Carbazolyl Electron Donor and Pyridinyl Electron Acceptor Containing Derivatives as Potential Host Materials for Green Organic Light-Emitting Diodes
Abstract
1. Introduction
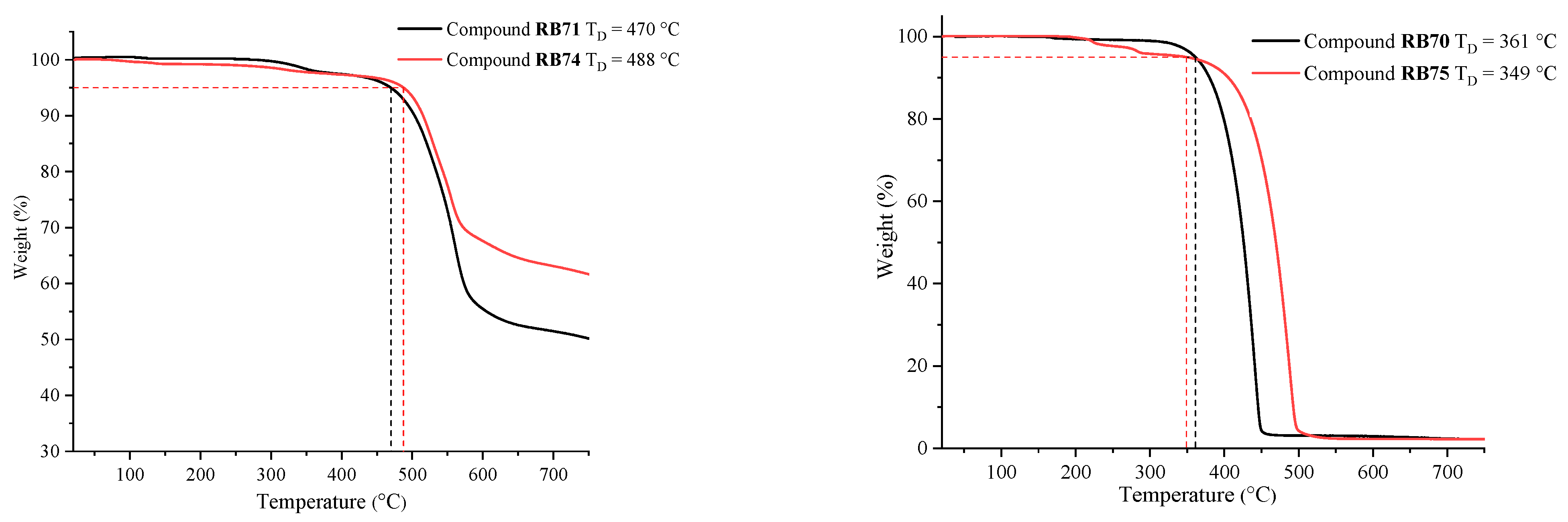
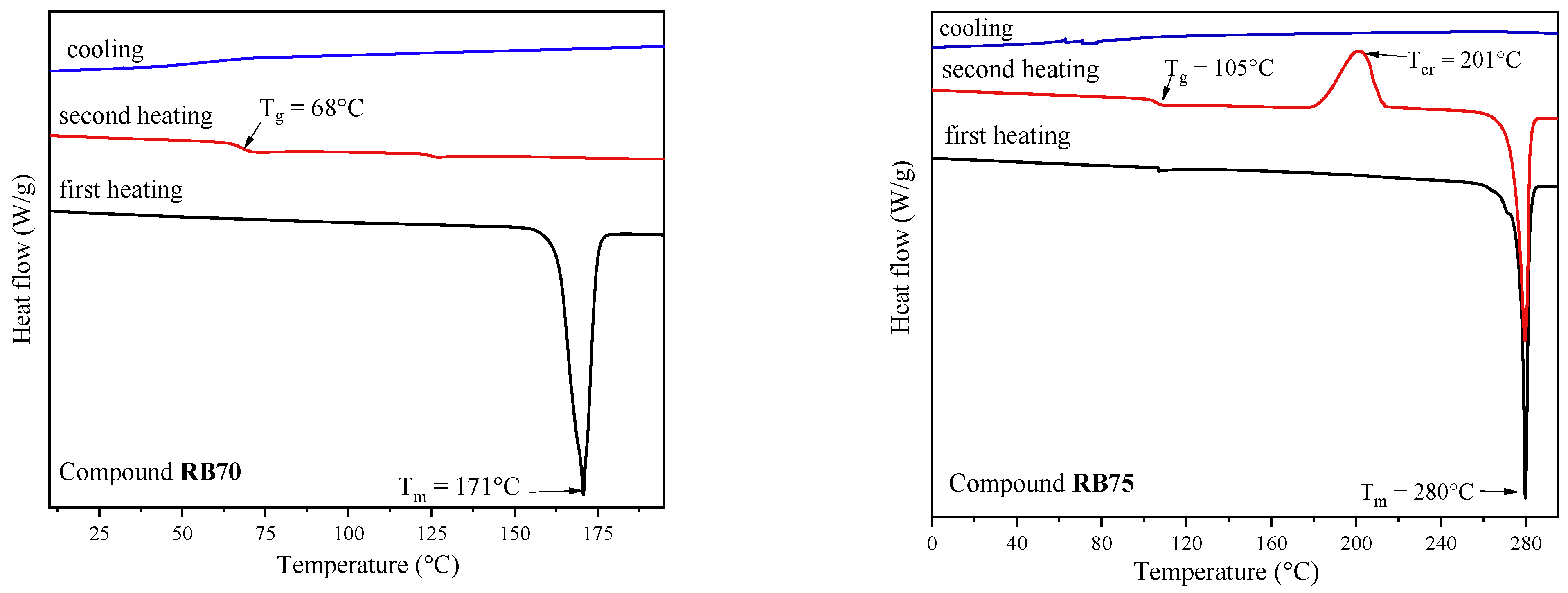
2. Results and Discussion
3. Experimental Methods
3.1. Instrumentation
3.2. Materials

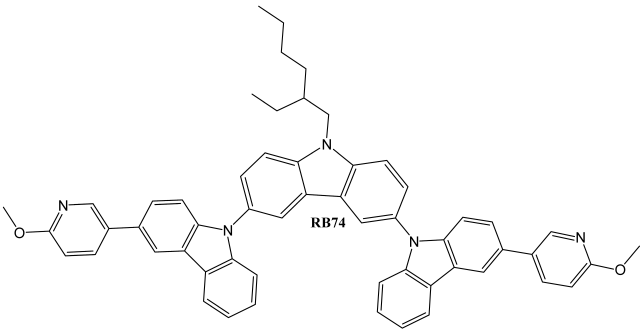
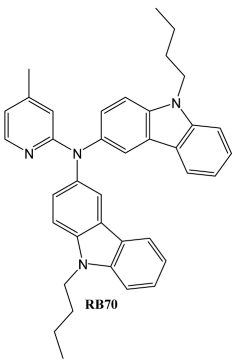
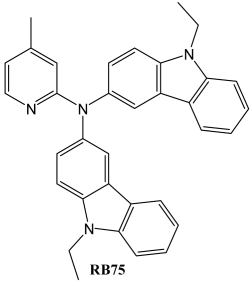
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, K.; Byeon, I.; Kim, Y.G.; Choi, J.; Kim, D. Nanostructures in Organic Light-Emitting Diodes: Principles and Recent Advances in the Light Extraction Strategy. Laser Photon Rev. 2024, 18, 2400547. [Google Scholar] [CrossRef]
- Yadav, S.; Mittal, P.; Negi, S. Architectural Design, Fabrication Techniques, Characteristics Parameters and Different Applications for OLED along with Some OTFT Driven OLEDs: A Review. Main Group Chem. 2024, 23, 1–16. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Jha, P.; Aswal, D.K.; Yakhmi, J.V. Organic Devices: Fabrication, Applications, and Challenges. J. Electron. Mater. 2022, 51, 447–485. [Google Scholar] [CrossRef]
- Yadav, S.; Mittal, P.; Negi, S. Review—Advancements and Perspectives of Organic LED: In Depth Analysis of Architectural Design, Characteristics Parameters, Fabrication Techniques, and Applications. ECS J. Solid. State Sci. Technol. 2023, 12, 046004. [Google Scholar] [CrossRef]
- Karzazi, Y.; Cornil, J.; Brédas, J.L. Theoretical Investigation of the Origin of Negative Differential Resistance in Substituted Phenylene Ethynylene Oligomers. Nanotechnology 2003, 14, 165–171. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, M.; Mishra, S.K.; Shrivastava, A.K. Recent Progress in Organic Light-Emitting Diodes. J. Nanoelectron. Optoelectron. 2019, 14, 1215–1224. [Google Scholar] [CrossRef]
- Mishra, N.; Mittal, P.; Kumar, B. Analytical Modeling for Static and Dynamic Response of Organic Pseudo All-p Inverter Circuits. J. Comput. Electron. 2019, 18, 1490–1500. [Google Scholar] [CrossRef]
- Salehi, A.; Fu, X.; Shin, D.; So, F. Recent Advances in OLED Optical Design. Adv. Funct. Mater. 2019, 29, 1808803. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, H.; Lee, S.; Shim, H.; Chun, Y.; Choi, W.; Kwack, J.; Han, D.; Song, M.; Kim, S.; et al. Low-Power Flexible Organic Light-Emitting Diode Display Device. Adv. Mater. 2011, 23, 3511–3516. [Google Scholar] [CrossRef]
- Miao, W.; Hsiao, F.; Sheng, Y.; Lee, T.; Hong, Y.; Tsai, C.; Chen, H.; Liu, Z.; Lin, C.; Chung, R.; et al. Microdisplays: Mini-LED, Micro-OLED, and Micro-LED. Adv. Opt. Mater. 2024, 12, 2300112. [Google Scholar] [CrossRef]
- Burn, P.L.; Lo, S.-C.; Samuel, I.D.W. The Development of Light-Emitting Dendrimers for Displays. Adv. Mater. 2007, 19, 1675–1688. [Google Scholar] [CrossRef]
- Liguori, R.; Nunziata, F.; Aprano, S.; Maglione, M.G. Overcoming Challenges in OLED Technology for Lighting Solutions. Electronics 2024, 13, 1299. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, J.; Yu, Y.; Yuan, J.; Luo, D.; Liu, B.; Liang, D. Recent Advances of Exciplex-Based White Organic Light-Emitting Diodes. Appl. Sci. 2018, 8, 1449. [Google Scholar] [CrossRef]
- Guo, K.; Tang, Z.; Chou, X.; Pan, S.; Wan, C.; Xue, T.; Ding, L.; Wang, X.; Huang, J.; Zhang, F.; et al. Printable Organic Light-Emitting Diodes for next-Generation Visible Light Communications: A Review. Adv. Photonics Nexus 2023, 2, 044001. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Multifunctional Materials in High-Performance OLEDs: Challenges for Solid-State Lighting. Chem. Mater. 2011, 23, 621–630. [Google Scholar] [CrossRef]
- Dumur, F.; Lepeltier, M.; Zamani Siboni, H.; Xiao, P.; Graff, B.; Lalevée, J.; Gigmes, D.; Aziz, H. Concentration-Insensitive Phosphorescent Organic Light Emitting Devices (PhOLEDs) for Easy Manufacturing. J. Lumin. 2014, 151, 34–40. [Google Scholar] [CrossRef]
- Qu, W.; Gao, Z.; Li, W.; Fan, X.; Shi, Y.; Miao, Y.; Wu, Z.; Huang, J.; Wang, H.; Wei, B. Carbazole/Triazine Based Host Materials for High-Performance Green PhOLEDs. Dyes Pigment. 2022, 199, 110086. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Rayabarapu, D.K.; Paulose, B.M.J.S.; Duan, J.-P.; Cheng, C.-H. New Iridium Complexes with Cyclometalated Alkenylquinoline Ligands as Highly Efficient Saturated Red-Light Emitters for Organic Light-Emitting Diodes. Adv. Mater. 2005, 17, 349–353. [Google Scholar] [CrossRef]
- Li, H.-C.; Chou, P.-T.; Hu, Y.-H.; Cheng, Y.-M.; Liu, R.-S. Synthesis, Characterization, and Photophysical Properties of Iridium Complexes with an 8-Phenylquinoline Framework. The First Six-Membered Chelated Iridium Complexes for Electroluminance. Organometallics 2005, 24, 1329–1335. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Zhou, G.-J.; Yu, X.-M.; Kwok, H.-S.; Tang, B.-Z. Amorphous Diphenylaminofluorene-Functionalized Iridium Complexes for High-Efficiency Electrophosphorescent Light-Emitting Diodes. Adv. Funct. Mater. 2006, 16, 838–846. [Google Scholar] [CrossRef]
- Keshari, H.; Ansari, N.; Chen, Y.-T.; Chao, Y.-Q.; Chang, C.-H.; Kumar, V.; Chetti, P.; Chaskar, A. Enhanced Efficiency in Green PhOLEDs Using a Simplified Three-Layer Architecture with Bipolar Carbazole–Quinazolinone Hosts. ACS Appl. Opt. Mater. 2024, 2, 2039–2050. [Google Scholar] [CrossRef]
- Hong, J.; Joo, C.W.; Sung, B.; Lee, J.; Hyeon, Y.J.; Kim, D.; Park, H.; Kim, J.; Lee, J.; Kim, Y.-H. Synthesis and Characterization of Bipolar Host Materials Based on Indolocarbazole Derivatives for Green Phosphorescent Organic Light-Emitting Diodes. Synth. Met. 2025, 311, 117845. [Google Scholar] [CrossRef]
- Goushi, K.; Kwong, R.; Brown, J.J.; Sasabe, H.; Adachi, C. Triplet Exciton Confinement and Unconfinement by Adjacent Hole-Transport Layers. J. Appl. Phys. 2004, 95, 7798–7802. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Liu, W.; Zhang, Z.; Li, J. Highly Efficient Top-Emitting Green Phosphorescent OLEDs with a Narrow Band and Slow Efficiency Roll-off for High-Definition Displays. Mater. Chem. Front. 2024, 8, 4106–4113. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, W.; Wang, X.; Zhou, H.; Huang, J.; Wei, B.; Wang, H. Triazine and Indancarbazole Based Bipolar Host Materials With Fluorene Bridge for Red Phosphorescent Oleds Have Excellent Device Performance. Chem. Asian J. 2025, e202401652. [Google Scholar] [CrossRef]
- Jesuraj, P.J.; Somasundaram, S.; Kamaraj, E.; Hafeez, H.; Lee, C.; Kim, D.; Won, S.H.; Shin, S.T.; Song, M.; Kim, C.-S.; et al. Intramolecular Charge Transfer-Based Spirobifluorene-Coupled Heteroaromatic Moieties as Efficient Hole Transport Layer and Host in Phosphorescent Organic Light-Emitting Diodes. Org. Electron. 2020, 85, 105825. [Google Scholar] [CrossRef]
- Jia, B.; Lian, H.; Sun, T.; Wei, J.; Yang, J.; Zhou, H.; Huang, J.; Dong, Q. New Bipolar Host Materials Based on Methyl Substituted Pyridazine for High-Performance Green and Red Phosphorescent OLEDs. Dyes Pigment. 2019, 168, 212–218. [Google Scholar] [CrossRef]
- Lade, J.; Lee, N.-Y.; Patil, B.; Deshpande, Y.Y.; Pownthurai, B.; Hsieh, C.-A.; Pingale, S.S.; Chen, L.-Y.; Chaskar, A. Novel Benzothiadiazine 1,1-Dioxide Based Bipolar Host Materials for Efficient Red Phosphorescent Organic Light Emitting Diodes. Org. Electron. 2021, 92, 106104. [Google Scholar] [CrossRef]
- Gao, W.; Wu, W.; Cao, S.; Han, B.; Li, N. A Simple Bipolar Host Material Based on Triphenylamine and Pyridine Featuring σ-Linkage for Efficient Solution-Processed Phosphorescent Organic Light-Emitting Diodes. Opt. Mater. 2022, 133, 112871. [Google Scholar] [CrossRef]
- Blazevicius, D.; Krucaite, G.; Shahnawaz, S.; Swayamprabha, S.S.; Zaleckas, E.; Jou, J.-H.; Grigalevicius, S. Easily Synthesized and Cheap Carbazole- or Phenoxazine-Based Hosts for Efficient Yellow Phosphorescent OLEDs. Opt. Mater. 2021, 118, 111251. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Xu, H.; Zhang, J.; Miao, Y.; Guo, K.; Shinar, R.; Shinar, J.; Wang, H.; Xu, B. Two Novel Bipolar Hosts Based on 1,2,4-Triazole Derivatives for Highly Efficient Red Phosphorescent OLEDs Showing a Small Efficiency Roll-Off. Org. Electron. 2019, 70, 272–278. [Google Scholar] [CrossRef]
- Jun, J.-W.; Lee, K.-M.; Kim, O.Y.; Lee, J.Y.; Hwang, S.-H. Synthesis of a Dibenzothiophene/Carboline/Carbazole Hybrid Bipolar Host Material for Green Phosphorescent OLEDs. Synth. Met. 2016, 213, 7–11. [Google Scholar] [CrossRef]
- Hong, M.; Ravva, M.K.; Winget, P.; Brédas, J.-L. Effect of Substituents on the Electronic Structure and Degradation Process in Carbazole Derivatives for Blue OLED Host Materials. Chem. Mater. 2016, 28, 5791–5798. [Google Scholar] [CrossRef]
- He, G.; Pfeiffer, M.; Leo, K.; Hofmann, M.; Birnstock, J.; Pudzich, R.; Salbeck, J. High-Efficiency and Low-Voltage p-i-n Electrophosphorescent Organic Light-Emitting Diodes with Double-Emission Layers. Appl. Phys. Lett. 2004, 85, 3911–3913. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Lai, S.; Liu, W.; Lee, C.; Lee, S.; Liu, Z. Synthesis of Multiaryl-Substituted Pyridine Derivatives and Applications in Non-doped Deep-Blue OLEDs as Electron-Transporting Layer with High Hole-Blocking Ability. Adv. Mater. 2010, 22, 527–530. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Zhou, Y.; Wu, W.; Zhang, L.; Li, H. Distinctive Electron Transport on Pyridine-Linked Molecular Junctions with Narrow Monolayer Graphene Nanoribbon Electrodes Compared with Metal Electrodes and Graphene Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 28217–28226. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Sivaraj, S.; Thanikachalam, V.; Anudeebhana, J. Multifunctional Pyridine Styrylphenanthroimidazoles: Electron Transport Materials for Blue FOLEDs with Low Efficiency Roll-off and Hosts for PHOLEDs with Low Turn-on Voltage. J. Mater. Chem. C Mater. 2021, 9, 10334–10346. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maruyama, T.; Zhou, Z.-H.; Ito, T.; Fukuda, T.; Yoneda, Y.; Begum, F.; Ikeda, T.; Sasaki, S. Pi.-Conjugated Poly(Pyridine-2,5-Diyl), Poly(2,2′-Bipyridine-5,5′-Diyl), and Their Alkyl Derivatives. Preparation, Linear Structure, Function as a Ligand to Form Their Transition Metal Complexes, Catalytic Reactions, n-Type Electrically Conducting Properties, Optical Properties, and Alignment on Substrates. J. Am. Chem. Soc. 1994, 116, 4832–4845. [Google Scholar] [CrossRef]
- Xiang, N.; Gao, Z.; Tian, G.; Chen, Y.; Liang, W.; Huang, J.; Dong, Q.; Wong, W.-Y.; Su, J. Novel Fluorene/Indole-Based Hole Transport Materials with High Thermal Stability for Efficient OLEDs. Dyes Pigment. 2017, 137, 36–42. [Google Scholar] [CrossRef]
- Ji, J.; Li, P.; Tian, Q.; Feng, W.; Wu, C. Three New Carbazole Derivatives with High Thermal Stability as Host for Efficient Green Phosphorescent Organic-Light Emitting Diodes. Dyes Pigment. 2019, 171, 107670. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Kim, Y. Progress in Organic Semiconducting Materials with High Thermal Stability for Organic Light-emitting Devices. InfoMat 2021, 3, 61–81. [Google Scholar] [CrossRef]
- Jeon, Y.P.; Kim, K.S.; Lee, K.K.; Moon, I.K.; Choo, D.C.; Lee, J.Y.; Kim, T.W. Blue Phosphorescent Organic Light-Emitting Devices Based on Carbazole/Thioxanthene-S,S-Dioxide with a High Glass Transition Temperature. J. Mater. Chem. C Mater. 2015, 3, 6192–6199. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Yu, H. Diphenylamino-Substituted Bicarbazole Derivative: Hole-Transporting Material with High Glass-Transition Temperature, Good Electron and Triplet Exciton Blocking Capabilities and Efficient Hole Injection. Chem. Phys. Lett. 2017, 674, 109–114. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Deng, Y.-L.; Liu, X.-Y.; Yuan, X.-D.; Jiang, Z.-Q.; Liao, L.-S. A Facile Way to Synthesize High-Triplet-Energy Hosts for Blue Phosphorescent Organic Light-Emitting Diodes with High Glass Transition Temperature and Low Driving Voltage. Dyes Pigment. 2015, 122, 6–12. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Z.; Yu, W.; Wang, Y.; Zhao, Z.; Gu, J.; Liu, Z.; Qu, B.; Xiao, L.; Chen, Z. A High Thermal Stability Terpyridine Derivative as the Electron Transporter for Long-Lived Green Phosphorescent OLED. Org. Electron. 2021, 89, 106048. [Google Scholar] [CrossRef]
- Jatautiene, E.; Simokaitiene, J.; Sych, G.; Volyniuk, D.; Ivaniuk, K.; Stakhira, P.; Fitio, V.; Petrovska, H.; Savaryn, V.; Nastishin, Y.; et al. Adjustment of Electronic and Emissive Properties of Indolocarbazoles for Non-Doped OLEDs and Cholesteric Liquid Crystal Lasers. Appl. Mater. Today 2021, 24, 101121. [Google Scholar] [CrossRef]
- Kumar, K.; Kesavan, K.K.; Kumar, S.; Banik, S.; Karmakar, A.; Chen, F.-R.; Jayakumar, J.; Jou, J.-H.; Ghosh, S. Pyridine-Annulated Functional Fused Indole as a Hole Transport Material for Solution-Processed OLEDs. ACS Appl. Opt. Mater. 2023, 1, 1930–1937. [Google Scholar] [CrossRef]
- Siddiqui, I.; Gautam, P.; Blazevicius, D.; Jayakumar, J.; Lenka, S.; Tavgeniene, D.; Zaleckas, E.; Grigalevicius, S.; Jou, J.-H. Bicarbazole-Benzophenone Based Twisted Donor-Acceptor Derivatives as Potential Blue TADF Emitters for OLEDs. Molecules 2024, 29, 1672. [Google Scholar] [CrossRef]
- Nghia, N.V.; Kim, J.; Kim, Y.; Lee, M.H. Triarylboryl-Functionalized Oxadiazole as a Host Material with Electron Transporting Property for Green PhOLEDs. Bull. Korean Chem. Soc. 2016, 37, 864–870. [Google Scholar] [CrossRef]
- Gautam, P.; Shahnawaz; Siddiqui, I.; Blazevicius, D.; Krucaite, G.; Tavgeniene, D.; Jou, J.-H.; Grigalevicius, S. Bifunctional Bicarbazole-Benzophenone-Based Twisted Donor–Acceptor–Donor Derivatives for Deep-Blue and Green OLEDs. Nanomaterials 2023, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.H. LXXIV.—Iodination in the Carbazole Series. J. Chem. Soc. 1926, 129, 546–553. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Zhang, B.; Xie, Z.; Forster, M.; Scherf, U. Polycarbazole-Based Networks Made by Photo-Crosslinking for Hole Transporting Layers of OLED Devices. Org. Electron. 2011, 12, 2253–2257. [Google Scholar] [CrossRef]
- Beresneviciute, R.; Tavgeniene, D.; Blazevicius, D.; Chen, K.-W.; Chen, Y.-H.; Grigalevicius, S.; Chang, C.-H. 9-(9-Alkylcarbazol-3-Yl)-3-(Methoxypyridin-3-Yl)Carbazoles as Host Materials for Very Efficient OLEDs. Opt. Mater. 2024, 157, 116273. [Google Scholar] [CrossRef]
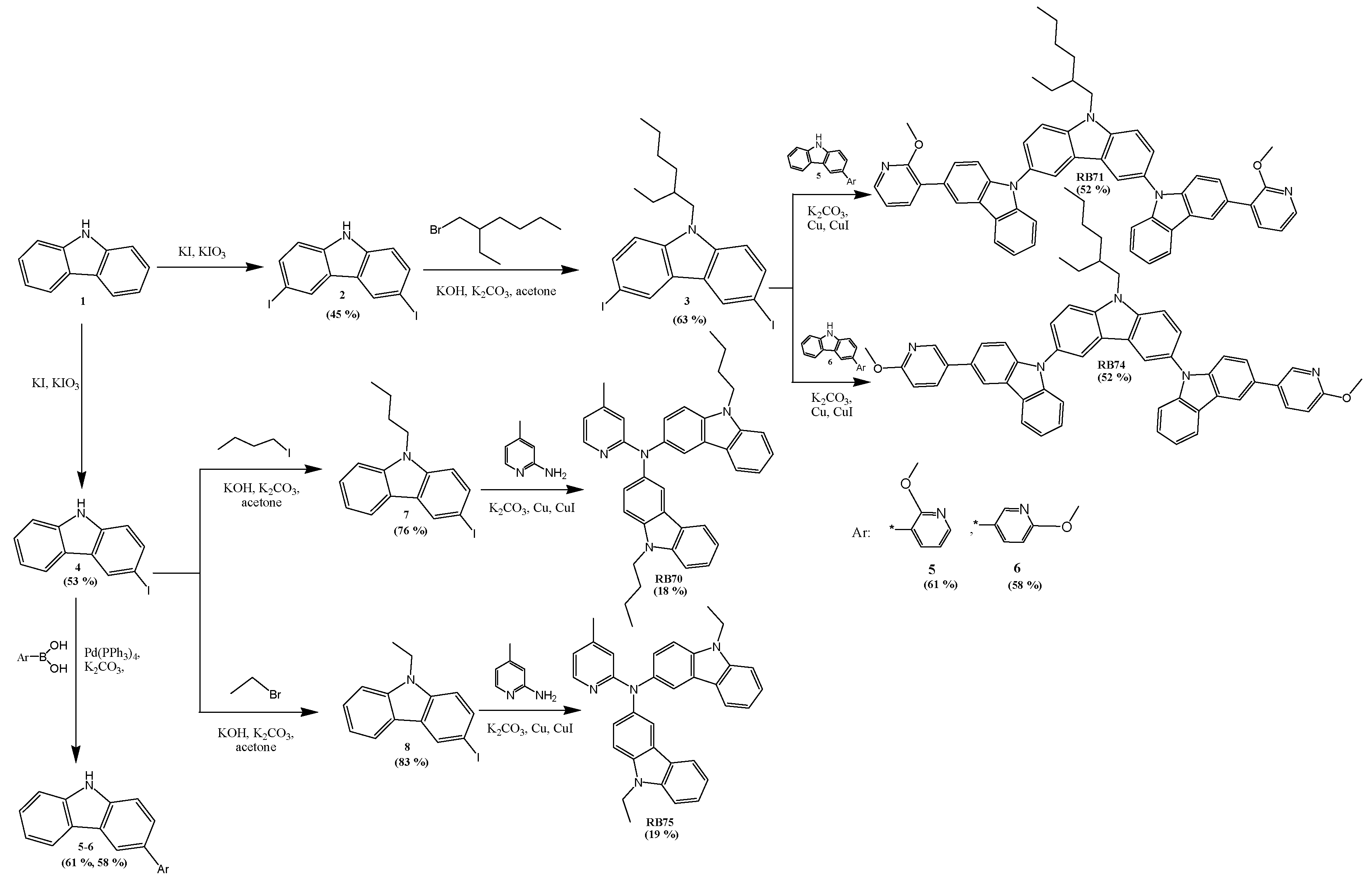



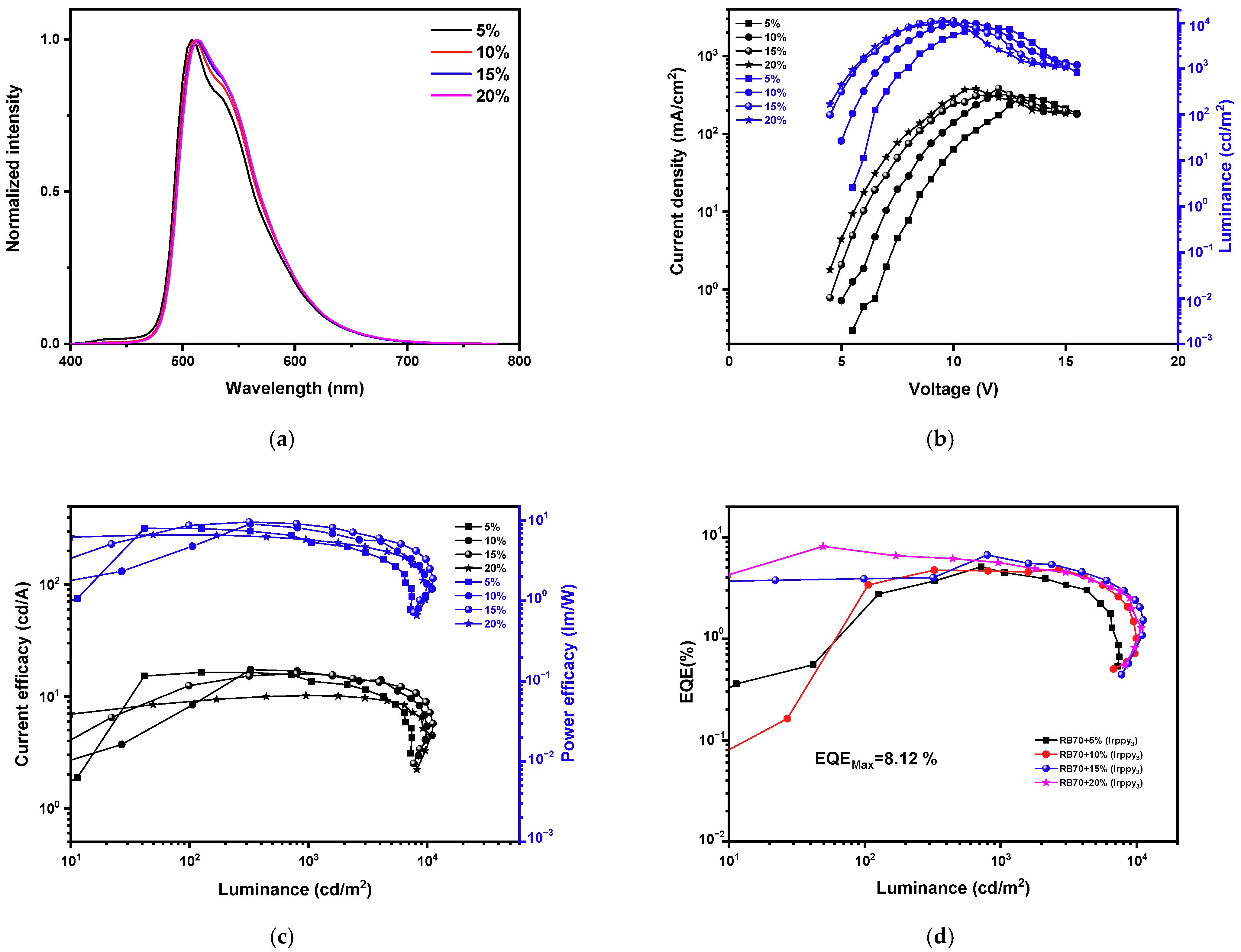
| Host | Dopant Ir(ppy)3, wt. % | Turn-on voltage, V | Power efficacy (PE), lm/W | Current efficacy (CE), cd/A | EQE, % | CIE (x, y) | Maximum luminance LMAX, cd/m2 |
| @100, 1000 cd/m2 and maximum | @100, 1000 cd/m2 | ||||||
| RB71 | 5 | 7.2 | 5.8/4.4/5.8 | 15.1/13.5/15.6 | 4.3/3.6/6.1 | (0.29, 0.61)/(0.29, 0.61) | 3307 |
| 10 | 5.6 | 7.2/5.3/7.3 | 15.1/13.2/15.3 | 4.6/3.8/5.8 | (0.30, 0.62)/(0.30, 0.61) | 3628 | |
| 15 | 5.0 | 1.2/4.2/4.2 | 3.1/11.4/11.4 | 1.9/4.8/4.8 | (0.30, 0.62)/(0.30, 0.62) | 5795 | |
| 20 | 4.3 | 7.9/5.2/8.0 | 12.7/10.5/12.7 | 3.8/2.3/5.4 | (0.30, 0.62)/(0.31, 0.62) | 4090 | |
| Host | Dopant Ir(ppy)3, wt. % | Turn-on voltage, V | Power efficacy (PE), lm/W | Current efficacy (CE), cd/A | EQE, % | CIE (x, y) | Maximum luminance LMAX, cd/m2 |
| @100, 1000 cd/m2 and maximum | @100, 1000 cd/m2 | ||||||
| RB70 | 5 | 5.4 | 8.0/5.6/8.0 | 16.1/14.0/16.5 | 5.3/4.3/5.3 | (0.29, 0.60)/(0.29, 0.60) | 7420 |
| 10 | 4.6 | 4.6/7.9/9.1 | 8.1/16.5/17.4 | 2.5/4.8/4.8 | (0.30, 0.61)/(0.30, 0.61) | 9944 | |
| 15 | 3.7 | 8.7/8.9/9.6 | 12.5/15.9/16.0 | 4.0/4.7/6.7 | (0.30, 0.62)/(0.30, 0.61) | 11,200 | |
| 20 | 3.5 | 6.6/5.8/6.6 | 8.9/10.2/10.4 | 2.8/3.0/8.1 | (0.30, 0.62)/(0.30, 0.62) | 10,800 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beresneviciute, R.; Kumar, A.; Blazevicius, D.; Lenka, S.; Hsieh, S.-T.; Tsai, M.-F.; Krucaite, G.; Tavgeniene, D.; Jou, J.-H.; Grigalevicius, S. Carbazolyl Electron Donor and Pyridinyl Electron Acceptor Containing Derivatives as Potential Host Materials for Green Organic Light-Emitting Diodes. Molecules 2025, 30, 1911. https://doi.org/10.3390/molecules30091911
Beresneviciute R, Kumar A, Blazevicius D, Lenka S, Hsieh S-T, Tsai M-F, Krucaite G, Tavgeniene D, Jou J-H, Grigalevicius S. Carbazolyl Electron Donor and Pyridinyl Electron Acceptor Containing Derivatives as Potential Host Materials for Green Organic Light-Emitting Diodes. Molecules. 2025; 30(9):1911. https://doi.org/10.3390/molecules30091911
Chicago/Turabian StyleBeresneviciute, Raminta, Anil Kumar, Dovydas Blazevicius, Sushanta Lenka, Song-Ting Hsieh, Ming-Feng Tsai, Gintare Krucaite, Daiva Tavgeniene, Jwo-Huei Jou, and Saulius Grigalevicius. 2025. "Carbazolyl Electron Donor and Pyridinyl Electron Acceptor Containing Derivatives as Potential Host Materials for Green Organic Light-Emitting Diodes" Molecules 30, no. 9: 1911. https://doi.org/10.3390/molecules30091911
APA StyleBeresneviciute, R., Kumar, A., Blazevicius, D., Lenka, S., Hsieh, S.-T., Tsai, M.-F., Krucaite, G., Tavgeniene, D., Jou, J.-H., & Grigalevicius, S. (2025). Carbazolyl Electron Donor and Pyridinyl Electron Acceptor Containing Derivatives as Potential Host Materials for Green Organic Light-Emitting Diodes. Molecules, 30(9), 1911. https://doi.org/10.3390/molecules30091911









