Dy3+ and Mn4+ Ions Co-Doped Stannate Phosphors for Applications in Dual-Mode Optical Thermometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure
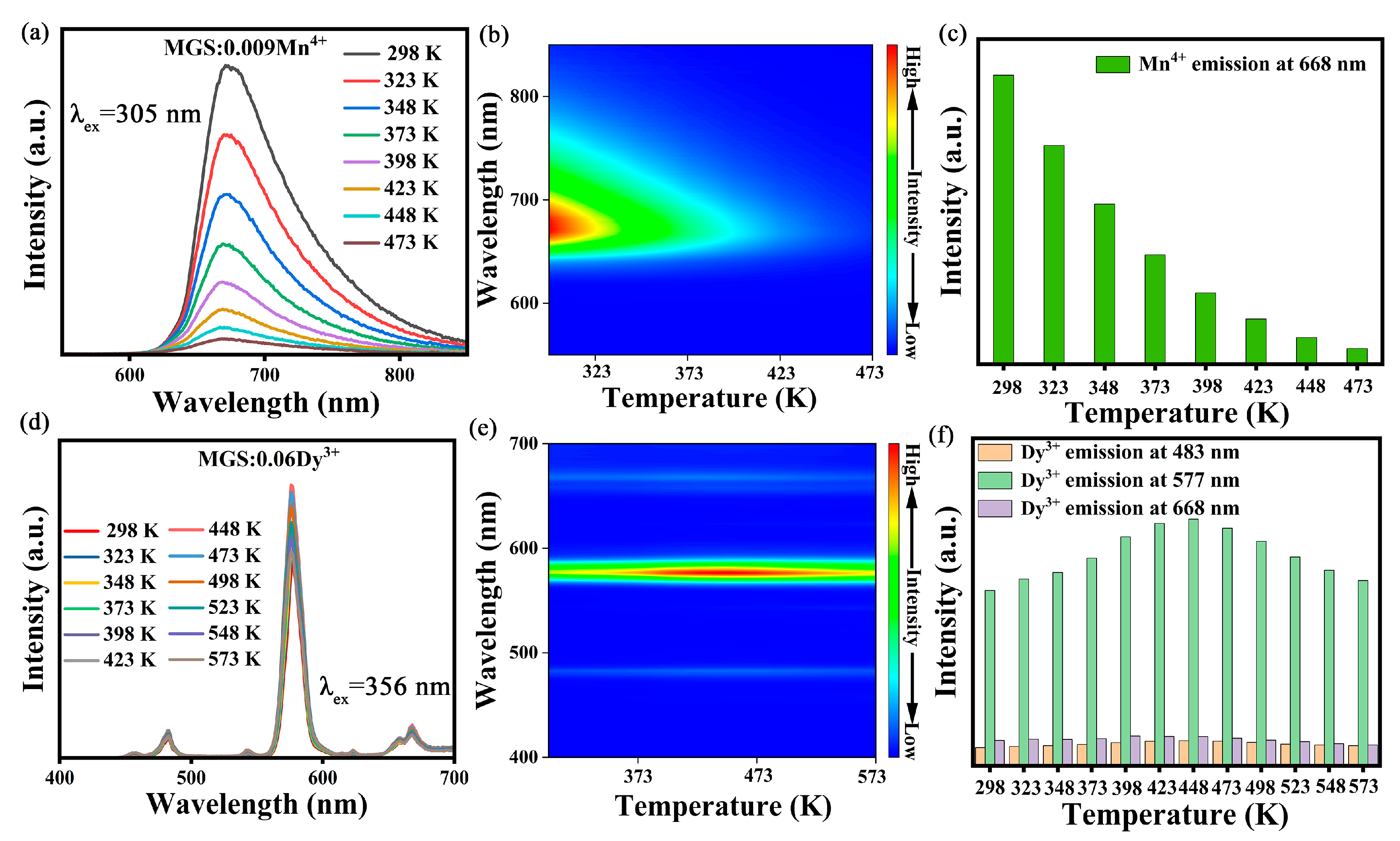
2.2. Optical Properties
2.3. Temperature Sensing Characterization
| Sample | Temperature Range (K) | Sr-Max (% K−1) | Mode | Ref. |
|---|---|---|---|---|
| La2LiSbO6:Mn4+,Dy3+ | 303–523 | 0.769 | FIR | [10] |
| Ca2LaNbO6:Mn4+,Eu3+ | 298–498 | 1.51 | FIR | [39] |
| SrLaLiTeO6:Mn4+,Dy3+ | 298–673 | 1.6 | FIR | [40] |
| MGS:0.06Dy3+,0.009Mn4+ | 298–473 | 1.82 | FIR | This work |
| Ca2GdSbO6:Eu3+, Mn4+ | 303–503 | 1.47 | FL | [22] |
| Ba2GdNbO6:Eu3+, Mn4+ | 303–483 | 1.73 | FL | [41] |
| MGS:0.06Dy3+,0.009Mn4+ | 298–473 | 2.75 | FL | This work |
3. Materials and Methods
3.1. Preparation of Materials
3.2. Characterization of Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.J.; Rosal, D.B.; Jaque, D.; Uchiyama, S.; Jin, D.Y. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [PubMed]
- Kucsko, G.; Maurer, P.C.; Yao, N.Y.; Kubo, M.; Noh, H.J.; Lo, P.K.; Park, H.; Lukin, M.D. Nanometre-scale thermometry in a living cell. Nature 2013, 500, 54–58. [Google Scholar] [PubMed]
- Liu, W.; Zhao, D.; Zhang, R.J.; Yao, Q.X.; Zhu, S.Y. Fluorescence lifetime-based luminescent thermometry material with lifetime varying over a factor of 50. Inorg. Chem. 2022, 61, 16468–16476. [Google Scholar] [PubMed]
- Venturini, F.; Bürgi, R.; Borisov, S.M.; Klimant, I. Optical temperature sensing using a new thermographic phosphor. Sensor Actuat. A-Phys. 2015, 233, 324–329. [Google Scholar] [CrossRef]
- Sun, S.; Qian, J.; Li, Z.; Huang, L.; Li, D. Sensitivity Enhancement of Thermometry in Tb3+-Doped KY(CO3)2:Sm3+ by Energy Transfer. Molecules 2025, 30, 767. [Google Scholar] [CrossRef]
- Trung, T.Q.; Dang, T.M.L.; Ramasundaram, S.; Toi, P.T.; Park, S.Y.; Lee, N.E. A Stretchable Strain-Insensitive Temperature Sensor Based on Free-Standing Elastomeric Composite Fibers for On-Body Monitoring of Skin Temperature. ACS Appl. Mater. Interfaces 2019, 11, 2317–2327. [Google Scholar]
- Qiang, K.; Ye, Y.; Mao, Q.; Chen, F.; Chu, L.; Liu, M.; Zhong, J. Dy3+, Mn4+ co-doped phosphors for synergistic luminescent dual-mode thermometer and high-resolution imaging. Mater. Design 2024, 241, 112906. [Google Scholar]
- Atabaev, T.S.; Sayatova, S.; Molkenova, A.; Taniguchi, I. Nitrogen-doped carbon nanoparticles for potential temperature sensing applications. Sens. Bio-Sens. Res. 2019, 22, 100253. [Google Scholar]
- Hu, F.Y.; Zhang, D.; Wu, Y.J.; Sun, C.M.; Xu, C.C.; Wang, Q.R.; Xie, Y.R.; Shi, Q.; Li, S.H.; Wang, K. Optical behaviors of Mn4+-modified cubic type ZnTiO3:Eu3+ nanocrystals: Application in optical thermometers based on fluorescence intensity ratio and lifetime. Spectrochim. Acta A 2024, 304, 123339. [Google Scholar]
- Song, Y.; Guo, N.; Li, J.; Xin, Y.; Lü, W.; Miao, Y. Dual-emissive Ln3+/Mn4+ Codoped double perovskite phosphor via site-beneficial occupation. Mater. Adv. 2021, 2, 1402–1412. [Google Scholar]
- Zheng, T.; Sójka, M.; Woźny, P.; Martín, I.R.; Lavín, V.; Zych, E.; Lis, S.; Du, P.; Luo, L.; Runowski, M. Supersensitive Ratiometric Thermometry and Manometry Based on Dual-Emitting Centers in Eu2+/Sm2+-Doped Strontium Tetraborate Phosphors. Adv. Opt. Mater. 2022, 10, 2201055. [Google Scholar]
- Wei, D.; Fang, L.; Zhou, X.; Xia, H.; Chen, B. Enhanced up-conversion luminescence in Na5Y9F32: Ho3+/Yb3+/Eu3+ single crystals by introducing of Gd3+ ions for highly sensitive temperature sensing. Mater. Today Chem. 2024, 36, 101970. [Google Scholar]
- Huang, Z.; Wu, F.; Zhang, Q.; Teng, Y.; Mu, Z. High sensitivity temperature sensing material In2Li3P3O12: Bi3+, Eu3+ based on effective energy transfer from Bi3+ to Eu3+. Ceram. Int. 2024, 50, 32180–32186. [Google Scholar]
- Ma, L.; Tian, G.; Xiao, H.; Jiang, L.; Du, Q.; Li, H.; Yang, B. A novel spinel-type Mg3Ga2SnO8 microwave dielectric ceramic with low εr and low loss. J. Eur. Ceram. Soc. 2024, 44, 5731–5737. [Google Scholar]
- Zhu, Q.; Huo, J.; Lin, Y.; Li, M.; Liu, W.; Gao, J.; Wang, Q. A new co-substitution strategy as a model to study a rare-earth-free spinel-type phosphor with red emissions and its application in light-emitting diodes. Inorg. Chem. 2020, 59, 433–442. [Google Scholar]
- Yang, Z.F.; Yang, L.L.; Ji, C.J.; Xu, D.H.; Zhang, C.Q.; Bu, H.X.; Tan, X.; Yun, X.Y.; Sun, J.Y. Studies on luminescence properties of double perovskite deep red phosphor La2ZnTiO6:Mn4+ for indoor plant growth LED applications. J. Alloys Compd. 2019, 802, 628–635. [Google Scholar]
- Cao, R.P.; Zhong, B.H.; Nie, J.H.; Chen, Y.Y.; Zhang, L.; Li, L.; Chen, T.; Wang, J. Synthesis, spectral characteristics and energy transfer of SrLa2Al2O7:Mn4+, Dy3+. J. Lumin. 2023, 264, 120163. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar]
- Han, M.; Liu, L.; Zhang, D.; Du, Y.; Zhao, L.; Wang, Y.; Lv, L. Tuning the morphology of Mg2TiO4: Mn4+ for luminescence performance and latent fingerprint visualization. J. Lumin. 2022, 252, 119417. [Google Scholar]
- Yadav, S.; Kumar, D.; Yadav, R.S.; Rai, S.B.; Singh, A.K. Structural and wavelength dependent optical properties of La1-xEuxCoO3 perovskite phosphor. Ceram. Int. 2022, 48, 30754–30766. [Google Scholar]
- Raghupathi, P.; Jamalaiah, B.C. Structure, morphology and optical analysis of Dy3+ -doped Li6AlGd(BO3)4 phosphors for lighting applications. J. Mol. Struct. 2022, 1268, 133695. [Google Scholar] [CrossRef]
- Li, G.X.; Xue, Y.; Mao, Q.N.; Pei, L.; He, H.; Liu, M.J.; Chu, L.; Zhong, J.S. Synergistic luminescent thermometer using co-doped Ca2GdSbO6:Mn4+/(Eu3+ or Sm3+) phosphors. Dalton Trans. 2022, 51, 4685–4694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, M.; Chen, B.; Huang, X.; Liu, M.; Liu, Y.; Su, K.; Min, X.; Mi, R.; Huang, Z. Optical thermometry based on fluorescence intensity ratio of Dy3+-Doped oxysilicate apatite warm white phosphor. Ceram. Int. 2023, 49, 4971–4978. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, R.; Yang, S.; Bu, H.; Zhao, J. Enhancing the Luminescence of La3Mg2NbO9:Mn4+ Phosphor through H3BO3 and Charge Compensator Co-Doping for Use in Plant Growth Lamps. Molecules 2024, 29, 1402. [Google Scholar] [CrossRef]
- Li, G.; Li, G.; Mao, Q.; Pei, L.; Yu, H.; Liu, M.; Chu, L.; Zhong, J. Efficient luminescence lifetime thermometry with enhanced Mn4+-activated BaLaCa1−xMgxSbO6 red phosphors. Chem. Eng. J. 2022, 430, 132923. [Google Scholar] [CrossRef]
- Wang, S.; Seto, T.; Liu, B.; Wang, Y.; Li, C.; Liu, Z.; Dong, H. Tremendous Acceleration of Plant Growth by Applying a New Sunlight Converter Sr4Al14−xGaxO25:Mn4+ Breaking Parity Forbidden Transition. Adv. Sci. 2023, 10, 2204418. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, M.; Sun, C.; Wang, R.; Yang, S.; Yan, J. A novel multifunctional Eu3+/Mn4+ co-doping double perovskite phosphor for applications in LEDs and optical thermometry. J. Mol. Struct. 2025, 1319, 139399. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Hussain, N.; Rubab, S.; Kumar, V. Spectroscopic characterizations and investigation of Judd-Ofelt intensity parameters of Dy3+-doped Ba2La8(SiO4)6O2 near white light emitting phosphor. Ceram. Int. 2023, 49, 15341–15348. [Google Scholar] [CrossRef]
- Nair, G.B.; Swart, H.C.; Dhoble, S.J. A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): Phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 2020, 109, 100622. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, M.; Sun, C.; Zhao, J.; Bu, H.; Yang, S.; Wang, R. Highly sensitive dual-mode temperature measurement utilizing completely opposite thermal quenching luminescence. Ceram. Int. 2024, 50, 44833–44842. [Google Scholar]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar]
- Huang, J.M.; Jiang, P.F.; Cheng, Z.; Wang, R.; Cong, R.H.; Yang, T. Structural confinement-induced highly efficient deep-red emission and negative thermal quenching performance in Mn4+-activated Ca7Mg2Ga6−yAlyO18:Mn4+ phosphors. Inorg. Chem. Front. 2023, 10, 2776. [Google Scholar]
- Jin, Y.; Hu, Y.; Wu, H.; Duan, H.; Chen, L.; Fu, Y.; Ju, G.; Mu, Z.; He, M. A deep red phosphor Li2MgTiO4:Mn4+ exhibiting abnormal emission: Potential application as color converter for warm w-LEDs. Chem. Eng. J. 2016, 288, 596–607. [Google Scholar]
- Long, J.; Xu, Y.; Huang, W.; Deng, C. Dual-mode optical thermometry based on La2MgTiO6: Mn4+, Dy3+ double perovskite phosphors. J. Mater. Sci. Mater. Electron. 2023, 34, 1613. [Google Scholar]
- Ding, Y.; Guo, N.; Lü, X.; Zhou, H.; Wang, L.; Ouyang, R.; Miao, Y.; Shao, B. None–rare–earth activated Ca14Al10Zn6O35:Bi3+, Mn4+ phosphor involving dual luminescent centers for temperature sensing. J. Am. Ceram. Soc. 2019, 102, 7436–7447. [Google Scholar]
- Hu, F.; Ren, S.; Wu, Y.; Sun, C.; Zhu, B.; Wang, Q.; Li, S.; Zhang, D. Dual-mode optical thermometer based on fluorescence intensity ratio of Eu3+/Mn4+ co-doping zinc titanate phosphors. Spectrochim. Acta 2023, 288, 122127. [Google Scholar]
- Yang, Z.F.; Ye, M.J.; Yang, S.Y.; Wang, R.X.; Sun, C.H.; Li, Y.F.; Wang, J.H. Efficient and abnormal thermal quenching Sm3+ activated perovskites-type niobate phosphor for plant growth lamp and WLEDs. Ceram. Int. 2024, 50, 21745–21754. [Google Scholar]
- Wang, P.; Mao, J.; Zhao, L.; Jiang, B.; Xie, C.; Lin, Y.; Chi, F.; Yin, M.; Chen, Y. Double perovskite A2LaNbO6:Mn4+, Eu3+ (A = Ba, Ca) phosphors: Potential applications in optical temperature sensing. Dalton Trans. 2019, 48, 10062–10069. [Google Scholar]
- Wu, Z.; Li, L.; Tian, G.; Wang, Y.; Ling, F.; Cao, Z.; Jiang, S.; Xiang, G.; Li, Y.; Zhou, X. High-sensitivity and wide-temperature-range dual-mode optical thermometry under dual-wavelength excitation in a novel double perovskite tellurate oxide. Dalton Trans. 2021, 50, 11412–11421. [Google Scholar]
- Luo, Y.X.; Chen, Y.; Li, L.J.; Chen, J.Y.; Pang, T.; Chen, L.P.; Guo, H. Three-mode fluorescence thermometers based on double perovskite Ba2GdNbO6:Eu3+, Mn4+ phosphors. Ceram. Int. 2023, 49, 38007–38014. [Google Scholar]
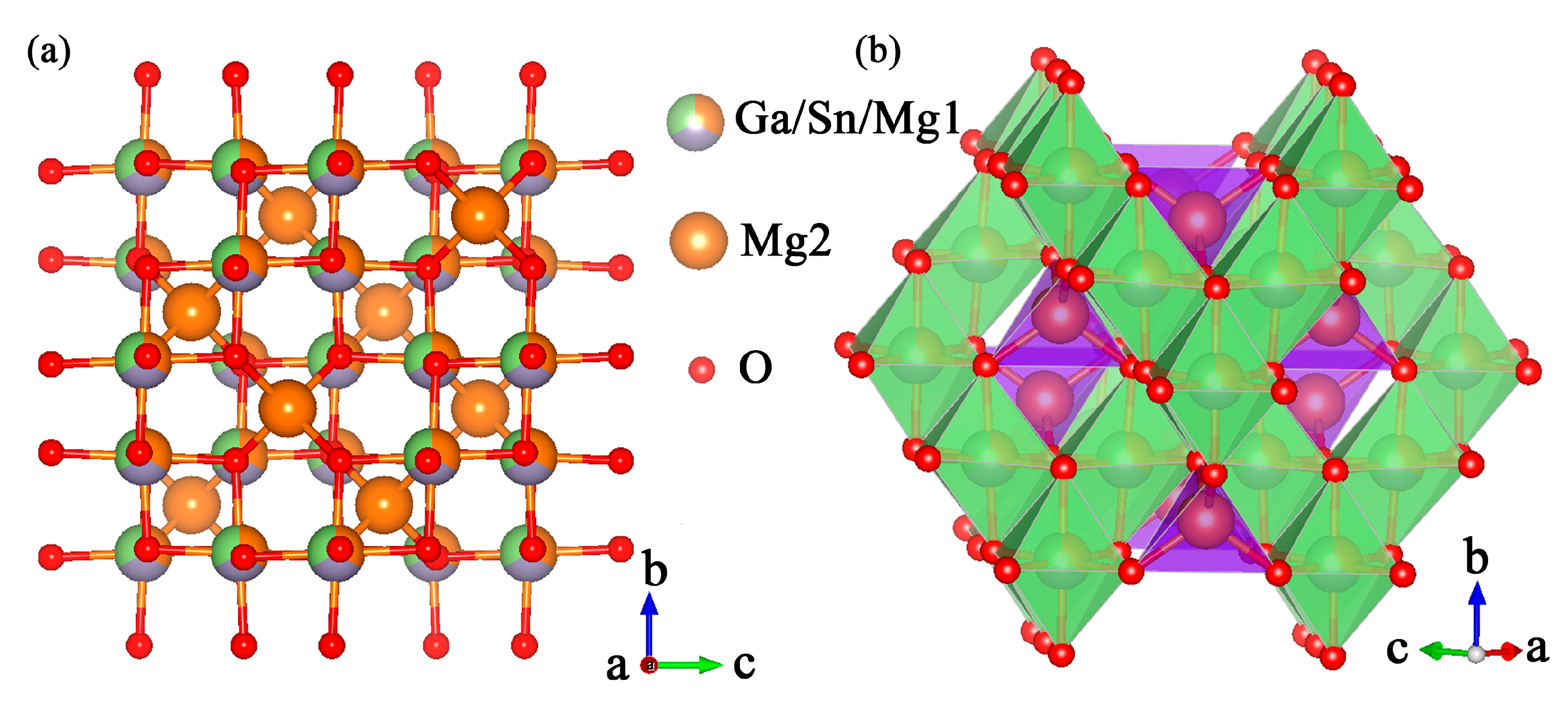

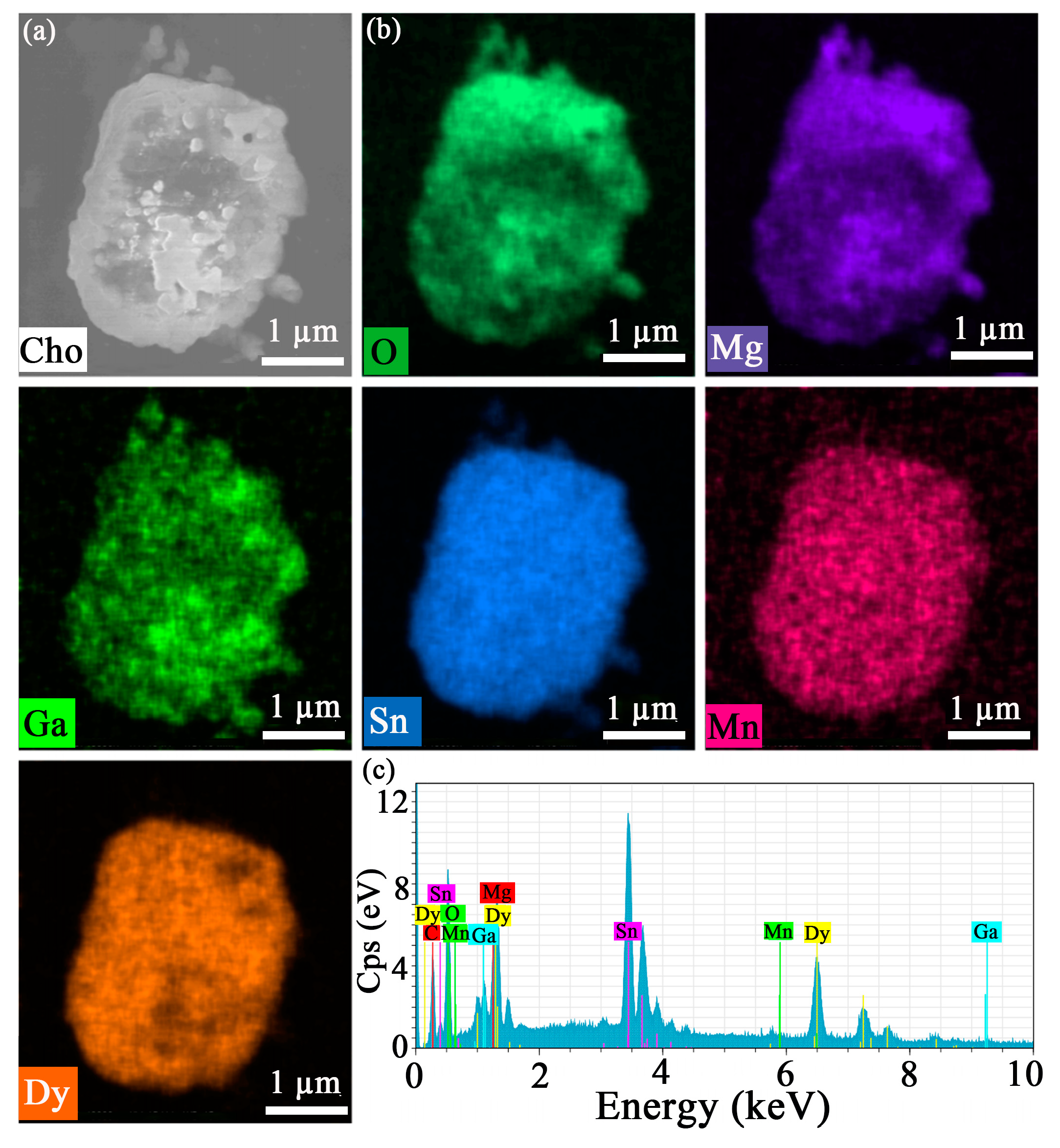
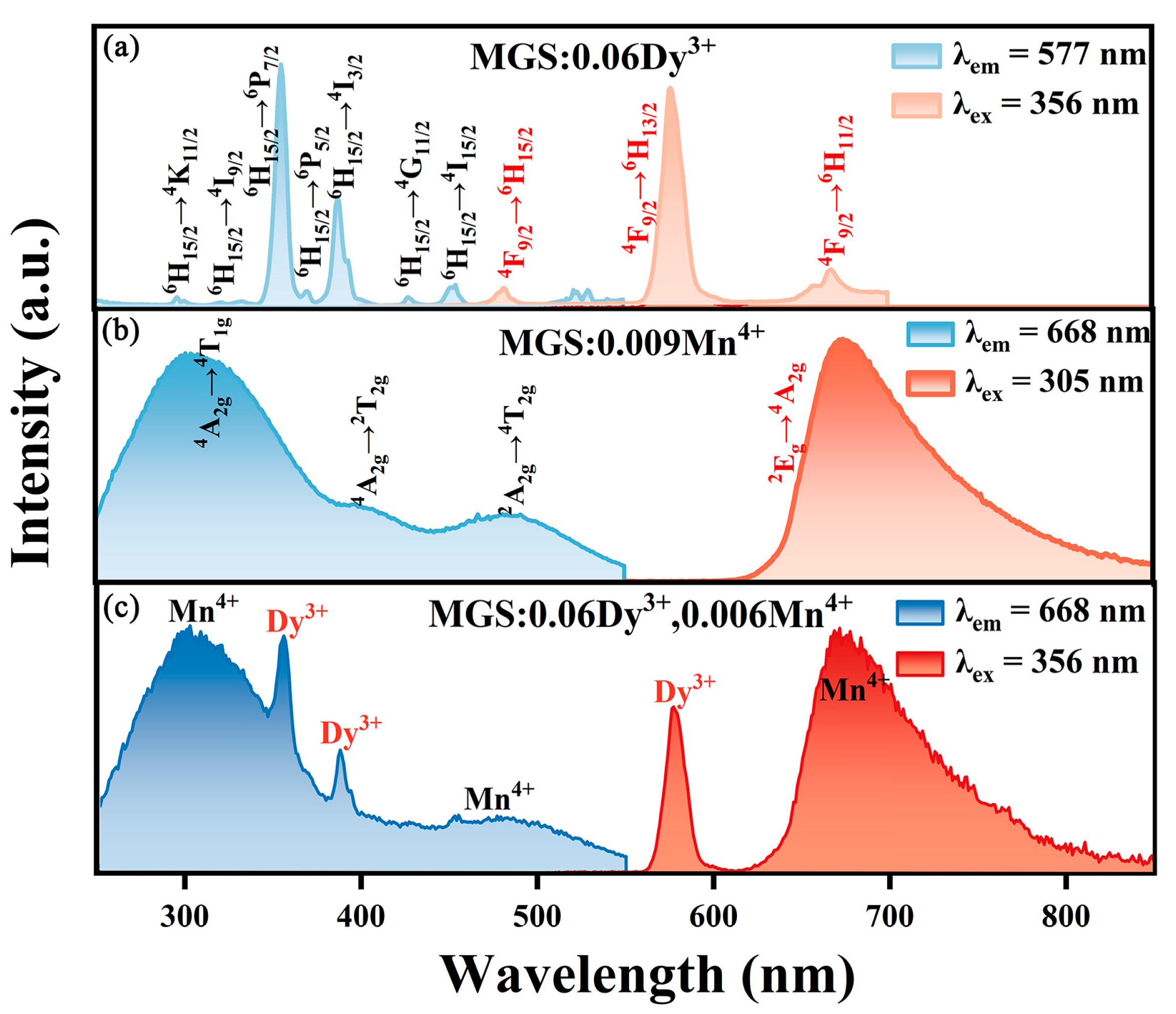
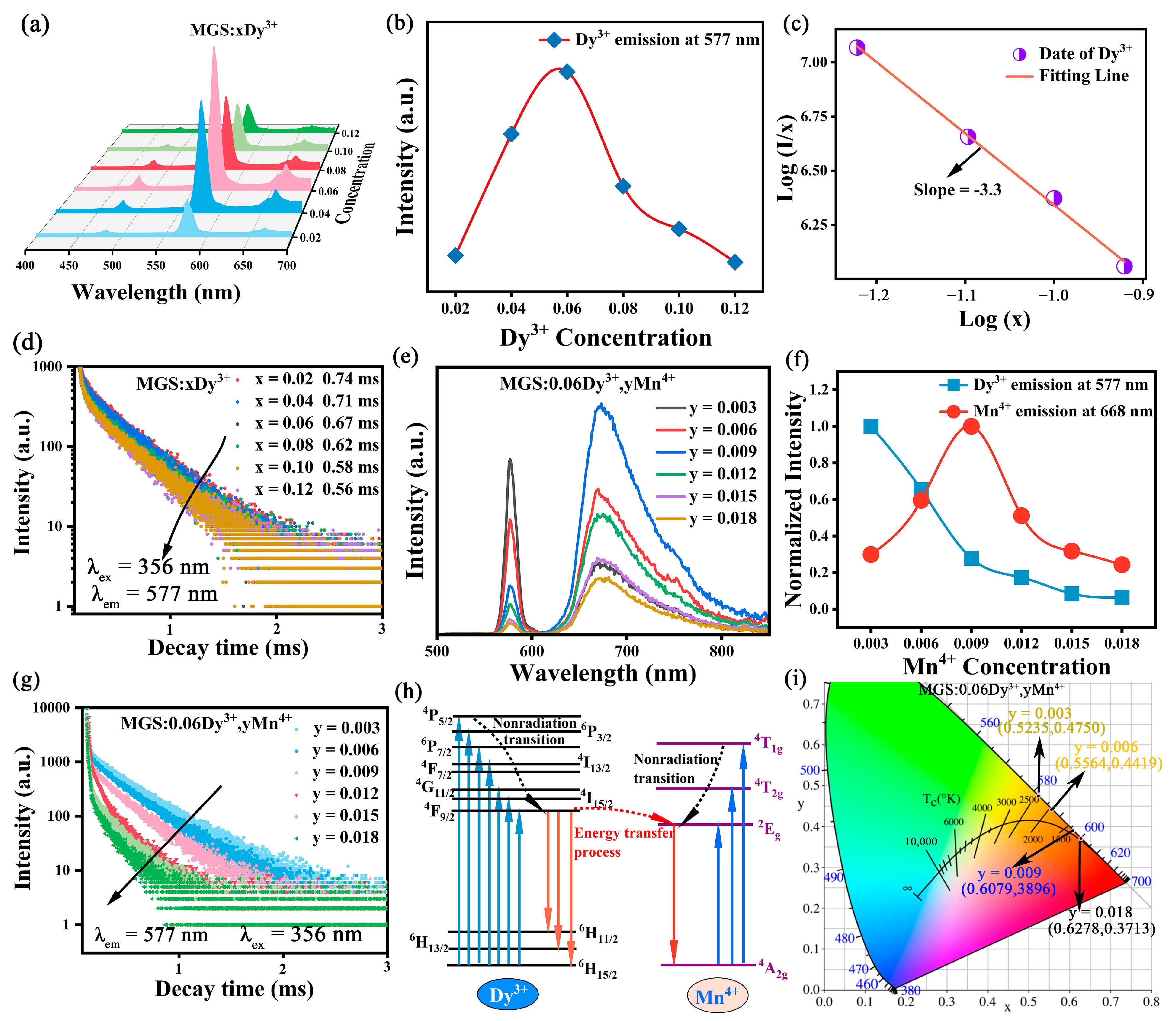


| Sample | MGS | MGS:0.009Mn4+ | MGS:0.06Dy3+ | MGS:0.06Dy3+,0.003Mn4+ |
|---|---|---|---|---|
| Space group | Fd-3m | Fd-3m | Fd-3m | Fd-3m |
| Symmetry | cubic | cubic | cubic | cubic |
| a/b/c, Å | 8.4570 | 8.4552 | 8.4573 | 8.4566 |
| V, Å3 | 604.85 | 604.46 | 604.92 | 604.77 |
| Z | 8 | 8 | 8 | 8 |
| α = β = γ ° | 90 | 90 | 90 | 90 |
| Rwp | 8.5 | 9.6 | 10.3 | 8.8 |
| Rp | 6.7 | 7.5 | 8.1 | 6.9 |
| χ2 | 2.25 | 2.83 | 3.20 | 2.32 |
| Samples | τ (ms) | CIE | Color Purity (%) |
|---|---|---|---|
| MGS:0.06Dy3+ | 0.67 | (0.4925, 0.4770) | 88.3 |
| MGS:0.06Dy3+,0.003Mn4+ | 0.52 | (0.5235, 0.4750) | 97.2 |
| MGS:0.06Dy3+,0.006Mn4+ | 0.44 | (0.5564, 0.4419) | 97.8 |
| MGS:0.06Dy3+,0.009Mn4+ | 0.41 | (0.6278, 0.3713) | 99.1 |
| MGS:0.06Dy3+,0.012Mn4+ | 0.29 | (0.6079, 0.3896) | 98.9 |
| MGS:0.06Dy3+,0.015Mn4+ | 0.21 | (0.6134, 0.3836) | 98.5 |
| MGS:0.06Dy3+,0.018Mn4+ | 0.14 | (0.6106, 0.3863) | 98.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, Z.; Su, Y.; Zhang, W.; Zheng, Y. Dy3+ and Mn4+ Ions Co-Doped Stannate Phosphors for Applications in Dual-Mode Optical Thermometry. Molecules 2025, 30, 1569. https://doi.org/10.3390/molecules30071569
Yang Z, Wang Z, Su Y, Zhang W, Zheng Y. Dy3+ and Mn4+ Ions Co-Doped Stannate Phosphors for Applications in Dual-Mode Optical Thermometry. Molecules. 2025; 30(7):1569. https://doi.org/10.3390/molecules30071569
Chicago/Turabian StyleYang, Zaifa, Zhide Wang, Yi Su, Wenyue Zhang, and Yu Zheng. 2025. "Dy3+ and Mn4+ Ions Co-Doped Stannate Phosphors for Applications in Dual-Mode Optical Thermometry" Molecules 30, no. 7: 1569. https://doi.org/10.3390/molecules30071569
APA StyleYang, Z., Wang, Z., Su, Y., Zhang, W., & Zheng, Y. (2025). Dy3+ and Mn4+ Ions Co-Doped Stannate Phosphors for Applications in Dual-Mode Optical Thermometry. Molecules, 30(7), 1569. https://doi.org/10.3390/molecules30071569






