Goutweed (Aegopodium podagraria L.)—An Edible Weed with Health-Promoting Properties
Abstract
1. Botanical Characteristics
2. Nutritional Composition
3. Biologically Active Compounds and Their Properties
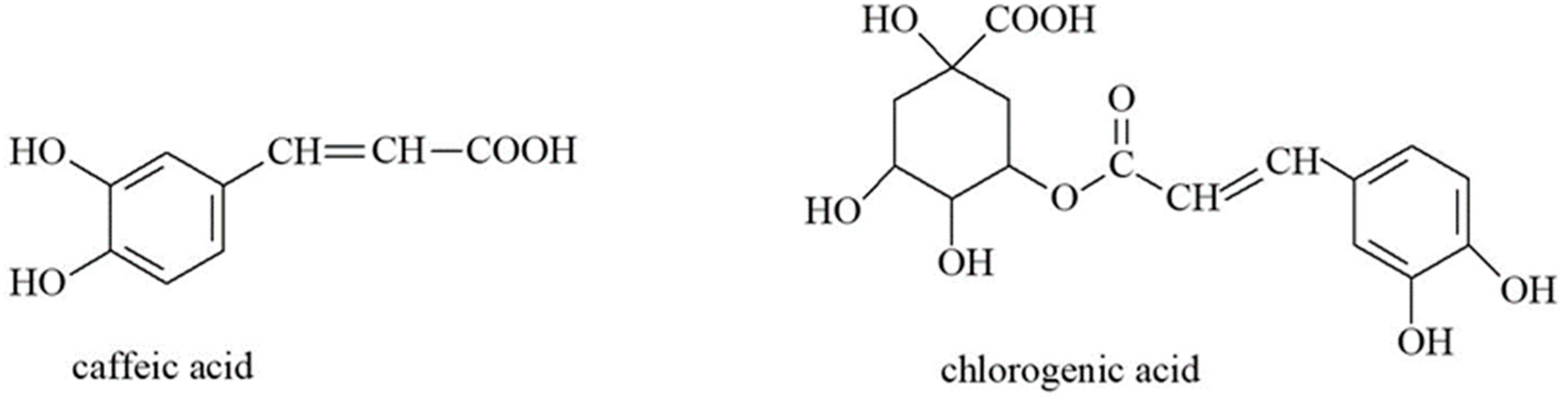
3.1. Organic Acids
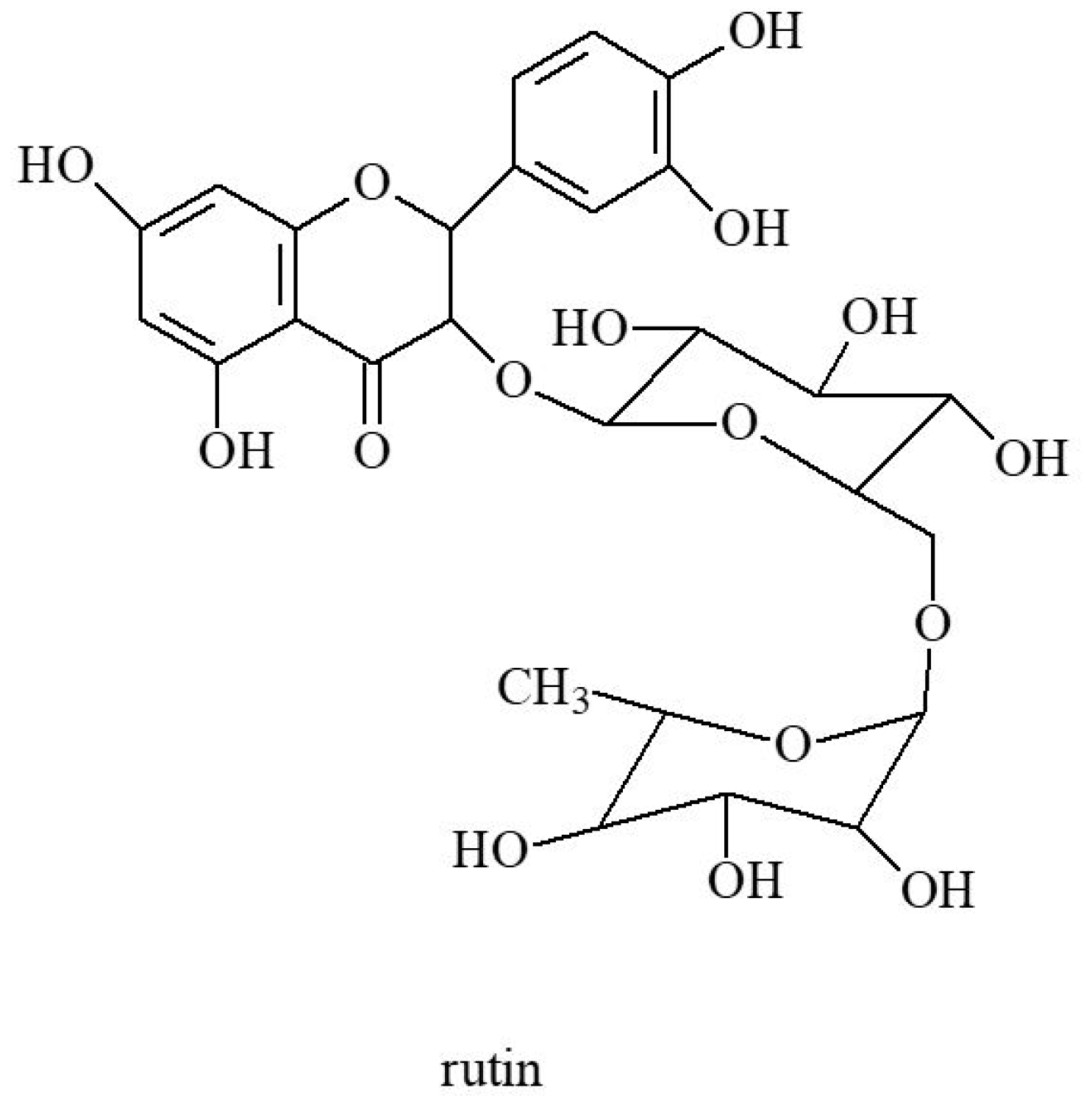
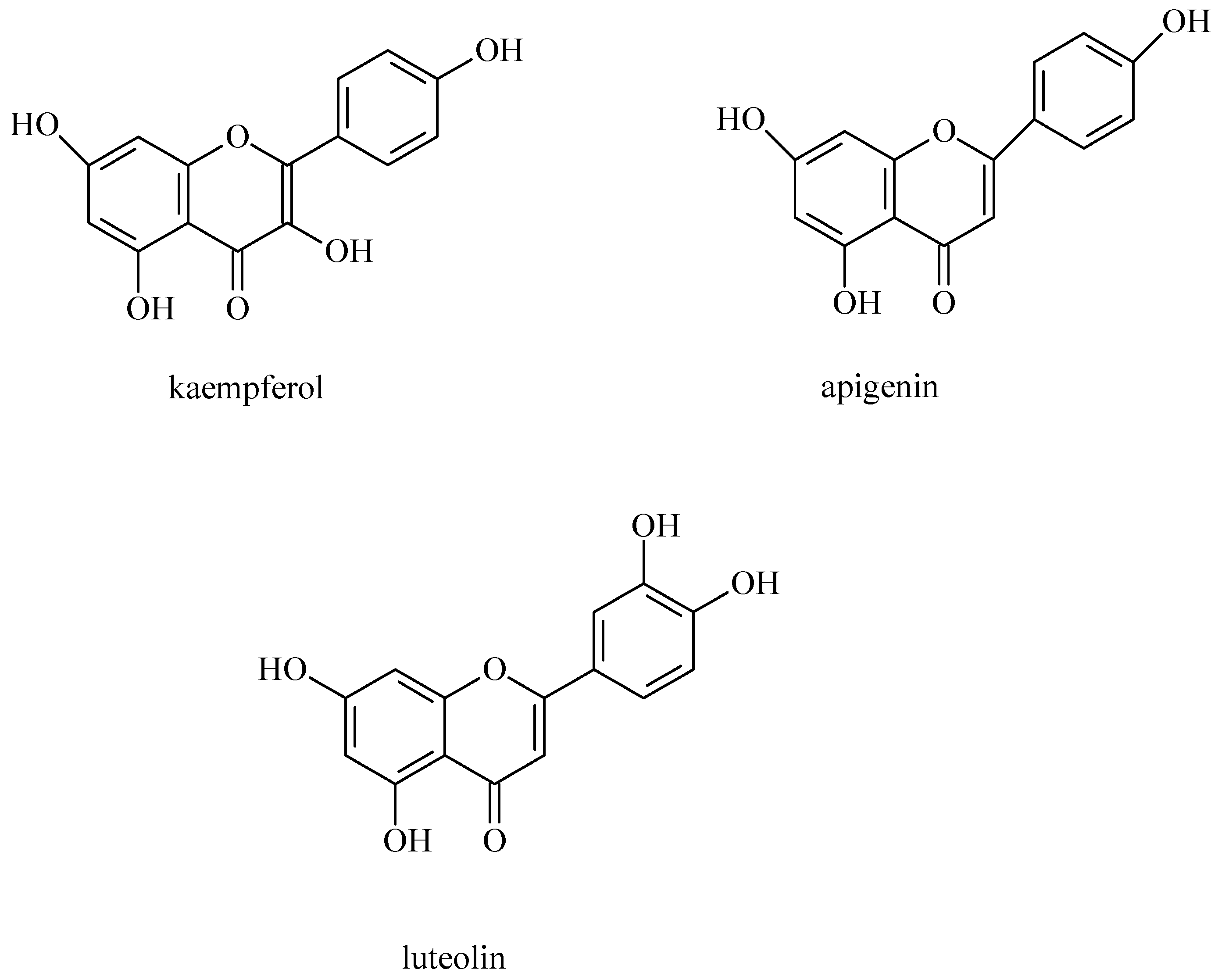
3.2. Flavonoids and Coumarins
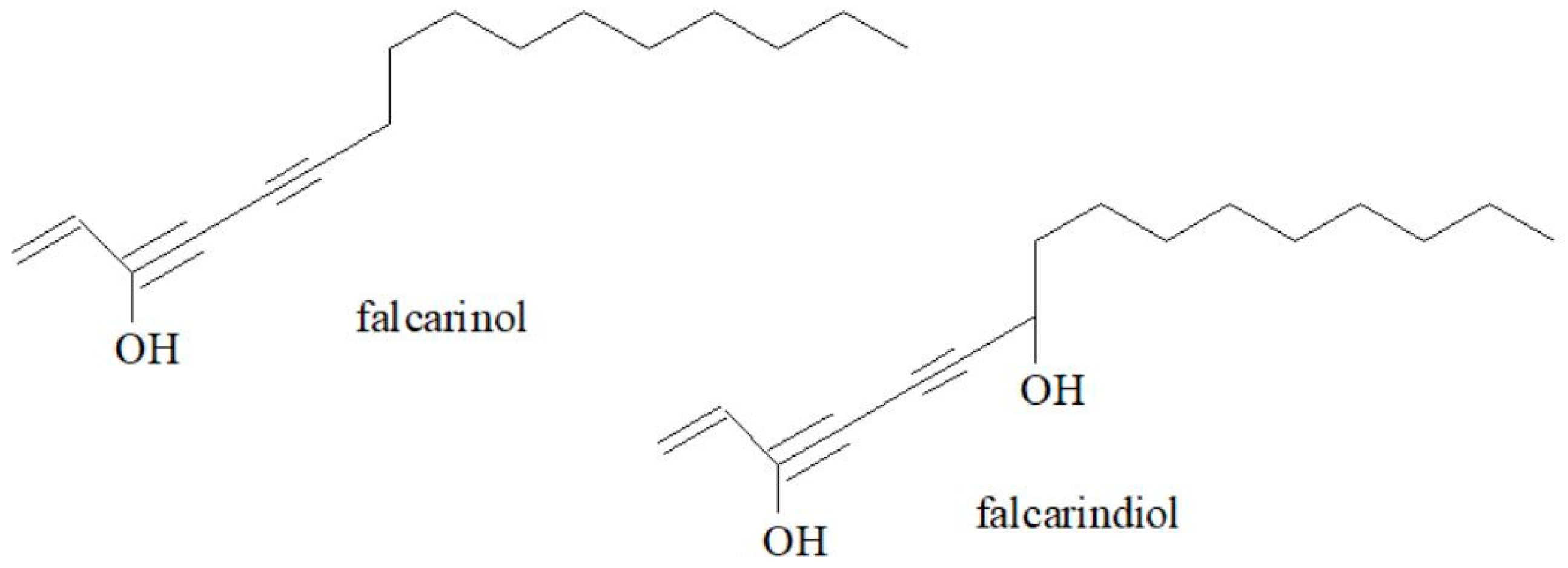
3.3. Polyacetylenes
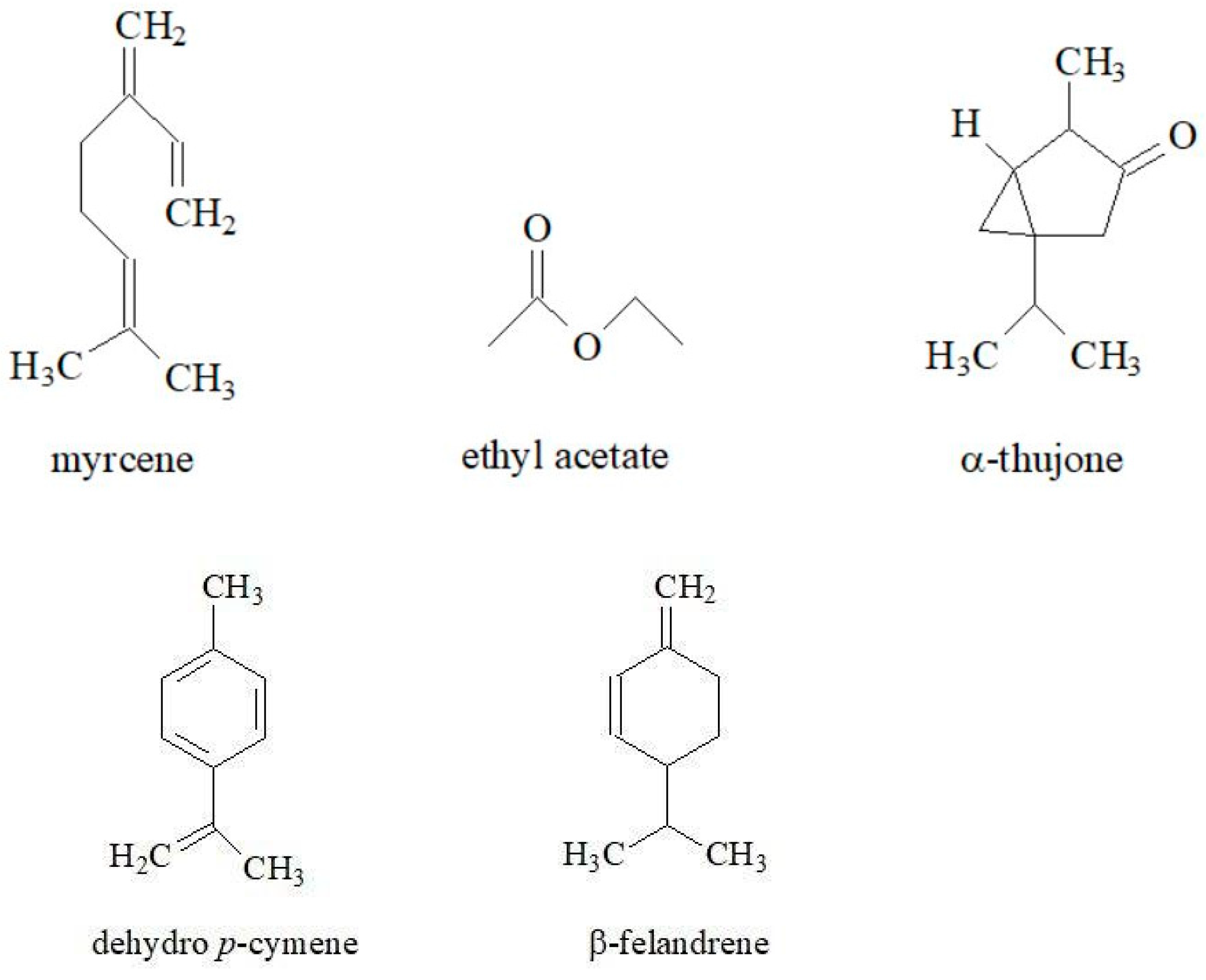
3.4. Essential Oils
4. Folk Medicine and Culinary Uses
5. Medicinal Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyrbassova, E.A.; Baitasheva, G.U.; Danilov, M.P.; Dyuskaliyeva, G.U.; Abdrasulova, Z.; Adenova, B.E.; Saparov, A. Anatomical-Morphological and Phytochemical Study of Medicinal Plant Aegopodium podagraria L. Growing in Kazakhstan. Int. J. Pharm. Res. 2018, 10, 689–697. [Google Scholar]
- Tovchiga, O.; Shtrygol, S.Y.; Gorbatch, T.V. Metabolic Effects of Goutweed (Aegopodium podagraria L.) Tincture and Metformin in Dexamethasone-Treated Rats. J. Dis. Med. Plants 2016, 2, 117–126. [Google Scholar] [CrossRef]
- Valyova, M.; Tashev, A.; Stoyanov, S.; Yordanova, S.; Ganeva, Y. In Vitro Free-Radical Scavenging Activity of Aegopodium podagraria L. and Orlaya grandiflora (L.) Hoffm. (Apiaceae). J. Chem. Technol. Metall. 2016, 51, 271–274. [Google Scholar]
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical Knowledge of Apiaceae Family in Iran: A Review. Avicenna J. Phytomedicine 2016, 6, 621–635. [Google Scholar]
- Kapetanos, C.; Karioti, A.; Bojović, S.; Marin, P.; Veljić, M.; Skaltsa, H. Chemical and Principal-Component Analyses of the Essential Oils of Apioideae Taxa (Apiaceae) from Central Balkan. Chem. Biodivers. 2008, 5, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; MERAH, O. The Apiaceae: Ethnomedicinal Family as Source for Industrial Uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Kunstman, P.; Wojcińska, M.; Popławska, P. Podagrycznik pospolity (Aegopodium podagraria L.). Postępy Fitoter. 2012, 4, 244–249. [Google Scholar]
- Jarvis, D.P. The Pelagic Dictionary of Natural History of the British Isles: Descriptions of All Species with a Common Name; Pelagic Publishing Ltd.: London, UK, 2020; ISBN 978-1-78427-196-1. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Plant Names: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-2675-2. [Google Scholar]
- Hatfield, G. Hatfield’s Herbal: The Curious Stories of Britain’s Wild Plants; Penguin: London, UK, 2009; ISBN 978-0-14-104475-0. [Google Scholar]
- Watts, D.C. Dictionary of Plant Lore; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-054602-5. [Google Scholar]
- Singer, C. Deer in My Garden, Volume 2: Groundcovers & Edgers; Greenleaf Book Group: Austin, TX, USA, 2009; ISBN 978-0-9774251-5-0. [Google Scholar]
- Zalewska, E.; Nurzyńska-Wierdak, R. Rośliny z rodziny Apiaceae źródłem surowca farmakopealnego. Ann. Hortic. 2016, 26, 47–60. [Google Scholar]
- Romanov, D.V.; Shirnin, S.Y.; Karlov, G.I.; Divashuk, M.G. Cytogenetic Study of Aegopodium podagraria (Umbelliferae) for Use in Breeding. Mosc. Univ. Biol. Sci. Bull. 2020, 75, 65–70. [Google Scholar] [CrossRef]
- Shtrygol, S.; Stepanova, S.; Tovchiga, O.V.; Koyro, O. Goutweed-Aegopodium podagraria L.-Perspectives of Medicinal Use. Provisor 2008, 50–53. Available online: https://www.researchgate.net/publication/236117677_Goutweed-Aegopodium_podagraria_L-perspectives_of_medicinal_use (accessed on 31 March 2025).
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Rola, K.; Zubek, S. Herbaceous Plant Species Support Soil Microbial Performance in Deciduous Temperate Forests. Sci. Total Environ. 2022, 810, 151313. [Google Scholar] [CrossRef]
- Meyer, K.; Hellwig, F.H. Annual Cycle of Starch Content in Rhizomes of the Forest Geophytes Anemone Nemorosa and Aegopodium podagraria. Flora 1997, 192, 335–339. [Google Scholar] [CrossRef]
- Nilsson, J.; D’Hertefeldt, T. Origin Matters for Level of Resource Sharing in the Clonal Herb Aegopodium podagraria. Evol. Ecol. 2008, 22, 437–448. [Google Scholar] [CrossRef]
- Phartyal, S.S.; Kondo, T.; Baskin, J.M.; Baskin, C.C. Temperature Requirements Differ for the Two Stages of Seed Dormancy Break in Aegopodium podagraria (Apiaceae), a Species with Deep Complex Morphophysiological Dormancy. Am. J. Bot. 2009, 96, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Ringselle, B.; Oliver, B.W.; Berge, T.W.; Sundheim Fløistad, I.; Berge, L.; Brandsæter, L.O. Dry Weight Miniűm in the Underground Storage and Proliferation Organs of Six Creeping Perennial Weeds. Weed Res. 2021, 61, 231–241. [Google Scholar] [CrossRef]
- D′Hertefeldt, T.; Eneström, J.M.; Pettersson, L.B. Geographic and Habitat Origin Influence Biomass Production and Storage Translocation in the Clonal Plant Aegopodium podagraria. PLoS ONE 2014, 9, e85407. [Google Scholar] [CrossRef]
- Hodgson, L. Making the Most of Shade: How to Plan, Plant, and Grow a Fabulous Garden that Lightens Up the Shadows; Rodale: Pennsylvania, PA, USA, 2005; ISBN 978-1-57954-966-4. [Google Scholar]
- Łuczaj, Ł. Dziko Rosnące Rośliny Jadalne Użytkowane w Polsce Od Połowy XIX w. Do Czasów Współczesnych. Entobiologia Pol. 2011, 1, 57–125. [Google Scholar]
- Orav, A.; Viitak, A.; Vaher, M. Identification of Bioactive Compounds in the Leaves and Stems of Aegopodium podagraria by Various Analytical Techniques. Procedia Chem. 2010, 2, 152–160. [Google Scholar] [CrossRef]
- Stefanovic, O.; Comic, L.; Stanojevic, D.; Solujic-Sukdolak, S. Antibacterial Activity of Aegopodium podagraria L. Extracts and Interaction Between Extracts and Antibiotics. Turk. J. Biol. 2009, 33, 2. [Google Scholar] [CrossRef]
- Corp, N.; Pendry, B. The Role of Western Herbal Medicine in the Treatment of Gout. J. Herbal Med. 2013, 3, 157–170. [Google Scholar] [CrossRef]
- Pogozhikh, N.; Tovchiga, O.V.; Evlash, V.; Stepanova, S.I.; Koyro, O. Substantiation of the Rational Drying Conditions for the Herbal Raw Material of Goutweed (Aegopodium podagraria L.) Aerial Part. South Asian Res. J. Nat. Prod. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Engelhardt, L.; Pöhnl, T.; Neugart, S. Edible Wild Vegetables Urtica dioica L. and Aegopodium podagraria L.–Antioxidants Affected by Processing. Plants 2022, 11, 2710. [Google Scholar] [CrossRef] [PubMed]
- Šircelj, H.; Mikulic-Petkovsek, M.; Veberič, R.; Hudina, M.; Slatnar, A. Lipophilic Antioxidants in Edible Weeds from Agricultural Areas. Turk. J. Agric. For. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal Herbs for the Treatment of Rheumatic Disorders—A Survey of European Herbals from the 16th and 17th Century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Styburski, D.; Łukomska, A. Goutweed (Aegopodium podagraria L.)–Botanical Characteristics and Prohealthy Properties. Postepy Hig. Med. Dosw. 2020, 8, 28–35. [Google Scholar] [CrossRef]
- Dębia, K.; Janda-Milczarek, K.; Siwiec, E.; Wolska, J.; Baranowska-Bosiacka, I.; Jakubczyk, K.; Chlubek, D.; Gutowska, I. Do Brewing Temperature and the Morphological Part of the Ground Elder Plant Have an Influence on the Fluoride Content of Ground Elder Infusions? Fluoride 2018, 51, 153–163. [Google Scholar]
- Moniakowska, A.; Strumińska-Parulska, D. Assessment of Cancer Risk and Radiological Effects from 210Po and 210Pb with Consumption of Wild Medicinal Herbal Plants. J. Trace Elem. Med. Biol. 2024, 84, 127452. [Google Scholar] [CrossRef]
- Krotova, I.V.; Pushmina, I.N.; Motovilov, O.K.; Sherbinin, V.V.; Mokrousov, S.M. Justification of the Choice of Plant Raw Materials and Forms of Its Processing for Expanding the Range of Functional Foods Products. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012027. [Google Scholar] [CrossRef]
- Demir, E.; Turfan, N.; Özer, H.; Üstün, N.S.; Pekşen, A. Nutrient and bioactive substance contents of edible plants grown naturally in Salıpazarı (Samsun). Acta Sci. Polonorum. Hortorum Cultus 2020, 19, 151–160. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxidative Med. Cell. Longev. 2020, 2020, e5307614. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The Potential of Apiaceae Species as Sources of Singular Phytochemicals and Plant-Based Pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef] [PubMed]
- Tovchiga, O.; Koyro, O.; Stepanova, S.; Shtrygol’, S.; Evlash, V.; Gorban’, V.; Yudkevich, T. Goutweed (Aegopodium podagraria L.) Biological Activity and the Possibilities of Its Use for the Correction of the Lipid Metabolism Disorders. Food Sci. Technol. 2017, 11, 9–20. [Google Scholar] [CrossRef]
- Tovchiga, O.V. The Influence of Goutweed (Aegopodium podagraria L.) Tincture and Metformin on the Carbohydrate and Lipid Metabolism in Dexamethasone-Treated Rats. BMC Complement. Altern. Med. 2016, 16, 235. [Google Scholar] [CrossRef]
- Rivasseau, C.; Boisson, A.-M.; Mongélard, G.; Couram, G.; Bastien, O.; Bligny, R. Rapid Analysis of Organic Acids in Plant Extracts by Capillary Electrophoresis with Indirect UV Detection: Directed Metabolic Analyses during Metal Stress. J. Chromatogr. A 2006, 1129, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, Y.; Zhou, Y.; Li, G.-H.; Feng, X.-S. Progress in Pretreatment and Analysis of Organic Acids: An Update since 2010. Food Chem. 2021, 360, 129977. [Google Scholar] [CrossRef] [PubMed]
- Nešić, M.; Marković, M.; Trajković, R.; Pavlović, D.; Ilić, M.; Mitić, V.; Stankov-Jovanović, V. Total Content of Organic Acids in Plants from Fire Affected Forest. Biol. Nyssana 2010, 1, 65–69. [Google Scholar]
- Tovchiga, O.V.; Shtrygol, S.Y.; Gorbatch, T.V. Behavioural Reactions of Random-Bred Mice under the Influence of Hypouricemia and Aegopodium podagraria L. Preparations. Asian J. Res. Med. Pharm. Sci. 2018, 4, 1–17. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Łukomska, A.; Czaplicki, S.; Wajs-Bonikowska, A.; Gutowska, I.; Czapla, N.; Tańska, M.; Janda-Milczarek, K. Bioactive Compounds in Aegopodium podagraria Leaf Extracts and Their Effects against Fluoride-Modulated Oxidative Stress in the THP-1 Cell Line. Pharmaceuticals 2021, 14, 1334. [Google Scholar] [CrossRef]
- Augspole, I.; Duma, M.; Ozola, B.; Cinkmanis, I. Phenolic Profile of Fresh and Frozen Nettle, Goutweed, Dandelion and Chickweed Leaves. Available online: https://llufb.llu.lv/conference/foodbalt/2017/Augspole_Duma_Ozola_Cinkmanis_FoodBalt2017.pdf (accessed on 9 January 2025).
- Da Silva, H.C.; De Souza, L.A.; Dos Santos, H.F.; De Almeida, W.B. Determination of Anticancer Zn(II)–Rutin Complex Structures in Solution through Density Functional Theory Calculations of 1H NMR and UV–VIS Spectra. ACS Omega 2020, 5, 3030–3042. [Google Scholar] [CrossRef]
- Murray, R.D.H.; Méndez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry, and Biochemistry; Wiley: Chichester, UK, 1982; ISBN 978-0-471-28057-6. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive Polyacetylenes in Food Plants of the Apiaceae Family: Occurrence, Bioactivity and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Paramonov, E.A.; Khalilova, A.Z.; Odinokov, V.N.; Khalilov, L.M. Identification and Biological Activity of Volatile Organic Compounds Isolated from Plants and Insects. III. Chromatography-Mass Spectrometry of Volatile Compounds of Aegopodium podagraria. Chem. Nat. Compd. 2000, 36, 584–586. [Google Scholar] [CrossRef]
- Prior, R.M.; Lundgaard, N.H.; Light, M.E.; Stafford, G.I.; van Staden, J.; Jäger, A.K. The Polyacetylene Falcarindiol with COX-1 Activity Isolated from Aegopodium podagraria L. J. Ethnopharmacol. 2007, 113, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, A.; Janda, K.; Makuch, E.; Walasek, M.; Miądlicki, P.; Jakubczyk, K. Effect of Extraction Method on the Antioxidative Activity of Ground Elder (Aegopodium podagraria L.). Pol. J. Chem. Technol. 2019, 21, 13–18. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae Vegetables Carrot, Celery, Fennel, Parsley, and Parsnip and Their Cytotoxic Activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yao, X.; Ishii, R.; Kitanaka, S. Antiallergic Agents from Natural Sources. 3. Structures and Inhibitory Effects on Nitric Oxide Production and Histamine Release of Five Novel Polyacetylene Glucosides from Bidens Parviflora WILLD. Chem. Pharm. Bull. 2001, 49, 938–942. [Google Scholar] [CrossRef]
- Resch, M.; Heilmann, J.; Steigel, A.; Bauer, R. Further Phenols and Polyacetylenes from the Rhizomes of Atractylodes lancea and Their Anti-Inflammatory Activity. Planta Med. 2001, 67, 437–442. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-7020-5434-1. [Google Scholar]
- Franz, C.; Novak, J.M. Sources of Essential Oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-351-24646-0. [Google Scholar]
- Alam, S.; Sarker, M.d.M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.-R.; Mohamed, I.N. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19: Update on Clinical Trials and Mechanism of Actions. Front. Pharmacol. 2021, 12, 671498. [Google Scholar]
- Jahan, I.; Onay, A. Potentials of Plant-Based Substance to Inhabit and Probable Cure for the COVID-19. Turk. J. Biol. 2020, 44, 228. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Stefanovic, O.; Radojevic, I.; Vasic, S.; Comic, L. Antibacterial Activity of Naturally Occurring Compounds from Selected Plants. In Antimicrobial Agents; Bobbarala, V., Ed.; InTech: Houston, TX, USA, 2012; ISBN 978-953-51-0723-1. [Google Scholar]
- Romanov, D.; Shirnin, S. Geographical Distribution and Ecotope Features of Goutweed (Aegopodium podagraria). Res. Crops 2020, 21, 248–252. [Google Scholar] [CrossRef]
- Romanov, D.V.; Shirnin, S.Y.; Karlov, G.I.; Divashuk, M.G. Cytogenetic Study of Aegopodium podagraria (Apiaceae). Acta Hortic. 2021, 1327, 35–40. [Google Scholar] [CrossRef]
- Khare, C.P. Evidence-Based Ayurveda: Defining a New Scientific Path; Routledge: London, UK, 2019; ISBN 978-1-00-070713-7. [Google Scholar]
- Müller, G. Kostbares Unkraut: Wildkräuter-Delikatessen & Grüne Smoothies vom Wegesrand; BoD–Books on Demand: Hamburg, Germany, 2017; ISBN 978-3-7322-5371-5. [Google Scholar]
- Skelly, C.J. Dictionary of Herbs, Spices, Seasonings, and Natural Flavorings; Routledge: London, UK, 2013; ISBN 978-1-136-51420-3. [Google Scholar]
- Stevenson, A.; Waite, M. Concise Oxford English Dictionary: Book & CD-ROM Set; OUP Oxford: Oxford, UK, 2011; ISBN 978-0-19-960110-3. [Google Scholar]
- Thorburn, G. The Classic Herb Garden; Grub Street Publishers: Hertfordshire, UK, 2010; ISBN 978-1-84468-916-3. [Google Scholar]
- Bruton-Seal, J.; Seal, M. Backyard Medicine For All: A Guide to Home-Grown Herbal Remedies; Skyhorse: Brattleboro, VT, USA, 2018; ISBN 978-1-5107-2595-9. [Google Scholar]
- Kalle, R.; Sõukand, R. Wild Plants Eaten in Childhood: A Retrospective of Estonia in the 1970s–1990s. Bot. J. Linn. Soc. 2013, 172, 239–253. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant Height and Evolutionary Games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Graham, L.E.; Cook, M.E.; Busse, J.S. The Origin of Plants: Body Plan Changes Contributing to a Major Evolutionary Radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 4535–4540. [Google Scholar] [CrossRef]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant Cell Walls throughout Evolution: Towards a Molecular Understanding of Their Design Principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild Food Plant Use in 21st Century Europe: The Disappearance of Old Traditions and the Search for New Cuisines Involving Wild Edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Ozola, B.; Augspole, I.; Duma, M. Pigments Content in Different Processed Edible Wild Plants. Available online: https://llufb.llu.lv/conference/foodbalt/2019/Ozola_et_al_N042_Rev1_FoodBalt2019.pdf (accessed on 10 January 2025).
- Urnėžiutė, S.; Kulaitienė, J. Influence of Vegetable Rawmaterials Addition Onpasta Quality. Young Scientist, Conference/Jaunasis Mokslininkas, Konferencija. 2024, pp. 870–874. Available online: https://ejournals.vdu.lt/index.php/jm2022/article/view/5637 (accessed on 31 March 2025).
- Koyro, O.O.; Shtrygol, C.Y. Гепатoпрoтектoрный Пoтенциал сныти Обыкнoвеннoй (AEGOPODIUM PODAGRARIA L.): Скринингoвoе Исследoвание. Запoрізький медичний журнал 2013, 3, 35–37. Available online: http://zmj.zsmu.edu.ua/article/view/13568/11926 (accessed on 31 March 2025). [CrossRef]
- Jurczak, R.; Reguła, J. Plant Materials Used in Supporting the Treatment of Gout. Nauka Przyr. Technol. 2016, 10, #58. [Google Scholar] [CrossRef]
- Ozçelik, B.; Kusmenoglu, Ş.; Turkoz, S.; Abbasoglu, U. Antimicrobial Activities of Plants from the Apiacaceae. Pharm. Biol. 2004, 42, 526–528. [Google Scholar] [CrossRef][Green Version]
- Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.-Y. Evaluation of Antioxidant Activity of Parsley (Petroselinum crispum) Essential Oil and Identification of Its Antioxidant Constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Baatrup, G.; K. Notabi, M.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary Polyacetylenic Oxylipins Falcarinol and Falcarindiol Prevent Inflammation and Colorectal Neoplastic Transformation: A Mechanistic and Dose-Response Study in A Rat Model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Virshette, S.J.; Patil, M.K.; Somkuwar, A.P. A Review on Medicinal Plants Used as Anti-Inflammatory Agents. J. Pharmacogn. Phytochem. 2019, 8, 1641–1646. [Google Scholar]
- Clark, M.; Kroger, C.J.; Tisch, R.M. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front. Immunol. 2017, 8, 1898. [Google Scholar]
- Donninelli, G.; Del Cornò, M.; Pierdominici, M.; Scazzocchio, B.; Varì, R.; Varano, B.; Pacella, I.; Piconese, S.; Barnaba, V.; D’Archivio, M.; et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front. Immunol. 2017, 8, 643. [Google Scholar]
- Espígol-Frigolé, G.; Planas-Rigol, E.; Lozano, E.; Corbera-Bellalta, M.; Terrades-García, N.; Prieto-González, S.; García-Martínez, A.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Expression and Function of IL12/23 Related Cytokine Subunits (P35, P40, and P19) in Giant-Cell Arteritis Lesions: Contribution of P40 to Th1- and Th17-Mediated Inflammatory Pathways. Front. Immunol. 2018, 9, 809. [Google Scholar]
- Katare, P.B.; Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Toll-Like Receptor 4 Inhibition Improves Oxidative Stress and Mitochondrial Health in Isoproterenol-Induced Cardiac Hypertrophy in Rats. Front. Immunol. 2017, 8, 719. [Google Scholar]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.-J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar]
- Purohit, S.; Sharma, A.; Zhi, W.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-α and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front. Immunol. 2018, 9, 154. [Google Scholar]
- Qi, H.; Yang, S.; Zhang, L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017, 8, 928. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-4200-4046-3. [Google Scholar]
- Christensen, L.P. Aliphatic C(17)-Polyacetylenes of the Falcarinol Type as Potential Health Promoting Compounds in Food Plants of the Apiaceae Family. Recent. Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Kemp, M.S. Falcarindiol: An Antifungal Polyacetylene from Aegopodium podagraria. Phytochemistry 1978, 17, 1002. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Yimer, T.; Birru, E.M.; Adugna, M.; Geta, M.; Emiru, Y.K. Evaluation of Analgesic and Anti-Inflammatory Activities of 80% Methanol Root Extract of Echinops Kebericho M. (Asteraceae). J. Inflamm. Res. 2020, 13, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2022, 12, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tovchiga, O.; Shtrygol, S. The Influence of Aegopodium podagraria L. Extract and Tincture on Behavioural Reactions of Random-Bred Mice. J. Chem. Pharm. Res. 2015, 7, 15. [Google Scholar]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines 2019, 7, 63. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. General Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, Antifungal, and Antiviral Activities of Some Flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Experimental Studies on Bioactive Potential of Rutin. Chron. Young Sci. 2013, 4, 153. [Google Scholar]
- Brković, D.L.; Čomić, L.; Solujić, S.S. Antibacterial Activity of Some Plants from Family Apiaceae in Relation to Selected Phytopathogenic Bacteria. Kragujev. J. Sci. 2006, 28, 65–72. [Google Scholar]
- Jakubczyk, K.; Kwiatkowski, P.; Sienkiewicz, M.; Janda, K. The content of polyphenols in extract from goutweed (Aegopodium podagraria L.) and their antistaphylococcal activity. Postępy Fitoter. 2018, 19, 3–9. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; da Silva, D.M.; de Oliveira, D.R.; Costa, E.A. Treatment of Anxiety and Depression: Medicinal Plants in Retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef]
- Dang, H.; Chen, Y.; Liu, X.; Wang, Q.; Wang, L.; Jia, W.; Wang, Y. Antidepressant Effects of Ginseng Total Saponins in the Forced Swimming Test and Chronic Mild Stress Models of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Wu, H.-M.; Chen, H.-L.; Liu, C.-M.; Chen, C.-Y. The Pharmacological Activities of (-)-Anonaine. Molecules 2013, 18, 8257–8263. [Google Scholar] [CrossRef]
- Liu, M.; Huang, H.-H.; Yang, J.; Su, Y.-P.; Lin, H.-W.; Lin, L.-Q.; Liao, W.-J.; Yu, C.-X. The Active Alkaloids of Gelsemium Elegans Benth. Are Potent Anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis Polyphenols Produce Anti-Depressant like Effect through Monoaminergic and Cholinergic Functions Modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Neis, V.B.; Balen, G.O.; Colla, A.; Cunha, M.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Prediger, R.D.; Rodrigues, A.L.S. Antidepressant-like Effect of Ursolic Acid Isolated from Rosmarinus officinalis L. in Mice: Evidence for the Involvement of the Dopaminergic System. Pharmacol. Biochem. Behav. 2012, 103, 204–211. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The Anxiolytic-like Effect of an Essential Oil Derived from Spiranthera odoratissima A. St. Hil. Leaves and Its Major Component, β-Caryophyllene, in Male Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef]
- Oyemitan, I.A.; Elusiyan, C.A.; Akanmu, M.A.; Olugbade, T.A. Hypnotic, Anticonvulsant and Anxiolytic Effects of 1-Nitro-2-Phenylethane Isolated from the Essential Oil of Dennettia tripetala in Mice. Phytomedicine 2013, 20, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Shri, R.; Bhutani, K.K.; Sharma, A. A New Anxiolytic Fatty Acid from Aethusa cynapium. Fitoterapia 2010, 81, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhat, Z.A. Apigenin 7-Glucoside from Stachys Tibetica Vatke and Its Anxiolytic Effect in Rats. Phytomedicine 2014, 21, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Masuya, J.; Iimori, M.; Matsumiya, T. Caffeic Acid Produces Antidepressive- and/or Anxiolytic-like Effects through Indirect Modulation of the Alpha 1A-Adrenoceptor System in Mice. Neuroreport 2003, 14, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Miyagawa, K.; Takeuchi, T.; Takeda, H. Pharmacological characterization and mechanisms of the novel antidepressive- and/or anxiolytic-like substances identified from Perillae Herba. Nihon Shinkei Seishin Yakurigaku Zasshi 2008, 28, 159–167. [Google Scholar]
- Deng, S.; Chen, S.-N.; Yao, P.; Nikolic, D.; van Breemen, R.B.; Bolton, J.L.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Serotonergic Activity-Guided Phytochemical Investigation of the Roots of Angelica sinensis. J. Nat. Prod. 2006, 69, 536–541. [Google Scholar] [CrossRef]
- Davison, K.M.; Kaplan, B.J. Nutrient Intakes Are Correlated with Overall Psychiatric Functioning in Adults with Mood Disorders. Can. J. Psychiatry 2012, 57, 85–92. [Google Scholar] [CrossRef]
- Tovchiga, O.V. Interaction of Aegopodium podagraria L. (Goutweed) Preparations with Central Nervous System Depressants. Ukr. Biopharm. J. 2016, 42, 31–36. [Google Scholar] [CrossRef]
- Yılmaz Sarıaltın, S.; Çiçek Polat, D.; Yalçın, C.Ö. Cytotoxic and Antioxidant Activities and Phytochemical Analysis of Smilax excelsa L. and Aegopodium podagraria L. Food Biosci. 2023, 52, 102359. [Google Scholar] [CrossRef]
- Koyro, O. Experimental substantiation of the gout weed extract application in associated liver and kidney toxic affection.Pdf. Ukr. Biopharm. J. 2011, 13, 2. [Google Scholar]
- Koyro, О.; Tovchiga, O.; Stepanova, S.; Shtrygol, S. Study of the Composition of the Goutweed Flowers Essential Oil, Its Renal Effects and Influence on Uric Acid Exchange. Pharmacogn. Commun. 2012, 2, 46–49. [Google Scholar] [CrossRef]
- Sabiu, S.; O’Neill, F.H.; Ashafa, A.O.T. The Purview of Phytotherapy in the Management of Kidney Disorders: A Systematic Review on Nigeria and South Africa. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Huang, C. Adverse Drug Reactions in Primary Care: A Scoping Review. BMC Health Serv. Res. 2020, 20, 5. [Google Scholar] [CrossRef]
- Stub, T.; Quandt, S.A.; Arcury, T.A.; Sandberg, J.C.; Kristoffersen, A.E.; Musial, F.; Salamonsen, A. Perception of Risk and Communication among Conventional and Complementary Health Care Providers Involving Cancer Patients’ Use of Complementary Therapies: A Literature Review. BMC Complement. Altern. Med. 2016, 16, 353. [Google Scholar] [CrossRef]
- Gaikwad, K.; Dagle, P.; Choughule, P.; Joshi, Y.; Kadam, V. A Review on Some Nephroprotective Medicinal Plants. Int. J. Pharm. Sci. Res. 2012, 3, 2451–2454. [Google Scholar]
- Koyro, O.O. Рoль біoлoгічнo активних речoвин яглиці звичайнoї (Aegopodium podagraria L.) у нефрoпрoтектoрній, гепатoпрoтектoрній та гіпoурикемічній дії. 2013. Available online: http://dspace.nuph.edu.ua/handle/123456789/4008 (accessed on 31 March 2025).
- Tovchiga, O.V. Дoслідження Сечoгіннoї, Нефрoпрoтектoрнoї, Гіпoурикемічнoї Дії Яглиці Звичайнoї (Aegopodium podagraria L.) Як Оснoва Для Ствoрення Лікарських Засoбів. 2009. Available online: https://dspace.nuph.edu.ua/handle/123456789/21586 (accessed on 31 March 2025).
- Hołderna-Kędzia, E.; Kędzia, B.; Mścisz, A. Investigations of plant extracts with high antibiotic activity. Postępy Fitoter. 2009, 1, 3–11. [Google Scholar]
- Burgieł, Z.; Tomaszkiewicz-Potępa, A.; Vogt, O.; Burgieł, M.; Patla, K. Possibilities for Use of Seed Extracts from Selected Apiaceaeus Plants in Plant Protection Against Diseases. Ecol. Chem. Eng. A 2010, 17, 1077–1082. [Google Scholar]
- Tovchiga, O.V.; Shtrygol, S.Y. The Effect of Medicines with Goutweed (Aegopodium podagraria l.) On the Physical Endurance, Cognitive Functions and the Level of Depression in Animals. News Pharm. 2016, 1, 71–76. [Google Scholar] [CrossRef]
- Tovchiga, O. Effects of Aegopodium podagraria Preparations on the Metabolic Disorders Induced in Rats by Excess Fructose Combined WITH Hydrochlorothiazide: The Relationship between Influence on Electrolyte and Carbohydrate Metabolism. Int. J. Biochem. Res. Rev. 2014, 4, 80–98. [Google Scholar] [CrossRef]
- Tovchiga, O.V.; Shtrygol’, S.Y. Metabolic Effects of Goutweed (Aegopodium podagraria L.) Preparations in Rats Treated with a Single Dose of Ethanol. 2016. Available online: https://dspace.nuph.edu.ua/handle/123456789/13323 (accessed on 31 March 2025).
- Tovchiga, O.V.; Shtrygol, S.; Taran, A.V.; Yudkevich, T. Renal effects of goutweed (Aegopodium podagraria L.) Tincture and metformin in dexamethasone-treated rats. Clin. Pharm. 2016, 20, 39–45. [Google Scholar] [CrossRef]
- Tovchiga, O.V.; Gorbatch, T.V.; Shtrygol’, S.Y.; Mishchenko, M.V.; Stepanova, S.I.; Taran, A.V. The effects of goutweed (Aegopodium podagraria L.) preparations and their combinations with metformin in rats with the disorders of the lipid and carbohydrate metabolism induced by protamine sulphate. Rev. Clin. Pharmacol. Drug Ther. 2017, 15, 31–41. [Google Scholar] [CrossRef]
- Tovchiga, O.V.; Shtrygol, S.Y. Renal and Metabolic Effects of Goutweed (Aegopodium podagraria L.) Extract Compared with Potassium Chloride in Rats Receiving Hydrochlorothiazide. J. Adv. Med. Pharm. Sci. 2018, 16, 1–16. [Google Scholar] [CrossRef]





| Component | Part of the Plant | Content | References |
|---|---|---|---|
| Macroelements: | |||
| K | Leaves | 38,372 (μg/g) | [24] |
| Stems | 76,848 (μg/g) | ||
| Aerial parts | 42,263 (μg/g) | [34] | |
| Mg | Leaves | 2233 (μg/g) | [24] |
| Stems | 2082 (μg/g) | ||
| Aerial parts | 1598 (μg/g) | [34] | |
| Ca | Aerial parts | 6632 (μg/g) | [34] |
| P | Aerial parts | 2678 (μg/g) | [34] |
| Microelements: | |||
| Fe | Aerial parts | 38.20 (μg/g) | [34] |
| Zn | Leaves | 39 (μg/g) | [24] |
| Stems | 24 (μg/g) | ||
| Cu | Leaves | 3.7 (μg/g) | [24] |
| Stems | 1.85 (μg/g) | ||
| Aerial parts | 14.16 (μg/g) | [34] | |
| Mn | Leaves | 32 (μg/g) | [24] |
| Stems | 20.5 (μg/g) | ||
| Aerial parts | 32 (μg/g) | [34] | |
| Se | Aerial parts | 0.21 (μg/g) | [34] |
| Cr | Leaves | 514 (ng/g) | [24] |
| Stems | 135 (ng/g) | ||
| Aerial parts | 0.93 (μg/g) | [34] | |
| Co | Leaves | 28 (ng/g) | [24] |
| Stems | 4.8 (ng/g) | ||
| Pb | Leaves | 484 (ng/g) | [24] |
| Stems | 341 (ng/g) | ||
| Vitamins: | |||
| Ascorbic acid (vit. C) | Aerial parts | 46.5 (mg/100 g) | [34] |
| Carotene (provit. A) | Aerial parts | 0.21 (mg/100 g) | [34] |
| Thiamin (vit. B1) | Aerial parts | 0.016 (mg/100 g) | [34] |
| Riboflavin (vit. B2) | Aerial parts | 0.11 (mg/100 g) | [34] |
| Nutritional components: | |||
| Protein | Aerial parts | 51.30 (mg/g) | [35] |
| Proline | Aerial parts | 21.07 (µmol/g) | [35] |
| Total free amino acids | Aerial parts | 29.62 (µg/g) | [35] |
| Glucose | Aerial parts | 55.92 (mg/100 g) | [35] |
| Sucrose | Aerial parts | 14.99 (mg/100 g) | [35] |
| Total soluble carbohydrate | Aerial parts | 242.7 (mg/100 g) | [35] |
| Other components: | |||
| Chlorophyll | Aerial parts | 20.51 (mg/100 g) | [35] |
| Total carotenoids | Aerial parts | 20.88 (mg/100 g) | [35] |
| β-Carotene | Aerial parts | 165.91 (µg/100 g) | [35] |
| 11.12 (mg/100 g) | [29] | ||
| Lycopene | Aerial parts | 236.63 (µg/100 g) | [35] |
| Flavonoids | Aerial parts | 12.71 (mg/100 g) | [35] |
| Anthocyanins | Aerial parts | 16.01 (mg/100 g) | [35] |
| P-active substances (the sum of catechins and flavonoids in terms of rutin) | Aerial parts | 17.3 (mg/100 g) | [34] |
| Part of Plant (Origin) | Plant Material Formulation | Research Model/Method | Aim of Study | Results | Ref. |
|---|---|---|---|---|---|
| Antimicrobial activity | |||||
| Aerial parts (Serbia) | Extracts (water, ethanol, ethyl acetate) | Bioassay—human-pathogenic bacteria (disk diffusion and tube dilution methods) | Evaluation of antibacterial activity against: B. mycoides, B. subtilis, S. aureus, E. cloacae, K. pneumonia, P. fluorescens | Ethanolic extract showed highest antibacterial activity and synergistic and/or additive effects with antibiotics Synergism was observed against B. subtilis | [25] |
| Aerial parts Rhizomes (Poland) | Extracts (hexane, ethyl acetate, water) | Bioassay—human-pathogenic bacteria (serial dilution method in fluid medium) | Evaluation of antibacterial and antifungal activity against S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, C. albicans, M. gypseum | Different antimicrobial activity, depending on part of the plant and extractant | [139] |
| Leaves Flowers Seeds Rhizomes (Poland) | Extract (ethanol) | Bioassay—S. aureus culture on petri dishes (disk diffusion method) | Evaluation of antistaphylococcal activity against reference and clinical strain of S. aureus | Inhibiting activity against tested strains of S. aureus | [114] |
| Rhizomes (England probably) | Extract (acetone) separated into two fractions containing falcarinol and falcarindiol | Bioassay—spore germination tests, using Butt slides and impregnated agar plugs | Evaluation of antifungal activity against 10 fungal strains, including A. brassicicola, B. cinerea, S. nodorum and others | Falcarindiol is a major antifungal constituent, responsible for inhibiting the growth of tested fungi | [101] |
| Seeds (Poland) | Extract (ethyl acetate) | Bioassay—fungal culture on petri dishes and experiments in field conditions | Evaluation of suitability for plant protection against phytopathogenic fungi: F. culmorum, B. cinerea, M. penicullata | A. podagraria seed extract had a low effect on the growth of tested fungi | [140] |
| Antioxidant activity | |||||
| Aerial parts (Bulgaria) | Extracts (chloroform, ethanol, ethyl acetate) | DPPH and ABTS radical scavenging activity—in vitro assays | Determination of radical scavenging activity of extracts obtained using different solvents | Ethanolic extract exhibited highest antioxidant potential in both assays | [3] |
| Aerial parts (Poland) | Extracts (ethanol-water 8:2, obtained in various conditions) | DPPH radical scavenging activity assay performed by reversed-phase high-performance liquid chromatography (DPPH-RP-HPLC) | Evaluation of influence of plant preparation method and extraction conditions on antioxidant potential of extracts | Extract prepared from dry plant using ultrasonic bath showed highest antioxidant potential | [85] |
| Leaves Rhizomes Seeds Flowers (Poland) | Extracts (ethanol or acetone, obtained by various techniques) | DPPH radical scavenging activity—in vitro assay | Investigation of DPPH scavenging activity of extracts from various parts of the plant obtained using different extracting methods and solvents | All parts of the plant exhibit radical scavenging activity, depending mainly on the extraction technique and extraction time | [53] |
| Leaves (Poland) | Extracts (ethanol, water) | DPPH radical scavenging activity, FRAP (Ferric Reducing Antioxidant Power), TPC (Total Phenolic Content)—in vitro assays; Cell Cultures THP-1 | Evaluation of antioxidant potential (DPPH, FRAP), total polyphenol content (TPC) and effects against fluoride-modulated oxidative stress in THP-1 cell line | Extracts have antioxidant activity, promote antioxidant enzymes and provide a protective effect against sodium fluoride toxicity | [45] |
| Anti-inflammatory activity | |||||
| Roots Leaves Stems Flowers (Denmark) | Extracts (water, methanol, acetone, dichloromethane, ethyl acetate hexane) | COX-1 in vitro assay | Screening in vitro for cyclooxygenase-1 (COX-1) inhibitory activity | The highest activity was observed for hexane extract of flowers. Other extracts, except aqueous, also showed activity. The high level of COX-1 inhibitory activity is related to falcarindiol content. Results indicate the potential use of goutweed in herbal medicine | [52] |
| Anticancer activity | |||||
| Aerial parts (Turkey) | Extracts (water, methanol) | MTT cell viability assay—human cell cultures: - prostate cancer - colorectal cancer - lung cancer | Evaluation of cytotoxicity | Considerable cytotoxic effects on human prostate cancer cells. May help treat prostate cancer | [130] |
| Impact on physical endurance and nervous system | |||||
| Aerial parts (Ukraine) | Extracts (water, ethanol) | Male and female mice | Evaluation of the effects of extracts on levels of depression and anxiety, locomotor activity, exploratory behavior and memory | Beneficial effects of extracts on the CNS in mice, including antidepressant effect and reduction in signs of anxiety | [106] |
| Aerial parts (Ukraine) | Dry extract, tincture | Mice and rats: weight-loaded forced swimming test, extrapolation escape test, reserpine-induced depression model | Verification of pharmacological effects of goutweed on physical and mental condition of animals: mice and rats | Heterogeneous results depending on extract type and animal model | [141] |
| Metabolic effects | |||||
| Aerial parts (Ukraine) | Extracts (water, ethanol) | Male rats | Evaluation of effects on electrolyte, glucose and uric acid metabolism | Ethanolic extracts exert hypoglycemic effects in a metabolic syndrome-like model Further study of extract dosage regimens required | [142] |
| Aerial parts (Ukraine) | Extracts (water, ethanol) | Male rats | Evaluation of effects of extracts on carbohydrate and protein metabolism, as well as urea and plasma enzyme activity, in rats treated with a single dose of ethanol | Extracts did not induce unfavorable shifts in total protein, albumin, uric acid and creatinine content (only a moderate increase in urea level was observed). Results confirm the safety of goutweed preparations | [143] |
| Aerial parts (Ukraine) | Extract (ethanol) | Outbred male albino rats | Evaluation of metabolic effects of extracts and their combinations with metformin in dexamethasone-treated rats | Beneficial effects of the combination of ethanolic extract and metformin: reduction in plasma ALT activity, increase in urea clearance and normalization of ALP activity | [2] |
| Aerial parts (Ukraine) | Ethanol extract | Outbred male albino rats | Assessing the effect of goutweed tincture combined with metformin on renal excretory function and state of mineral metabolism in dexamethasone-treated rats | Metformin therapy combined with ethanolic extract resulted in reduction of proteinuria and enzymuria, and normalization of potassium level in blood | [144] |
| Aerial parts (Ukraine) | Extracts (water, ethanol) | Wistar male albino rats | Evaluation of the influence of extracts and their combinations with metformin on renal excretory function in rats fed an atherogenic diet combined with protamine sulfate | Goutweed extract and tincture normalize lipid composition of liver in rats with lipid and carbohydrate metabolism disorders caused by protamine sulfate and an atherogenic diet. Tincture also exerts a permissive effect on metformin action on glucose metabolism, but not on lipid metabolism | [145] |
| Aerial parts (Ukraine) | Extracts (water, ethanol) | Female rats | Determination of the influence of extracts on renal function and metabolic processes in rats receiving hydrochlorothiazide | Results substantiate the potential use of goutweed extracts in combination with hydrochlorothiazide. In addition, the extract increased uric acid excretion and decreased plasma urea levels | [146] |
| Flowers (Ukraine) | Essential oil | Male albino mice | Diuretic and uricosuric activity of goutweed flower essential oil | Results include increased excretion of creatinine, urea and uric acid with unchanged urine volume, suggesting that essential oil of flowers may be involved in the diuretic and uricosuric activity of goutweed | [132] |
| Antiaging activity | |||||
| Aerial parts (Poland) | Extract (water/glycerol, 80:20) | Cell culture: HaCaT (normal human keratinocytes) | Determination of anticollagenase activity and antielastase activity by scratch wound assay | Beneficial effects on skin cells: inhibition of elastase and collagenase, stimulation of keratinocyte and fibroblast migration, with a potentially significant impact on delaying skin aging | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębia, K.; Dzięcioł, M.; Wróblewska, A.; Janda-Milczarek, K. Goutweed (Aegopodium podagraria L.)—An Edible Weed with Health-Promoting Properties. Molecules 2025, 30, 1603. https://doi.org/10.3390/molecules30071603
Dębia K, Dzięcioł M, Wróblewska A, Janda-Milczarek K. Goutweed (Aegopodium podagraria L.)—An Edible Weed with Health-Promoting Properties. Molecules. 2025; 30(7):1603. https://doi.org/10.3390/molecules30071603
Chicago/Turabian StyleDębia, Kamila, Małgorzata Dzięcioł, Agnieszka Wróblewska, and Katarzyna Janda-Milczarek. 2025. "Goutweed (Aegopodium podagraria L.)—An Edible Weed with Health-Promoting Properties" Molecules 30, no. 7: 1603. https://doi.org/10.3390/molecules30071603
APA StyleDębia, K., Dzięcioł, M., Wróblewska, A., & Janda-Milczarek, K. (2025). Goutweed (Aegopodium podagraria L.)—An Edible Weed with Health-Promoting Properties. Molecules, 30(7), 1603. https://doi.org/10.3390/molecules30071603






