A N, S-Containing Graphene Oxide Composite for the Adsorptive Removal of p-Nitrophenol from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of the Samples
2.1.1. Scanning Electron Microscopy (SEM)
2.1.2. FT-IR Spectroscopy
2.1.3. XPS
2.1.4. Nitrogen Adsorption–Desorption Isotherms
2.1.5. TGA
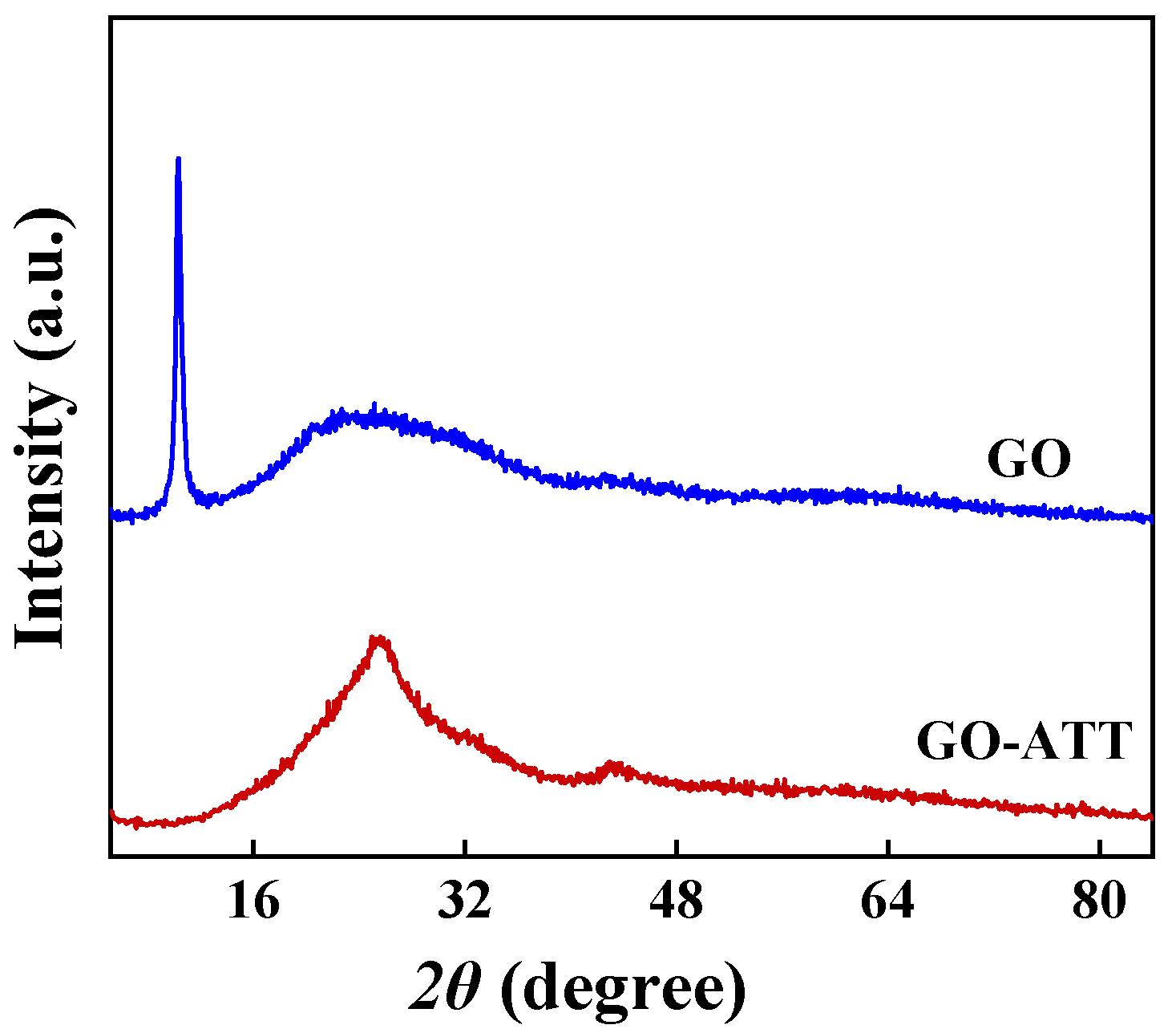
2.1.6. XRD
2.2. Process Optimization and Adsorption Modeling
2.2.1. Adsorption Performances
2.2.2. Effect of Adsorption Conditions
Effect of Contact Time
Effect of pH
Adsorption Kinetics
Adsorption Isotherms
Adsorption Thermodynamics
2.3. Reusability of GO-ATT
2.4. Adsorption Mechanism
3. Methodology
3.1. Preparation of GO-ATT Composite
3.2. Characterization of Materials
3.3. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Gu, P.; Wan, H.; Zhu, Y.; Li, N.; Chen, D.; Marcomini, A.; Xu, Q.; Lu, J. Preparation of new triptycene- and pentiptycene-based crosslinked polymers and their adsorption behavior towards aqueous dyes and phenolic organic pollutants. Sep. Purif. Technol. 2021, 278, 119495. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, C.; Duan, W.; Lang, D.; Pan, B. Coupling adsorption and degradation in p-nitrophenol removal by biochars. J. Clean. Prod. 2020, 271, 122550. [Google Scholar] [CrossRef]
- Bi, C.; Zhao, B.; Zheng, W.; Sun, M.; Kan, W.; Wang, L.; Sun, L.; Wang, X.; Zhao, M. Highly efficient adsorption and capture of prevalent phenolic contaminants from the real samples by trifluoromethyl-functionalized covalent organic frameworks. Sep. Purif. Technol. 2024, 339, 126631. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, E114–E122. [Google Scholar] [CrossRef]
- Serra, A.; Artal, R.; Pozo, M.; Garcia-Amoros, J.; Gomez, E. Simple Environmentally-Friendly Reduction of 4-Nitrophenol. Catalysts 2020, 10, 458. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: Adsorption behavior and mechanism. J. Hazard. Mater. 2021, 403, 123666. [Google Scholar] [CrossRef]
- Sas, O.G.; Sánchez, P.B.; González, B.; Domínguez, Á. Removal of phenolic pollutants from wastewater streams using ionic liquids. Sep. Purif. Technol. 2020, 236, 116310. [Google Scholar] [CrossRef]
- Cao, X.; Wang, K.; Feng, X. Removal of phenolic contaminants from water by pervaporation. J. Membr. Sci. 2021, 623, 119043. [Google Scholar] [CrossRef]
- Tang, W.; Chen, J.; Yin, Z.; Sheng, W.; Lin, F.; Xu, H.; Cao, S. Complete removal of phenolic contaminants from bismuth-modified TiO2 single-crystal photocatalysts. Chin. J. Catal. 2021, 42, 347–355. [Google Scholar] [CrossRef]
- Bahadi, S.A.; Drmosh, Q.A.; Onaizi, S.A. Adsorptive removal of organic pollutants from aqueous solutions using novel GO/bentonite/MgFeAl-LTH nanocomposite. Environ. Res. 2024, 248, 118218. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalination Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, D.; Koh, K.Y.; Chen, J.P. A new carbon nanotube modified by nano CaO2 for removal of chromate and phosphate from aqueous solutions. Chem. Eng. J. 2022, 446, 136845. [Google Scholar] [CrossRef]
- Lekshmi, R.; Rejiniemon, T.S.; Sathya, R.; Kuppusamy, P.; Al-mekhlafi, F.A.; Wadaan, M.A.; Rajendran, P. Adsorption of heavy metals from the aqueous solution using activated biomass from Ulva flexuosa. Chemosphere 2022, 306, 135479. [Google Scholar]
- Qu, Y.; Qin, L.; Liu, X.; Yang, Y. Magnetic Fe3O4/ZIF-8 composite as an effective and recyclable adsorbent for phenol adsorption from wastewater. Sep. Purif. Technol. 2022, 294, 121169. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Zhu, L.; Gao, F.; Xu, X.; Yang, J. Removal of p-Nitrophenol from simulated sewage using steel slag: Capability and mechanism. Environ. Res. 2022, 212, 113450. [Google Scholar] [CrossRef]
- Shao, L.; Wan, H.A.; Wang, L.; Wang, J.; Liu, Z.; Wu, Z.; Zhan, P.; Zhang, L.; Ma, X.; Huang, J. N-doped highly microporous carbon derived from the self-assembled lignin/chitosan composites beads for selective CO2 capture and efficient p-nitrophenol adsorption. Sep. Purif. Technol. 2023, 313, 123440. [Google Scholar] [CrossRef]
- Kordić, B.B.; Jović, B.D.; Kovačević, M.M.; Tričković, J.S. Influence of selected amides and adsorbent particle size on the adsorption of p-nitrophenol on granulated activated carbon. Environ. Technol. 2022, 43, 171–182. [Google Scholar] [CrossRef]
- Qin, Y.; Luo, J.; Zhao, Y.; Yao, C.; Li, Y.; An, Q.; Xiao, Z.; Zhai, S. Dual-wastes derived biochar with tailored surface features for highly efficient p-nitrophenol adsorption. J. Clean. Prod. 2022, 353, 131571. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Deng, H.; Qiao, N.; Zhang, D.; Lin, H.; Chen, Y. Modified graphene oxide composite aerogels for enhanced adsorption behavior to heavy metal ions. J. Environ. Chem. Eng. 2021, 9, 106008. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Y.; Xie, H.; Wang, B. Anaerobic reduction of graphene oxide induces the release of sorbed organic contaminants and enhances environmental risk. J. Hazard. Mater. 2024, 465, 133316. [Google Scholar] [CrossRef]
- Ortun, H.; Karapinar, N. Adsorption Performance of Cobalt, Manganese, and Iron Modified Graphene Oxide for Bromophenol Blue Removal from Water. Russ. J. Phys. Chem. A 2021, 95, S179–S188. [Google Scholar] [CrossRef]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Wakawa, Y.M.; Li, J.; Akbour, R.A.; Yap, P.-S.; Lau, S.Y.; Jeevanandam, J. Recent developments in the utilization of modified graphene oxide to adsorb dyes from water: A review. J. Ind. Eng. Chem. 2023, 117, 21–37. [Google Scholar] [CrossRef]
- Mohammadi, A.; Doctorsafaei, A.H.; Zia, K.M. Alginate/calix 4 arenes modified graphene oxide nanocomposite beads: Preparation, characterization, and dye adsorption studies. Int. J. Biol. Macromol. 2018, 120, 1353–1361. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Qureshi, R.B.; Muntha, S.T.; Farooq, M.; Siddiq, M. Facile synthesis of graphene oxide-silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manag. 2019, 230, 199–211. [Google Scholar] [CrossRef]
- Haydari, I.; Aziz, K.; Kaya, S.; Daştan, T.; Ouazzani, N.; Mandi, L.; Aziz, F. Green synthesis of reduced graphene oxide and their use on column adsorption of phenol from olive mill wastewater. Process Saf. Environ. Prot. 2023, 170, 1079–1091. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Polyethylene glycol functionalized reduced graphene oxide coupled with zinc oxide composite adsorbent for removal of phenolic wastewater. Environ. Res. 2022, 214, 114044. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Bibi, S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. New material of polyacrylic acid-modified graphene oxide composite for phenol remediation from synthetic and real wastewater. Environ. Technol. Innov. 2022, 27, 102795. [Google Scholar] [CrossRef]
- Tran, M.L.; Tran, T.T.V.; Juang, R.-S.; Nguyen, C.H. Graphene oxide crosslinked chitosan composites for enhanced adsorption of cationic dye from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2023, 142, 104678. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Peng, J.; Zhang, G. Selective adsorption of Ag+ by silica nanoparticles modified with 3-Amino-5-mercapto-1,2,4-triazole from aqueous solutions. J. Mol. Liq. 2017, 241, 292–300. [Google Scholar] [CrossRef]
- Miao, C.; Xun, X.; Dodd, L.J.; Niu, S.; Wang, H.; Yan, P.; Wang, X.-C.; Li, J.; Wu, X.; Hasell, T.; et al. Inverse vulcanization with SiO2-embedded elemental sulfur for superhydrophobic, anticorrosion, and antibacterial coatings. ACS Appl. Polym. Mater. 2022, 4, 4901–4911. [Google Scholar] [CrossRef]
- Ortiz, S.N.C.; Cabanzo, R.; Mejia-Ospino, E. Crude oil/water emulsion separation using graphene oxide and amine-modified graphene oxide particles. Fuel 2019, 240, 162–168. [Google Scholar] [CrossRef]
- Liao, J.; Yin, K.; Chen, X.; Huang, B. Defect-rich N doped porous carbon derived from Camellia shells for chlorobenzene adsorption. New J. Chem. 2024, 48, 10273–10283. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, J.; Song, H.; Yang, F.; Shen, L.; Zhu, L.; Zeng, Z.; Li, X.; Wang, G. Enhanced adsorption of dyes by functionalized UiO-66 nanoparticles: Adsorption properties and mechanisms. J. Mol. Struct. 2023, 1292, 136111. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Tao, Y. Insight into the fate of 8-hydroxyquinoline and o-aminophenol in the treatment of antibiotic production wastewater by adsorption method: Synergistic or competitive adsorption. J. Water Process Eng. 2024, 58, 104777. [Google Scholar] [CrossRef]
- Cui, Y.; Kang, W.; Hu, J. Effectiveness and mechanisms of the adsorption of phenol from wastewater onto N-doped graphene oxide aerogel. J. Water Process Eng. 2023, 53, 103665. [Google Scholar] [CrossRef]
- Guo, L.; Hao, L.; Gao, T.; Wang, C.; Wu, Q.; Wang, Z. p-Phenylenediamine-modified graphene oxide as a sorbent for solid-phase extraction of phenylurea herbicides, nitroimidazoles, chlorophenols, phenylurea insecticides and phthalates. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, W.; Liu, X.; Cao, M.; Gao, M.; Li, Y.; Yang, H. Self-propagating combustion synthesized magnetic cobalt carbohydrate-based adsorbents for tetracycline elimination. Process Saf. Environ. Prot. 2023, 175, 845–853. [Google Scholar] [CrossRef]
- Xu, A.; Gong, Y.; Sun, Q.; Li, L.; Wang, F.; Xiao, Z.; Liu, R. Recoverable cellulose composite adsorbents for anionic/cationic dyes removal. Int. J. Biol. Macromol. 2023, 238, 124022. [Google Scholar] [CrossRef]
- Yang, L.; Bao, L.; Dong, T.; Xie, H.; Wang, X.; Wang, H.; Wu, J.; Hao, C. Adsorption properties of cellulose/guar gum/biochar composite hydrogel for Cu2+, Co2+ and methylene blue. Int. J. Biol. Macromol. 2023, 242, 125021. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Esmaeili, A.; Entezari, M.H. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradiation. J. Colloid Interface Sci. 2014, 432, 19–25. [Google Scholar] [CrossRef]
- Dan, S.; Bagheri, H.; Shahidizadeh, A.; Hashemipour, H. Performance of graphene Oxide/SiO2 Nanocomposite-based: Antibacterial Activity, dye and heavy metal removal. Arab. J. Chem. 2023, 16, 104450. [Google Scholar] [CrossRef]
- Liyanage, C.D.; Kumar, H.; Perera, I.; Abeykoon, P.G.; Chen, F.; Joya, J.S.; Suib, S.L.; Adamson, D.H. Synthesis of graphene oxide: Effect of sonication during oxidation. Carbon 2024, 223, 119047. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Y.; Zhu, K.; Wang, Q.; Wakeel, M.; Wahid, A.; Alharbi, N.S.; Chen, C. Investigation of the adsorption mechanisms of Pb(II) and 1-naphthol by β-cyclodextrin modified graphene oxide nanosheets from aqueous solution. J. Colloid Interface Sci. 2018, 530, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Cao, V.D.; Nguyen, V.H.; Hoang, B.N.; Vo, D.-V.N.; Nguyen, T.D.; Bach, L.G. MIL-53 (Fe) derived magnetic porous carbon as a robust adsorbent for the removal of phenolic compounds under the optimized conditions. J. Environ. Chem. Eng. 2020, 8, 102902. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, H.; Lu, Y. Effective solid phase extraction for the enrichment of p-nitrophenol in water using microwave-assisted synthesized fly ash@ p-nitrophenol surface molecular imprinted polymer. J. Mater. Sci. 2023, 58, 4399–4415. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Sheng, J.; Xu, J.; Shi, Y.; Jiang, H. Adsorption and co-adsorption mechanisms of p-nitrophenol and Pb(II) on magnetic carbon aerogel in water. Environ. Sci. Water Res. Technol. 2022, 8, 820–835. [Google Scholar] [CrossRef]
- Nakhjiri, M.T.; Marandi, G.B.; Kurdtabar, M. Preparation of magnetic double network nanocomposite hydrogel for adsorption of phenol and p-nitrophenol from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105039. [Google Scholar] [CrossRef]
- Lang, D.; Shi, M.; Xu, X.; He, S.; Yang, C.; Wang, L.; Wu, R.; Wang, W.; Wang, J. DMAEMA-grafted cellulose as an imprinted adsorbent for the selective adsorption of 4-nitrophenol. Cellulose 2021, 28, 6481–6498. [Google Scholar] [CrossRef]
- Aaddouz, M.; Azzaoui, K.; Akartasse, N.; Mejdoubi, E.; Hammouti, B.; Taleb, M.; Sabbahi, R.; Alshahateet, S.F. Removal of methylene blue from aqueous solution by adsorption onto hydroxyapatite nanoparticles. J. Mol. Struct. 2023, 1288, 135807. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Ma, Z.; Gao, J.; Wu, Y. Synthesis of Ag-HKUST-1 composites and adsorption performance of neutral red dye. Inorg. Chem. Commun. 2024, 167, 112757. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, H.; Mei, Y.; Ye, L.; Zhuang, S.; Pu, N.; Wang, L. Performance and mechanism of sulfonamide-antibiotic adsorption by Ti3C2 MXene. New J. Chem. 2024, 48, 16742–16752. [Google Scholar] [CrossRef]
- Feng, K.; Hao, Z.; Zhao, W.; Wang, T.; Liu, X.; Zhai, N.; Wang, W. Convert waste to MOF: Nitro-MIL-53(Al) synthesized from waste acid leachate for highly efficient capture of p-nitrophenol. J. Environ. Chem. Eng. 2023, 11, 110239. [Google Scholar] [CrossRef]
- Rai, P.; Gautam, R.K.; Banerjee, S.; Rawat, V.; Chattopadhyaya, M.C. Synthesis and characterization of a novel SnFe2O4 @activated carbon magnetic nanocomposite and its effectiveness in the removal of crystal violet from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 2281–2291. [Google Scholar] [CrossRef]
- Bonetto, L.R.; Ferrarini, F.; de Marco, C.; Crespo, J.S.; Guegan, R.; Giovanela, M. Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J. Water Process Eng. 2015, 6, 11–20. [Google Scholar] [CrossRef]
- Xing, T.; Wu, Y.; Wang, Q.; Sadrnia, A.; Behmaneshfar, A.; Dragoi, E.N. Adsorption of ibuprofen using waste coffee derived carbon architecture: Experimental, kinetic modeling, statistical and bio-inspired optimization. Environ. Res. 2023, 231, 116223. [Google Scholar] [CrossRef]
- Azimi, E.B.; Badiei, A.; Ghasemi, J.B. Efficient removal of malachite green from wastewater by using boron-doped mesoporous carbon nitride. Appl. Surf. Sci. 2019, 469, 236–245. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Zhang, C.; Luan, J.; Yu, X.; Chen, W. Characterization and adsorption performance of graphene oxide—Montmorillonite nanocomposite for the simultaneous removal of Pb2+ and p-nitrophenol. J. Hazard. Mater. 2019, 378, 120739. [Google Scholar] [CrossRef]
- Mashhour, D.M.; Ibrahim, S.M.; Al-Hossainy, A.F.; El-Aal, M.A. Glycogen-assisted biosynthesis of MnO2 for adsorptive elimination of methylene blue from water. J. Mol. Struct. 2024, 1313, 138665. [Google Scholar] [CrossRef]
- Li, Y.; Wei, L.; Ou, C.; Wu, Q.; Liao, Z.; Zha, X. Loofah sponge immobilized ZIF-8 for efficient adsorption removal of U (VI). Inorg. Chem. Commun. 2024, 167, 112838. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Wu, T.; Sun, D.; Li, Y. Adsorption of p-nitrophenol from aqueous solutions using nanographite oxide. Colloids Surf. A Physicochem. Eng. Asp. 2015, 464, 78–88. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chowd, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.-H.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar] [CrossRef]















| Samples | GO-ATT | GO-ATT-PNP | |
|---|---|---|---|
| Binding Energy (eV) | |||
| C | C=C/C-C | 284.80 | 284.80 |
| C-S/C-N | 285.78 | 285.55 | |
| C-O | 287.25 | 286.68 | |
| C=O | 288.73 | 288.27 | |
| O | C-O | 533.59 | 533.79 |
| C=O | 531.47 | 531.01 | |
| -NO2 | - | 532.48 | |
| N | C=N | 399.28 | 399.36 |
| C-N | 400.67 | 400.72 | |
| -NO2 | - | 405.59 | |
| S | C-S (S 2p3/2) | 164.19 | 164.16 |
| C-S (S 2p1/2) | 165.29 | 165.31 | |
| Adsorbent | Adsorbent Dosage | C0 (mg L−1) | qmax (mg g−1) | Ref. |
|---|---|---|---|---|
| CGA | 0.015 g/0.015 L | 5000 | 46.8 | [17] |
| MPC | 0.75 g/L | 26 | 31.15 | [45] |
| FA@PNP-SMIP | 0.01 g/0.05 L | 160 | 102.5 | [46] |
| Steel slag | 0.02 g/0.025 L | 100 | 109.66 | [15] |
| 10% Fe-MCA | 0.002 g/0.02 L | 250 | 141 | [47] |
| DNNH | 0.6 g/L | 15 | 22.10 | [48] |
| CD-MIP | 0.01 g/0.025 L | 50 | 26.87 | [49] |
| GO-ATT | 0.005 g/0.02 L | 50 | 40 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Shi, T.-T.; Hu, W.-G.; Gao, G.-J.; Liu, Y.-P.; Yu, J.-G. A N, S-Containing Graphene Oxide Composite for the Adsorptive Removal of p-Nitrophenol from Aqueous Solutions. Molecules 2025, 30, 2046. https://doi.org/10.3390/molecules30092046
Yang B, Shi T-T, Hu W-G, Gao G-J, Liu Y-P, Yu J-G. A N, S-Containing Graphene Oxide Composite for the Adsorptive Removal of p-Nitrophenol from Aqueous Solutions. Molecules. 2025; 30(9):2046. https://doi.org/10.3390/molecules30092046
Chicago/Turabian StyleYang, Bi, Tao-Tao Shi, Wei-Guo Hu, Guan-Jin Gao, Yi-Ping Liu, and Jin-Gang Yu. 2025. "A N, S-Containing Graphene Oxide Composite for the Adsorptive Removal of p-Nitrophenol from Aqueous Solutions" Molecules 30, no. 9: 2046. https://doi.org/10.3390/molecules30092046
APA StyleYang, B., Shi, T.-T., Hu, W.-G., Gao, G.-J., Liu, Y.-P., & Yu, J.-G. (2025). A N, S-Containing Graphene Oxide Composite for the Adsorptive Removal of p-Nitrophenol from Aqueous Solutions. Molecules, 30(9), 2046. https://doi.org/10.3390/molecules30092046







