Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains
Abstract
1. Introduction
2. Results and Discussion
2.1. Enological Parameters and Fermentation Dynamics
2.2. Key Volatile Compounds
2.3. Odorant Series Values and E-Nose Data Matrices
3. Materials and Methods
3.1. Grape Must and Fermentation Conditions
3.2. Yeasts and Inoculation Conditions
3.2.1. Preparation of Starter Culture of ADY in Free Format
3.2.2. Preparation of Immobilized L. thermotolerans Starter Culture
3.3. Analytical Methods
3.4. Electronic Nose Measurement
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADY | Active dry yeast |
| ANOVA | Analysis of Variance |
| BC | Biocapsules of Lachancea thermotolerans |
| CV | Cross-validation |
| E-nose | Electronic nose |
| EI | Electron impact |
| f | Frequency |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GSH | Glutathione |
| HG | Homogeneous groups |
| LSD | Least significant difference |
| LT | Lachancea thermotolerans |
| LV | Latent variable |
| m | Mass |
| MP | Metschnikowia pulcherrima |
| MPS | Multi-Purpose Sampler |
| MS | Mass spectrometry |
| OAV | Odor Activity Value |
| OPT | Odor perception threshold |
| OS | Odorant series |
| PCA | Principal Component Analysis |
| PCR | Principal component regression |
| PDMS | Polydimethylsiloxane |
| PLS-DA | Partial least squares discriminant analysis |
| QMB | Quartz crystal microbalances |
| RMSEC | Root mean square error in calibration |
| RMSECV | Root mean square error in cross-validation |
| RPD | Residual prediction deviation |
| SBSE | Stir Bar Sorptive Extraction |
| SC | Saccharomyces cerevisiae |
| TD | Thermal Desorption |
| TDU | Thermal Desorption Unit |
| TPC | Triphenylcorrole |
| VIP | Variables of importance in projection |
| WY | Wild yeast (spontaneous fermentation procedure) |
References
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. Advances in wine fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Benito, S.; Marquina, D.; Santos, A. Microsatellite typing of Lachancea thermotolerans for wine fermentation monitoring. Int. J. Food Microbiol. 2023, 394, 110186. [Google Scholar] [CrossRef]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous yeast interactions in dual-starter fermentations may improve the varietal expression of Moschofilero wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Mallard, J.; Ballester, J.; David, V.; Vichy, S.; Tourdot-Maréchal, R.; Alexandre, H.; Roullier-Gall, C. Influence of spontaneous, “pied de cuve” and commercial dry yeast fermentation strategies on wine molecular composition and sensory properties. Food Res. Int. 2023, 174, 113648. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Assessing the impact of commercial Lachancea thermotolerans immobilized in biocapsules on wine quality: Odor active compounds and organoleptic properties. Fermentation 2024, 10, 303. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; You, Y. Mechanisms and effects of non-Saccharomyces yeast fermentation on the aromatic profile of wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Muñoz-Redondo, J.M.; Puertas, B.; Cantos-Villar, E.; Jiménez-Hierro, M.J.; Carbú, M.; Garrido, C.; Moreno-Rojas, J.M. Impact of sequential inoculation with the non-Saccharomyces T. delbrueckii and M. pulcherrima combined with Saccharomyces cerevisiae strains on chemicals and sensory profile of rosé wines. J. Agric. Food Chem. 2021, 69, 1598–1609. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. The life of Saccharomyces and non-Saccharomyces yeasts in drinking wine. Microorganisms 2023, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Sanarica, L.; Zara, I.; Pisarra, C.; Gambacorta, G.; Natrella, G.; Cardinale, M. Cultivar-dependent effects of non-Saccharomyces yeast starter on the oenological properties of wines produced from two autochthonous grape cultivars in Southern Italy. Foods 2022, 11, 3373. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Chemometric differentiation of white wines from a low-aromatic grape obtained by spontaneous fermentation, enriched with non-Saccharomyces, or with a high-glutathione-producing Saccharomyces yeast. Fermentation 2023, 9, 1023. [Google Scholar] [CrossRef]
- Prieto, N.; Rodríguez-Méndez, M.L.; Leardi, R.; De Saja, J.A. Application of multi-way analysis to UV-visible spectroscopy, gas chromatography, and electronic nose data for wine ageing evaluation. Anal. Chim. Acta 2012, 719, 43–51. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Modesti, M.; Moreno-García, J.; Rodríguez-Moreno, M.; Catini, A.; Capuano, R.; Di Natale, C.; Bellincontro, A.; Moreno, J. Differentiation through E-nose and GC-FID data modeling of rosé sparkling wines elaborated via traditional and Charmat methods. J. Sci. Food. Agric. 2023, 105, 1439–1447. [Google Scholar] [CrossRef]
- Cozzolino, D.; Dambergs, R.G. Instrumental analysis of grape must and wine. In Managing Wine Quality: Viticulture and Wine Quality; Woodhead Publishing: Sawston, UK, 2010; pp. 134–161. [Google Scholar]
- Gamboa, J.C.R.; Albarracín, E.S.A.; da Silva, A.; Ferreira, T.A.E. Electronic nose dataset for detection of wine spoilage thresholds. Data Brief 2019, 25, 104202. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; Araujo, I.C.; Albarracín, E.S.; Durán, A.C. Validation of the rapid detection approach for enhancing the electronic nose systems performance, using different deep learning models and support vector machines. Sens. Actuators B Chem. 2021, 327, 128921. [Google Scholar] [CrossRef]
- Alfieri, G.; Modesti, M.; Riggi, R.; Bellincontro, A. Recent advances and future perspectives in the E-nose technologies addressed to the wine industry. Sensors 2024, 24, 2293. [Google Scholar] [CrossRef]
- An, J.; Wilson, D.I.; Deed, R.C.; Kilmartin, P.A.; Young, B.R.; Yu, W. The importance of outlier rejection and significant explanatory variable selection for Pinot Noir wine soft sensor development. Curr. Res. Food Sci. 2023, 6, 100514. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cowey, G.; Lattey, K.A.; Godden, P.; Cynkar, W.U.; Dambergs, R.G.; Janik, L.; Gishen, M. Relationship between wine scores and visible–near-infrared spectra of Australian red wines. Anal. Bioanal. Chem. 2008, 391, 975–981. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Fine wine recognition and appreciation: It is time to change the paradigm of wine tasting. Food Res. Int. 2023, 174, 113668. [Google Scholar] [CrossRef]
- Parr, W.V.; White, K.G.; Heatherbell, D.A. The Nose Knows: Influence of Colour on Perception of Wine Aroma. J. Wine Res. 2003, 14, 79–101. [Google Scholar] [CrossRef]
- Barwich, A.S. Smellosophy, What the Nose Tells the Mind; Harvard University Press: Cambridge, MA, USA, 2020. [Google Scholar]
- González-Caballero, V.; Sánchez, M.T.; López, M.I.; Pérez-Marín, D. First steps towards the development of a non-destructive technique for the quality control of wine grapes during on-vine ripening and on arrival at the winery. J. Food Eng. 2010, 101, 158–165. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.; Dambergs, R. Application of electronic noses in the wine industry. In Handbook on Mass Spectrometry: Instrumentation, Data and Analysis, and Applications; Lang, J.K., Ed.; Hauppauge: New York, NY, USA, 2009; pp. 435–445. ISBN 978-1-60741-580-0. [Google Scholar]
- Gonzalez-Viejo, C.; Fuentes, S. Digital Assessment and Classification of Wine Faults Using a Low-Cost Electronic Nose, Near-Infrared Spectroscopy and Machine Learning Modelling. Sensors 2022, 22, 2303. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An integrative view of the role of Lachancea thermotolerans in wine technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione production by non-Saccharomyces yeast and its impact on winemaking: A review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of odor-active volatiles and odor contribution based on binary interaction effects in mango and vodka cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef]
- Guclu, G.; Sevindik, O.; Kelebek, H.; Selli, S. Determination of volatiles by odor activity value and phenolics of cv. Ayvalik early-harvest olive oil. Foods 2016, 5, 46. [Google Scholar] [CrossRef]
- Abreu, T.; Perestrelo, R.; Bordiga, M.; Locatelli, M.; Coïsson, J.D.; Câmara, J.S. The Flavor Chemistry of FortifiedWines—A Comprehensive Approach. Foods 2021, 10, 1239. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Orbanić, F.; Horvat, I.; Lukić, I.; Ilak Peršurić, A.S.; Bubola, M.; Plavša, T.; Radeka, S. Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging. Appl. Sci. 2024, 14, 8729. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of yeast strain on odor-active compounds in Fiano wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Zhang, X.K.; Liu, P.T.; Zheng, X.W.; Li, Z.F.; Sun, J.P.; Fan, J.S.; Ding, Z.Y. The role of indigenous yeasts in shaping the chemical and sensory profiles of wine: Effects of different strains and varieties. Molecules 2024, 29, 4279. [Google Scholar] [CrossRef]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of industrial yeast derivative products on the modification of wine aroma compounds and sensorial profile. A review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef]
- Nedović, V.; Gibson, B.; Mantzouridou, T.F.; Bugarski, B.; Djordjević, V.; Kalušević, A.; Yilmaztekin, M. Aroma formation by immobilized yeast cells in fermentation processes. Yeast 2015, 32, 173–216. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Yang, Y.; Frank, S.; Wei, X.; Wang, X.; Li, Y.; Steinhaus, M.; Tao, Y. Molecular rearrangement of four typical grape free terpenes in the wine environment. J. Agric. Food Chem. 2023, 71, 721–728. [Google Scholar] [CrossRef]
- Li, J.; Hong, M.; Qi, B. Impact of Torulaspora delbrueckii during fermentation on aromatic profile of Vidal Blanc icewine. Front. Microbiol. 2022, 13, 860128. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Xiong, S.; Tao, Y. Utilization efficiency of Ehrlich pathway-related amino acid affected higher alcohol acetate production of non-Saccharomyces yeasts during alcoholic fermentation. Food Biosci. 2024, 61, 104963. [Google Scholar] [CrossRef]
- Puig-Pujol, A.; Bertran, E.; García-Martínez, T.; Capdevila, F.; Mínguez, S.; Mauricio, J.C. Application of a New Organic Yeast Immobilization Method for Sparkling Wine Production. Am. J. Enol. Vitic. 2013, 64, 386–394. [Google Scholar] [CrossRef]
- Fernández-Fernández, E.; Rodríguez-Nogales, J.M.; Vila-Crespo, J.; Falqué-López, E. Application of Immobilized Yeasts for Improved Production of Sparkling Wines. Fermentation 2022, 8, 559. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Ma, Y.; Li, T.; Xu, X.; Ji, Y.; Jiang, X.; Shi, X.; Wang, B. Investigation of Volatile Compounds, Microbial Succession, and Their Relation During Spontaneous Fermentation of Petit Manseng. Front. Microbiol. 2021, 12, 717387. [Google Scholar] [CrossRef]
- Capone, S.; Tufariello, M.; Francioso, L.; Montagna, G.; Casino, F.; Leone, A.; Siciliano, P. Aroma analysis by GC/MS and electronic nose dedicated to Negroamaro and Primitivo typical Italian Apulian wines. Sens. Actuators B Chem. 2013, 179, 259–269. [Google Scholar] [CrossRef]
- Wang, B.; Deng, J.; Jiang, H.; Chen, Q. Electronic nose signals-based deep learning models to realize high-precision monitoring of simultaneous saccharification and fermentation of cassava. Microchem. J. 2022, 182, 107929. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- OIV. Available online: https://www.oiv.int/en (accessed on 27 January 2025).
- Roldán, A.M.; Sánchez-García, F.; Pérez-Rodríguez, L.; Palacios, V.M. Influence of Different Vinification Techniques on Volatile Compounds and the Aromatic Profile of Palomino Fino Wines. Foods 2021, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Capuano, R.; Mansi, A.; Paba, E.; Marcelloni, A.M.; Chiominto, A.; Proietto, A.R. A pilot study for Legionella pneumophila volatilome characterization using a gas sensor array and GC/MS techniques. Sensors 2023, 23, 1401. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Cain, W.S.; Abraham, M.H.; Gil-Lostes, J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol. Behav. 2008, 95, 658–667. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The effect of yeast, sugar and sulfur dioxide on the volatile compounds in wine. Fermentation 2023, 9, 541. [Google Scholar] [CrossRef]
- Ogawa, M.; Vararu, F.; Moreno-Garcia, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Analyzing the minor volatilome of Torulaspora delbrueckii in an alcoholic fermentation. Eur. Food Res. Technol. 2022, 248, 613–624. [Google Scholar] [CrossRef]
- Pardo, E.; Rico, J.; Gil, J.V.; Orejas, M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered Saccharomyces cerevisiae wine strain. Microb. Cell Fact. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dai, F.; Yao, J.; Li, Z.; Huang, Z.; Liu, H.; Zhu, Z. Characterization of the volatile profile of Feijoa (Acca sellowiana) fruit at different ripening stages by HS-SPME-GC/MS. LWT Food Sci. Technol. 2023, 184, 115011. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Zhang, S.; Petersen, M.A.; Liu, J.; Toldam-Andersen, T.B.; Ebeler, S.E.; Hopfer, H. Influence of pre-fermentation treatments on wine volatile and sensory profile of the new disease-tolerant cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Du, G.; Gao, Y.T.; Wang, L.W.; Meng, D.; Li, B.J.; Brennan, C.; Wang, M.Y.; Zhao, H.; Wang, S.Y.; et al. The effect of carbonic maceration during winemaking on the color, aroma and sensory properties of ‘Muscat Hamburg’ wine. Molecules 2019, 24, 3120. [Google Scholar] [CrossRef]
- Zhu, L.X.; Zhang, M.M.; Shi, Y.; Duan, C.Q. Evolution of the aromatic profile of traditional Msalais wine during industrial production. Int. J. Food Prop. 2019, 22, 911–924. [Google Scholar] [CrossRef]
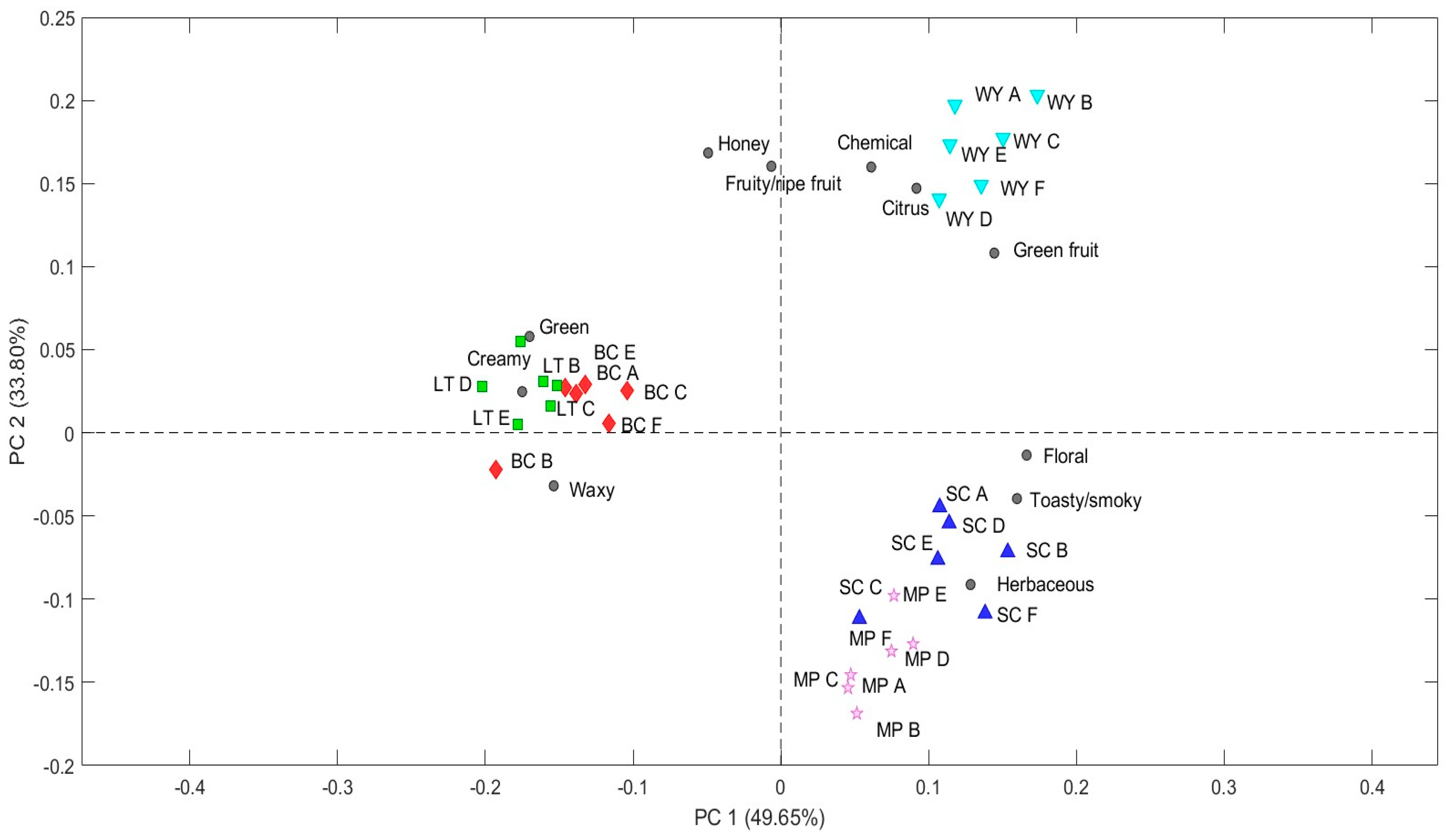
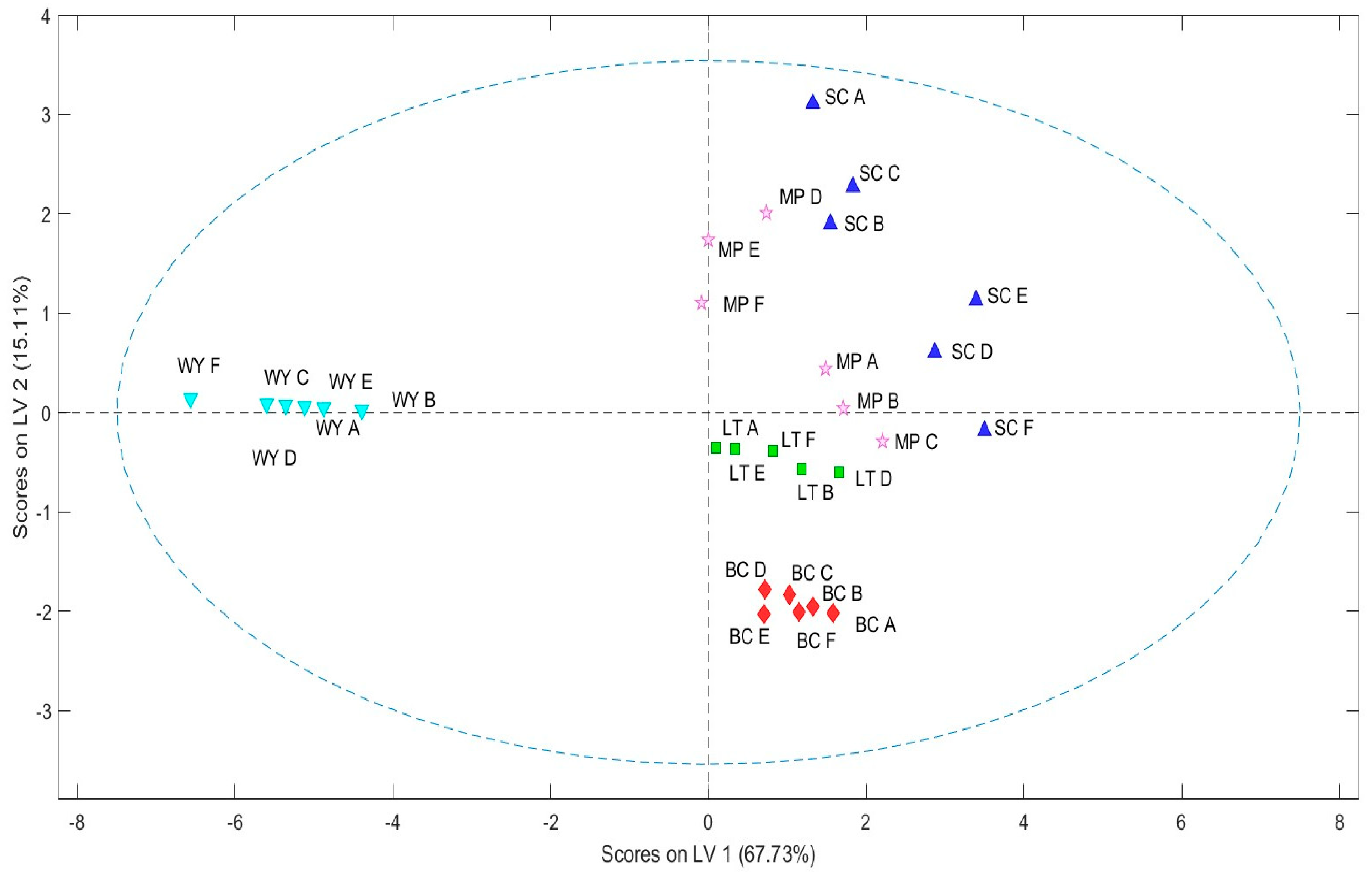
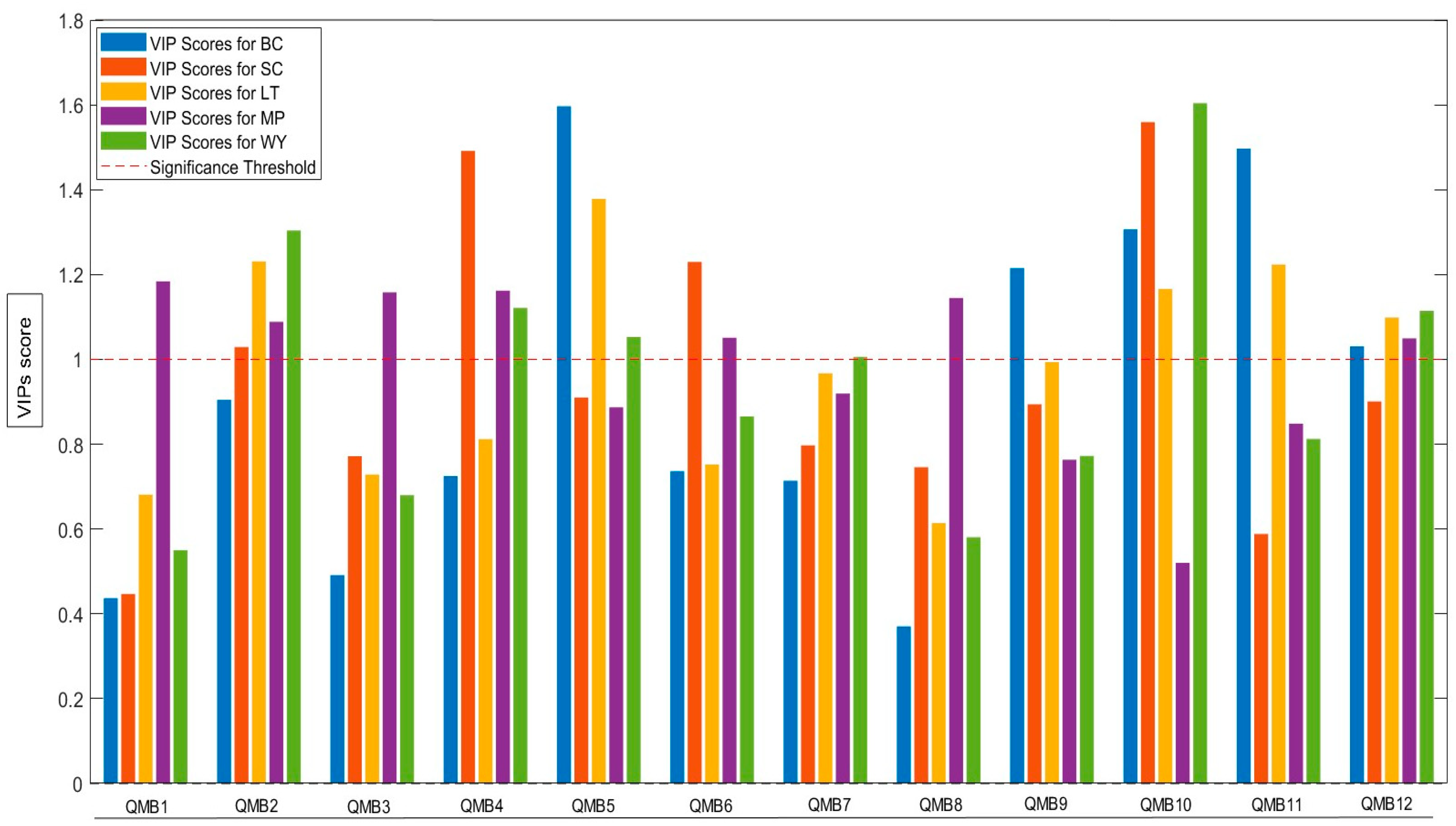
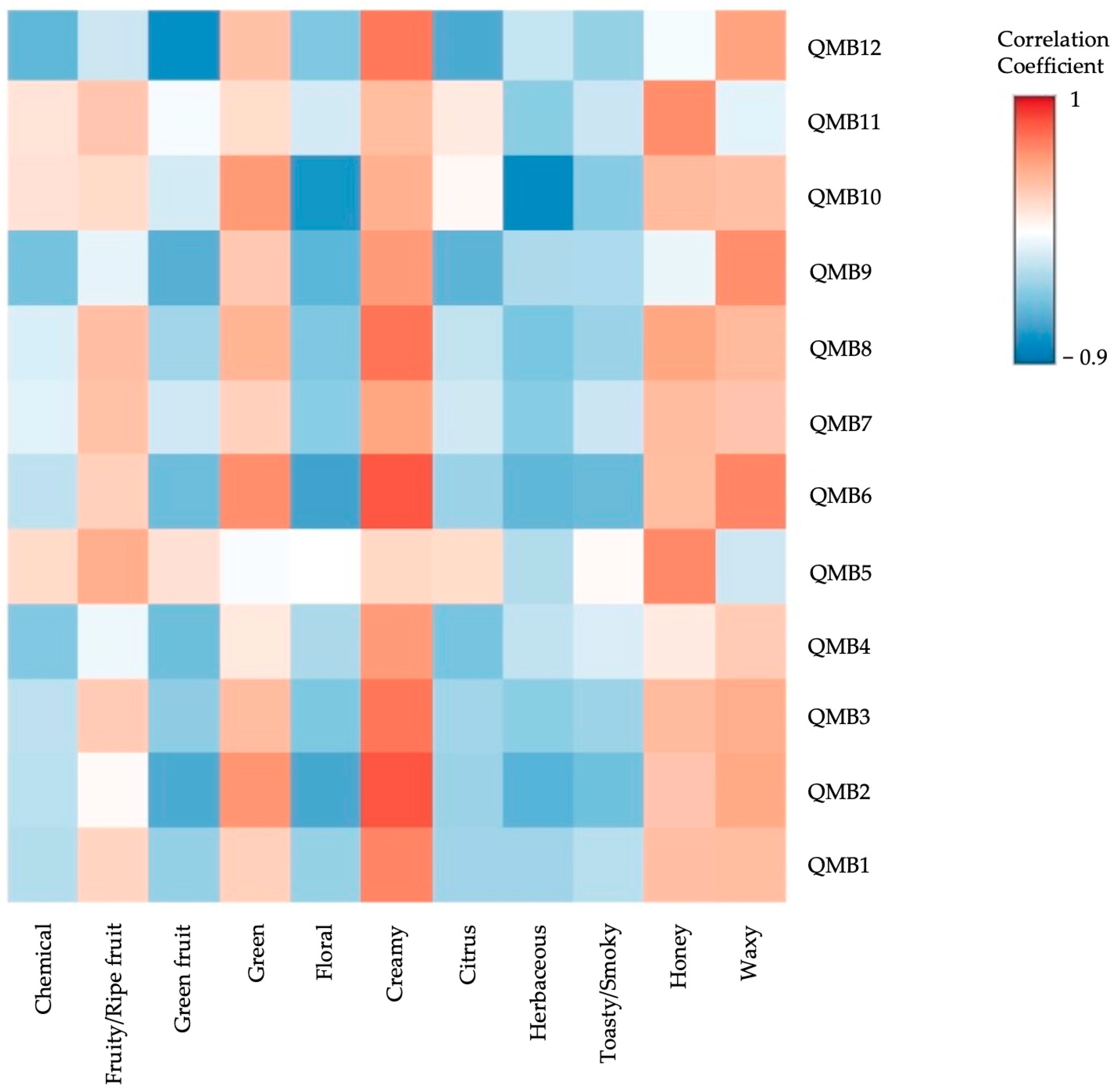
| WY | SC | MP | LT | BC | |
|---|---|---|---|---|---|
| Ethanol (% v/v) | 13.95 ± 0.05 c | 13.75 ± 0.27 c | 14.10 ± 0.01 c | 12.30 ± 0.33 a | 12.9 ± 0.5 b |
| pH | 3.23 ± 0.01 b | 3.29 ± 0.00 c | 3.18 ± 0.01 a | 3.39 ± 0.03 d | 3.40 ± 0.02 d |
| Volatile acidity (g L−1) | 0.27 ± 0.01 a | 0.46 ± 0.00 c | 0.27 ± 0.03 a | 0.39 ± 0.00 b | 0.44 ± 0.02 c |
| Total acidity (g L−1) | 7.70 ± 0.05 c | 6.30 ± 0.08 a | 7.17 ± 0.04 b | 9.5 ± 0.2 e | 8.80 ± 0.08 d |
| Reducing sugars (g L−1) | 0.17 ± 0.00 a | 0.14 ± 0.00 a | 0.22 ± 0.00 ab | 0.6 ± 0.2 b | 2.4 ± 0.7 c |
| Lactic acid (g L−1) | 0.23 ± 0.02 a | 0.18 ± 0.00 a | 0.25 ± 0.01 a | 4.7 ± 0.5 c | 4.0 ± 0.4 b |
| Malic acid (g L−1) | 0.88 ± 0.07 bc | 0.84 ± 0.02 d | 0.81 ± 0.01 cd | 0.44 ± 0.02 a | 0.55 ± 0.09 ab |
| Gluthatione (mg L−1) | 0.64 ± 0.04 b | 1.99 ± 0.19 c | 0.25 ± 0.01 a | 6.61 ± 0.12 e | 3.2 ± 0.7 d |
| Compounds | WY | SC | MP | LT | BC | HG | OS |
|---|---|---|---|---|---|---|---|
| Acetates (9) | |||||||
| Ethyl acetate | 7.83 ± 0.09 *c | 5.7 ± 0.2 *b | 5.0 ± 0.2 *a | 11.5 ± 0.9 *e | 9.4 ± 0.4 *d | 4 | 1,2,4 |
| Butyl acetate | 0.00034 ± 0.00009 a | 0.0002 ± 0.0001 a | 0.00035 ± 0.00004 a | 0.00081 ± 0.00009 b | 0.0008 ± 0.0001 b | 2 | 2 |
| Isoamyl acetate | 24 ± 2 *c | 19 ± 3 *b | 9.6 ± 0.9 *a | 11 ± 2 *a | 18 ± 2 *b | 3 | 2 |
| (Z)-3-Hexenyl acetate | 0.5 ± 0.2 *c | 0.75 ± 0.07 *d | 0.0001 ± 0 a | 0.22 ± 0.06 *b | 0.28 ± 0.03 *b | 4 | 2,4 |
| Hexyl acetate | 0.7 ± 0.3 *b | 1.1 ± 0.2 *c | 0.0005 ± 0 a | 0.11 ± 0.02 a | 0.15 ± 0.05 a | 3 | 2,3 |
| Octyl acetate | 0.115 ± 0.005 ab | 0.121 ± 0.007 b | 0.112 ± 0.002 a | 0.113 ± 0.007 ab | 0.12 ± 0.01 ab | 2 | 5,11 |
| Ethyl phenylacetate | 0.031 ± 0.004 c | 0.009 ± 0.002 a | 0.023 ± 0.005 b | 0.051 ± 0.004 e | 0.041 ± 0.008 d | 5 | 5,10 |
| 2-Phenylethyl acetate | 15 ± 1 *d | 3.6 ± 0.2 *c | 2.4 ± 0.2 *b | 1.3 ± 0.2 *a | 2.2 ± 0.1 *b | 4 | 5,10 |
| Geranyl acetate | 0.30 ± 0.09 *b | 0.21 ± 0.04*a | 0.54 ± 0.03 *d | 0.41 ± 0.03 *c | 0.45 ± 0.04 *c | 3 | 5 |
| Ethyl esters (13) | |||||||
| Ethyl lactate | 0.17 ± 0.02 a | 0.166 ± 0.007 a | 0.21 ± 0.02 *a | 0.95 ± 0.09 *c | 0.80 ± 0.07 *b | 3 | 2 |
| Ethyl isobutyrate | 1.9 ± 0.2 *c | 0.60 ± 0.09 *a | 1.08 ± 0.06 *b | 3.9 ± 0.2 *e | 3.5 ± 0.3 *d | 5 | 2 |
| Ethyl butyrate | 1.9 ± 0.2 *a | 1.9 ± 0.3 *a | 2.6 ± 0.3 *b | 1.8 ± 0.2 *a | 1.7 ± 0.1 *a | 2 | 2 |
| Ethyl 2-methylbutyrate | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.16 ± 0.02 b | 2 | 2 |
| Ethyl 3-methylbutyrate | 1.7 ± 0.3 *b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 2 | 2,3 |
| Diethyl succinate | 0.118 ± 0.04 c | 0.079 ± 0.007 b | 0.08 ± 0.02 b | 0.0 ± 0.0 a | 0.02 ± 0.02 a | 3 | 2 |
| Ethyl hexanoate | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 1 | 2,3 |
| Ethyl heptanoate | 0.087 ± 0.005 d | 0.055 ± 0.005 b | 0.0 ± 0.0 a | 0.058 ± 0.007 bc | 0.062 ± 0.006 c | 4 | 2,3 |
| Ethyl octanoate | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 1 | 2,11 |
| Ethyl decanoate | 0.113 ± 0.005 ab | 0.12 ± 0.02 b | 0.114 ± 0.009 ab | 0.106 ± 0.004 a | 0.15 ± 0.02 c | 3 | 2,11 |
| Ethyl dodecanoate | 0.0069 ± 0.0004 a | 0.0063 ± 0.0003 a | 0.0069 ± 0.0003 a | 0.007 ± 0.002 a | 0.020 ± 0.003 b | 2 | 11 |
| Ethyl tetradecanoate | 0.0042 ± 0.0003 b | 0.0041 ± 0.0002 b | 0.0042 ± 0.0003 b | 0.0034 ± 0.0004 a | 0.0042 ± 0.0004 b | 2 | 5,6 |
| Ethyl hexadecanoate | 0.0064 ± 0.0008 b | 0.0049 ± 0.0005 a | 0.008 ± 0.002 c | 0.0040 ± 0.0007 a | 0.0052 ± 0.0005 ab | 3 | 2,6,11 |
| Other esters (3) | |||||||
| Cis-3-Hexenyl butyrate | 9 ± 1 *c | 7 ± 1 *a | 8.6 ± 0.8 *bc | 8 ± 1 *ab | 7.1 ± 0.8 *a | 3 | 4 |
| 2-Phenylethyl butanoate | 0.004 ± 0.002 b | 0.000005 ± 0.000000 a | 0.010 ± 0.002 c | 0.004 ± 0.002 b | 0.009 ± 0.001 c | 3 | 5 |
| E-Methyldihydrojasmonate | 0.012 ± 0.007 ab | 0.02 ± 0.02 b | 0.02 ± 0.01 b | 0.008 ± 0.002 a | 0.011 ± 0.002 ab | 2 | 5 |
| Alcohols (10) | |||||||
| Methanol | 0.062 ± 0.005 a | 0.09 ± 0.01 b | 0.067 ± 0.004 a | 0.060 ± 0.003 a | 0.118 ± 0.007 c | 3 | 1 |
| 1-Propanol | 0.027 ± 0.002 b | 0.023 ± 0.001 a | 0.029 ± 0.001 b | 0.083 ± 0.003 c | 0.082 ± 0.005 c | 3 | 1,4 |
| Isobutanol | 1.6 ± 0.1 *b | 0.79 ± 0.02 *a | 2.34 ± 0.04 *d | 1.87 ± 0.09 *c | 1.93 ± 0.08 *c | 4 | 1 |
| 2-Methyl-1-butanol | 1.80 ± 0.07 *b | 1.56 ± 0.05 *a | 2.56 ± 0.05 *d | 2.42 ± 0.06 *c | 2.7 ± 0.1 *e | 5 | 1 |
| 3-Methyl-1-butanol | 10.5 ± 0.3 *c | 9.1 ± 0.3 *a | 10.0 ± 0.1 *b | 10.6 ± 0.4 *cd | 10.8 ± 0.3 *d | 4 | 1 |
| 2-Phenylethanol | 6 ± 1 *ab | 5.0 ± 0.3 *a | 6.2 ± 0.4 *b | 8.2 ± 0.7 *c | 8.7 ± 0.5 *c | 3 | 5 |
| Hexanol | 0.10 ± 0.01 a | 0.092 ± 0.007 a | 0.094 ± 0.006 a | 0.09 ± 0.01 a | 0.118 ± 0.006 b | 2 | 4 |
| 2-Ethyl-1-hexanol | 0.0017 ± 0.0004 a | 0.0021 ± 0.0004 ab | 0.0017 ± 0.0002 a | 0.0024 ± 0.0006 b | 0.0024 ± 0.0004 b | 2 | 7 |
| Dodecanol | 0.006 ± 0.003 cd | 0.0053 ± 0.0005 bc | 0.0074 ± 0.0008 d | 0.004 ± 0.001 ab | 0.0032 ± 0.0008 a | 4 | 11 |
| 2-Methoxy-4-vinylphenol | 0.7 ± 0.1 *b | 0.8 ± 0.2 *bc | 0.83 ± 0.02 *c | 0.000008 ± 0.0 a | 0.000008 ± 0.0 a | 3 | 9 |
| Lactones (4) | |||||||
| γ-Butyrolactone | 0.40 ± 0.07 *a | 0.48 ± 0.02 *b | 0.71 ± 0.04 *c | 0.41 ± 0.05 *a | 0.39 ± 0.03 *a | 3 | 6 |
| γ-Crotonolactone | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1 | 6 |
| γ-Nonalactone | 0.20 ± 0.03 *c | 0.17 ± 0.02 b | 0.39 ± 0.03 *d | 0.14 ± 0.03 a | 0.21 ± 0.02 *c | 4 | 6,2 |
| β-Damascenone | 47 ± 2 *b | 62 ± 9 *d | 55 ± 4 *c | 38 ± 3 *a | 43 ± 5 *ab | 4 | 5,8 |
| Carbonyl compounds (10) | |||||||
| Acetaldehyde | 7 ± 1 *ab | 9.5 ± 0.9 *b | 6.4 ± 0.4 *a | 20 ± 4 *c | 21 ± 2 *c | 3 | 1,2 |
| 1,1-Diethoxyethane | 0.0 ± 0.0 a | 0.010 ± 0.003 a | 0.0 ± 0.0 a | 8 ± 2 *b | 1.45 ± 0.04 *a | 2 | 1,4 |
| Acetoin | 1.2 ± 0.1 *a | 0.99 ± 0.06 *a | 1.06 ± 0.07 *a | 4.8 ± 0.3 *b | 5.7 ± 0.6 *c | 3 | 6 |
| Hexanal | 0.33 ± 0.08 *ab | 0.24 ± 0.09 *a | 0.48 ± 0.08 *c | 0.46 ± 0.07 *c | 0.39 ± 0.06 *bc | 3 | 4 |
| Furfural | 0.7 ± 0.2 *a | 0.6 ± 0.2 *a | 0.7 ± 0.1 *a | 0.57 ± 0.04 *a | 0.9 ± 0.2 *b | 2 | 1,9 |
| Benzaldehyde | 0.0 ± 0.0 a | 0.002 ± 0.002 b | 0.0 ± 0.0 a | 0.003 ± 0.000 b | 0.007 ± 0.002 c | 3 | 2 |
| Octanal | 0.0 ± 0.0 a | 0.33 ± 0.02 *b | 0.4 ± 0.1 *bc | 0.6 ± 0.1 *d | 0.4 ± 0.1 *c | 4 | 7 |
| Nonanal | 2.9 ± 0.3 *a | 3.3 ± 0.3 *b | 3.6 ± 0.3 *bc | 3.7 ± 0.2 *c | 3.4 ± 0.2 *b | 3 | 7 |
| 2-Phenylacetaldehyde | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 8 ± 1 *b | 10.3 ± 0.7 *c | 3 | 4,10 |
| Decanal | 4.4 ± 0.4 *a | 5.2 ± 0.2 *b | 5.0 ± 0.6 *b | 6.3 ± 0.5 *c | 6.1 ± 0.2 *c | 3 | 8,11 |
| Terpenes and derivatives (5) | |||||||
| Limonene | 468 ± 27 *b | 348 ± 30 *a | 322 ± 44 *a | 352 ± 20 *a | 320 ± 34 *a | 2 | 1,7 |
| E-Geranyl acetone | 0.024 ± 0.008 d | 0.017 ± 0.003 bc | 0.022 ± 0.005 cd | 0.011 ± 0.002 a | 0.015 ± 0.003 ab | 4 | 5 |
| Z-Geranyl acetone | 0.0302 ± 0.0009 a | 0.030 ± 0.002 a | 0.031 ± 0.001 a | 0.030 ± 0.002 a | 0.0299 ± 0.0009 a | 1 | 5 |
| Nerolidol | 0.0 ± 0.0 a | 0.0062 ± 0.0004 c | 0.002 ± 0.002 b | 0.0001 ± 0.0001 a | 0.0 ± 0.0 a | 3 | 4,5 |
| Farnesol | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 1 | 5 |
| Miscellaneous (3) | |||||||
| 2,3-Butanediol levo | 0.7 ± 0.2 *b | 0.57 ± 0.05 *ab | 0.69 ± 0.08 *b | 0.49 ± 0.05 a | 0.47 ± 0.08 *a | 2 | 2,6 |
| 2,3-Butanediol meso | 0.3 ± 0.1 *b | 0.17 ± 0.01 a | 0.25 ± 0.02 *b | 0.19 ± 0.01 a | 0.19 ± 0.02 a | 2 | 2,6 |
| 2-Pentylfuran | 1.0 ± 0.2 *c | 0.5 ± 0.2 *b | 0.66 ± 0.06 *b | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 3 | 3 |
| Odorant Series | Mean | Min | Max | SD | RMSEC | RMSEC CV | R2 Cal | R2 CV | RPD |
|---|---|---|---|---|---|---|---|---|---|
| Chemical | 389.43 | 291.34 | 522.09 | 60.67 | 31.89 | 40.12 | 0.71 | 0.56 | 1.51 |
| Fruity/ripe fruit | 41.26 | 28.66 | 53.35 | 7.17 | 2.92 | 3.9 | 0.82 | 0.70 | 1.84 |
| Green fruit | 1.23 | 0.14 | 3.7 | 1.25 | 0.3 | 0.38 | 0.94 | 0.90 | 3.29 |
| Green | 22.31 | 12.76 | 37.35 | 9.22 | 2.91 | 3.71 | 0.89 | 0.83 | 2.49 |
| Floral | 61.18 | 42.85 | 84.94 | 10.13 | 4.27 | 5.25 | 0.81 | 0.72 | 1.93 |
| Creamy | 4.26 | 2.29 | 7.74 | 1.93 | 0.66 | 0.82 | 0.87 | 0.81 | 2.35 |
| Citrus | 365.81 | 263 | 507.1 | 62.63 | 32.56 | 41.36 | 0.72 | 0.56 | 1.51 |
| Herbaceous | 54.34 | 38.1 | 81.87 | 9.78 | 4.43 | 5.38 | 0.78 | 0.69 | 1.82 |
| Toasty/smoky | 1.15 | 0.52 | 1.84 | 0.41 | 0.22 | 0.27 | 0.70 | 0.53 | 1.52 |
| Honey | 8.61 | 2.24 | 16.64 | 4.99 | 1.42 | 1.84 | 0.91 | 0.85 | 2.71 |
| Waxy | 5.66 | 4.09 | 7.36 | 0.83 | 0.44 | 0.54 | 0.69 | 0.55 | 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castells, R.; Modesti, M.; Moreno-García, J.; Catini, A.; Capuano, R.; Di Natale, C.; Bellincontro, A.; Moreno, J. Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains. Molecules 2025, 30, 1584. https://doi.org/10.3390/molecules30071584
Muñoz-Castells R, Modesti M, Moreno-García J, Catini A, Capuano R, Di Natale C, Bellincontro A, Moreno J. Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains. Molecules. 2025; 30(7):1584. https://doi.org/10.3390/molecules30071584
Chicago/Turabian StyleMuñoz-Castells, Raquel, Margherita Modesti, Jaime Moreno-García, Alexandro Catini, Rosamaria Capuano, Corrado Di Natale, Andrea Bellincontro, and Juan Moreno. 2025. "Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains" Molecules 30, no. 7: 1584. https://doi.org/10.3390/molecules30071584
APA StyleMuñoz-Castells, R., Modesti, M., Moreno-García, J., Catini, A., Capuano, R., Di Natale, C., Bellincontro, A., & Moreno, J. (2025). Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains. Molecules, 30(7), 1584. https://doi.org/10.3390/molecules30071584








