The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds
Abstract
1. Introduction
2. Pyrazoles, Pyrazolines and Fused Scaffolds
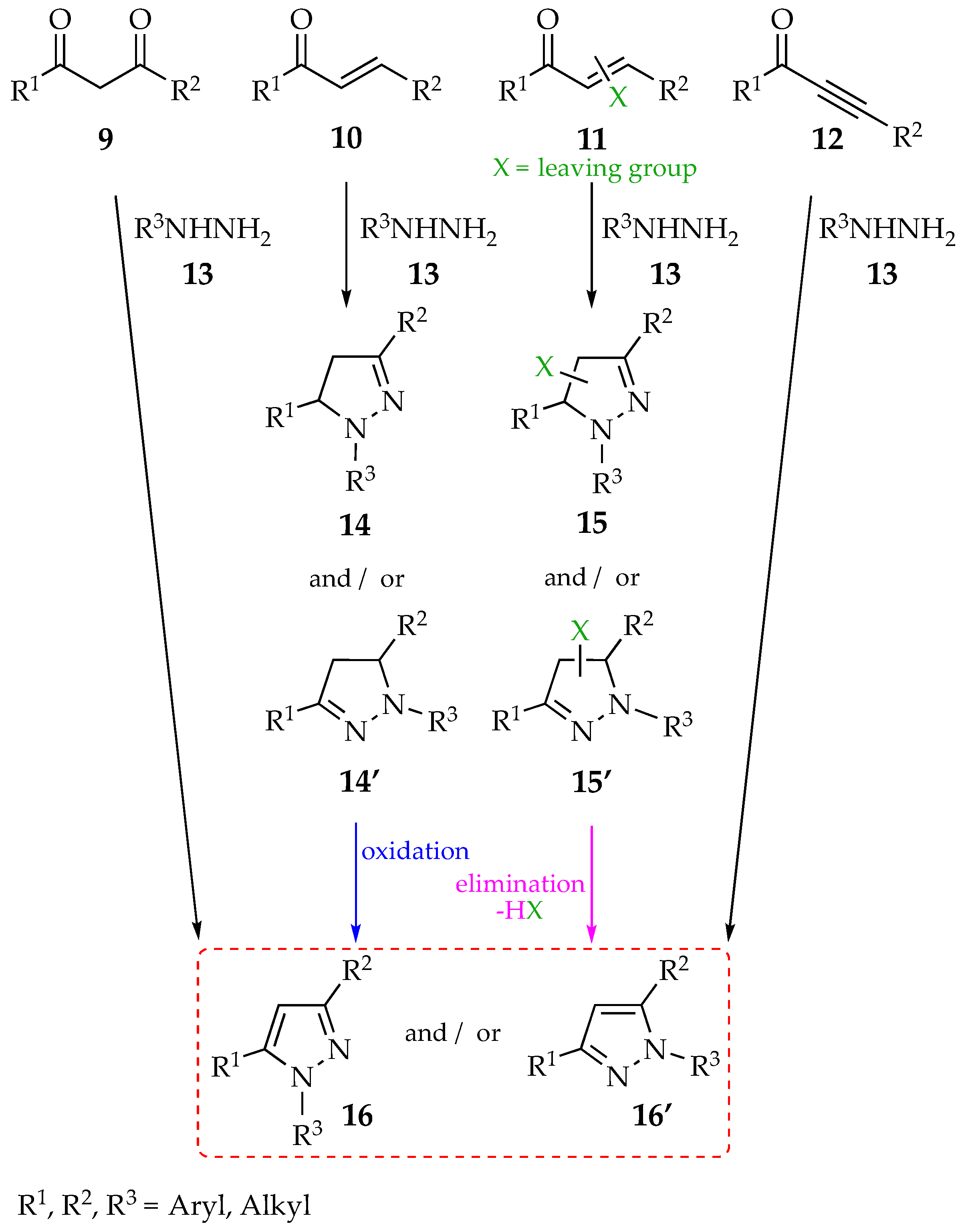
2.1. Synthesis of Pyrazoles and Pyrazolines
2.2. Synthesis of Indazoles
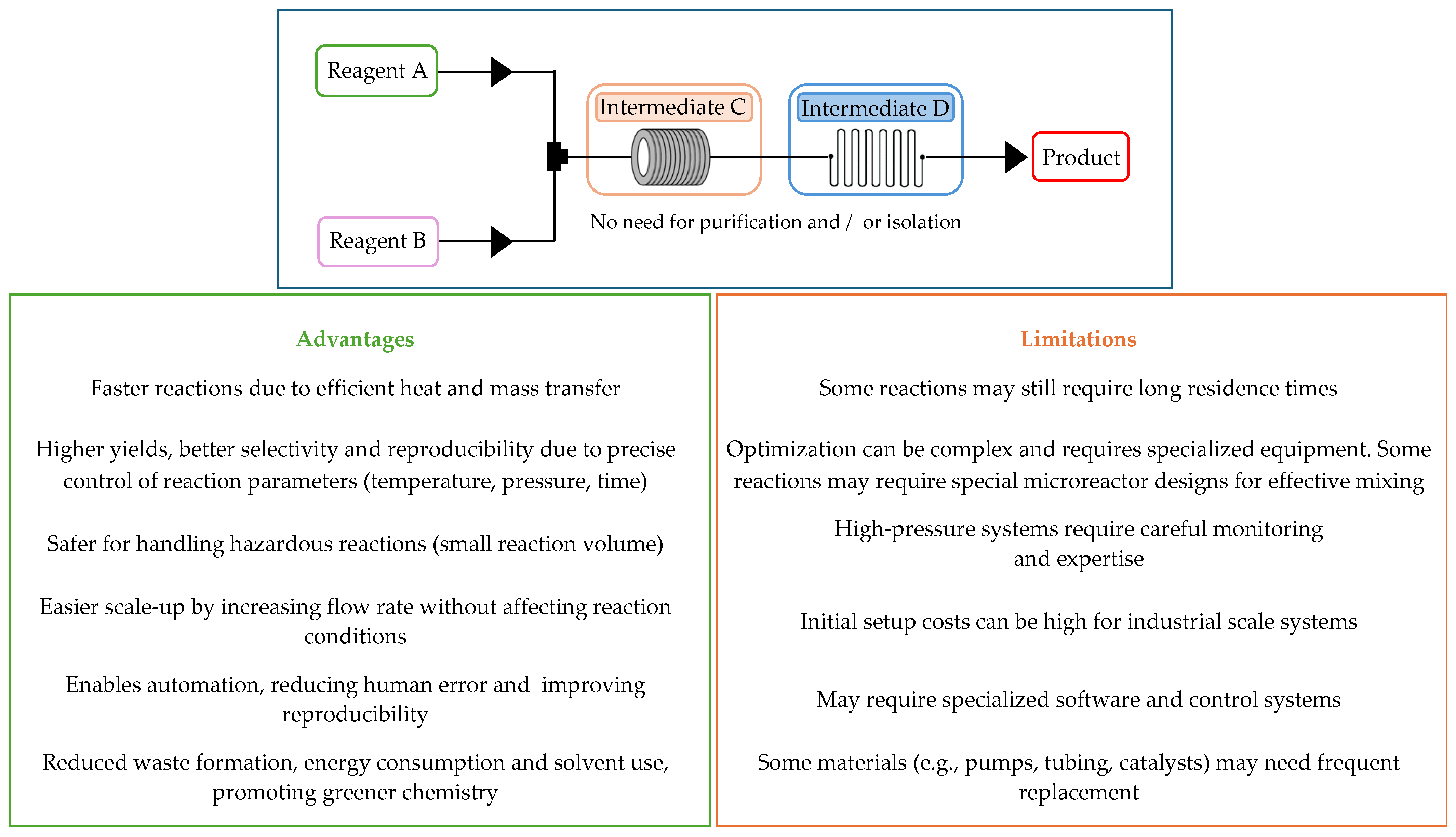
3. Principles of Flow Chemistry
4. Flow Chemistry in the Synthesis of Pyrazoles and Related Derivatives
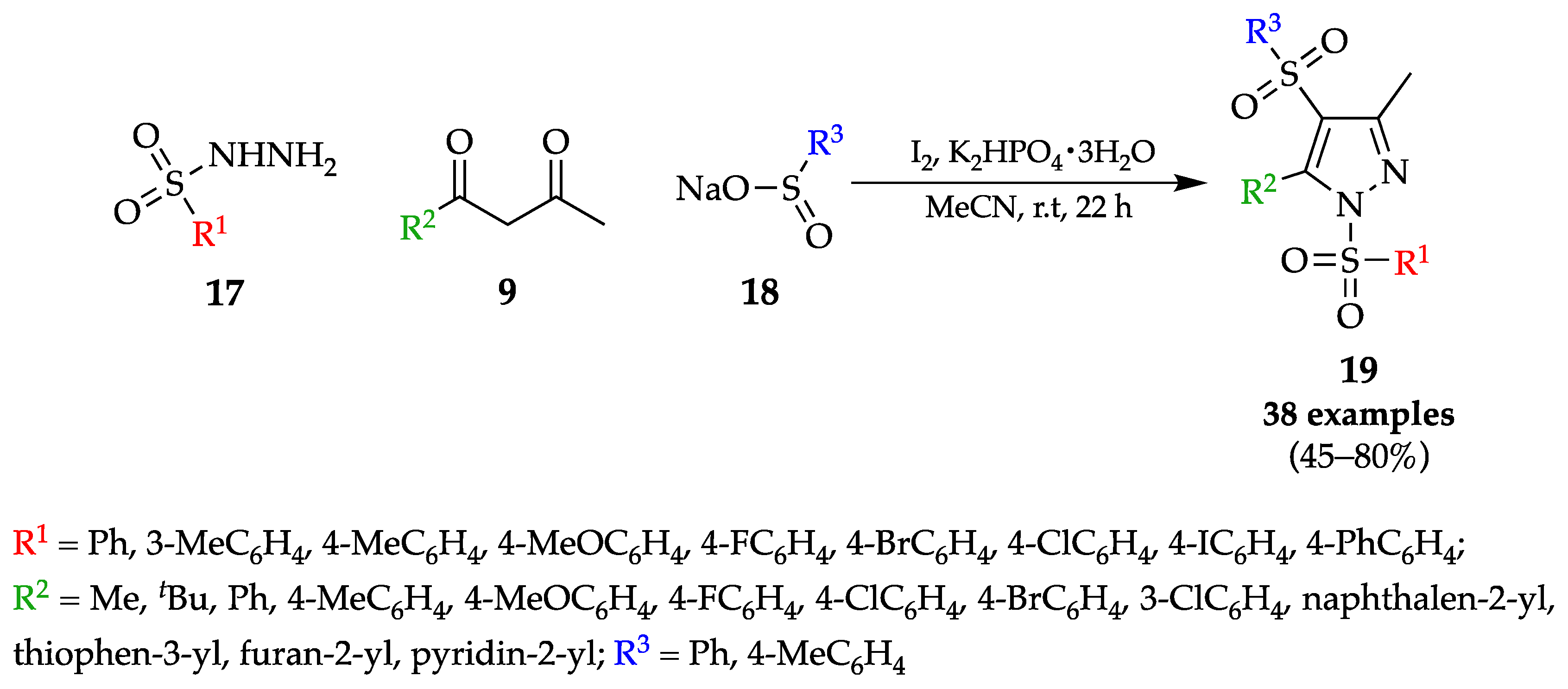
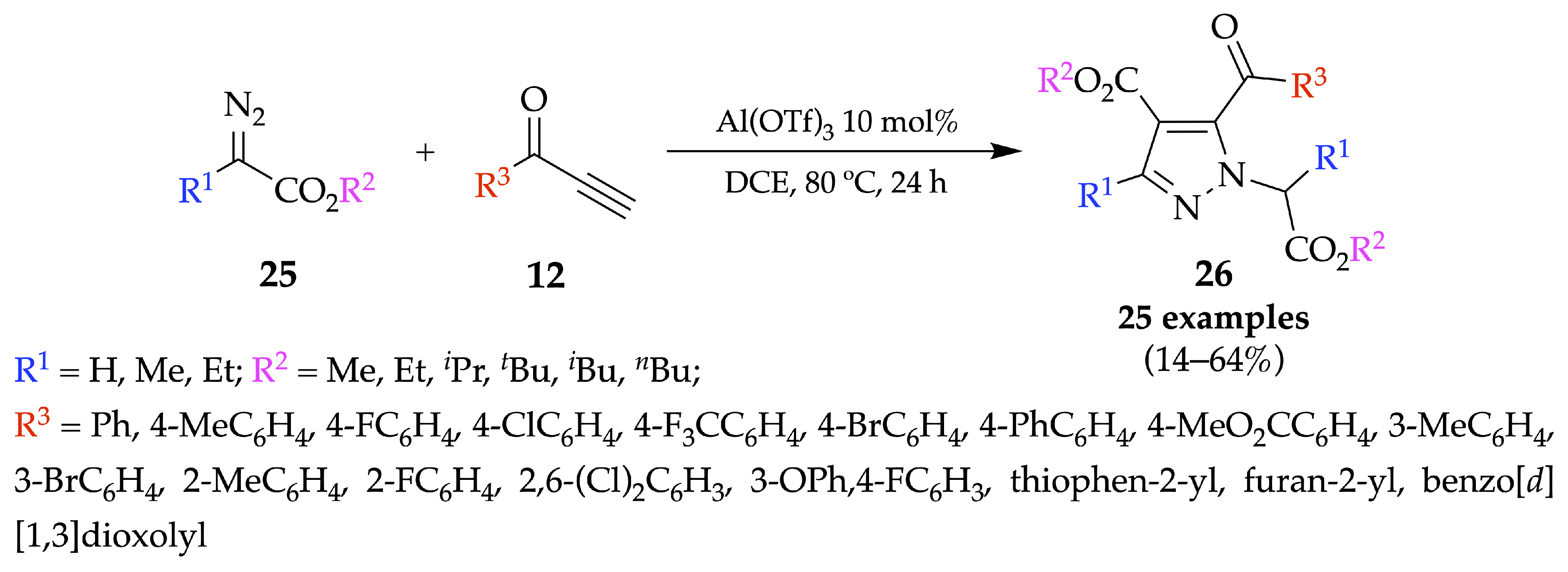
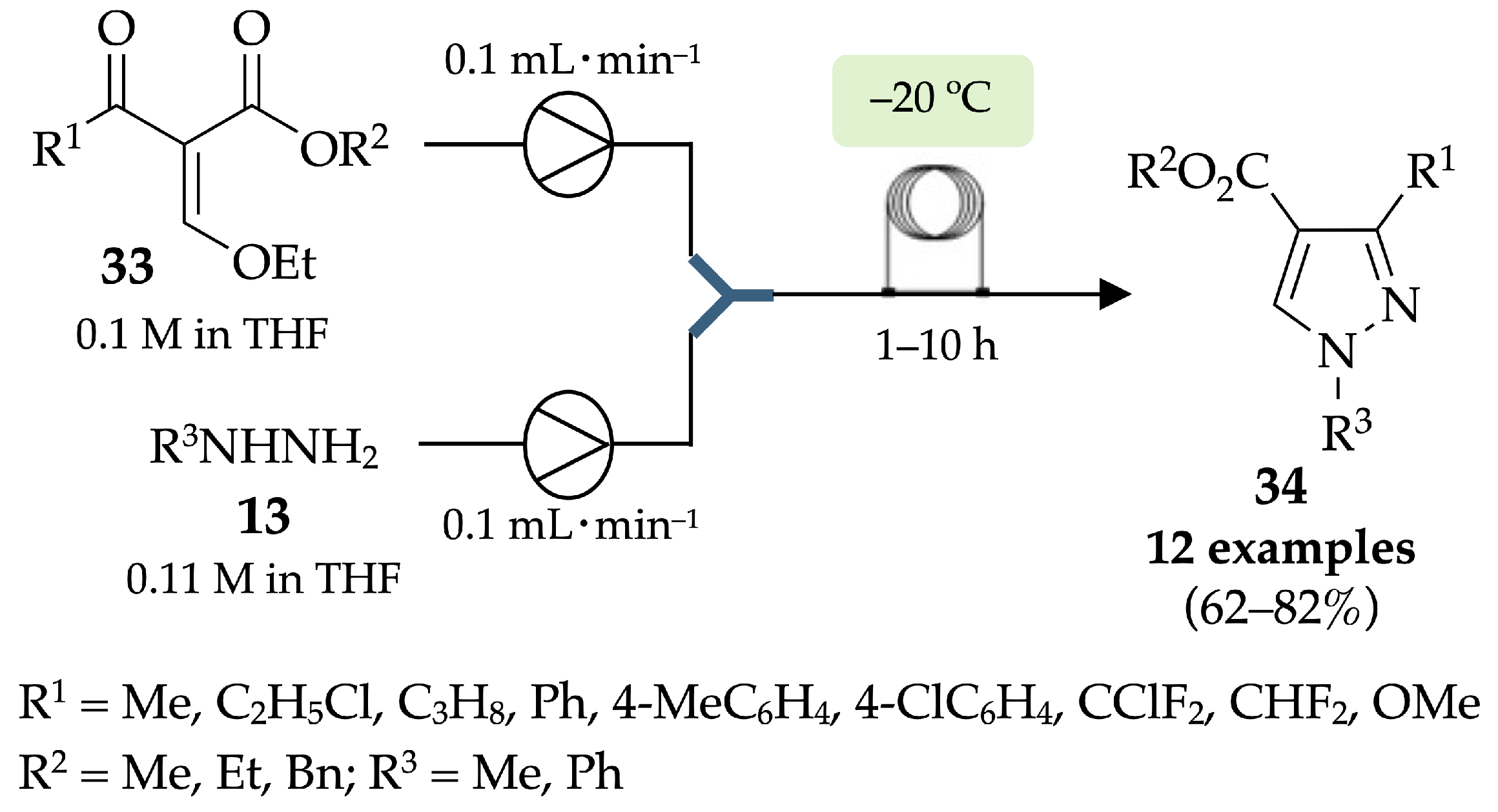
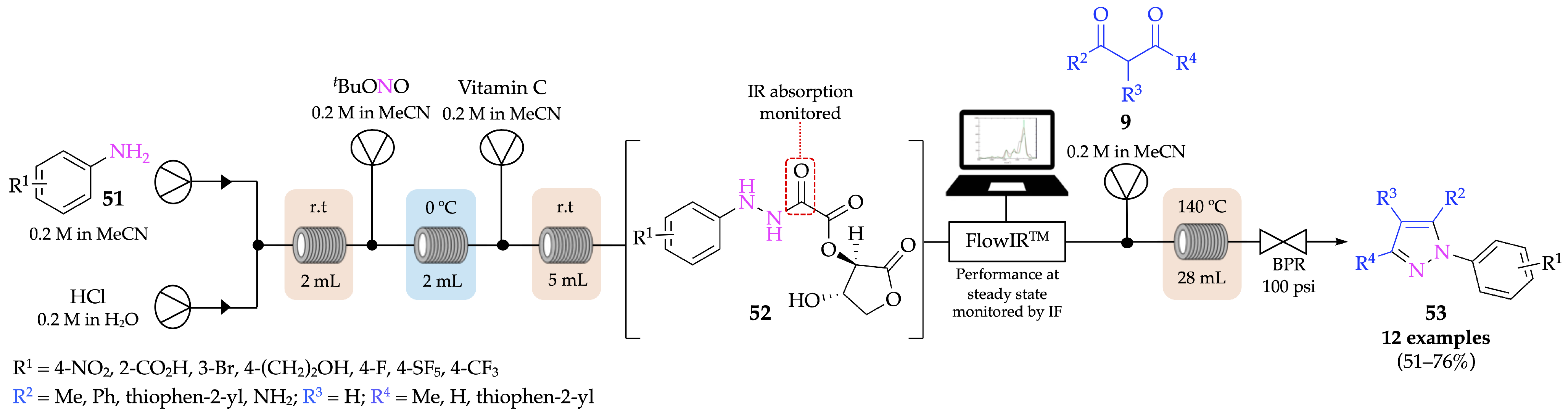
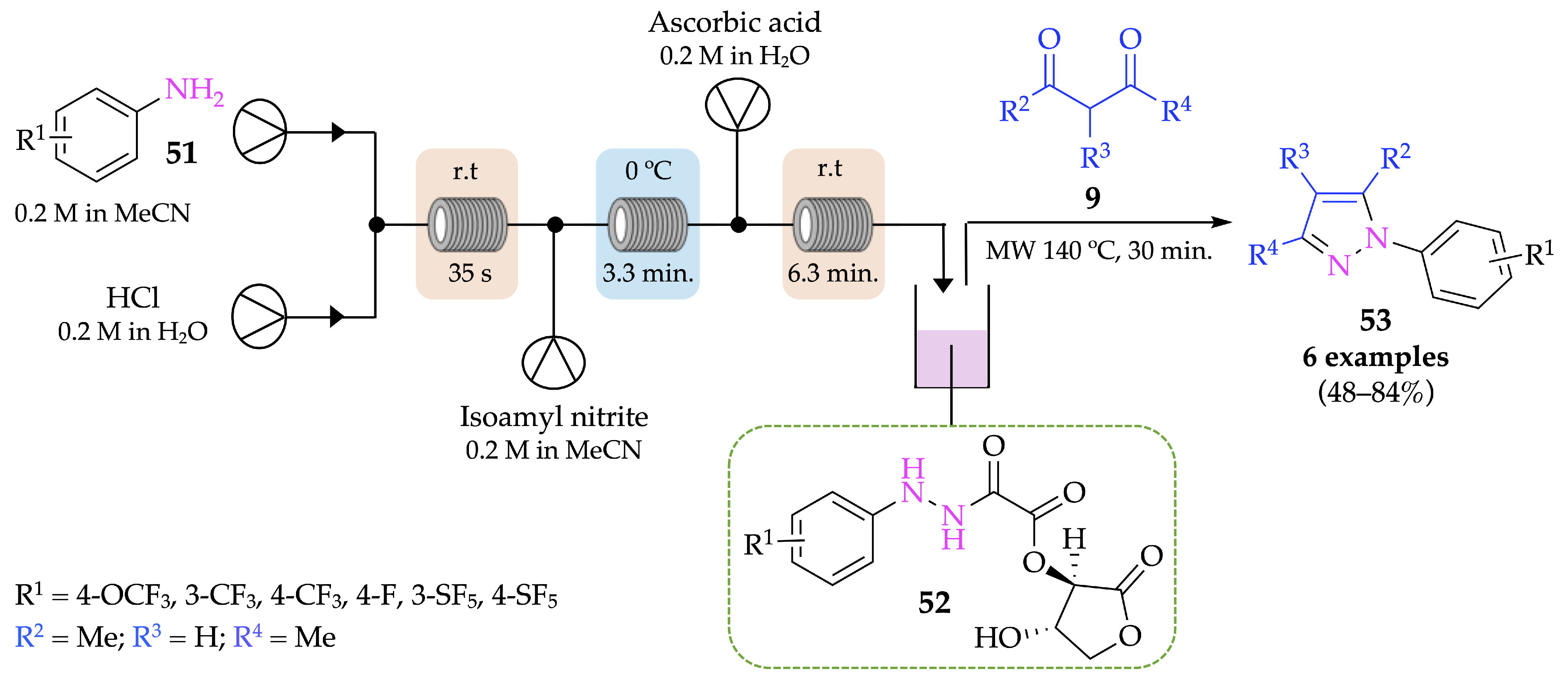
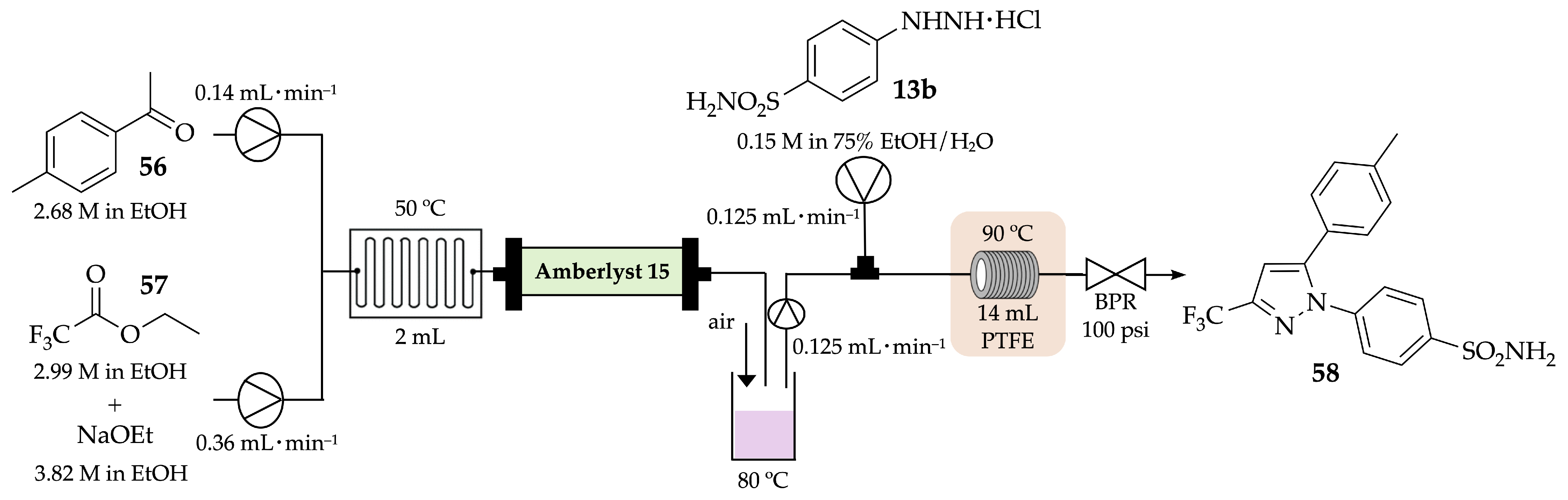
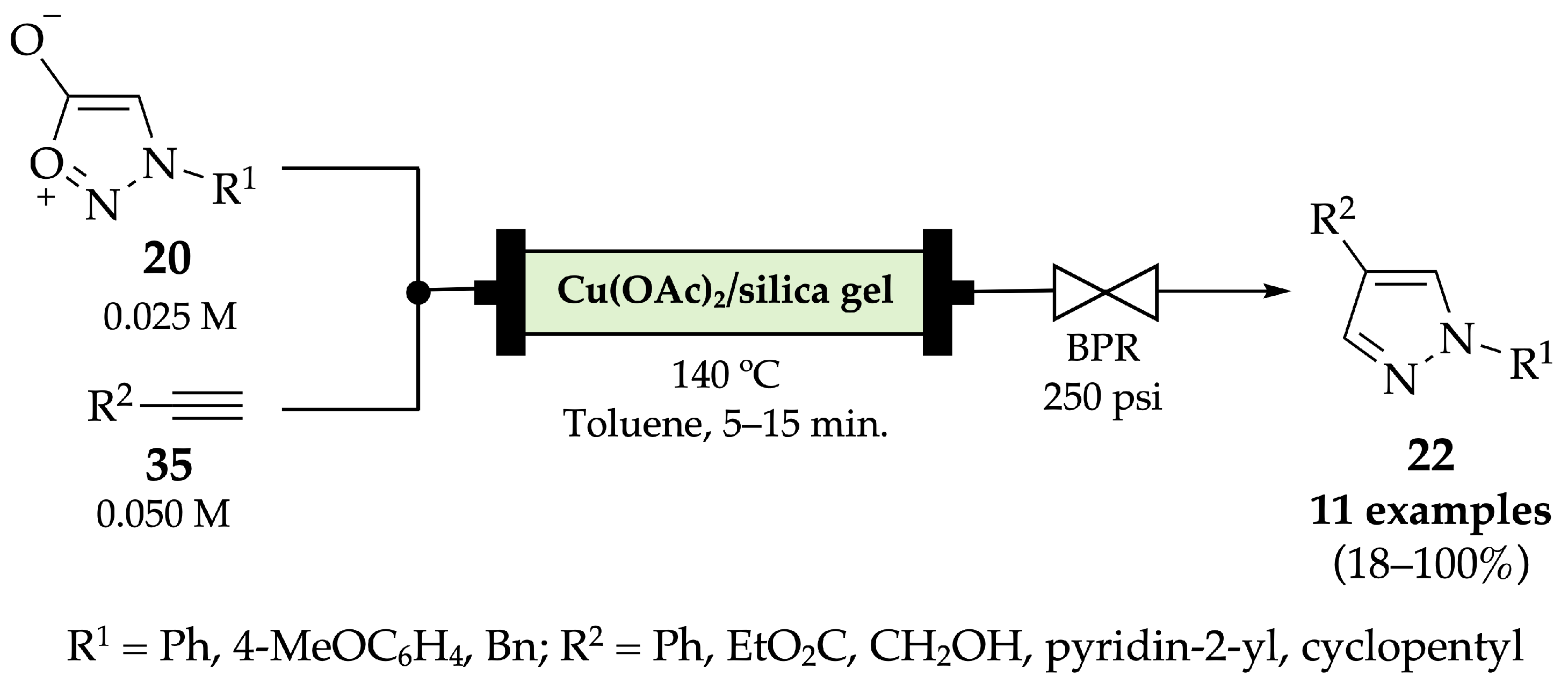
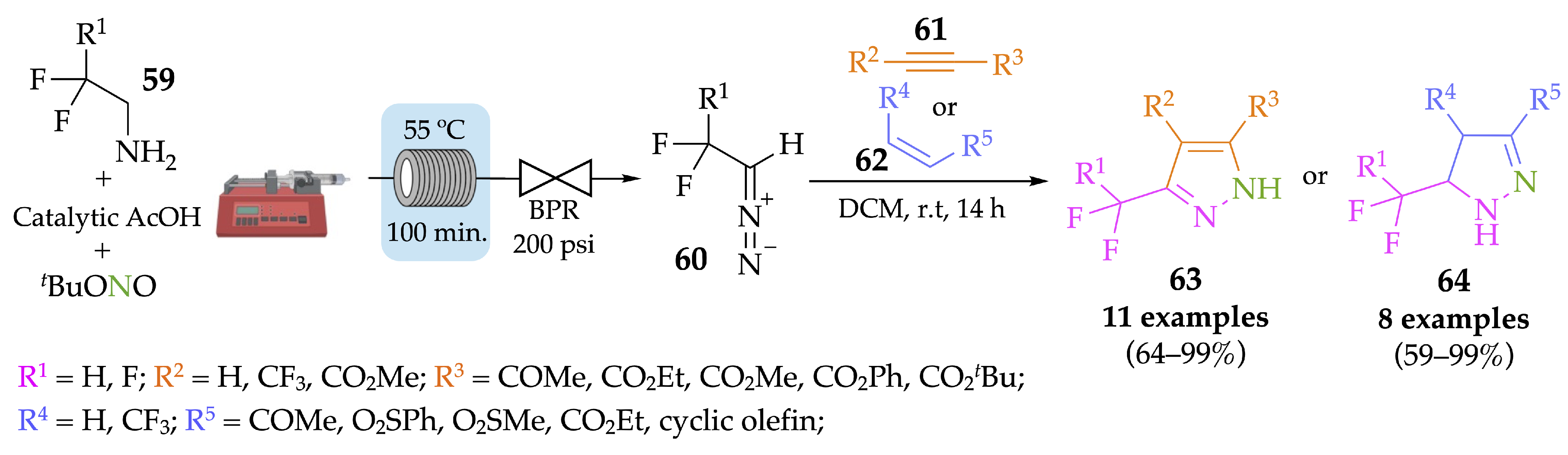
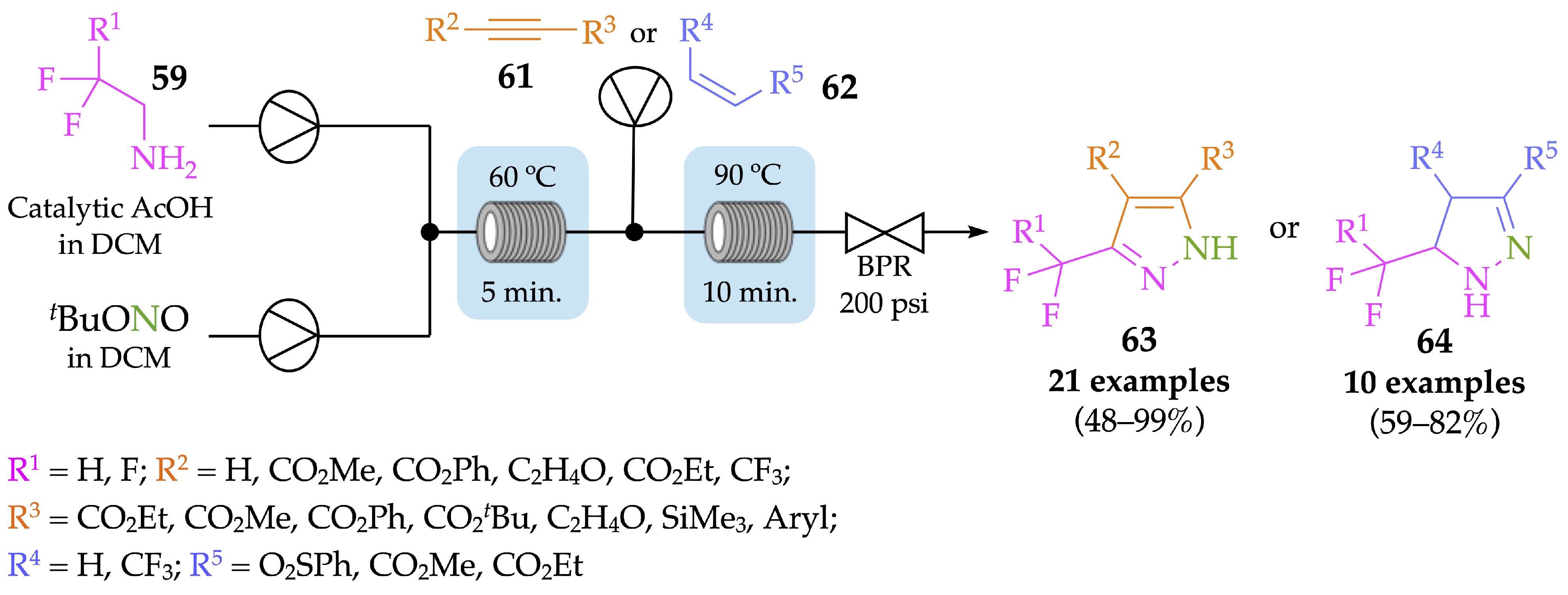
4.1. Flow Synthesis of Pyrazoles and Pyrazolines
4.2. Flow Synthesis of Pyrazole-Fused Scaffolds
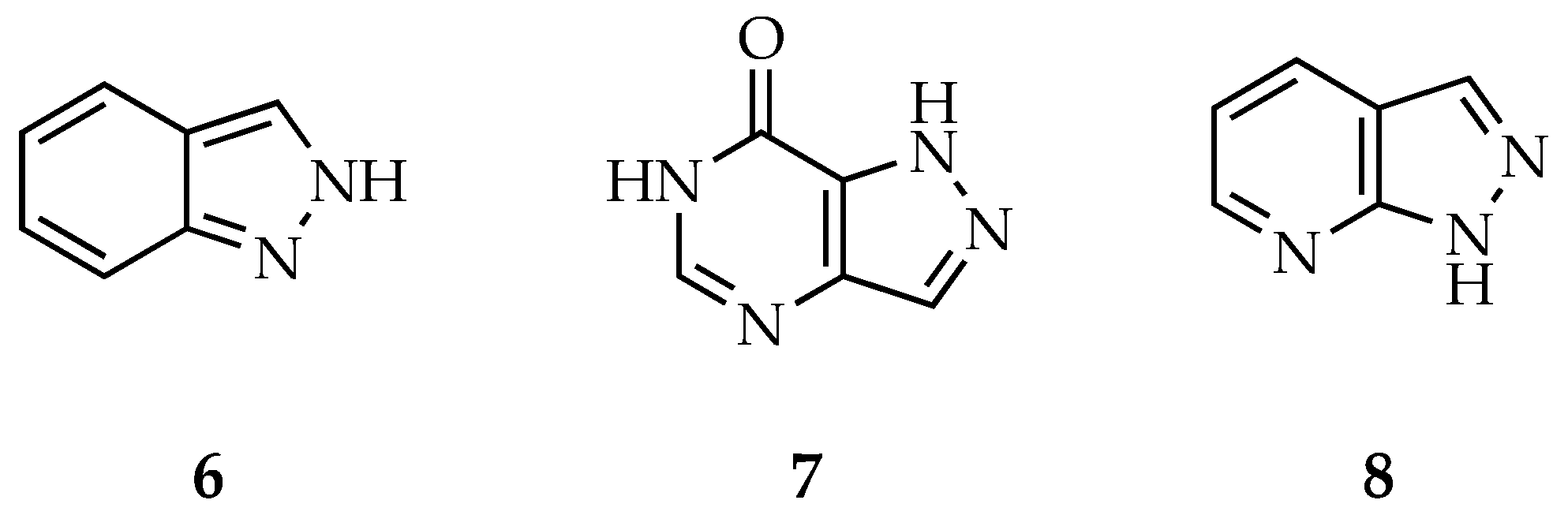
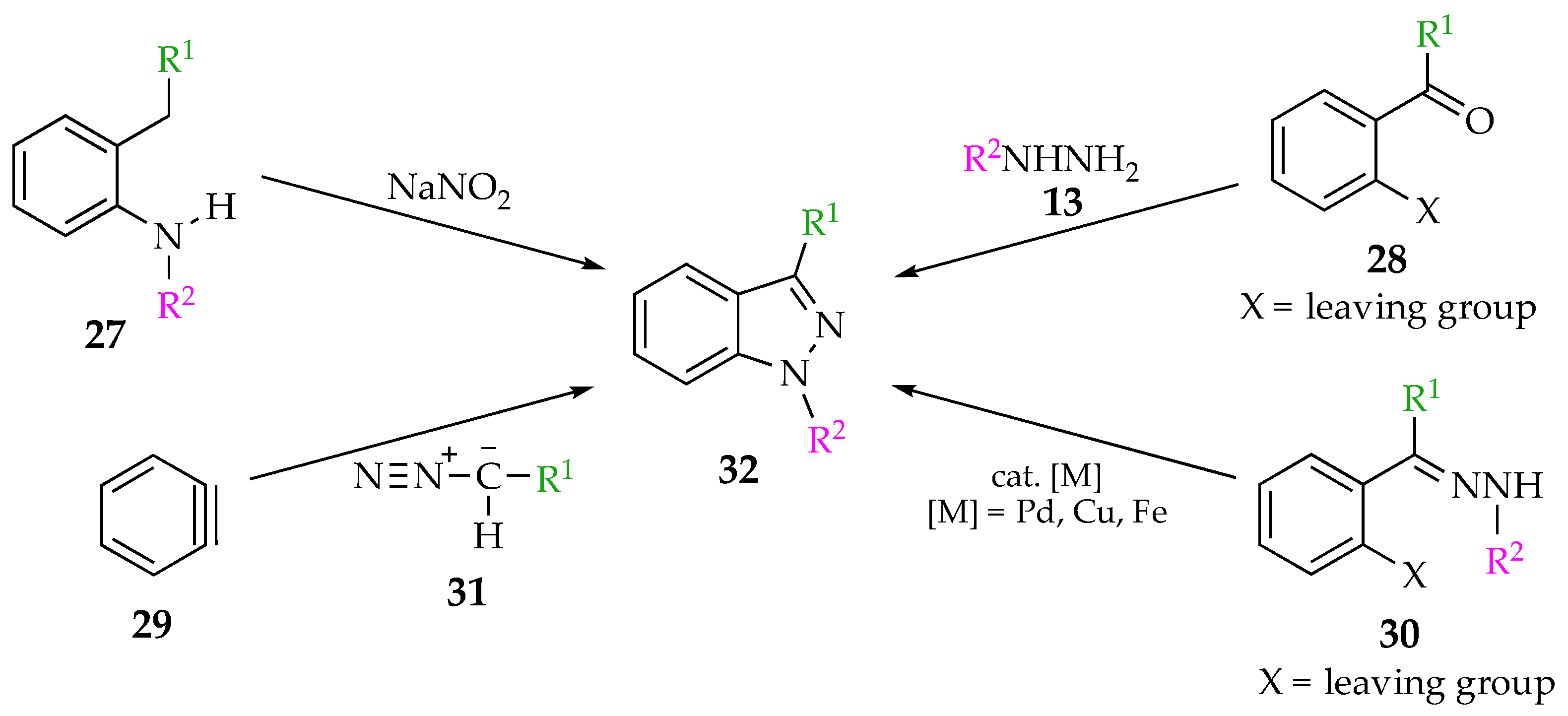
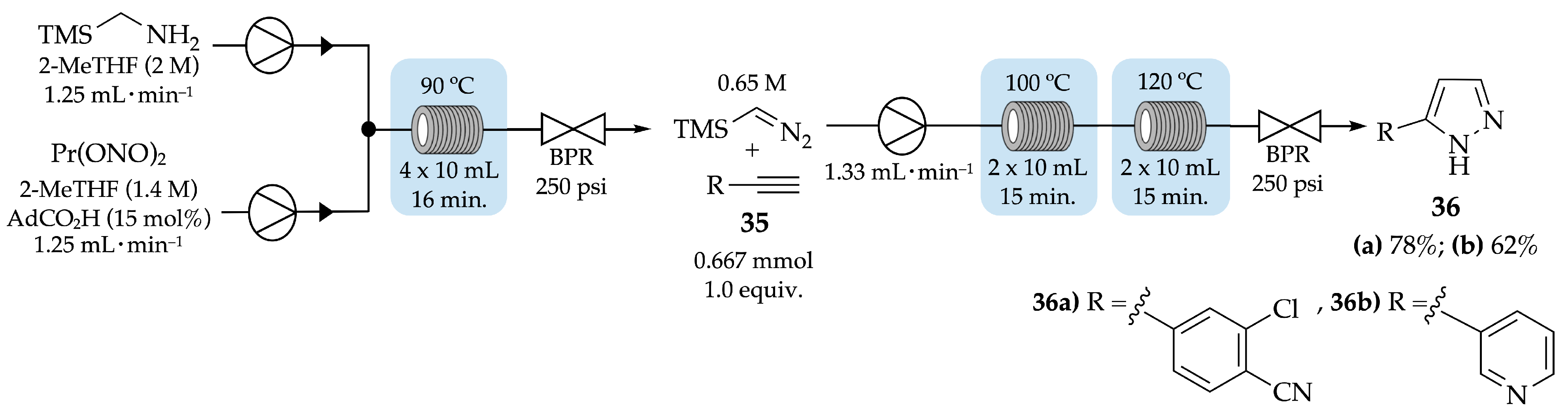
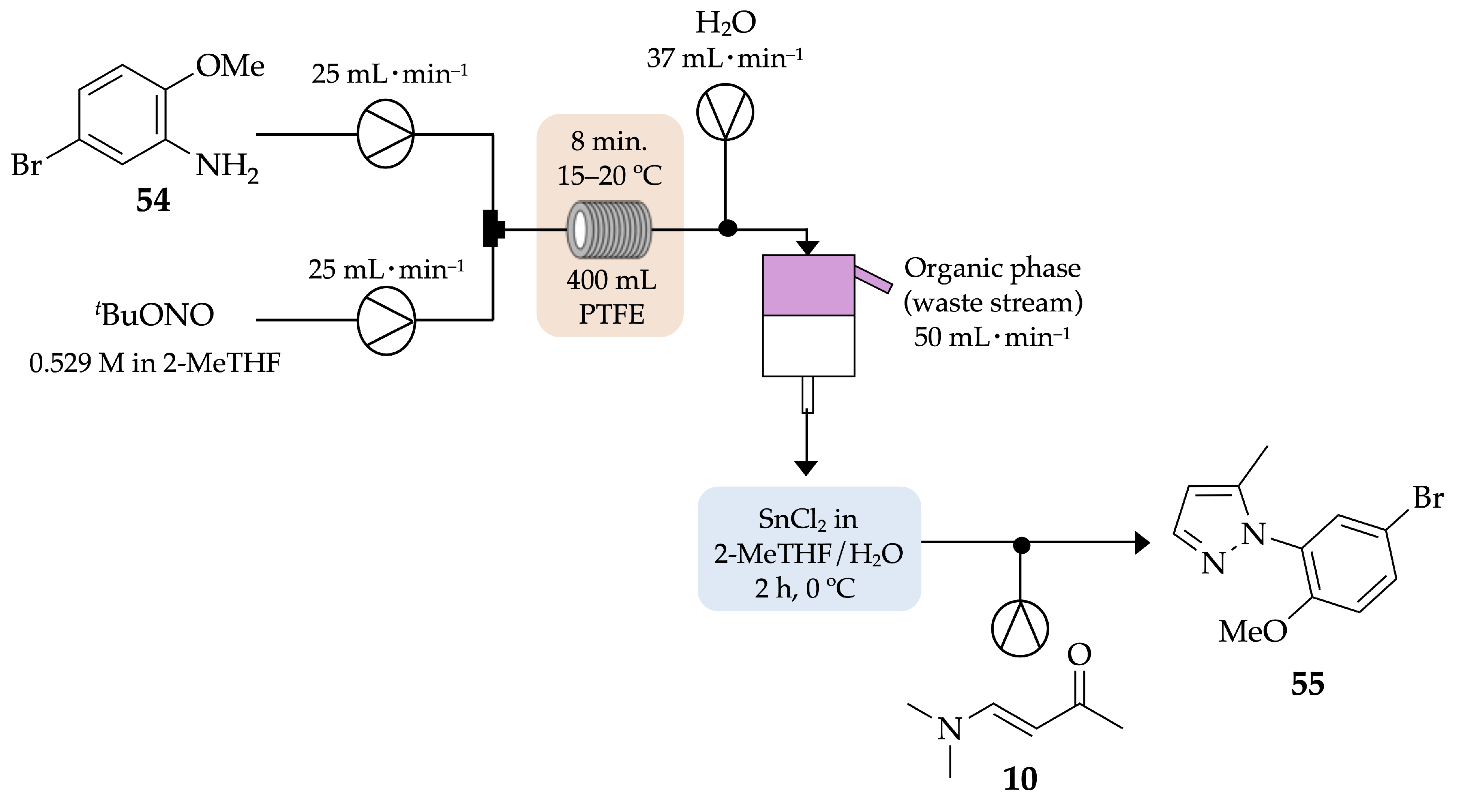
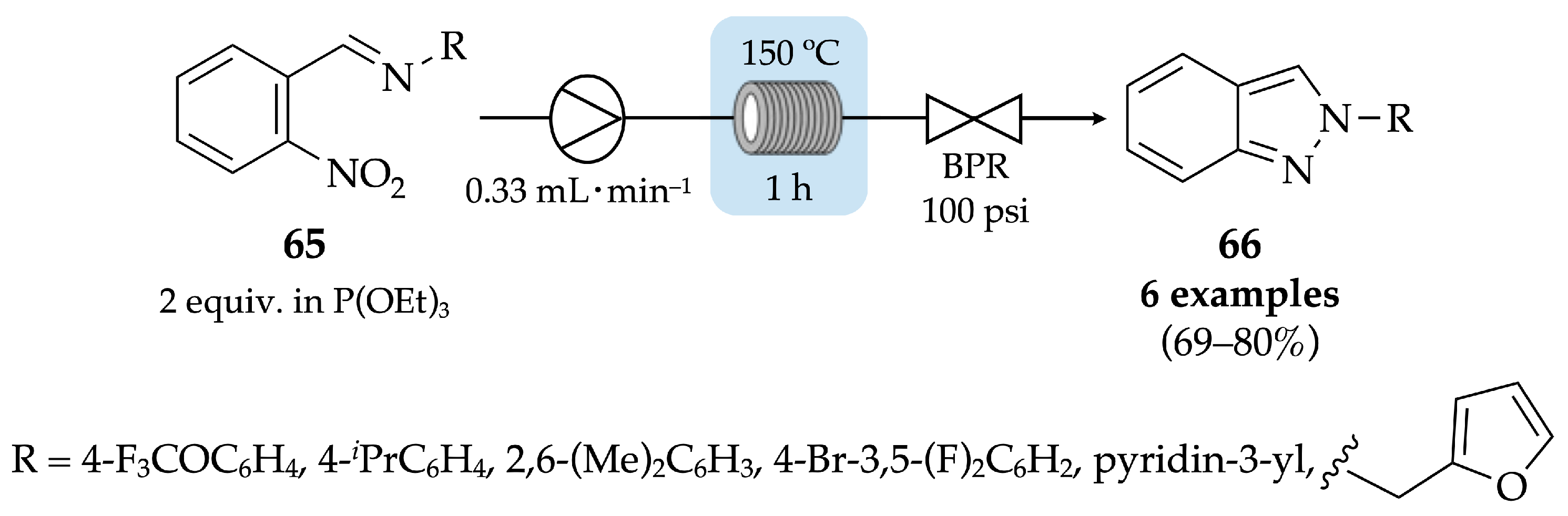
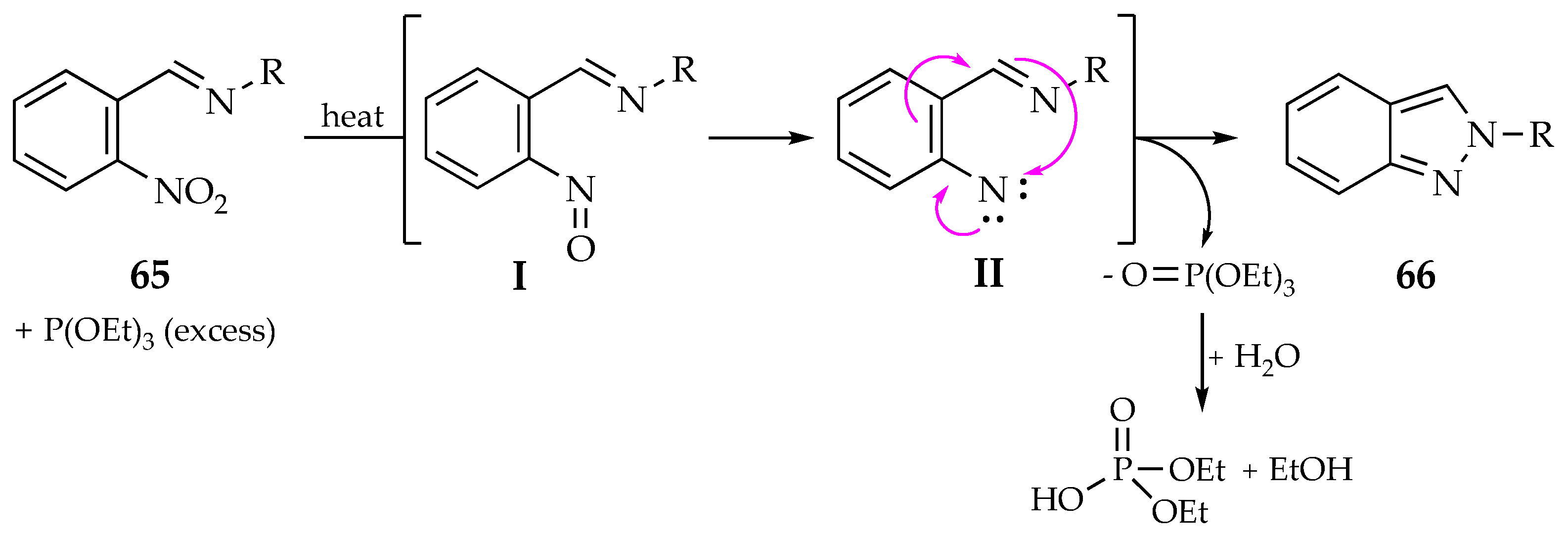
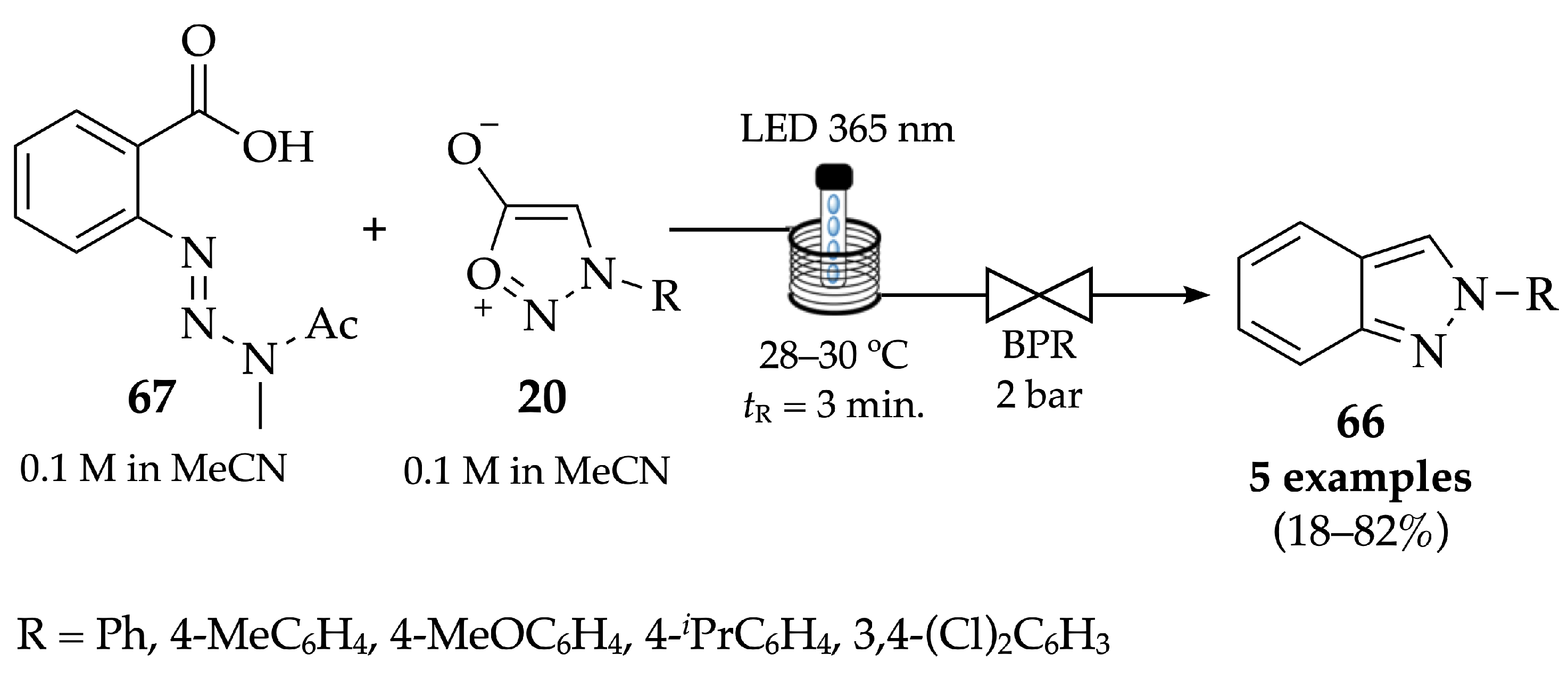
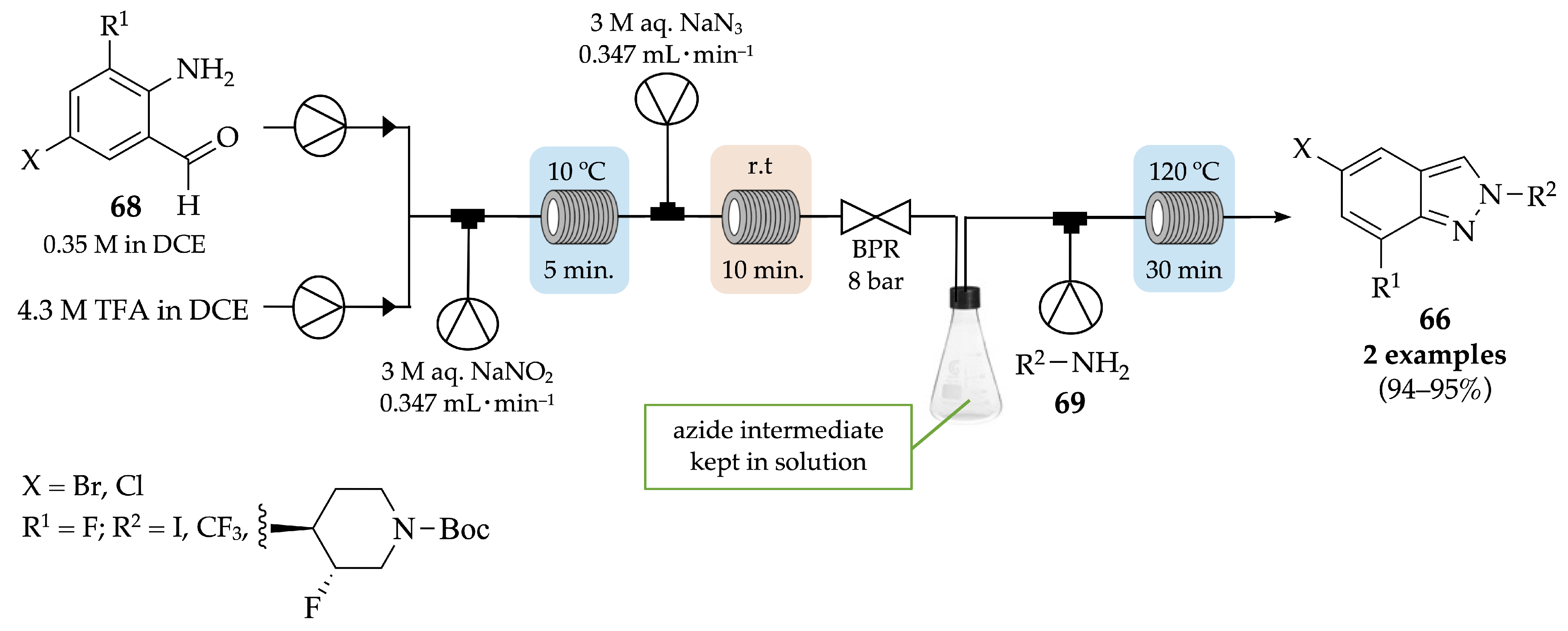
4.2.1. Indazoles
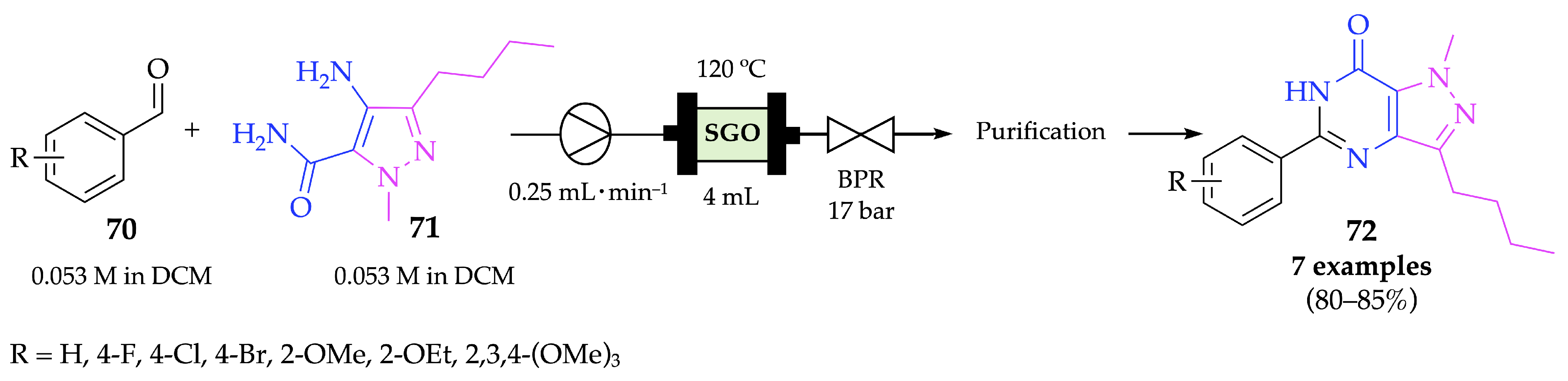
4.2.2. Pyrazolopyrimidinones
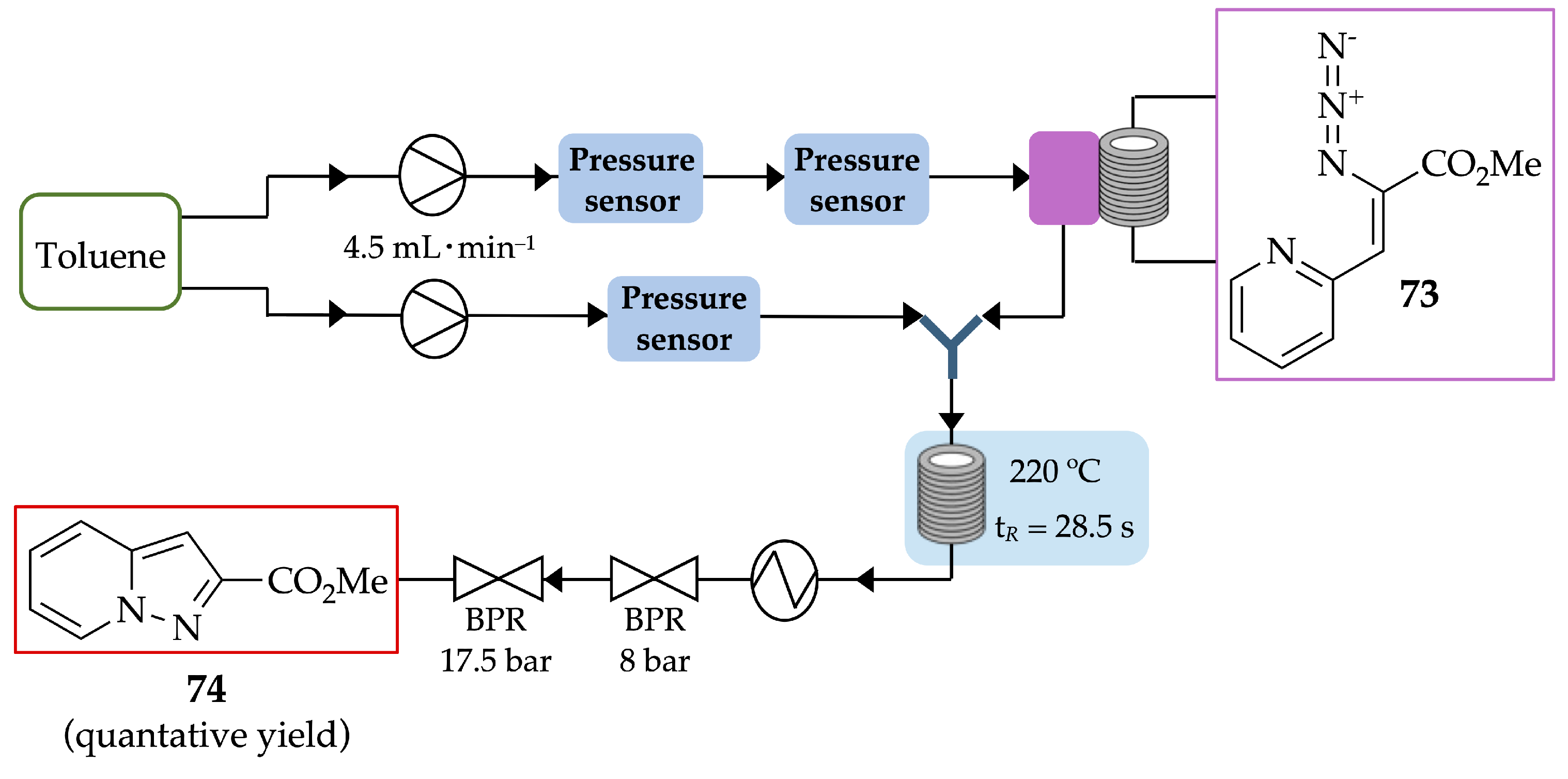
4.2.3. Pyrazolopyridines
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPR | Back pressure regulator |
| Bn | Benzyl |
| Bu | Butyl |
| Bz | Benzoyl |
| cat | Catalyst |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DIEA | N,N-Diisopropylethylamine |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| equiv | Molar equivalent |
| Et | Ethyl |
| EVI2 | Ethyl viologen diiodide |
| EWG | Electron withdrawing group |
| FEP | Fluorinated ethylene-propylene |
| 1H NMR | Proton nuclear magnetic resonance spectroscopy |
| iBu | iso-Butyl |
| iPr | iso-Propyl |
| IR | Infrared |
| LED | Light emitting diode |
| Me | Methyl |
| MW | Microwave |
| NMP | N-Methyl-2-pyrrolidone |
| PDE-5 | Phosphodiesterase 5 |
| PFA | Perfluoroalkoxy |
| Ph | Phenyl |
| PTFE | Polytetrafluoroethylene |
| p-TSA | para-Toluenesulfonic acid |
| r.t | Room temperature |
| SGO | Sulfonated graphene oxide |
| iBu | t-Butyl |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TLR | Toll-like receptor |
| TMEDA | N,N,N’,N’-Tetramethylethylenediamine |
| TMS | Tetramethylsilane |
| tR | Residence time |
References
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole Containing Natural Products: Synthetic Preview and Biological Significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Ali, A.; Ali, A.; Thiriveedhi, A.; Bakht, M.A.; Yusuf, M.; Salahuddin; Afzal, O.; Altamimi, A.S.A. Pyrazoline Containing Compounds as Therapeutic Targets for Neurodegenerative Disorders. ACS Omega 2022, 7, 38207–38245. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Sawant, S.; Juvale, K. A Comprehensive Review on the Indazole Based Derivatives as Targeted Anticancer Agents. J. Mol. Struct. 2023, 1284, 135327. [Google Scholar] [CrossRef]
- Hassani, I.A.E.; Rouzi, K.; Assila, H.; Karrouchi, K.; Ansar, M. Recent Advances in the Synthesis of Pyrazole Derivatives: A Review. Reactions 2023, 4, 478–504. [Google Scholar] [CrossRef]
- Capaldo, L.; Wen, Z.; Noël, T. A Field Guide to Flow Chemistry for Synthetic Organic Chemists. Chem. Sci. 2023, 14, 4230–4247. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, X.; Pan, Y.; Zuo, T.; Ning, Z.; Li, C.; Zhang, Z. Recent Developments of Automated Flow Chemistry in Pharmaceutical Compounds Synthesis. J. Flow Chem. 2023, 13, 385–404. [Google Scholar] [CrossRef]
- Cherkasov, N.; Adams, S.J.; Bainbridge, E.G.A.; Thornton, J.A.M. Continuous Stirred Tank Reactors in Fine Chemical Synthesis for Efficient Mixing, Solids-Handling, and Rapid Scale-Up. React. Chem. Eng. 2023, 8, 266–277. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Laybourn, A.; Robertson, K.; Slater, A.G. Quid Pro Flow. J. Am. Chem. Soc. 2023, 145, 4355–4365. [Google Scholar] [CrossRef]
- Abrigach, F.; Touzani, R. Pyrazole Derivatives with NCN Junction and Their Biological Activity: A Review. Med. Chem. 2016, 6, 292–298. [Google Scholar] [CrossRef]
- Alam, J.; Alam, O.; Alam, P.; Naim, M.J. A Review on Pyrazole Chemical Entity and Biological Activity. Int. J. Pharm. Sci. Res. 2015, 6, 1433–1442. [Google Scholar]
- Gomes, P.M.O.; Ouro, P.M.S.; Silva, A.M.S.; Silva, V.L.M. Styrylpyrazoles: Properties, Synthesis and Transformations. Molecules 2020, 25, 5886. [Google Scholar] [CrossRef]
- Mal, S.; Malik, U.; Mahapatra, M.; Mishra, A.; Pal, D.; Paidesetty, S.K. A Review on Synthetic Strategy, Molecular Pharmacology of Indazole Derivatives, and Their Future Perspective. Drug Dev. Res. 2022, 83, 1469–1504. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhuang, Y.; Chen, Q. Current Scenario of Pyrazole Hybrids with in Vivo Therapeutic Potential against Cancers. Eur. J. Med. Chem. 2023, 257, 115495. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Pinto, D.C.G.A.; Rodríguez, P.; Gómez, M.; Jagerovic, N.; Callado, L.F.; Cavaleiro, J.A.S.; Elguero, J.; Fernández-Ruiz, J. Synthesis and Pharmacological Evaluation of New (E)- and (Z)-3-Aryl-4-Styryl-1H-Pyrazoles as Potential Cannabinoid Ligands. Arkivoc 2010, 2010, 226–247. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; El-Gamal, M.I.; Gamal El-Din, M.M.; Oh, C.H. Design, Synthesis, in Vitro Anticancer Evaluation, Kinase Inhibitory Effects, and Pharmacokinetic Profile of New 1,3,4-Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety. J. Enzyme Inhib. Med. Chem. 2019, 34, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, S. Synthesis and Anti-Inflammatory, Analgesic, Ulcerogenic and Lipid Peroxidation Activities of 3,5-Dimethyl Pyrazoles, 3-Methyl Pyrazol-5-ones and 3,5-Disubstituted Pyrazolines. Indian J. Chem. 2005, 44, 2532–2537. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Nasralla, S.N.; El-Agroudy, E.J.; Hamouda, N.; El-Fattah, A.A.; Bekhit, S.A.; Amagase, K.; Ibrahim, T.M. Investigation of the Anti-Inflammatory and Analgesic Activities of Promising Pyrazole Derivative. Eur. J. Pharm. Sci. 2022, 168, 106080. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Elguero, J.; Silva, A.M.S. Current Progress on Antioxidants Incorporating the Pyrazole Core. Eur. J. Med. Chem. 2018, 156, 394–429. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, B.N.; Umashankara, M.; Dinesh, B.; Girish, K.; Baba, A.R. Development of 5-(Aryl)-3-Phenyl-1H-Pyrazole Derivatives as Potent Antimicrobial Compounds. Asian J. Chem. 2019, 31, 45–50. [Google Scholar] [CrossRef]
- Secci, D.; Bolasco, A.; Chimenti, P.; Carradori, S. The State of the Art of Pyrazole Derivatives as Monoamine Oxidase Inhibitors and Antidepressant/Anticonvulsant Agents. Curr. Med. Chem. 2011, 18, 5114–5144. [Google Scholar] [CrossRef] [PubMed]
- Datar, P. Development of Pyrazole Compounds as Antidiabetic Agent: A Review. Drug Des. Discov. 2014, 11, 686–703. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Pinto, D.C.G.A.; Jagerovic, N.; Callado, L.F.; Cavaleiro, J.A.S.; Elguero, J. Synthesis and Pharmacological Evaluation of Chlorinated N-Alkyl-3- and -5-(2-Hydroxyphenyl)Pyrazoles as CB1 Cannabinoid Ligands. Monatsh. Chem. 2007, 138, 797–811. [Google Scholar] [CrossRef]
- Chernyshov, V.V.; Popadyuk, I.I.; Yarovaya, O.I.; Salakhutdinov, N.F. Nitrogen-Containing Heterocyclic Compounds Obtained from Monoterpenes or Their Derivatives: Synthesis and Properties. Top. Curr. Chem. 2022, 380, 42. [Google Scholar] [CrossRef]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a Versatile Heterocycle: Pyrazoline—A Review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef]
- Noori, Z.; Solà, M.; Viñas, C.; Teixidor, F.; Poater, J. Unraveling Aromaticity: The Dual Worlds of Pyrazole, Pyrazoline, and 3D Carborane. Beilstein J. Org. Chem. 2025, 21, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liang, Y.J.; Lu, R.; Chuai, X.H.; Yi, Z.H.; Zhao, Y.; Zhang, H.J. Synthesis and Properties of Photoluminescence and Electroluminescence of Pyrazoline Derivatives. Synth. Met. 2004, 140, 37–41. [Google Scholar] [CrossRef]
- Portilla, J. Current Advances in Synthesis of Pyrazole Derivatives: An Approach Toward Energetic Materials. J. Heterocycl. Chem. 2024, 61, 2026–2039. [Google Scholar] [CrossRef]
- Lee, B.H.; Jaung, J.Y.; Jang, S.C.; Yi, S.C. Synthesis and Optical Properties of Push–Pull Type Tetrapyrazinoporphyrazines. Dyes Pigm. 2005, 65, 159–167. [Google Scholar] [CrossRef]
- Secrieru, A.; O’Neill, P.M.; Cristiano, M.L.S. Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules 2019, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, D.; Kuang, J.; Ma, Y. Transition Metal-Free De Novo Synthesis of Sulfonated Pyrazoles from Sulfonyl Hydrazides, 1,3-Diketones, and Sodium Sulfinates at Room Temperature. J. Org. Chem. 2021, 86, 9289–9298. [Google Scholar] [CrossRef] [PubMed]
- Lakeland, C.P.; Watson, D.W.; Harrity, J.P.A. Exploiting Synergistic Catalysis for an Ambient Temperature Photocycloaddition to Pyrazoles. Chem. Eur. J. 2020, 26, 155. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Wang, K.-H.; Yang, M.; Zhao, P.; Wang, F.; Wang, J.; Huang, D.; Hu, Y. Synthesis of Difluoromethylated Pyrazoles by the [3 + 2] Cycloaddition Reaction of Difluoroacetohydrazonoyl Bromides. J. Org. Chem. 2022, 87, 498–511. [Google Scholar] [CrossRef]
- Zhao, P.; Zeng, Z.; Feng, X.; Liu, X. Multisubstituted Pyrazole Synthesis via [3 + 2] Cycloaddition/Rearrangement/N-H Insertion Cascade Reaction of α-Diazoesters and Ynones. Chin. Chem. Lett. 2021, 32, 132–135. [Google Scholar] [CrossRef]
- Song, J.J.; Yee, N.K. A Novel Synthesis of 2-Aryl-2H-Indazoles via a Palladium-Catalyzed Intramolecular Amination Reaction. Org. Lett. 2000, 2, 519–521. [Google Scholar] [CrossRef]
- Batt, D.G.; Petraitis, J.J.; Houghton, G.C.; Modi, D.P.; Cain, G.A.; Corjay, M.H.; Mousa, S.A.; Bouchard, P.J.; Forsythe, M.S.; Harlow, P.P.; et al. Disubstituted Indazoles as Potent Antagonists of the Integrin Avβ3. J. Med. Chem. 2000, 43, 41–58. [Google Scholar] [CrossRef]
- Wu, C.; Fang, Y.; Larock, R.C.; Shi, F. Synthesis of 2H-Indazoles by the [3 + 2] Cycloaddition of Arynes and Sydnones. Org. Lett. 2010, 12, 2234–2237. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.-C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry 2022, 4, 940–968. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous Manufacturing—The Green Chemistry Promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Why Flow Means Green—Evaluating the Merits of Continuous Processing in the Context of Sustainability. Curr. Opin. Green Sust. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Das, A.; Ishitani, H.; Kobayashi, S. Toward Continuous-Flow Synthesis of Biologically Interesting Pyrazole Derivatives. Adv. Synth. Catal. 2019, 361, 5127–5132. [Google Scholar] [CrossRef]
- Audubert, C.; Gamboa Marin, O.J.; Lebel, H. Batch and Continuous-Flow One-Pot Processes Using Amine Diazotization to Produce Silylated Diazo Reagents. Angew. Chem. Int. Ed. 2017, 56, 6294–6297. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, S.B.; Georgiádes, Á.; Ozsvár, D.; Fülöp, F. Continuous-Flow Synthesis of 3,5-Disubstituted Pyrazoles via Sequential Alkyne Homocoupling and Cope-Type Hydroamination. RSC Adv. 2019, 9, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Feng, X.; Bao, M. Synthesis of 3,5-Disubstituted Pyrazoles via Cope-Type Hydroamination of 1,3-Dialkynes. J. Org. Chem. 2013, 78, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Paquin, P.; DeGrâce, N.; Bélanger-Chabot, G.; Paquin, J.-F. Synthesis of Substituted Pentafluorosulfanylpyrazoles Under Flow Conditions. J. Org. Chem. 2024, 89, 3552–3562. [Google Scholar] [CrossRef]
- Kandasamy, M.; Ganesan, B.; Hung, M.; Lin, W. Fast and Efficient Continuous Flow Method for the Synthesis of Ynones and Pyrazoles. Eur. J. Org. Chem. 2019, 2019, 3183–3189. [Google Scholar] [CrossRef]
- Burke, A.; Spiccio, S.; Di Filippo, M.; Baumann, M. Photochemical Synthesis of Pyrazolines from Tetrazoles in Flow. SynOpen 2023, 7, 69–75. [Google Scholar] [CrossRef]
- Burke, A.; Di Filippo, M.; Spiccio, S.; Schito, A.M.; Caviglia, D.; Brullo, C.; Baumann, M. Antimicrobial Evaluation of New Pyrazoles, Indazoles and Pyrazolines Prepared in Continuous Flow Mode. Int. J. Mol. Sci. 2023, 24, 5319. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Q.; Xu, Z.; Houk, K.N.; Zheng, K. Photochemical Skeletal Editing of Pyridines to Bicyclic Pyrazolines and Pyrazoles. J. Am. Chem. Soc. 2024, 146, 21389–21400. [Google Scholar] [CrossRef]
- Battilocchio, C.; Deadman, B.J.; Nikbin, N.; Kitching, M.O.; Baxendale, I.R.; Ley, S.V. A Machine-Assisted Flow Synthesis of SR48692: A Probe for the Investigation of Neurotensin Receptor-1. Chem. Eur. J. 2013, 19, 7917–7930. [Google Scholar] [CrossRef]
- Poh, J.-S.; Browne, D.L.; Ley, S.V. A Multistep Continuous Flow Synthesis Machine for the Preparation of Pyrazoles via a Metal-Free Amine-Redox Process. React. Chem. Eng. 2016, 1, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Groves, L.M.; Schotten, C.; Beames, J.; Platts, J.A.; Coles, S.J.; Horton, P.N.; Browne, D.L.; Pope, S.J.A. From Ligand to Phosphor: Rapid, Machine-Assisted Synthesis of Substituted Iridium(III) Pyrazolate Complexes with Tuneable Luminescence. Chem. Eur. J. 2017, 23, 9407–9418. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Widlicka, D.; Boucher, S.; Hayward, C.; Lucas, J.; Murray, J.C.; O’Neil, B.T.; Pfisterer, D.; Samp, L.; VanAlsten, J.; et al. Telescoped Flow Process for the Syntheses of N-Aryl Pyrazoles. Org. Process Res. Dev. 2012, 16, 2031–2035. [Google Scholar] [CrossRef]
- Scholtz, C.; Riley, D.L. Improved Batch and Flow Syntheses of the Nonsteroidal Anti-Inflammatory COX-2 Inhibitor Celecoxib. React. Chem. Eng. 2021, 6, 138–146. [Google Scholar] [CrossRef]
- Comas-Barceló, J.; Blanco-Ania, D.; Van Den Broek, S.A.M.W.; Nieuwland, P.J.; Harrity, J.P.A.; Rutjes, F.P.J.T. Cu-Catalysed Pyrazole Synthesis in Continuous Flow. Catal. Sci. Technol. 2016, 6, 4718–4723. [Google Scholar] [CrossRef]
- Mertens, L.; Hock, K.J.; Koenigs, R.M. Fluoroalkyl-Substituted Diazomethanes and Their Application in a General Synthesis of Pyrazoles and Pyrazolines. Chem. Eur. J. 2016, 22, 9542–9545. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Jamison, T.F. A Unified Continuous Flow Assembly-Line Synthesis of Highly Substituted Pyrazoles and Pyrazolines. Angew. Chem. Int. Ed. 2017, 56, 8823–8827. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.; Di Filippo, M.; Baumann, M. Synthesis of 2H-Indazoles via the Cadogan Reaction in Batch and Flow Mode. Tetrahedron Lett. 2021, 86, 153522. [Google Scholar] [CrossRef]
- Bracken, C.; Batsanov, A.S.; Baumann, M. Development of a Continuous Photochemical Benzyne-Forming Process. SynOpen 2021, 5, 29–35. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, C.; Larock, R.C.; Shi, F. Synthesis of 2H-Indazoles by the [3 + 2] Dipolar Cycloaddition of Sydnones with Arynes. J. Org. Chem. 2011, 76, 8840–8851. [Google Scholar] [CrossRef]
- Lehmann, H.; Ruppen, T.; Knoepfel, T. Scale-Up of Diazonium Salts and Azides in a Three-Step Continuous Flow Sequence. Org. Process Res. Dev. 2022, 26, 1308–1317. [Google Scholar] [CrossRef]
- Shaaban, M.A.; Elshaier, Y.A.M.M.; Hammad, A.H.; Farag, N.A.; Hassan Haredy, H.; AbdEl-Ghany, A.A.; Mohamed, K.O. Design and Synthesis of Pyrazolo[3,4-d]Pyrimidinone Derivatives: Discovery of Selective Phosphodiesterase-5 Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127337. [Google Scholar] [CrossRef]
- Tageldin, G.N.; Ibrahim, T.M.; Fahmy, S.M.; Ashour, H.M.; Khalil, M.A.; Nassra, R.A.; Labouta, I.M. Synthesis, Modeling and Biological Evaluation of Some Pyrazolo[3,4-d]Pyrimidinones and Pyrazolo[4,3-e][1,2,4]Triazolo[4,3-a]Pyrimidinones as Anti-Inflammatory Agents. Bioorg. Chem. 2019, 90, 102844. [Google Scholar] [CrossRef]
- Abdellatif, K.; Abdelall, E.; Abdelgawad, M.; Ahmed, R.; Bakr, R. Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]Pyrimidin-4-One Derivatives. Molecules 2014, 19, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Beyzaei, H.; Aryan, R.; Moghaddam-Manesh, M.; Ghasemi, B.; Karimi, P.; Samareh Delarami, H.; Sanchooli, M. Evaluation and Structure-Activity Relationship Analysis of a New Series of 4-Imino-5H-Pyrazolo[3,4-d]Pyrimidin-5-Amines as Potential Antibacterial Agents. J. Mol. Struct. 2017, 1144, 273–279. [Google Scholar] [CrossRef]
- Sagar, S.R.; Agarwal, J.K.; Pandya, D.H.; Dash, R.P.; Nivsarkar, M.; Vasu, K.K. Design, Synthesis and Biological Evaluation of Novel Pyrazolo-Pyrimidinones as DPP-IV Inhibitors in Diabetes. Bioorg. Med. Chem. Lett. 2015, 25, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- El-Mekabaty, A.; Etman, H.A.; Mosbah, A.; Fadda, A.A. Synthesis, In Vitro Cytotoxicity and Bleomycin-Dependent DNA Damage Evaluation of Some Heterocyclic-Fused Pyrimidinone Derivatives. ChemistrySelect 2020, 5, 4856–4861. [Google Scholar] [CrossRef]
- Bruzziches, R.; Francomano, D.; Gareri, P.; Lenzi, A.; Aversa, A. An Update on Pharmacological Treatment of Erectile Dysfunction with Phosphodiesterase Type 5 Inhibitors. Expert Opin. Pharmacother. 2013, 14, 1333–1344. [Google Scholar] [CrossRef]
- Lee, S.S.; Oh, C.H. Synthesis of Sildenafil and Its Derivatives Bearing Pyrazolo-pyrimidinones Scaffold. Bull. Korean Chem. Soc. 2024, 45, 759–763. [Google Scholar] [CrossRef]
- Sthalam, V.K.; Mahajan, B.; Karra, P.R.; Singh, A.K.; Pabbaraja, S. Sulphonated Graphene Oxide Catalyzed Continuous Flow Synthesis of Pyrazolo Pyrimidinones, Sildenafil and Other PDE-5 Inhibitors. RSC Adv. 2022, 12, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Donaire-Arias, A.; Montagut, A.M.; Puig De La Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo[3,4-b]Pyridines: Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.G.; Lévesque, F.; Seeberger, P.H. Continuous Flow Thermolysis of Azidoacrylates for the Synthesis of Heterocycles and Pharmaceutical Intermediates. Chem. Commun. 2011, 47, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Tsoung, J.; Bogdan, A.R.; Kantor, S.; Wang, Y.; Charaschanya, M.; Djuric, S.W. Synthesis of Fused Pyrimidinone and Quinolone Derivatives in an Automated High-Temperature and High-Pressure Flow Reactor. J. Org. Chem. 2017, 82, 1073–1084. [Google Scholar] [CrossRef]




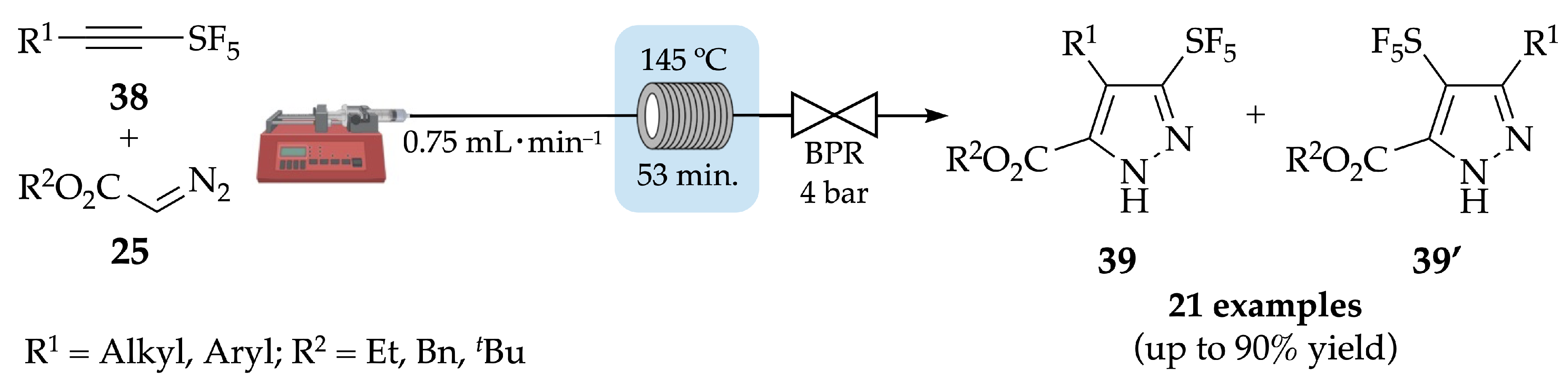

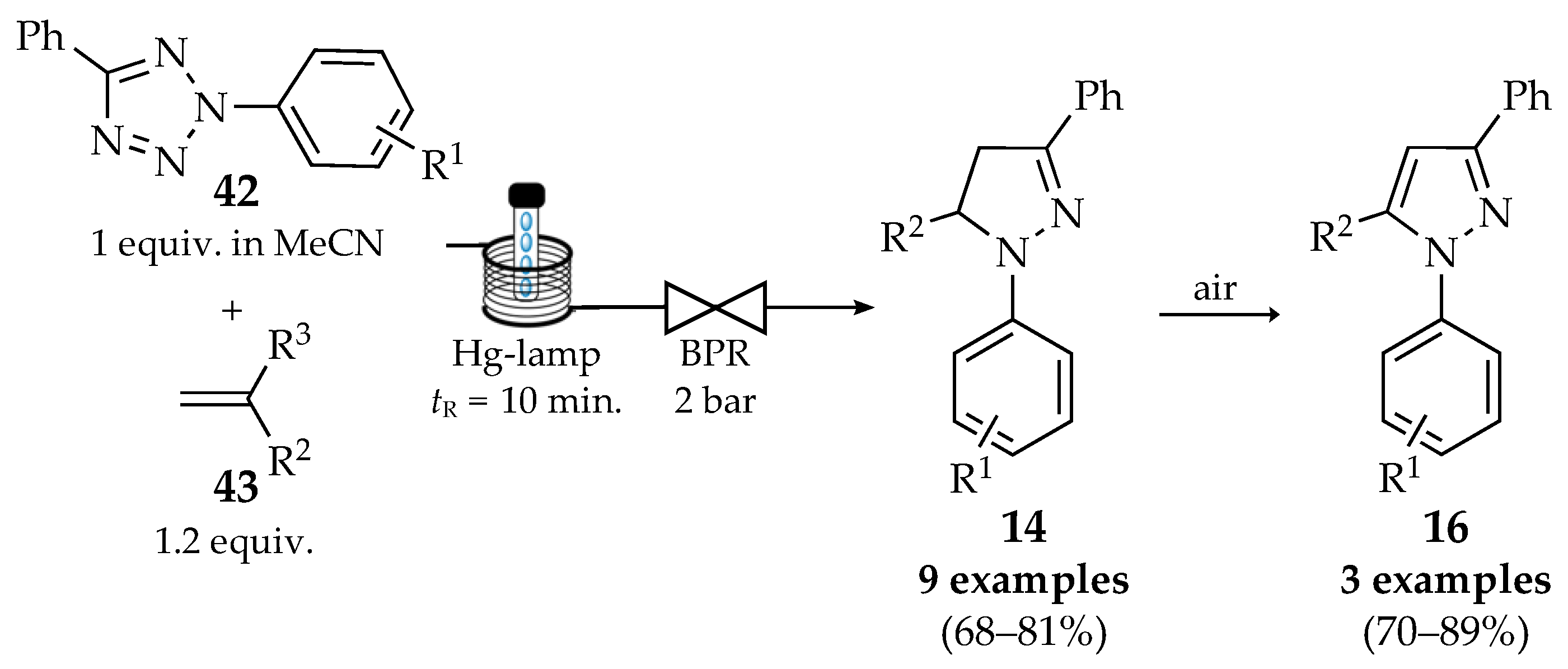
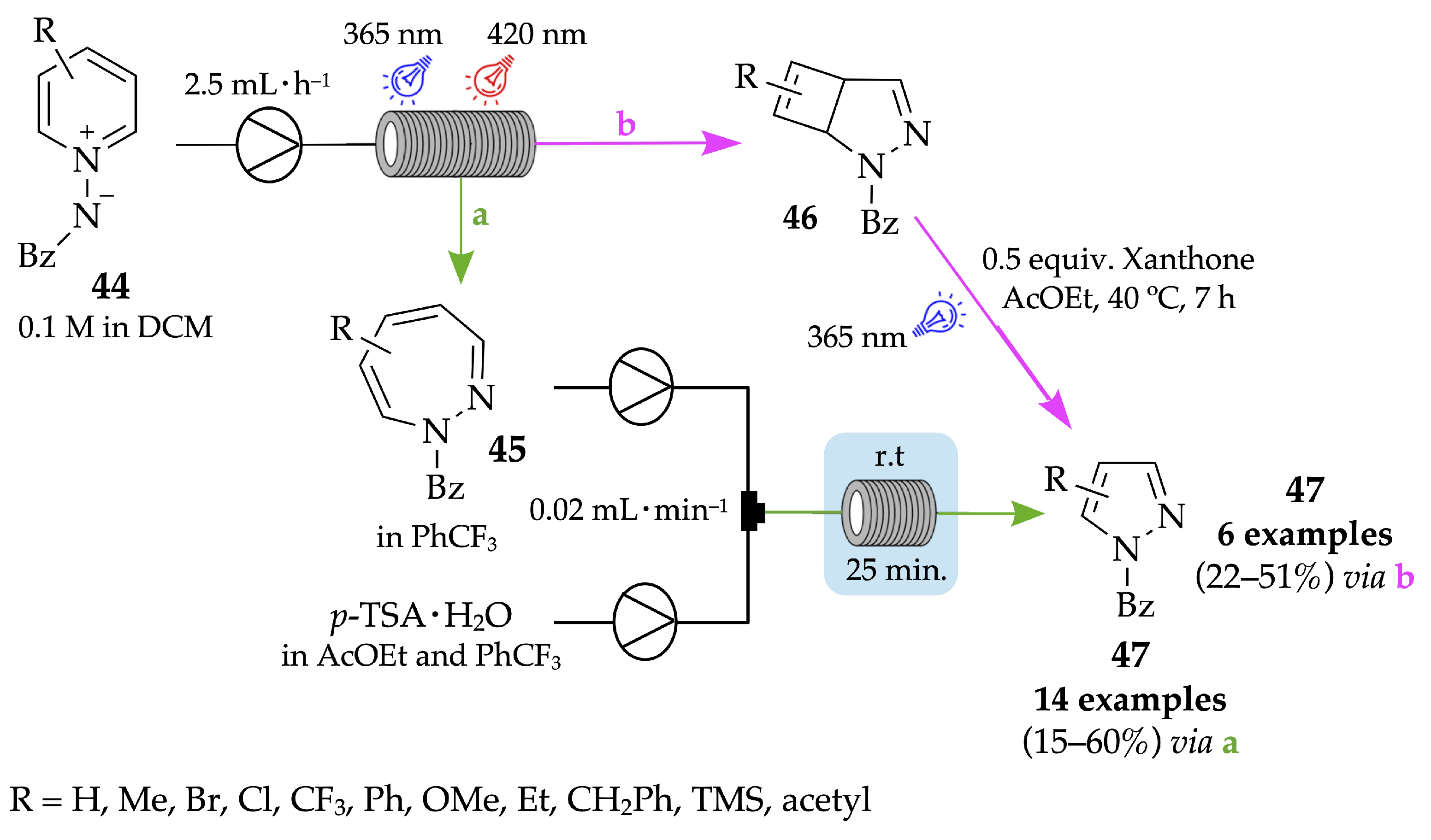
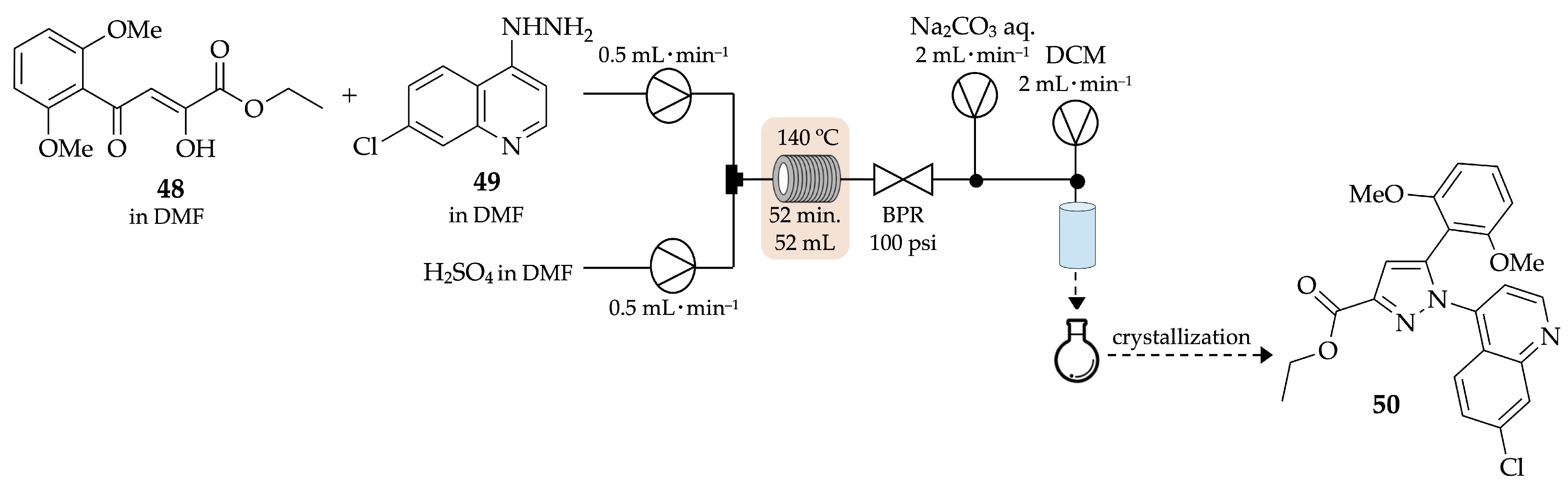

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, C.M.; Silva, A.M.S.; Silva, V.L.M. The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds. Molecules 2025, 30, 1582. https://doi.org/10.3390/molecules30071582
Correia CM, Silva AMS, Silva VLM. The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds. Molecules. 2025; 30(7):1582. https://doi.org/10.3390/molecules30071582
Chicago/Turabian StyleCorreia, Catarina M., Artur M. S. Silva, and Vera L. M. Silva. 2025. "The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds" Molecules 30, no. 7: 1582. https://doi.org/10.3390/molecules30071582
APA StyleCorreia, C. M., Silva, A. M. S., & Silva, V. L. M. (2025). The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds. Molecules, 30(7), 1582. https://doi.org/10.3390/molecules30071582








