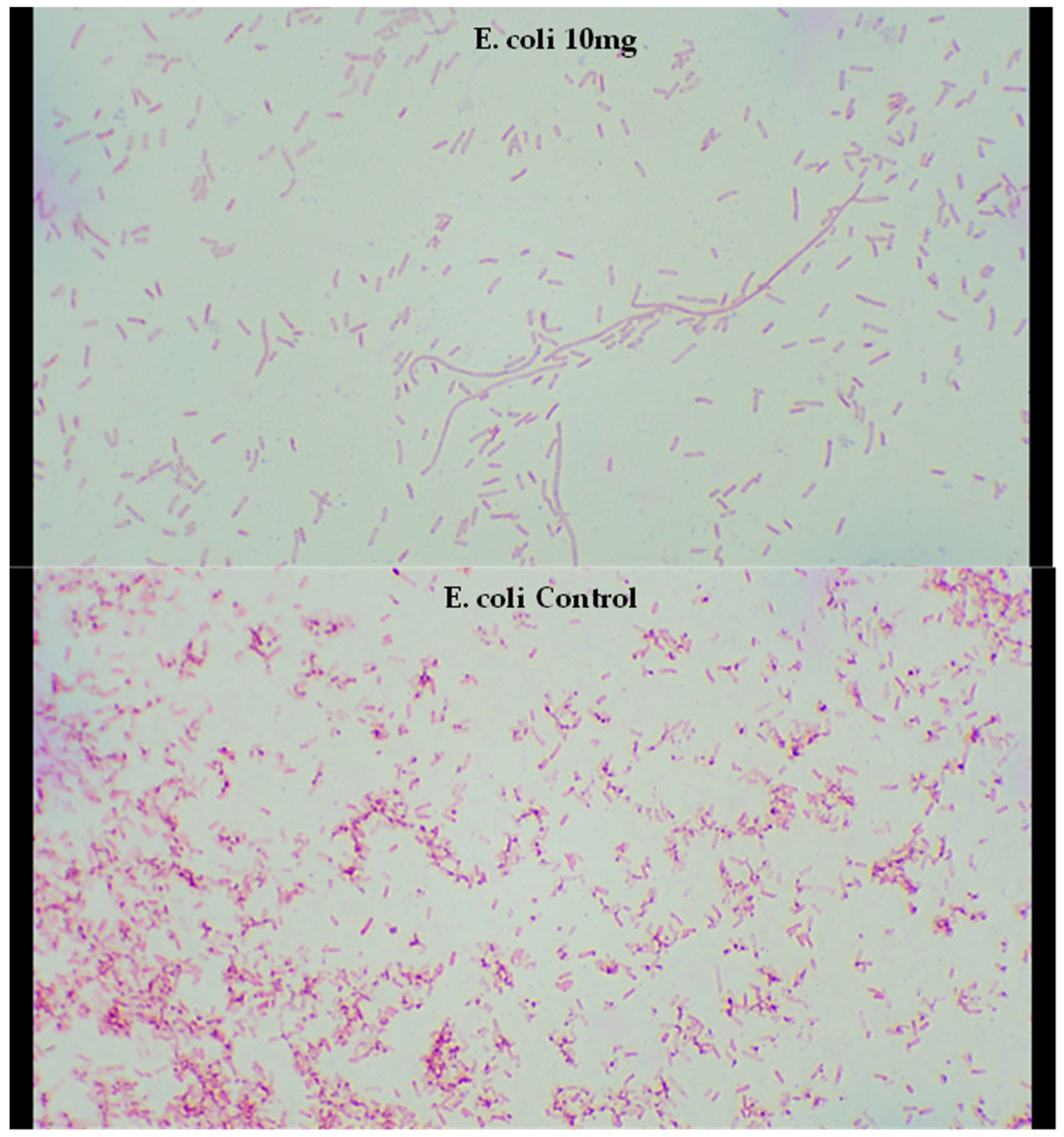
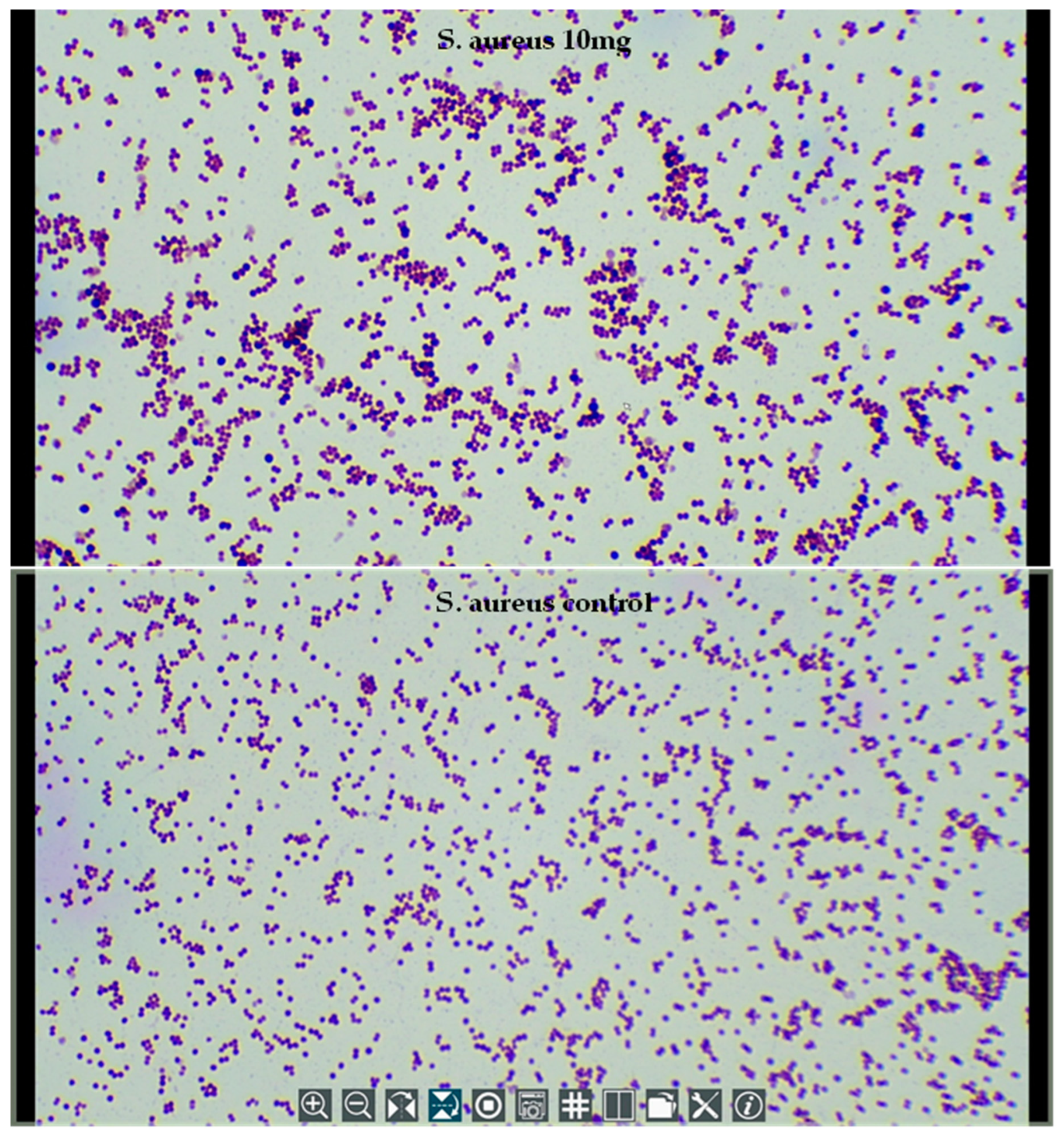
2.5. Microscopy of Treated Bacteria
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. Microbial Strains
3.3. Isolation of Cranberry Volatiles by Hydrodistillation Technique
3.4. GC-MS Method Development and Optimization
3.5. Antioxidant Activity Determination
3.6. Disc Diffusion Assay for Antimicrobial Screening
3.7. Minimum Inhibitory and Bactericidal Concentrations (MIC and MBC)
3.8. Structural Morphological Changes of the Bacteria
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elangovan, S.; Mudgil, P. Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. Antibiotics 2023, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus Infection. In Treasure Island (FL); StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Mueller, M.; Tainter, C.R. Escherichia coli Infection. In Treasure Island (FL); StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Blomquist, K.C.; Nix, D.E. A Critical Evaluation of Newer β-Lactam Antibiotics for Treatment of Pseudomonas aeruginosa Infections. Ann. Pharmacother. 2021, 55, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Speck, F.G. Medicine Practices of the Northeastern Algonquians; Creative Media Partners, LLC.: London, UK, 2022. [Google Scholar]
- Weber, J.T. Traditional Uses and Beneficial Effects of Various Species of Berry-Producing Plants in Eastern Canada. Botany 2022, 100, 175–182. [Google Scholar] [CrossRef]
- Balawejder, M.; Piechowiak, T.; Kapusta, I.; Chęciek, A.; Matłok, N. In Vitro Analysis of Selected Antioxidant and Biological Properties of the Extract from Large-Fruited Cranberry Fruits. Molecules 2023, 28, 7895. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin-Rich Haskap (Lonicera caerulea L.) Berry Extracts Reduce Nitrosamine-Induced DNA Damage in Human Normal Lung Epithelial Cells. Food Chem. Toxicol. 2020, 141, 111404. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Saracila, M.; Panaite, T.D.; Oancea, A.G.; Vlaicu, P.A.; Grosu, I.A. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants 2023, 12, 1084. [Google Scholar] [CrossRef]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Janulis, V. Phytogenotypic Anthocyanin Profiles and Antioxidant Activity Variation in Fruit Samples of the American Cranberry (Vaccinium macrocarpon Aiton). Antioxidants 2022, 11, 250. [Google Scholar] [CrossRef]
- Xue, L.; Liu, C.; Ma, H.; Seeram, N.P.; Neto, C.C. Anti-Inflammatory Activities of Cranberry Fruit Extracts in Human THP-1 Monocytes Are Influenced by Their Phytochemical Composition. ACS Food Sci. Technol. 2022, 2, 75–83. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.-Y.O.; Blumberg, J. Chronic Consumption of a Low Calorie, High Polyphenol Cranberry Beverage Attenuates Inflammation and Improves Glucoregulation and HDL Cholesterol in Healthy Overweight Humans: A Randomized Controlled Trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Nantz, M.P.; Rowe, C.A.; Muller, C.; Creasy, R.; Colee, J.; Khoo, C.; Percival, S.S. Consumption of Cranberry Polyphenols Enhances Human Γδ-T Cell Proliferation and Reduces the Number of Symptoms Associated with Colds and Influenza: A Randomized, Placebo-Controlled Intervention Study. Nutr. J. 2013, 12, 161. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Occhipinti, A.; Luganini, A.; Maffei, M.E.; Gribaudo, G. Inhibition of Herpes Simplex Type 1 and Type 2 Infections by Oximacro®, a Cranberry Extract with a High Content of A-Type Proanthocyanidins (PACs-A). Antivir. Res. 2016, 132, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Gallique, M.; Wei, K.; Maisuria, V.B.; Okshevsky, M.; McKay, G.; Nguyen, D.; Tufenkji, N. Cranberry-Derived Proanthocyanidins Potentiate β-Lactam Antibiotics against Resistant Bacteria. Appl. Environ. Microbiol. 2021, 87, e00127-21. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods 2020, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Lorenzo, G.; Côté, J.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Cancer Chemopreventive Effect of Fractions from Cranberry Products. Food Res. Int. 2012, 45, 320–330. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Bolaños-Carrillo, M.A.; Carmona-Ruiz, D.; Lopéz-Macay, A.; Hernández-Sánchez, F.; Montés-Sánchez, D.; Escuadra-Landeros, M.; Gaitán-Cepeda, L.A.; Maldonado-Frías, S.; Yáñez-Ocampo, B.R.; et al. The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants 2023, 12, 2330. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway. Antioxidants 2020, 9, 871. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Duan, W.; Sun, P.; Chen, L.; Gao, S.; Shao, W.; Li, J. Comparative Analysis of Fruit Volatiles and Related Gene Expression between the Wild Strawberry Fragaria Pentaphylla and Cultivated Fragaria × Ananassa. Eur. Food Res. Technol. 2018, 244, 57–72. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, B.; Yuan, F. Volatile Composition of Eight Blueberry Cultivars and Their Relationship with Sensory Attributes. Flavour Fragr. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New Insights into Plant Isoprenoid Metabolism. Mol. Plant 2012, 5, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Howard, L.; Brownmiller, C.; Gu, I.; Lee, S.-O.; Mauromoustakos, A. Inhibitory Effects of Cranberry Polyphenol and Volatile Extracts on Nitric Oxide Production in LPS Activated RAW 264.7 Macrophages. Food Funct. 2019, 10, 7091–7102. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Dincheva, I.; Badjakov, I.; Gochev, V.; Tsolova, V. Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes. Molecules 2019, 24, 3355. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Howard, L.; Lee, S.-O. Chemical Composition of Volatile Extracts from Black Raspberries, Blueberries, and Blackberries and Their Antiproliferative Effect on A549 Non-Small-Cell Lung Cancer Cells. Life 2022, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Beema Shafreen, R.; Dymerski, T.; Namieśnik, J.; Jastrzębski, Z.; Vearasilp, S.; Gorinstein, S. Interaction of Human Serum Albumin with Volatiles and Polyphenols from Some Berries. Food Hydrocoll. 2017, 72, 297–303. [Google Scholar] [CrossRef]
- Overton, S.V.; Manura, J.J. Note 35_Volatile Organics Composition of Cranberries. Available online: https://www.sisweb.com/referenc/applnote/app-35.htm (accessed on 15 December 2024).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Karimnejad, M.; Ghavam, M. Comparison of Quantity, Quality and Antibacterial Activity of Essential Oil Mentha longifolia (L.) L. under Different Traditional and Modern Extraction Methods. PLoS ONE 2024, 19, e0301558. [Google Scholar] [CrossRef]
- Feyzi, P.; Kamali, H.; Yazdani, A.; Hashemimoghadam, H. Comparison of solvent extraction and hydrodistillation of essential oil from Biebersteinia multifida DC. Conjunction with gas chromatography—Mass spectroscopy. North Khorasan Univ. Med. Sci. 2013, 4, 35–41. [Google Scholar] [CrossRef]
- Ghavam, M. A GC-MC Analysis of Chemical Compounds and Identification of the Antibacterial Characteristics of the Essential Oil of Two Species Exclusive to Iranian Habitats: New Chemotypes. PLoS ONE 2022, 17, e0273987. [Google Scholar] [CrossRef]
- Yitbarek, R.M.; Admassu, H.; Idris, F.M.; Fentie, E.G. Optimizing the Extraction of Essential Oil from Cinnamon Leaf (Cinnamomum verum) for Use as a Potential Preservative for Minced Beef. Appl. Biol. Chem. 2023, 66, 47. [Google Scholar] [CrossRef]
- Ibi, A.; Kyuka, C. Sources, Extraction and Biological Activities of Cinnamaldehyde. Trends Pharm. Sci. 2022, 8, 263–282. [Google Scholar] [CrossRef]
- Qasim, M.; Islam, W.; Rizwan, M.; Hussain, D.; Noman, A.; Khan, K.A.; Ghramh, H.A.; Han, X. Impact of Plant Monoterpenes on Insect Pest Management and Insect-Associated Microbes. Heliyon 2024, 10, e39120. [Google Scholar] [CrossRef]
- Salamci, E.; Aslan, İ.; Çalmaşur, O.; Kazaz, C. Fumigant toxicity of norbornanetetraacetate, cyclooctanetetraacetate and 7-oxanorbornene derivative against the stored product insect pest Sitophilus granarius (L.). Fresenius Environ. Bull. 2009, 18, 642–646. [Google Scholar]
- de Lange, E.S.; Kyryczenko-Roth, V.; Johnson-Cicalese, J.; Davenport, J.; Vorsa, N.; Rodriguez-Saona, C. Increased nutrient availability decreases insect resistance in cranberry. Agric. For. Entomol. 2019, 21, 326–335. [Google Scholar] [CrossRef]
- Neto, C.C.; Dao, C.A.; Salvas, M.R.; Autio, W.R.; Vanden Heuvel, J.E. Variation in Concentration of Phenolic Acid Derivatives and Quercetin Glycosides in Foliage of Cranberry That May Play a Role in Pest Deterrence. J. Am. Soc. Hortic. Sci. 2010, 135, 494–500. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Sun, B.; Zhao, M.; Zheng, F.; Huang, M.; Sun, X.; Li, H. Intracellular Antioxidant Effect of Vanillin, 4-Methylguaiacol and 4-Ethylguaiacol: Three Components in Chinese Baijiu. RSC Adv. 2017, 7, 46395–46405. [Google Scholar] [CrossRef]
- Nam, H.; Kim, M.-M. Eugenol with Antioxidant Activity Inhibits MMP-9 Related to Metastasis in Human Fibrosarcoma Cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef]
- Taha, K.F.; El-kashoury, E.-S.A.; Ezzat, S.M.; Saleh, N.A. Antimicrobial and antioxidant activity of volatile constituents of the leaves of Tecoma smithii Will. Wats. Glob. J. Med. Plant Res. 2016, 4, 16–22. [Google Scholar]
- Sripahco, T.; Khruengsai, S.; Charoensup, R.; Tovaranonte, J.; Pripdeevech, P. Chemical Composition, Antioxidant, and Antimicrobial Activity of Elsholtzia Beddomei C. B. Clarke Ex Hook. f. Essential Oil. Sci. Rep. 2022, 12, 2225. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-Picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Cho, H.-S.; Chun, J.-Y. Antioxidant Activity of β-Cyclodextrin Inclusion Complexes Containing Trans-Cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci. Biotechnol. 2021, 30, 807–814. [Google Scholar] [CrossRef]
- Siswadi, S.; Saragih, G.S. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar] [CrossRef]
- Kim, E.-N.; Trang, N.M.; Kang, H.; Kim, K.H.; Jeong, G.-S. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264.7 Cells. Cells 2022, 11, 3596. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural Characterization of N-Hexadecanoic Acid from the Leaves of Ipomoea Eriocarpa and Its Antioxidant and Antibacterial Activities. Biomass Conv. Bioref. 2024, 14, 14547–14558. [Google Scholar] [CrossRef]
- Santos, C.C.D.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; De Almeida, A.A.C.; De Oliveira, G.A.L.; Costa, J.P.; De Sousa, D.P.; De Freitas, R.M.; De Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Williams, D.N.; Saar, J.S.; Bleicher, V.; Rau, S.; Lienkamp, K.; Rosenzweig, Z. Poly(Oxanorbornene)-Coated CdTe Quantum Dots as Antibacterial Agents. ACS Appl. Bio Mater. 2020, 3, 1097–1104. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, Y.; Yang, T.; Chen, L.; Pei, X.; Zhang, Y.; Ma, T.; Xie, Y.; Wang, Y.; Li, H.; et al. Supplementation of Dietary Heptadecanoic Acid Enhances Anti-Listeria Monocytogenes Response in Macrophages. J. Funct. Foods 2024, 119, 106359. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Kim, J.-I.; Choi, H.-J.; Lee, J.-S. Anticancer and Antimicrobial Activities of 13(E)-Labd-13-Ene-8α,15-Diol from Brachyglottis Monroi. J. Appl. Biol. Chem. 2013, 56, 49–51. [Google Scholar] [CrossRef]
- Xu, W.; Shi, D.; Chen, K.; Popovich, D.G. TLC-Bioautography-Guided Isolation and Assessment of Antibacterial Compounds from Manuka (Leptospermum scoparium) Leaf and Branch Extracts. Molecules 2024, 29, 717. [Google Scholar] [CrossRef]
- Pereira, W.A.; Pereira, C.D.S.; Assunção, R.G.; Da Silva, I.S.C.; Rego, F.S.; Alves, L.S.R.; Santos, J.S.; Nogueira, F.J.R.; Zagmignan, A.; Thomsen, T.T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Ge, L.; Li, Z.; Chen, B.; Liao, S.; Lu, L.; Wu, Q.; Jiang, X.; An, Y.; Wang, Z.; et al. Antibacterial Activity of Cinnamon Essential Oil and Its Main Component of Cinnamaldehyde and the Underlying Mechanism. Front. Pharmacol. 2024, 15, 1378434. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2015, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Bayati, F.A.; Mohammed, M.J. Isolation, Identification, and Purification of Cinnamaldehyde from Cinnamomum zeylanicum Bark Oil. An Antibacterial Study. Pharm. Biol. 2009, 47, 61–66. [Google Scholar] [CrossRef]
- Kadiasi, N.; Tako, R.; Ibraliu, A.; Stanys, V.; Gruda, N.S. Principal Component and Hierarchical Cluster Analysis of Major Compound Variation in Essential Oil among Some Red Oregano Genotypes in Albania. Agronomy 2024, 14, 1419. [Google Scholar] [CrossRef]
- Visvalingam, J.; Hernandez-Doria, J.D.; Holley, R.A. Examination of the Genome-Wide Transcriptional Response of Escherichia coli O157:H7 to Cinnamaldehyde Exposure. Appl. Environ. Microbiol. 2013, 79, 942–950. [Google Scholar] [CrossRef]
- Rezende, D.A.C.S.; Souza, R.V.; Magalhães, M.L.; Silva Caetano, A.R.; Sousa Carvalho, M.S.; De Souza, E.C.; De Lima Guimarães, L.G.; Nelson, D.L.; Batista, L.R.; Das Graças Cardoso, M. Characterization of the Biological Potential of the Essential Oils from Five Species of Medicinal Plants. Am. J. Plant Sci. 2017, 08, 154–170. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Revisiting DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Assay as a Useful Tool in Antioxidant Evaluation: A New IC100 Concept to Address Its Limitations. J. Food Bioact. 2019, 7, 36–42. [Google Scholar] [CrossRef]




| RT | Compound Identified | Steam Distillation | Clevenger Hydrodistillation | Molecular Weight | ||||
|---|---|---|---|---|---|---|---|---|
| EB | MQ | ST ++ | EB | MQ | ST | |||
| 4.78 | Hexanal | 1.89 | 0.79 | N/A | N/A | N/A | N/A | 100 |
| 5.69 | 3-Furaldehyde | 2.69 | N/A | N/A | N/A | N/A | N/A | 96 |
| 7.19 | Styrene | 0.61 | 0.75 | N/A | N/A | N/A | N/A | 104 |
| 7.49 | Heptanal | 0.29 | 0.43 | N/A | N/A | N/A | N/A | 114 |
| 9.33 | Benzaldehyde | 1.87 | N/A | N/A | N/A | N/A | N/A | 106 |
| 9.47 | 5-methylfurfural | 3.44 | 0.58 | N/A | N/A | N/A | N/A | 110 |
| 10.57 | Octanal | 1.88 | 2.48 | N/A | N/A | N/A | N/A | 128 |
| 11.23 | O-cymene | N/A | N/A | N/A | N/A | N/A | N/A | 152 |
| 11.32 | Benzeneethanol, β-ethenyl-α-methyl- | 1.59 | N/A | N/A | N/A | N/A | N/A | 162 |
| 11.78 | Isophorone | 0.34 | 0.46 | N/A | N/A | N/A | N/A | 138 |
| 11.94 | Benzyl alcohol | N/A | N/A | N/A | N/A | 1.27 | N/A | 108 |
| 11.96 | Unknown | 1.33 | N/A | N/A | N/A | N/A | N/A | 150 |
| 12.57 | Ethyl sorbate | N/A | N/A | N/A | 1.08 | 1.45 | N/A | 140 |
| 12.74 | Unknown | 1.80 | 1.21 | N/A | N/A | N/A | N/A | 152 |
| 12.86 | Unknown | N/A | N/A | N/A | N/A | N/A | 1.52 | 97 |
| 12.97 | Unknown | 1.01 | N/A | N/A | N/A | N/A | N/A | 152 |
| 13.03 | 7-oxanorbornene | N/A | N/A | N/A | 3.86 | 51.4 | Trace | 96 |
| 13.28 | 4-Vinyl-o-xylene | 0.39 | 0.21 | N/A | N/A | N/A | N/A | 132 |
| 13.61 | Cis Geraniol | 0.47 | 0.44 | N/A | N/A | N/A | N/A | 154 |
| 13.75 | Nonanal | 1.72 | 3.23 | N/A | N/A | N/A | N/A | 142 |
| 15.09 | Ketoisophorone | 0.26 | 0.28 | N/A | N/A | N/A | N/A | 170 |
| 15.67 | Unknown | 1.44 | 1.46 | N/A | N/A | N/A | N/A | 136 |
| 15.83 | Unknown | 1.59 | N/A | N/A | N/A | N/A | N/A | 150 |
| 16.20 | Dodecene | N/A | N/A | N/A | 0.33 | N/A | 1.07 | 168 |
| 16.33 | alpha terpineol | 4.97 | 16.4 | N/A | N/A | N/A | N/A | 154 |
| 16.88 | Dodecane | N/A | N/A | N/A | 1.77 | N/A | N/A | 170 |
| 17.03 | Cyclocitral | 0.57 | 0.61 | N/A | N/A | N/A | N/A | 152 |
| 17.35 | Benzenepropanal | 14.2 | 17.8 | N/A | 0.24 | 2.15 | N/A | 136 |
| 17.83 | 2-Decenal | 6.13 | 5.25 | N/A | N/A | N/A | N/A | 154 |
| 18.14 | Cinnamaldehyde (E) | 12.8 | 27.8 | N/A | N/A | N/A | N/A | 132 |
| 18.24 | Unknown | N/A | N/A | N/A | 0.57 | 2.09 | N/A | 210 |
| 18.90 | Unknown | 6.60 | 3.62 | N/A | N/A | N/A | N/A | 150 |
| 18.90 | Tridecene | N/A | N/A | N/A | 3.36 | 2.70 | N/A | 182 |
| 19.34 | Ethyl phenethyl ketone | N/A | N/A | N/A | 0.53 | 1.76 | N/A | 134 |
| 19.42 | Longipinane | 0.59 | 0.83 | N/A | N/A | N/A | N/A | 206 |
| 19.65 | Unknown | 1.20 | N/A | N/A | N/A | N/A | N/A | 250 |
| 19.87 | 1,1-Diethoxynonae | N/A | N/A | N/A | N/A | N/A | 0.25 | 216 |
| 19.99 | 5-methylheptanol | N/A | N/A | N/A | N/A | N/A | 0.48 | 130 |
| 20.03 | Tetradecene | N/A | N/A | N/A | 0.71 | 1.01 | N/A | 196 |
| 20.11 | Methoxyacetic acid | N/A | N/A | N/A | N/A | N/A | 0.68 | 90 |
| 20.97 | Geranyl acetone | N/A | N/A | N/A | 0.57 | 0.94 | Trace | 194 |
| 21.00 | Unknown | 1.11 | 0.60 | N/A | N/A | N/A | N/A | 244 |
| 21.22 | Unknown | 2.18 | 0.94 | N/A | N/A | N/A | N/A | 190 |
| 21.31 | Pentadecane | N/A | N/A | N/A | N/A | 0.66 | N/A | 212 |
| 21.53 | beta Ionone | 1.09 | 1.41 | N/A | N/A | N/A | N/A | 192 |
| 21.54 | Germacrene d | N/A | N/A | N/A | N/A | 0.58 | Trace | 204 |
| 21.61 | Ionone | N/A | N/A | N/A | 0.35 | N/A | 0.33 | 192 |
| 21.92 | 1-(2,3,6-trimethylphenyl-3-buten-2-one | N/A | N/A | N/A | N/A | 1.13 | N/A | 188 |
| 21.92 | 1-(2,3,6-trimethylphenyl-3-buten-2-one | 5.53 | 0.88 | N/A | N/A | N/A | N/A | 188 |
| 22.25 | Pentadecene | N/A | N/A | N/A | N/A | 1.99 | 1.36 | 210 |
| 22.39 | Unknown | 2.12 | 0.45 | N/A | N/A | N/A | N/A | 188 |
| 22.51 | Dodecanoic acid | N/A | N/A | N/A | 0.84 | N/A | 1.44 | 200 |
| 22.58 | Unknown | 1.15 | N/A | N/A | N/A | N/A | N/A | 278 |
| 22.75 | Hexadecene | N/A | N/A | N/A | 0.73 | 2.93 | 1.27 | 224 |
| 22.81 | Megastigmatrienone | 3.06 | 0.62 | N/A | N/A | N/A | N/A | 190 |
| 23.41 | Megastigmatrienone (Isomer) | 2.37 | 0.36 | N/A | N/A | N/A | N/A | 190 |
| 23.49 | Unknown | N/A | N/A | N/A | 0.87 | N/A | 1.33 | 356 |
| 23.79 | Hexadecane | N/A | N/A | N/A | 0.96 | 2.18 | 0.59 | 226 |
| 23.86 | Eudesm-7(11)-en-4-ol | 1.57 | 0.27 | N/A | N/A | N/A | N/A | 222 |
| 24.01 | Heptadecane | N/A | N/A | N/A | 2.91 | 1.06 | Trace | 240 |
| 24.05 | Unknown | N/A | 3.46 | N/A | N/A | N/A | N/A | 250 |
| 24.59 | Tetradecanol | N/A | N/A | N/A | 1.79 | 0.28 | 1.62 | 214 |
| 24.67 | Pentadecanol | N/A | N/A | N/A | N/A | 0.84 | 1.62 | 228 |
| 24.79 | Tetradecanoic acid | N/A | N/A | N/A | N/A | N/A | 10.8 | 228 |
| 24.87 | Heptadecene | N/A | N/A | N/A | 2.09 | 2.28 | N/A | 238 |
| 25.04 | Octadecene | N/A | N/A | N/A | 0.52 | 1.75 | 1.54 | 252 |
| 25.15 | Unknown | 2.22 | 1.40 | N/A | N/A | N/A | N/A | 278 |
| 25.49 | Phytol (isomer) | N/A | N/A | N/A | 3.76 | 1.14 | 13.8 | 296 |
| 25.87 | 2-Methyl-7-octadecyne | N/A | N/A | N/A | 0.95 | N/A | 2.77 | 264 |
| 26.00 | 2-Methyl-7-octadecyne (isomer) | N/A | N/A | N/A | 1.57 | 0.55 | 4.99 | 264 |
| 26.45 | Methyl palmitate | N/A | N/A | N/A | 2.88 | 4.20 | 1.76 | 270 |
| 26.85 | Hexadecanoic acid | N/A | N/A | N/A | 4.44 | N/A | 4.54 | 256 |
| 27.07 | Unknown | N/A | N/A | N/A | N/A | N/A | 2.38 | 286 |
| 27.10 | Heptadecanoic acid | N/A | N/A | N/A | 2.03 | 2.64 | N/A | 282 |
| 27.45 | Unknown | 0.40 | N/A | N/A | N/A | N/A | N/A | 286 |
| 27.51 | Kaur-15-ene | N/A | N/A | N/A | N/A | 0.56 | N/A | 272 |
| 27.95 | Octadecanol | N/A | N/A | N/A | 5.58 | N/A | 5.06 | 270 |
| 27.96 | Kaur-15-ene (isomer) | 0.30 | 0.35 | N/A | N/A | N/A | N/A | 272 |
| 27.99 | Kaur-16-ene | 3.49 | 0.62 | N/A | 6.27 | N/A | 5.06 | 272 |
| 28.14 | Methyl Linolenate | N/A | N/A | N/A | 2.41 | N/A | 0.70 | 292 |
| 28.28 | Phytol | 1.79 | 4.00 | N/A | 8.76 | 2.89 | 6.70 | 296 |
| 28.45 | Heniecosane | N/A | N/A | N/A | N/A | 0.93 | N/A | 296 |
| 28.58 | Linoleic acid | N/A | N/A | N/A | N/A | 0.93 | N/A | 280 |
| 28.78 | Linoleic acid (isomer) | N/A | N/A | N/A | 2.42 | 2.58 | N/A | 280 |
| 28.95 | Docosane | N/A | N/A | N/A | 1.49 | 1.30 | 20.9 | 380 |
| 29.38 | Unknown | N/A | N/A | N/A | N/A | 1.17 | 3.44 | 306 |
| 29.55 | Methyl strictate | N/A | N/A | N/A | 32.3 | N/A | Trace | 328 |
| 29.87 | Unknown | N/A | N/A | N/A | 1.10 | 0.67 | 2.19 | 280 |
| Total % Area | 100.0 | 100.0 | N/A | 100.0 | 100.0 | 100.0 | ||
| RT | Compounds | Steam Distillation | Clevenger Hydrodistillation | Molecular Weight | ||||
|---|---|---|---|---|---|---|---|---|
| EB | MQ | ST ++ | EB | MQ | ST | |||
| 4.13 | Acetone, diethyl acetal | N/A | N/A | N/A | N/A | 0.55 | N/A | 132 |
| 4.79 | Hexanal | 1.28 | 1.85 | N/A | N/A | N/A | N/A | 100 |
| 6.55 | p-Xylene | Trace | 0.33 | N/A | N/A | N/A | N/A | 106 |
| 7.20 | Styrene | Trace | 0.70 | N/A | N/A | N/A | N/A | 227 |
| 9.27 | Unknown | 0.85 | Trace | N/A | N/A | N/A | N/A | 164 |
| 10.20 | Unknown | 0.96 | 0.82 | N/A | N/A | N/A | N/A | 138 |
| 10.98 | Unknown | Trace | 0.22 | N/A | N/A | N/A | N/A | 136 |
| 11.36 | Unknown | Trace | 0.74 | N/A | N/A | N/A | N/A | 136 |
| 12.31 | Unknown | Trace | 0.89 | N/A | N/A | N/A | N/A | 138 |
| 12.76 | Trans-p-mentha-2,8-dienol | 0.26 | 0.87 | N/A | N/A | N/A | N/A | 152 |
| 12.92 | Furfural diethyl acetal | N/A | N/A | N/A | N/A | 0.91 | N/A | 170 |
| 13.23 | 1,5,5-trimethyl-6-methylenecyclohexene | Trace | 0.82 | N/A | N/A | N/A | N/A | 136 |
| 13.62 | Citrylidene ethanol | Trace | 2.11 | N/A | N/A | N/A | N/A | 182 |
| 13.78 | Nonanal | 1.78 | 2.81 | N/A | N/A | N/A | N/A | 142 |
| 13.95 | 2-Methylcumarone | Trace | 1.51 | N/A | N/A | N/A | N/A | 124 |
| 14.94 | trans Sabinol | 3.36 | 1.08 | N/A | N/A | N/A | N/A | 152 |
| 15.72 | Borneol | 0.98 | 1.46 | N/A | N/A | N/A | N/A | 154 |
| 16.00 | Terpinen-4-ol | 1.81 | 2.69 | N/A | N/A | N/A | N/A | 154 |
| 16.24 | Dodecene | N/A | N/A | N/A | 1.05 | 0.39 | 0.86 | 168 |
| 16.34 | alpha terpineol | 8.39 | 25.2 | N/A | N/A | N/A | N/A | 154 |
| 16.49 | Myrtenol | 2.27 | 9.28 | N/A | N/A | N/A | N/A | 152 |
| 16.63 | Unknown | 1.19 | N/A | N/A | N/A | N/A | N/A | 150 |
| 17.03 | Unknown | 0.76 | 1.13 | N/A | N/A | N/A | N/A | 100 |
| 17.81 | 2-Furancarboxaldehyde | N/A | N/A | N/A | Trace | Trace | 1.98 | 96 |
| 17.99 | Unknown | 2.49 | 2.59 | N/A | N/A | N/A | N/A | 150 |
| 18.26 | p-Ethylguaicol | N/A | 6.64 | N/A | N/A | N/A | N/A | 152 |
| 18.61 | 2-Tridecene-E | N/A | N/A | N/A | 0.30 | 0.50 | Trace | 182 |
| 18.75 | Unknown | 0.88 | Trace | N/A | N/A | N/A | N/A | 256 |
| 18.76 | 2-Tridecene-E (isomer) | N/A | N/A | N/A | 0.51 | 0.69 | N/A | 182 |
| 18.90 | Tridecene (isomer) | N/A | N/A | N/A | 0.38 | 0.69 | Trace | 182 |
| 19.31 | Unknown | 1.13 | 0.75 | N/A | N/A | N/A | N/A | 150 |
| 19.67 | Unknown | 0.74 | 1.42 | N/A | N/A | N/A | N/A | 224 |
| 20.02 | Tetradecene | N/A | N/A | N/A | 1.95 | 1.16 | 3.24 | 196 |
| 21.16 | m-Eugenol | Trace | 3.81 | N/A | N/A | N/A | N/A | 164 |
| 21.55 | γ-Selinene | 1.89 | 3.26 | N/A | 0.64 | 0.89 | 1.26 | 204 |
| 21.86 | Unknown | 0.60 | 2.85 | N/A | N/A | N/A | N/A | 266 |
| 21.87 | Phenol, 2,4-ditertbutyl | N/A | N/A | N/A | 2.78 | 1.46 | Trace | 206 |
| 22.02 | Delta Amorphene | N/A | N/A | N/A | 0.67 | 1.15 | 0.39 | 204 |
| 22.25 | Unknown | N/A | N/A | N/A | 2.09 | 1.25 | 0.60 | 256 |
| 22.74 | Tridecanol | N/A | N/A | N/A | 3.07 | 0.88 | 3.34 | 200 |
| 23.86 | Eudesm-7(11)-en-4-ol | 49.8 | 22.9 | N/A | 3.33 | 1.20 | 2.63 | 222 |
| 24.23 | Unknown | N/A | N/A | N/A | N/A | 1.53 | N/A | 268 |
| 24.58 | Unknown | N/A | N/A | N/A | N/A | 1.93 | N/A | 306 |
| 24.79 | Unknown | N/A | N/A | N/A | 5.29 | 1.53 | N/A | 306 |
| 25.03 | Octadecene | N/A | N/A | N/A | 3.83 | 2.42 | 1.88 | 252 |
| 25.63 | Unknown | N/A | N/A | N/A | 1.34 | N/A | N/A | 322 |
| 26.22 | Unknown | N/A | N/A | N/A | 1.58 | 1.57 | N/A | 344 |
| 27.00 | Hexadecanoic acid | N/A | N/A | N/A | Trace | 0.34 | 12.8 | 256 |
| 27.10 | Eicosene | N/A | N/A | N/A | 6.25 | 5.82 | 2.72 | 280 |
| 27.44 | Methyl linoleate | N/A | N/A | N/A | 16.6 | 2.34 | 11.3 | 294 |
| 28.10 | Heneicosane | N/A | N/A | N/A | 1.63 | 1.64 | 2.28 | 296 |
| 28.28 | Phytol | N/A | N/A | N/A | 2.04 | 1.58 | 0.40 | 296 |
| 28.56 | Unknown | N/A | N/A | N/A | 0.88 | 0.77 | 12.4 | 298 |
| 28.98 | Docosene | N/A | N/A | N/A | 19.3 | 3.17 | 3.05 | 308 |
| 29.17 | Unknown | 7.99 | Trace | N/A | N/A | N/A | N/A | 294 |
| 29.62 | Labd-(13E)-8,15-diol | N/A | N/A | N/A | 23.9 | 63.6 | 36.8 | 306 |
| 29.66 | Unknown | 10.6 | 1.37 | N/A | N/A | N/A | N/A | 280 |
| 29.94 | Tetracosane | N/A | N/A | N/A | 0.57 | Trace | 1.99 | 338 |
| Total % Area | 100.0 | 100.0 | N/A | 100.0 | 100.0 | 100.0 | ||
| B. cereus | S. aureus | E. coli | P. aeruginosa | C. albicans | |
|---|---|---|---|---|---|
| EB leaf (SD) | 8.00 | 7.00 | 7.00 | 6.00 | 8.00 |
| MQ leaf (SD) | 6.00 | 7.00 | 6.00 | 6.00 | 7.00 |
| EB vine (SD) | 7.00 | 6.00 | 7.00 | 6.00 | 6.00 |
| MQ vine (SD) | 8.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| EB leaf (CM) | 15.33 | 14.67 | 11.33 | 7.67 | 32.33 |
| MQ leaf (CM) | 14.33 | 12.33 | 14.33 | 8.00 | 30.00 |
| ST leaf (CM) | 11.33 | 11.67 | 6.00 | 6.00 | 6.00 |
| EB vine (CM) | 8.67 | 10.33 | 6.00 | 9.33 | 13.33 |
| MQ vine (CM) | 7.67 | 8.67 | 6.00 | 7.00 | 19.33 |
| ST vine (CM) | 7.33 | 7.33 | 6.00 | 6.00 | 11.67 |
| Positive controls Erythromycin | 33.67 | 30.00 | N/A | N/A | N/A |
| Tobramycin | N/A | N/A | 22.00 | 24.33 | N/A |
| Miconazole nitrate | N/A | N/A | N/A | N/A | 10.00 |
| Organism | Cinnamaldehyde (mg/mL) | Cinnamaldehyde (Literature) (mg/mL) [59,62] | Alpha Terpineol (mg/mL) | Alpha Terpineol (Literature) (mg/mL) [59] |
|---|---|---|---|---|
| B. cereus (MIC) | 0.250 | 0.031 | 5 | N/A |
| B. cereus (MBC) | 0.250 | N/A | 5 | N/A |
| S. aureus (MIC) | 0.250 | 0.62 | 5 | 0.747 |
| S. aureus (MBC) | 1.000 | 5 | 5 | 1.868 |
| E. coli (MIC) | 0.500 | 1.25 | 2.5 | 0.747 |
| E. coli (MBC) | 0.500 | 5 | 2.5 | 1.868 |
| P. aeruginosa (MIC) | 0.667 | 0.31 | >5 | N/A |
| P. aeruginosa (MBC) | 0.500 | 2.5 | >5 | N/A |
| C. albicans (MIC) | 0.250 | 0.08 | 2.5 | N/A |
| C. albicans (MFC) | 0.250 | 0.31 | 2.5 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aborah, M.; Scarano, F.; Neto, C. Gas Chromatography/Mass Spectrometry Chemical Profiling of Volatile Compounds from Cranberry Plant Byproducts as Potential Antibacterials, Antifungals, and Antioxidants. Molecules 2025, 30, 2047. https://doi.org/10.3390/molecules30092047
Aborah M, Scarano F, Neto C. Gas Chromatography/Mass Spectrometry Chemical Profiling of Volatile Compounds from Cranberry Plant Byproducts as Potential Antibacterials, Antifungals, and Antioxidants. Molecules. 2025; 30(9):2047. https://doi.org/10.3390/molecules30092047
Chicago/Turabian StyleAborah, Martin, Frank Scarano, and Catherine Neto. 2025. "Gas Chromatography/Mass Spectrometry Chemical Profiling of Volatile Compounds from Cranberry Plant Byproducts as Potential Antibacterials, Antifungals, and Antioxidants" Molecules 30, no. 9: 2047. https://doi.org/10.3390/molecules30092047
APA StyleAborah, M., Scarano, F., & Neto, C. (2025). Gas Chromatography/Mass Spectrometry Chemical Profiling of Volatile Compounds from Cranberry Plant Byproducts as Potential Antibacterials, Antifungals, and Antioxidants. Molecules, 30(9), 2047. https://doi.org/10.3390/molecules30092047







