Investigation into the Feasibility of a Synergistic Photocatalytic Degradation Process for Fracturing Flowback Fluid Streams Utilizing O3 and Ti/Ni Composite Materials
Abstract
1. Introduction
2. Results and Discussion
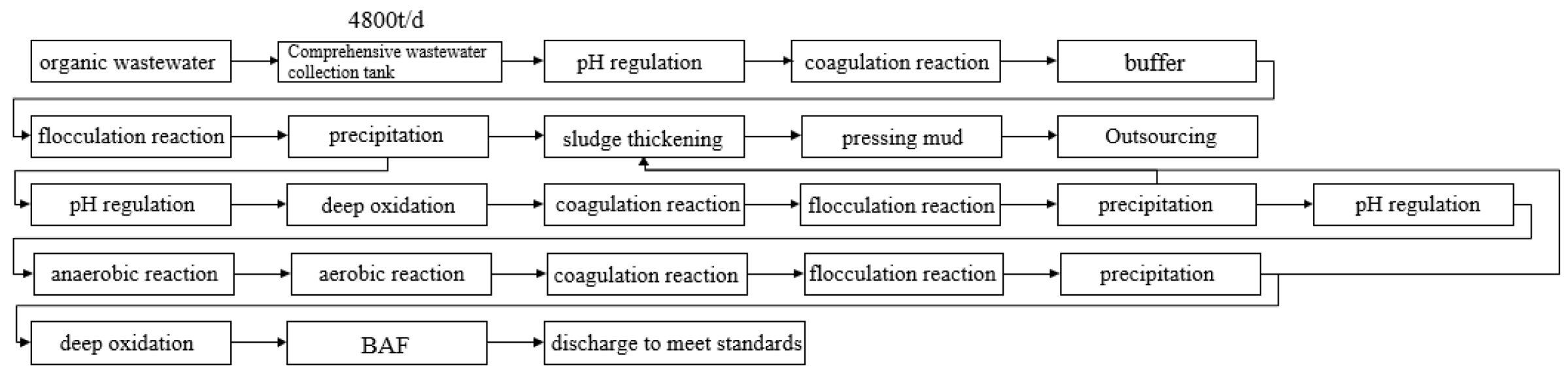
2.1. Creating Technological Process
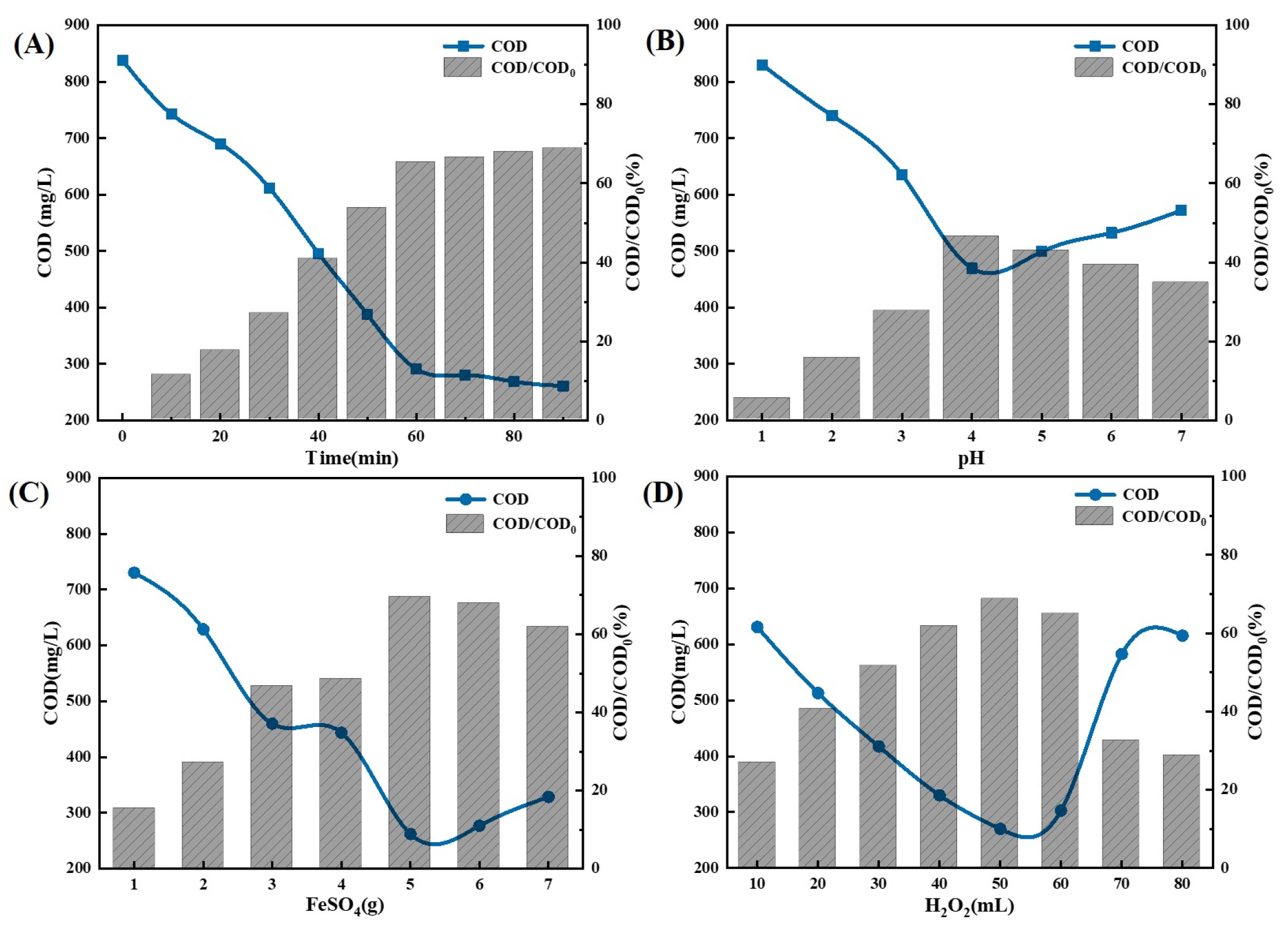
2.1.1. Optimization of Flocculating Settling Factors
2.1.2. Study on the Filtration Effect of Microporous Filter Membrane
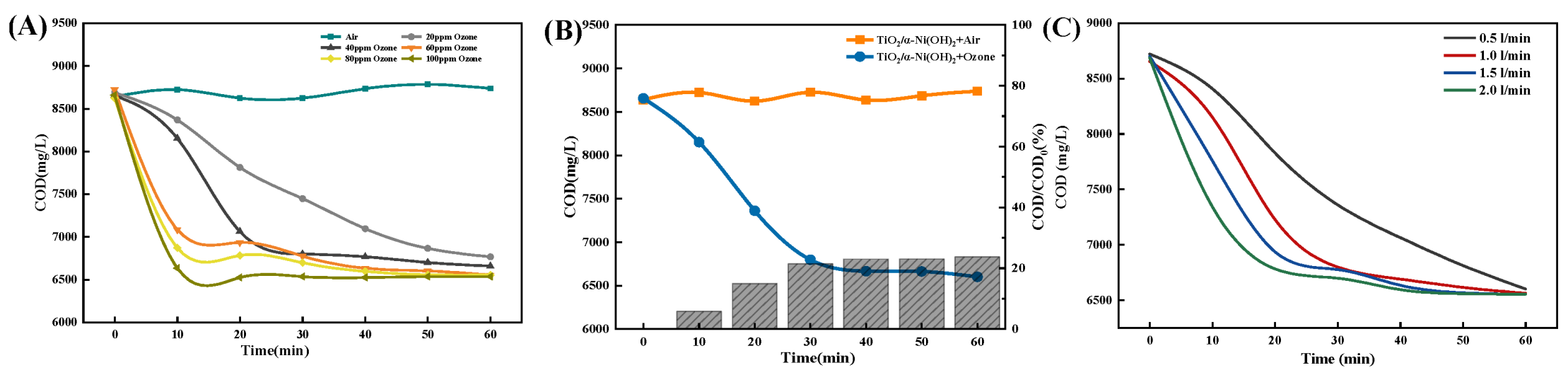
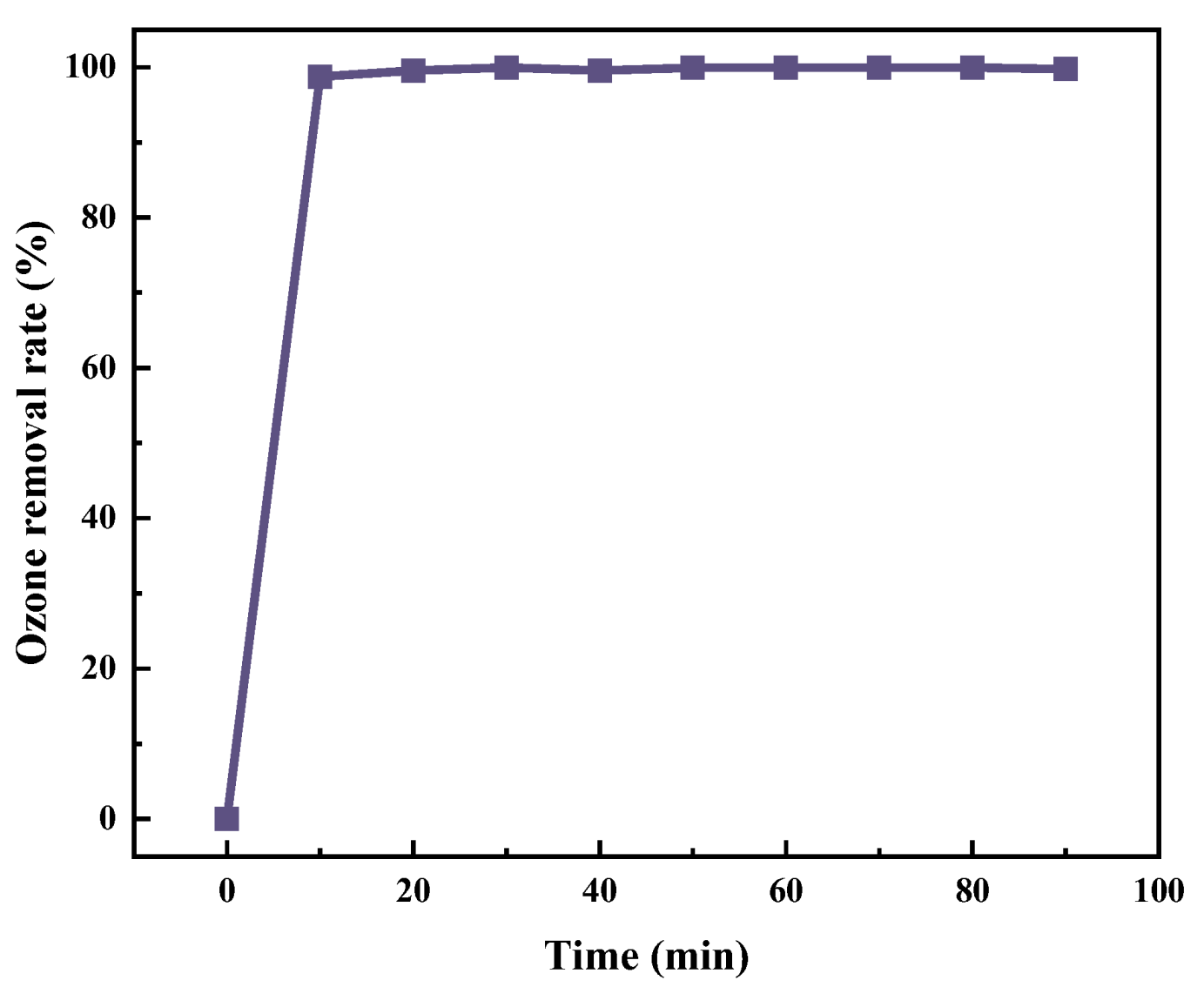
2.1.3. Optimization of Ozone Aeration Factors
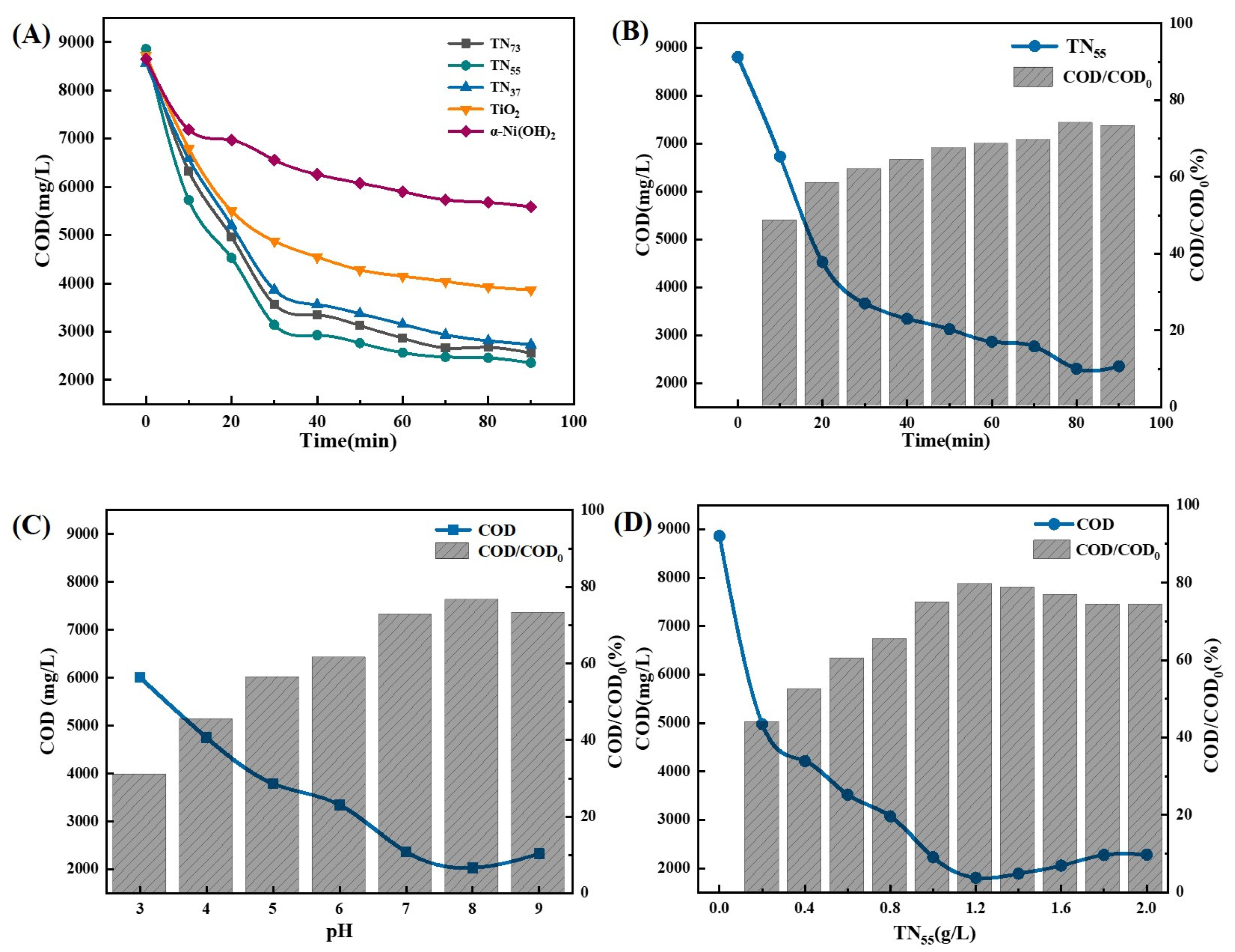
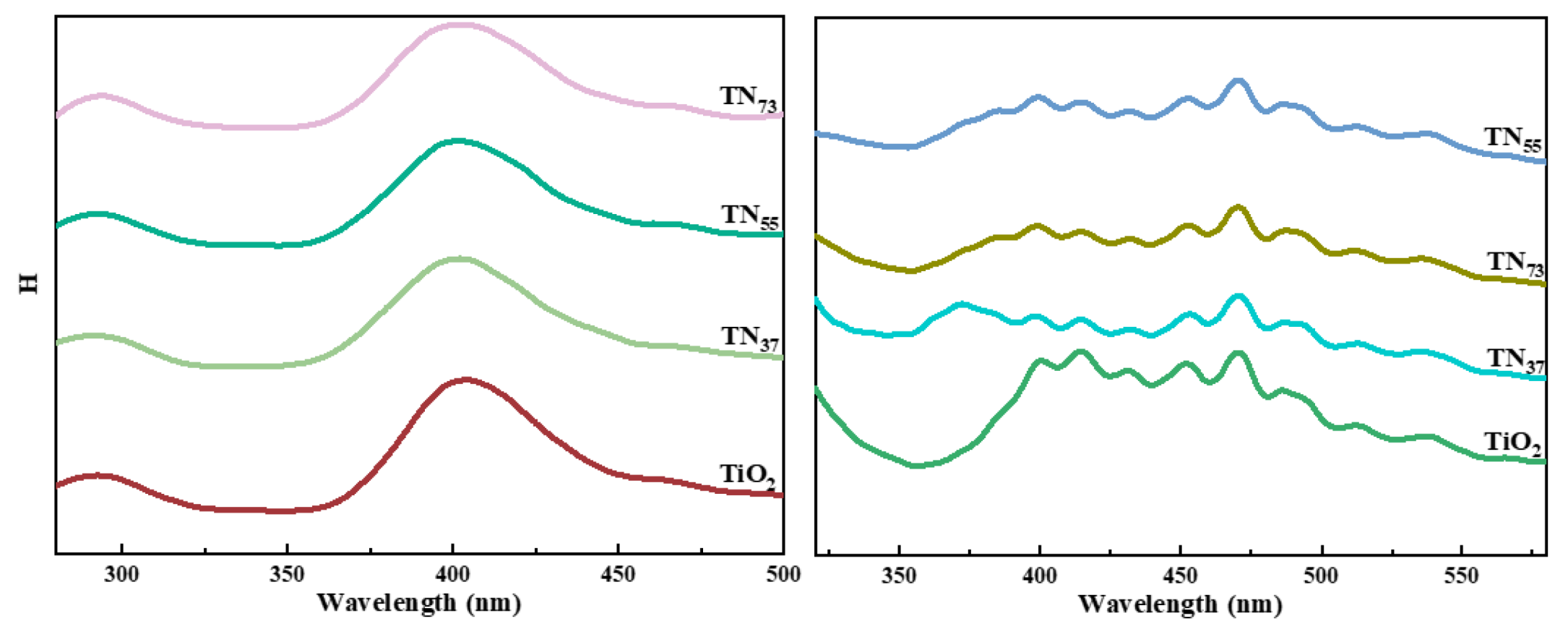
2.1.4. Optimization of Photocatalytic Factors
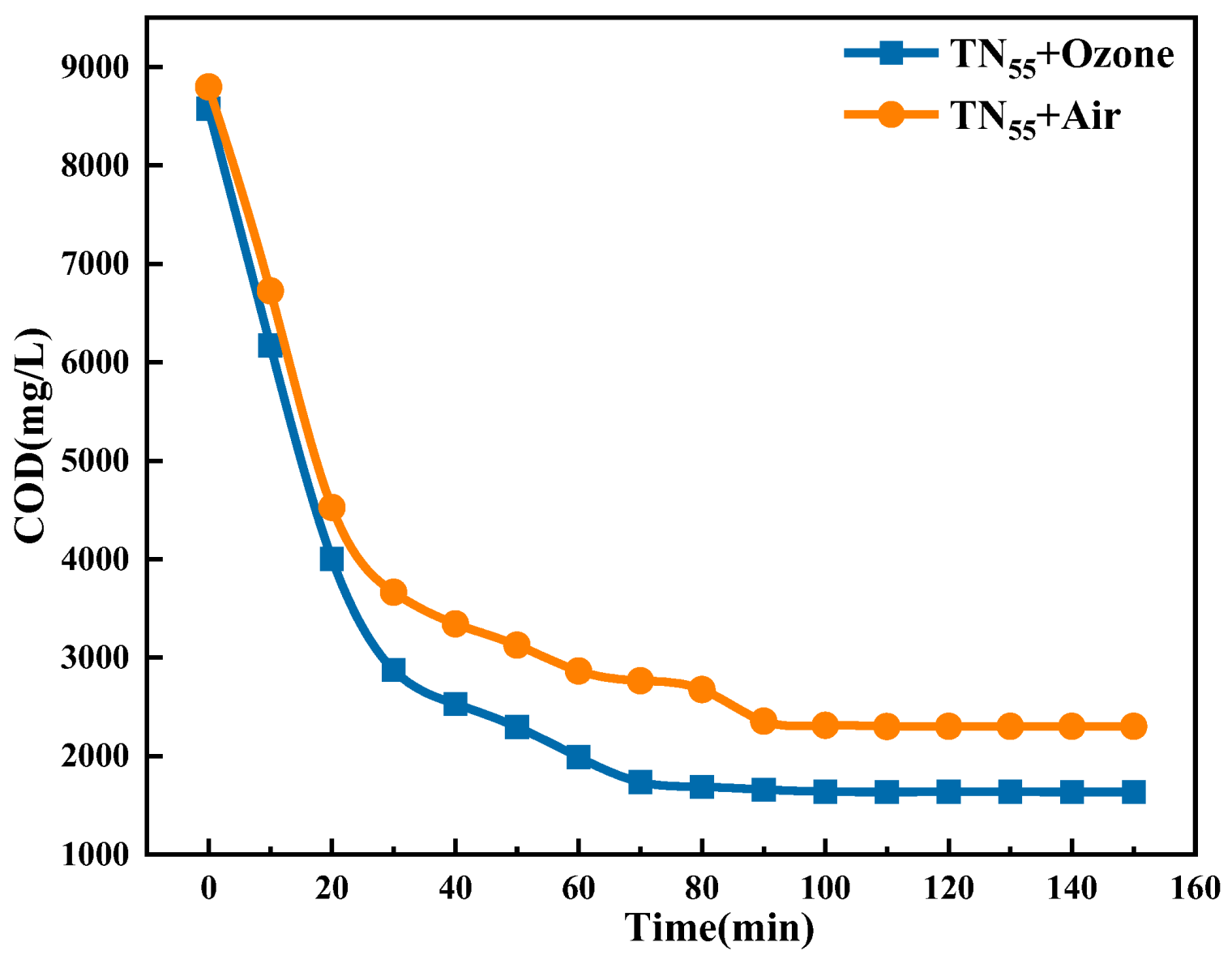
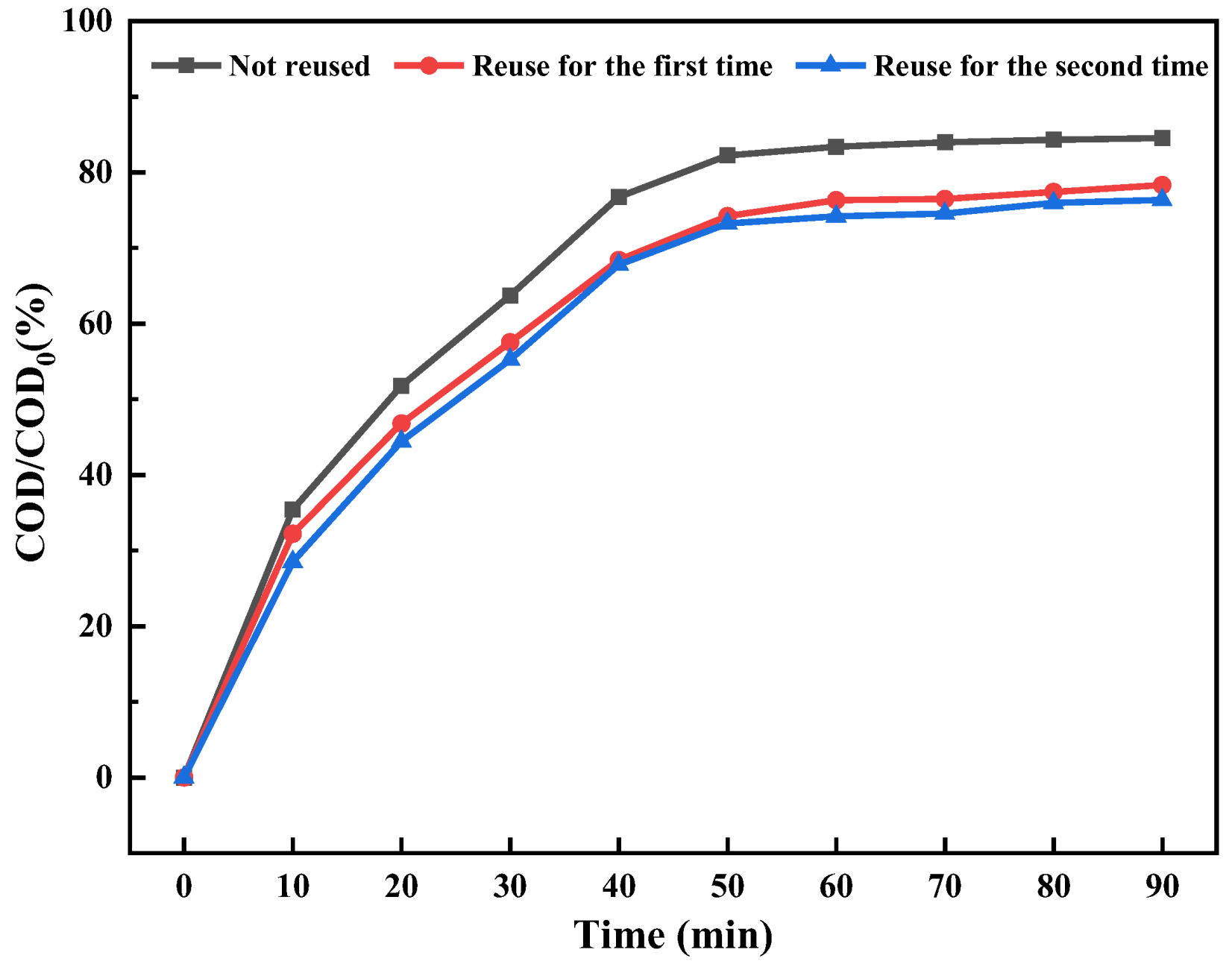
2.1.5. The Impact of Ozone Aeration on the Efficiency of Ti/Ni Composites in Breaking Down Fracturing Flowback Fluids
2.1.6. Substitutability of Ozone Synergistic Ti/Ni Composite Photocatalysis for Electrochemical Oxidation
2.1.7. Substitutability Study of Ozone Synergistic Ti/Ni Composite Photocatalysis for Fenton Oxidation
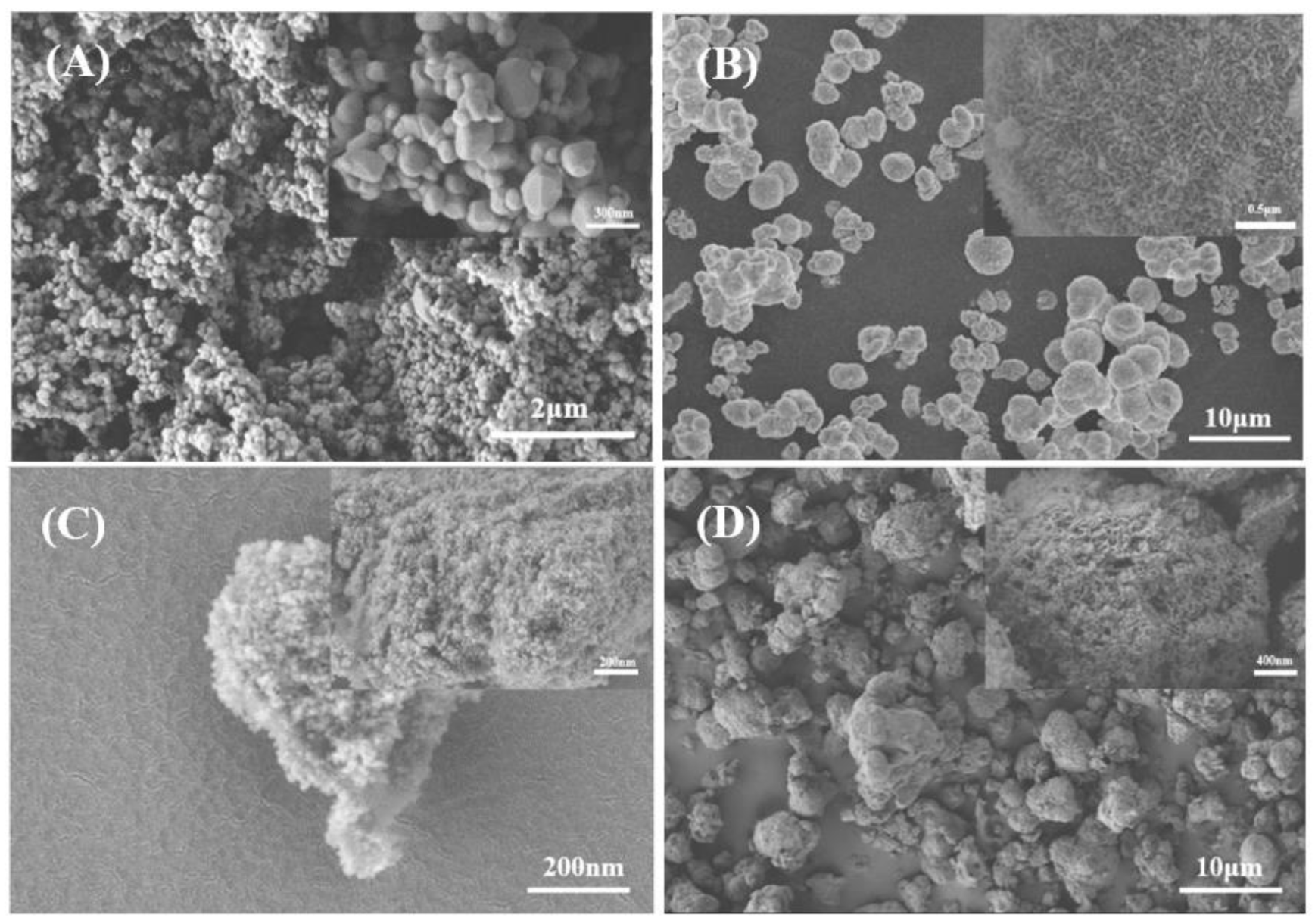
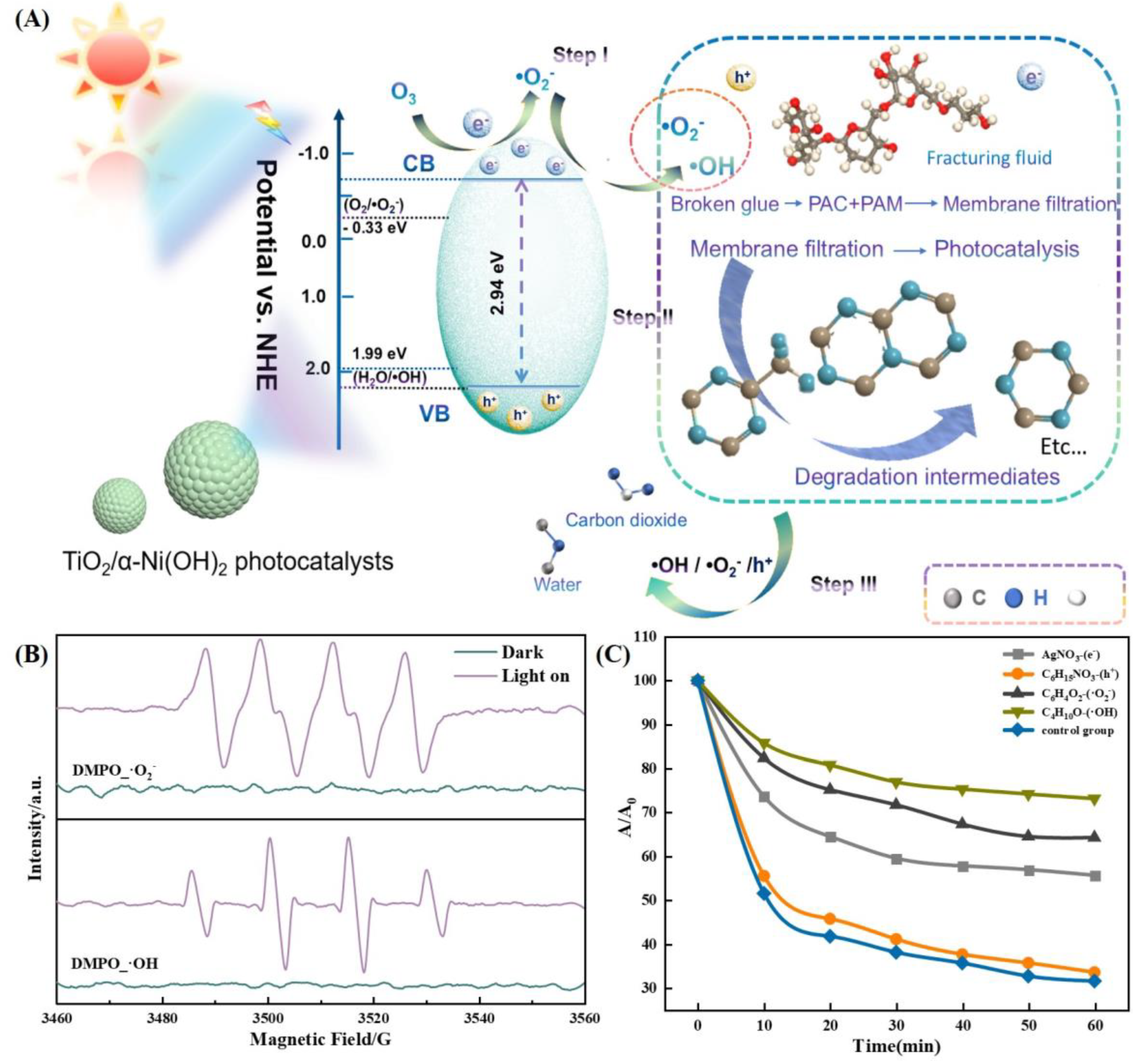
2.2. Mechanistic Analysis
3. Materials and Methods
3.1. Real Fracturing Flowback Fluid Composition Detection
3.2. Synthesis Method of Photocatalyst
3.3. Experimental Methods for Each Process
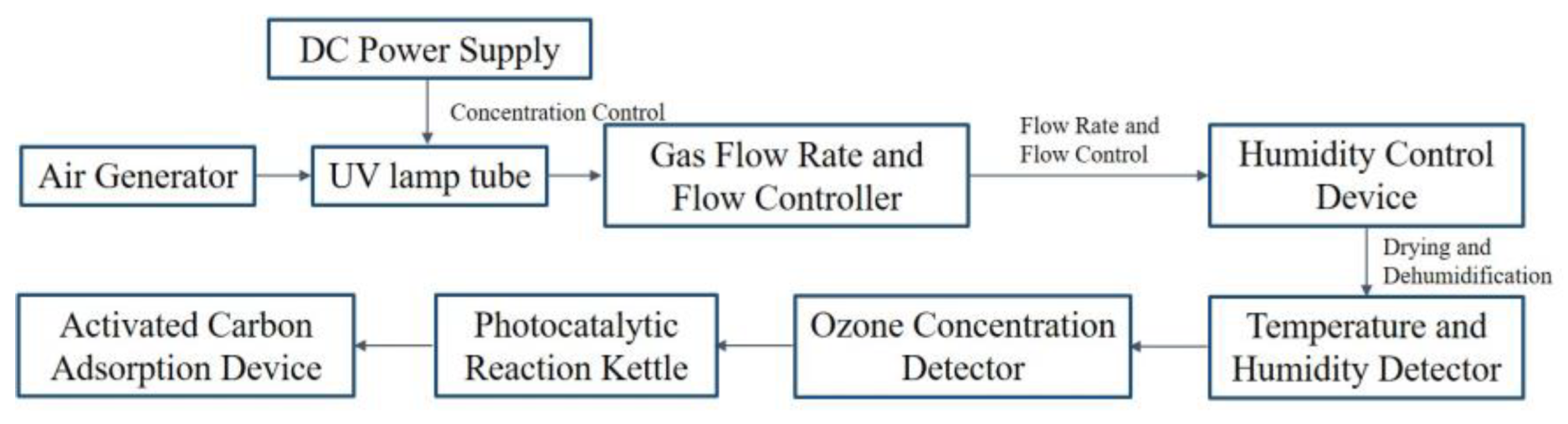
3.3.1. Experimental Methods for Ozone Aeration Steps
3.3.2. Experimental Methods for Flocculating Settling Steps
3.3.3. Experimental Methods for Microporous Filter Membrane Steps
3.3.4. Experimental Methods for Photocatalytic Steps
3.3.5. Experimental Methods for Substitutability Study
3.4. Laboratory Equipment and Chemicals
4. Conclusions
- (1)
- Coral-like anatase TiO2/α-Ni(OH)2 particles were synthesized using a hydrothermal method to enhance the photocatalytic performance and the ability to adsorb pollutants. The degradation effect was optimized when the mass ratio of Ti/Ni was 1:1.
- (2)
- The 90 min photodegradation treatment system, which combines flocculation and sedimentation, membrane separation, and ozone synergism, can produce a degradation rate of 98.42% for the original fluid return. This degradation rate is 24.18% greater than that achieved by single-material catalytic treatment. This approach efficiently substitutes the procedures of evaporation, concentration, and electrocatalytic degradation in the initial treatment phase.
- (3)
- The results of this study can provide valuable insights for the development and improvement of methods used to treat and optimize the discharge of shale gas fracturing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COD | Chemical Oxygen Demand |
| BOD | Biochemical Oxygen Demand |
| SS | Suspended Solids |
| PTFE | Polytetrafluoroethylen |
| XRD | X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| BJH | Barrett-Joyner-Halenda method |
| ESR | Electron Spin Resonance |
References
- Lester, Y.; Ferrer, I.; Thurman, E.M.; Sitterley, K.A.; Korak, J.A.; Aiken, G.; Linden, K.G. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Sci. Total Environ. 2015, 512–513, 637–644. [Google Scholar]
- Freedman, D.E.; Riley, S.M.; Jones, Z.L.; Rosenblum, J.S.; Sharp, J.O.; Spear, J.R.; Cath, T.Y. Biologically active filtration for fracturing flowback and produced water treatment. J. Water Process Eng. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [PubMed]
- Ferrer, I.; Thurman, E. Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends Environ. Anal. Chem. 2015, 5, 18–25. [Google Scholar]
- NB/T 14002/3-2015; Shale Gas Reservoir Remodeling Part III: Fracturing Fluid Recovery and Treatment Methods. National Energy Administration: Beijing, China, 2015.
- Manikandan, S.; Saraswathi, R. Electrocoagulation technique for removing Organic and Inorganic pollutants (COD) from the various industrial effluents: An overview. Environ. Eng. Res. 2023, 28, 220231. [Google Scholar]
- Uzelac, M.M.; Srđenović Čonić, B.; Kladar, N.; Armaković, S.; Armaković, S.J. Removal of hydrochlorothiazide from drinking and environmental water: Hydrolysis, direct and indirect photolysis. Energy Environ. 2023, 34, 1243–1257. [Google Scholar]
- Liu, W.; Sun, L.; Tao, S. Removal of Ca2+, Mg2+, Ba2+ and Sr2+ from shale gas flowback water from the Sichuan Basin in China by chemical softening under the guidance of OLI Stream Analyzer: Precipitation behavior and optimization study. Water Sci. Technol. 2020, 82, 194–206. [Google Scholar]
- Lin, M.; Du, Y.; Chen, K.; Yan, H. Study on the treatment of oil and gas field fracturing drainback by compound catalytic oxidation technology. Environ. Sci. Manag. 2007, 8, 115–118. [Google Scholar]
- GB 8978-1996; Comprehensive Sewage Discharge Standards. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 1998.
- Tan, B.; He, Z.; Fang, Y.; Zhu, L. Removal of organic pollutants in shale gas fracturing flowback and produced water: A review. Sci. Total Environ. 2023, 883, 163478. [Google Scholar]
- Yu, X.; Shi, S.; Xu, Z.; Zhao, L.; Li, M.; Chen, G. Application of fracturing fluid recycling technology. Chem. Environ. Prot. 2021, 41, 395–398. [Google Scholar]
- Lu, J.; Shen, Y.; Wang, J.; Xu, L.; Xie, Y.; Li, C.; Wang, L. Research on reinjection treatment technology of oilfield acidized fracturing return fluid. Appl. Chem. Eng. 2022, 51, 1685–1690. [Google Scholar]
- Zhang, Q.; Liang, H.; Bai, J.; Li, C. Progress of CdS-based photocatalytic materials. Chem. Bull. 2023, 86, 1181–1187. [Google Scholar]
- Ma, D.; Tang, J.; He, G.; Xue, Y.; Pan, S.; Liu, F.; Zhao, J. Enhancing photocatalytic degradation of rhodamine B with visible-light-driven HCl-assisted Bi2O3 photocatalysts: Activity, mechanism, and pathways. Mater. Sci. Semicond. Process. 2024, 181, 108672. [Google Scholar] [CrossRef]
- Dao, P.; Du, K.; Liu, G.; Li, G.; Tayyib, M.; Wang, K. Synthesis and photocatalytic properties of silicon-doped TiO2 nanotubes. Mater. Lett. 2024, 354, 135322. [Google Scholar]
- Jiang, S.; Jia, Y.; Yang, X.; Ou, Q.; Liu, Y.; Zhang, S.; Huang, D.; Wang, H. FeCo-LDH/g-C3N4 S-scheme photocatalyst with visible light response and enhanced photocatalytic performance. J. Photochem. Photobiol. A Chem. 2024, 456, 115870. [Google Scholar] [CrossRef]
- Yang, H.; Tang, X.; Li, R.; Liu, X.; Zhang, P.; Gong, Y. Tunable microscopic aggregation morphology of α-Ni(OH)2 for enhanced photocatalytic degradation of fracturing flowback fluid with ozone synergy. Front. Mater. 2022, 9, 995276. [Google Scholar]
- Liu, Y.; Wang, Y.; Yang, H.; Kong, L.Q.; Dou, M.Y.; Cui, Y.; Dou, J.M. Modification of electron transfer paths in CdS-TiO2 Z-type heterojunction by cobalt-based complexes for boosting visible-light photocatalytic hydrogen evolution. J. Alloys Compd. 2024, 1002, 175324. [Google Scholar]
- Baig, U.; Uddin, M.K.; Sajid, M. Surface Modification of TiO2 nanoparticles using Conducting Polymer Coating: Spectroscopic, Structural, Morphological Characterization and Interaction with Dye Molecules. Mater. Today Commun. 2020, 25, 101534. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, J.; Wang, Z.; Li, B.; Yan, Y.; Meng, M. Magnetic induced fabrication of core-shell structure Fe3O4@TiO2 photocatalytic membrane: Enhancing photocatalytic degradation of tetracycline and antifouling performance. J. Environ. Chem. Eng. 2021, 9, 6. [Google Scholar]
- Das, S.; Dutta, S.; Tama, A.M.; Basith, M.A. Nanostructured LaFeO3-MoS2 for efficient photodegradation and photocatalytic hydrogen evolution. Mater. Sci. Eng. B 2021, 271, 115295. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, H.; Lin, C.; Fang, D.; Tie, M.; Wang, J.; Zhang, Z. Immobilized Z-scheme Ag|AgBr/Ag/FeTiO3 nanocomposite film photocatalyst for visible-light-driven photocatalytic degradation of norfloxacin with simultaneous hydrogen evolution and mechanism perspective. J. Environ. Chem. Eng. 2023, 11, 111621. [Google Scholar]
- Xiong, Q.; Ma, X.; Zhao, L.; Lv, D.; Xie, L.; Jiang, L.; He, J.; Zhu, H.; Wang, J. Facile synthesis of Bi3O(OH)(AsO4)2 and simultaneous photocatalytic oxidation and adsorption of Sb(III) from wastewater. Chemosphere 2024, 359, 142308. [Google Scholar] [PubMed]
- Yang, Y.; Bian, S.; He, W.; Qiu, M.; Han, S.; Zhang, L.; Yan, G.; Zhang, X. Highly efficient Bi2Fe4O9-ZnIn2S4 Z-scheme heterojunction for piezo-photocatalytic overall water splitting under near-infrared light up to 1200 nm. J. Alloys Compd. 2024, 1002, 175315. [Google Scholar]
- Niu, H.; Xie, X.; Wu, X. Synthesis of α-Ni(OH)2 and characterization of its properties. Mater. Guide 2013, 27, 31–35. [Google Scholar]
- Wang, K.; Fan, X.; Chen, S.; Deng, J.; Zhang, L.; Jing, M.; Li, J.; Gou, L.; Li, D.; Ma, Y. 3D Co-Doping α-Ni(OH)2 Nanosheets for Ultrastable, High-Rate Ni-Zn Battery. Small 2023, 19, e2206287. [Google Scholar]
- Ren, Y.; Gao, L. From three-dimensional flower-like α-Ni(OH)2 nanostructures to hierarchical porous NiO nanoflowers: Microwave-assisted fabrication and supercapacitor properties (Article). J. Am. Ceram. Soc. 2010, 93, 3560–3564. [Google Scholar]
- Kotok, K. Comparative Investigation of Electrochemically Synthesized (α+β) Layered Nickel Hydroxide with Mixture of α-Ni(OH)2 and β-Ni(OH)2. East. Eur. J. Enterp. Technol. 2018, 92, 16–22. [Google Scholar]
- Jia, D.; Gao, H.; Dong, W.; Fan, S.; Dang, R.; Wang, G. Hierarchical α-Ni(OH)2 Composed of Ultrathin Nanosheets with Controlled Interlayer Distances and Their Enhanced Catalytic Performance(Article). ACS Appl. Mater. Interfaces 2017, 9, 20476–20483. [Google Scholar]
- Nema, S.; Sharma, A.; Rathore, V.K.; Chakraborty, M. Removal of Pharmaceuticals from Aqueous Solutions by Photodegradation Using TiO2 and Sn/Zn/Fe-Doped TiO2 as Photocatalyst Under Ultraviolet and Visible Light. Int. J. Environ. Res. 2024, 18, 12. [Google Scholar]
- Krzosa, R.; Makowski, Ł.; Orciuch, W.; Özcan-Taşkın, G.; Adamek, R.; Wojasiński, M. Characterization of structures and properties of TiO2 powders. Powder Technol. 2023, 421, 118437. [Google Scholar]
- Butkovskyi, A.; Bruning, H.; Kools, S.A.; Rijnaarts, H.H.; Van Wezel, A.P. Organic pollutants in shale gas flowback and produced waters: Identification, potential ecological impact, and implications for treatment strategies. Environ. Sci. Technol. 2017, 51, 4740–4754. [Google Scholar] [CrossRef] [PubMed]
- Akyon, B.; Stachler, E.; Wei, N.; Bibby, K. Microbial Mats as a Biological Treatment Approach for Saline Wastewaters: The Case of Produced Water from Hydraulic Fracturing. Environ. Sci. Technol. 2015, 49, 6172. [Google Scholar] [CrossRef] [PubMed]
- Bozkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Chen, M.; Sun, X.; Lozano-Blanco, G.; Tatarchuk, B.J. Tatarchuk, XPS and FTIR investigations of the transient photocatalytic decomposition of surface carbon contaminants from anatase TiO2 in UHV starved water/oxygen environments. Appl. Surf. Sci. 2021, 570, 151147. [Google Scholar]
- Zhang, J.; Zhang, H.; Zhu, X.; Song, Z.; Kang, H.; Liu, H.; Deng, W.; Hou, G.; Li, G.; Huang, Z. Progress of UiO-66 composites for adsorption and photocatalytic oxidation of typical organic pollutants. Adv. Chem. Eng. 2023, 42, 445–456. [Google Scholar]
- Xie, X.; Fan, P.; Liu, C. Progress of montmorillonite-based composite photocatalytic materials for organic wastewater treatment. Fudan J. (Nat. Sci. Ed.) 2022, 61, 238–248. [Google Scholar]
- Li, H.; Dong, X.; Sun, B.; Zhou, G. Progress in controllable preparation of TiO2/MXene nanocomposites and their application in photocatalysis and electrochemistry. Mater. Eng. 2021, 49, 54–62. [Google Scholar]
- Zhang, H.; Zhang, X.; Peng, L.; Huang, S. Research progress of composite semiconductor nanomaterials for photocatalytic applications. Mod. Chem. Ind. 2023, 43, 36–41. [Google Scholar]
- GB 3838-2002; Environmental Quality Standard for Surface Water. Ministry of Ecology and Environment: Beijing, China, 2002.
- Cibrev, D.; Jankulovska, M.; Lana-Villarreal, T.; Gómez, R. Potentiostatic reversible photoelectrochromism: An effect appearing in nanoporous TiO2/Ni(OH)2 thin films. ACS Appl. Mater. Interfaces 2014, 6, 10304–10312. [Google Scholar] [CrossRef]
- Zhao, Y.; Shu, Y.; Linghu, X.; Liu, W.; Di, M.; Zhang, C.; Shan, D.; Yi, R.; Wang, B. Modification engineering of TiO2-based nanoheterojunction photocatalysts. Chemosphere 2024, 346, 140595. [Google Scholar] [CrossRef]
- Zabara, M.A.; Kaplan, B.Y. Facet-Dependent Interfacial and Photoelectrochemical Properties of TiO2 Nanoparticles. Adv. Mater. Interfaces 2023, 10, 2300555. [Google Scholar]
- Xie, J.; Dong, L.; Song, H.; Yang, J.; Zhu, X. Preparation of doped TiO2 nanomaterials and their applications in photocatalysis. Bull. Mater. Sci. 2023, 46, 13. [Google Scholar]



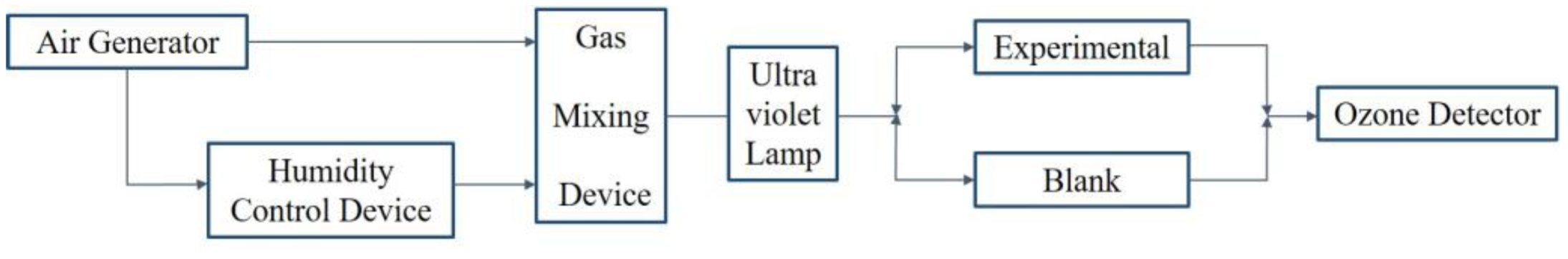






| Number | Factors | ||
|---|---|---|---|
| A | B | C | |
| Time (min) | Concentration (ppm) | Current Velocity (L/min) | |
| 1 | 30 | 20 | 0.5 |
| 2 | 40 | 40 | 1 |
| 3 | 50 | 60 | 1.5 |
| COD/COD0 range | 3.25 | 6.32 | 3.6 |
| Number | Factors | ||
|---|---|---|---|
| A | B | C | |
| Time (min) | pH | TN55 (g/L) | |
| 1 | 70 | 7 | 1 |
| 2 | 80 | 8 | 1.2 |
| 3 | 90 | 9 | 1.4 |
| COD/COD0 range | 2.77 | 1.93 | 3.7 |
| Processing Steps | Not |
Flocculating Settling |
Membrane Filtration | Complete | |
|---|---|---|---|---|---|
| Composition | |||||
| pH | 6.8 | 7 | 7 | 8 | |
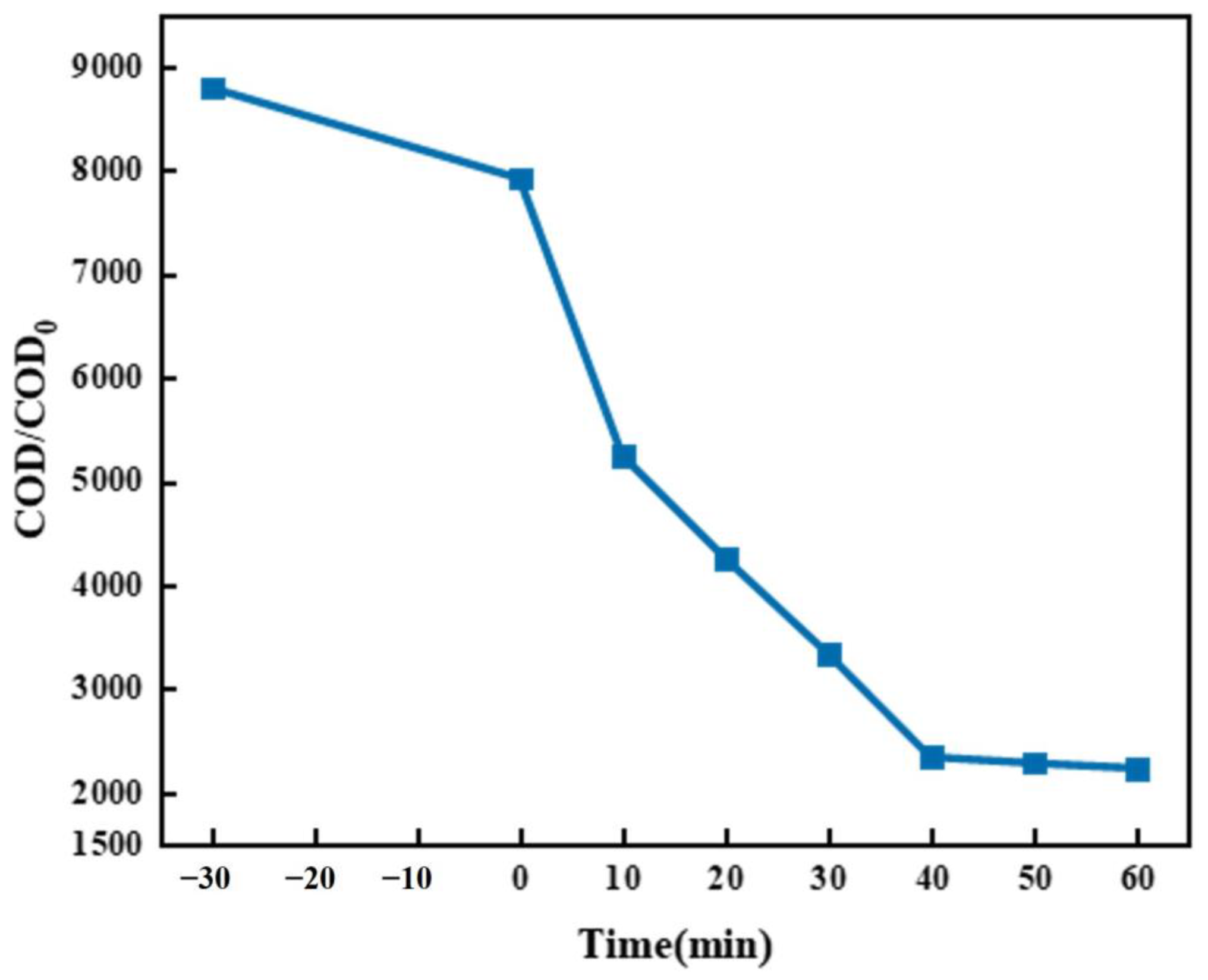
| COD | 8796 | 2551 | 878 | 135 | |
| SS | 896 | 15 | 10 | 10 | |
| BOD5 | 67 | - | - | 17 | |
| Na+ | 4812 | 2332 | 2335 | 2312 | |
| K+ | 981 | 364 | 362 | 361 | |
| Ca2+ | 723 | 473 | 477 | 466 | |
| Mg2+ | 336 | 256 | 245 | 247 | |
| Cl− | 7351 | 3768 | 3782 | 3714 | |
| SO42− | 326 | 251 | 235 | 264 | |
| NO3− | 69 | 34 | 38 | 35 | |
| Composition | Numerical Value (mg/L) | Composition | Numerical Value (mg/L) |
|---|---|---|---|
| pH | 6.8 | Ca2+ | 723 |
| COD | 8796 | Mg2+ | 336 |
| SS | 896 | Cl− | 7351 |
| BOD5 | 67 | SO42− | 326 |
| Na+ | 4812 | NO3− | 69 |
| K+ | 981 |
| Name of Instrument | Models | Manufacturer |
|---|---|---|
| Electronic balances | YJ-DTF | Shanghai Yajin Electronic Technology Co., Ltd. (Shanghai, China) |
| Magnetic Heating Stirrers | HJ-6 | Jinshui Yidu Instruments Co., Ltd. (Jinshui, China) |
| Electric Thermostatic Drying Oven | DHG-9030A | Shanghai Niyue Instrument Co., Ltd. (Shanghai, China) |
| Electric Thermostatic Water Bath | DZKW-4 | Beijing Optical Century Instrument Co., Ltd. (Beijing, China) |
| Vacuum Drying Oven | DZF-6050 | Shanghai Niyue Instrument Co., Ltd. (Shanghai, China) |
| Benchtop High-Speed Centrifuge | TG16G | Hunan Kaida Scientific Instrument Co., Ltd. (Changsha, China) |
| 300 W Long-Arc Xenon Lamp | GXZ300 | Shanghai Jiguang Special Lighting Electric Appliances Factory (Shanghai, China) |
| 70 mL Hydrothermal Synthesis Autoclave | KH-70 | Shanghai Zhize Biotechnology Development Co., Ltd. (Shanghai, China) |
| High-Pressure Quartz-Windowed Photocatalytic Reactor | PQ253 | Beijing Perfectlight Technology Co., Ltd. (Beijing, China) |
| Multifunctional Rapid Digestion System | SJ-16X | Henan Suijing Environmental Technology Co., Ltd. (Luoyang, China) |
| Chemical Oxygen Demand (COD) Spectrophotometric Analysis System | GNST-900 | Henan Suijing Environmental Technology Co., Ltd. (Luoyang, China) |
| Multi-Purpose X-ray Diffractometer (XRD) System | X’pertPRO | Netherlands PANalytical (Almelo, The Netherlands) |
| X-ray Photoelectron Spectrometer (XPS) | Thermo-Scientific TM ESCALAB250Xi | Thermo Fisher Scientific (Waltham, MA, USA) |
| Ultraviolet-Visible Diffuse Reflectance Spectrometer (UV-Vis DRS) | UV-2600 | Japan SHIMADZU (Kyoto, Japan) |
| High-Resolution Field Emission Scanning Electron Microscope (HR FE-SEM) | Gemini Sigma360 | German Carl Zeiss AG (Oberkochen, Germany) |
| Scanning Electron Microscope (SEM) | SU8010 | Japan Hitachi (Chiyoda, Japan) |
| pH Meter | PHS-25 | Shanghai Yuefeng Instruments and Meters Co., Ltd. (Shanghai, China) |
| Ion Chromatograph (IC) | 881 | Swiss Metrohm (Herisau, Switzerland) |
| Spectrophotometric Water Quality Analyzer | CleverChem 380 | German DeChem-Tech. GmbH (Hamburg, Germany) |
| BOD Rapid Analyzer | SQ-K80 | Shangqing Technology Co., Ltd. (Shanghai, China) |
| Thermostatic Magnetic Stirring Water Bath | DF-101S | Gongyi Yuhua Instruments Co., Ltd. (Gongyi, China) |
| Hydrophilic Mixed Cellulose Esters (MCE) Membrane Filter | / | Delvstlab (Jiaxing, China) |
| 250 mL Sintered Glass Filter Assembly | / | Gaode Glassware Co., Ltd. (Wenzhou, China) |
| Programmable DC Power Supply | MS-6050 | Dongguan Maihao Electronic Technology Co., Ltd. (Dongguan, China) |
| High-Precision UV Photometric Ozone Analyzer | 2B 106 L | Beijing Tonglin Technology Co., Ltd. (Beijing, China) |
| Laboratory-Grade Zero Air Generator | OML-3000A | Sichuan Oumeili Technology Co., Ltd. (Chengdu, China) |
| UV-C Disinfection Lamp | 225-VUV (185 nm) | Guangzhou Langpu Optoelectronic Technology Co., Ltd. (Guangzhou, China) |
| High-Precision Thermal Mass Flow Meter | CX-GMFM | Shanghai Jishen Instruments and Meters Co., Ltd. (Shanghai, China) |
| Reagent Name | Grade | Manufacturer |
|---|---|---|
| Ni(NO3)2·6H2O | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| CH₄N2O | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| CH3CH2OH | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Ti(SO4)2 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| H2O2 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| * GW-3 | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| XLW-32 | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| XLW-30G | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Magnacide575 | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Claytreat-3C | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Claymaster-5C | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Inflo-251G | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| BF-7L | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| BC-31 | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| * GBW-5 | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| PAC | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| PFS | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| PAS | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| PFC | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| PAM | T.P. | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| H2SO4 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| HgSO4 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| K2Cr2O7 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Ag2SO4 | AR | Chengdu Kelong Chemical Reagents Factory (Chengdu, China) |
| Raw Fracturing Flowback Fluid | / | Surig Gas Field(Ordos, China) |
| FeSO4·7H2O | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| AgNO3 | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| C6H15NO3 | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| C6H4O2 | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| C4H10O | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| C8H5O4K | SP | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Gong, Y.; Chen, X.; Li, R.; Chen, Y.; Li, M.; Tang, X. Investigation into the Feasibility of a Synergistic Photocatalytic Degradation Process for Fracturing Flowback Fluid Streams Utilizing O3 and Ti/Ni Composite Materials. Molecules 2025, 30, 1568. https://doi.org/10.3390/molecules30071568
Yang H, Gong Y, Chen X, Li R, Chen Y, Li M, Tang X. Investigation into the Feasibility of a Synergistic Photocatalytic Degradation Process for Fracturing Flowback Fluid Streams Utilizing O3 and Ti/Ni Composite Materials. Molecules. 2025; 30(7):1568. https://doi.org/10.3390/molecules30071568
Chicago/Turabian StyleYang, Huohai, Yeqi Gong, Xin Chen, Renze Li, Yuhang Chen, Mingjun Li, and Xinrui Tang. 2025. "Investigation into the Feasibility of a Synergistic Photocatalytic Degradation Process for Fracturing Flowback Fluid Streams Utilizing O3 and Ti/Ni Composite Materials" Molecules 30, no. 7: 1568. https://doi.org/10.3390/molecules30071568
APA StyleYang, H., Gong, Y., Chen, X., Li, R., Chen, Y., Li, M., & Tang, X. (2025). Investigation into the Feasibility of a Synergistic Photocatalytic Degradation Process for Fracturing Flowback Fluid Streams Utilizing O3 and Ti/Ni Composite Materials. Molecules, 30(7), 1568. https://doi.org/10.3390/molecules30071568








