Supramolecular Double-Helical Polymers: Supramolecular Chiral Induction and Asymmetric Catalysis
Abstract
1. Introduction
2. Results and Discussion
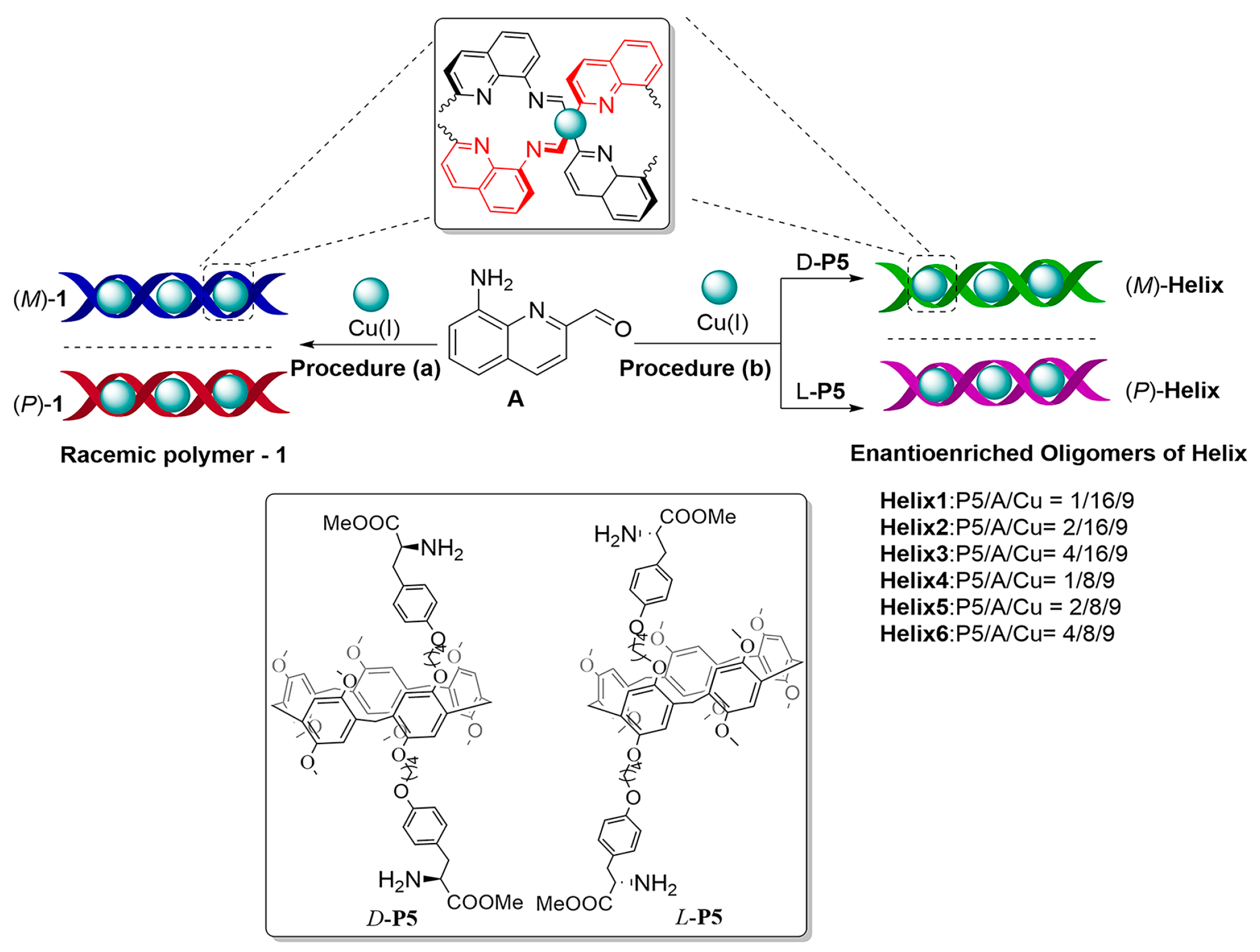
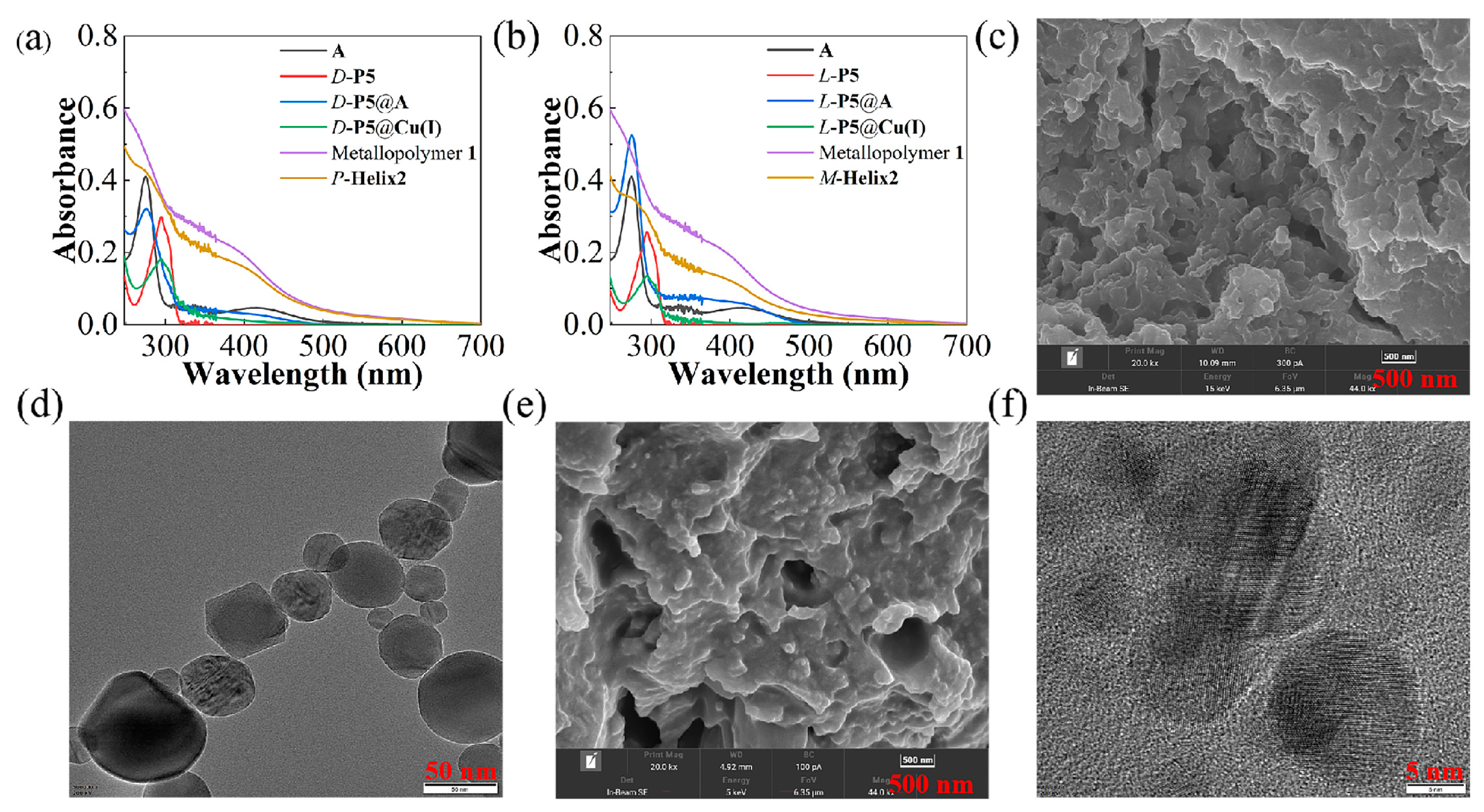
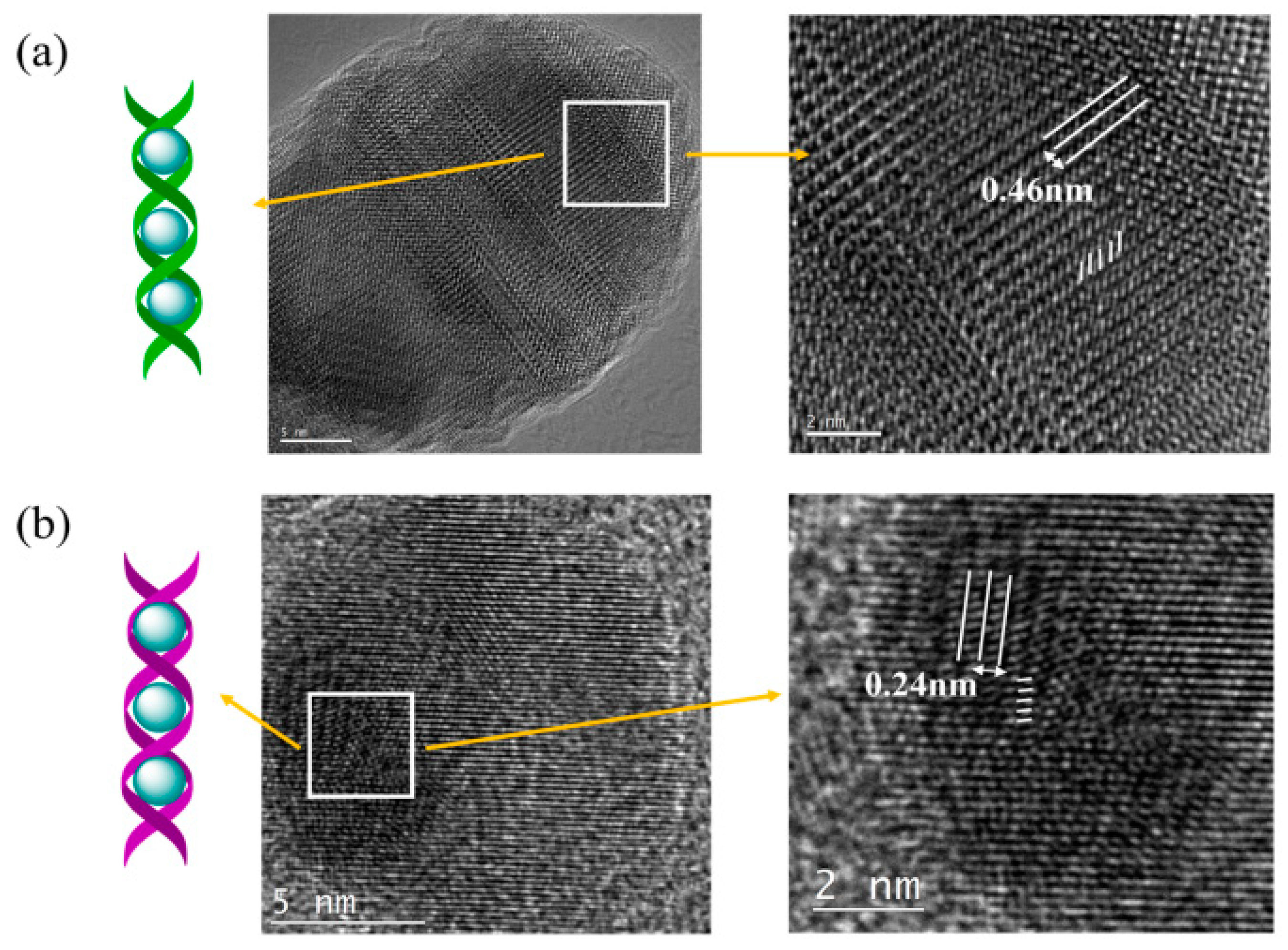
2.1. Self-Assembly and Characterization of Helix
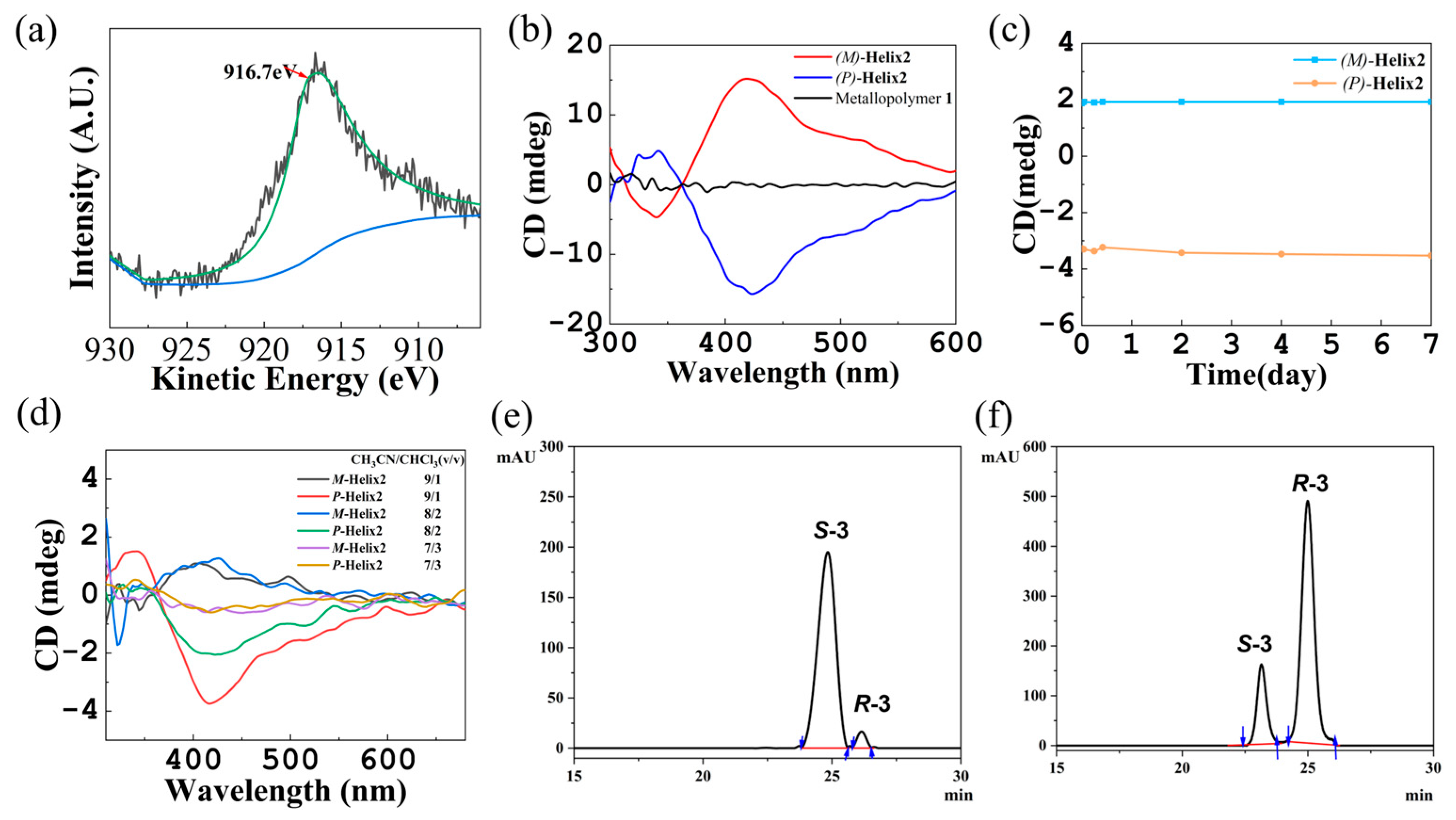
2.2. Supramolecular Chirality
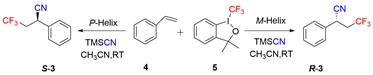
2.3. Asymmetric Catalysis
3. Materials and Methods
3.1. General
3.2. Synthesis of A and Tyrosine-Functionalized Pillar[5]arene P5
3.3. Typical Self-Assembly Procedure of Helix2
3.4. Trifluoromethylation of Styrene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Kan, J.L.; Yao, B.J.; Dong, Y.B. Synthesis of Chiral Covalent Organic Frameworks via Asymmetric Organocatalysis for Heterogeneous Asymmetric Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202115044. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, C.; Hong, T.; Zhang, Z.; Xie, C.; Li, S. Regulation of Chiral Phosphoric Acid Catalyzed Asymmetric Reaction through Crown Ether Based Host–Guest Chemistry. Org. Lett. 2022, 24, 7955–7960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yuan, C.; Sun, M.; Dong, J.; Liu, Y.; Cui, Y. Construction of Monophosphine–Metal Complexes in Privileged Diphosphine-Based Covalent Organic Frameworks for Catalytic Asymmetric Hydrogenation. J. Am. Chem. Soc. 2023, 145, 6100–6111. [Google Scholar] [CrossRef]
- Wang, G.; Peng, D.; Sun, Y.; Chen, C. Interplay of Supramolecular Chemistry and Photochemistry with Palladium-Catalyzed Ethylene Polymerization. CCS Chem. 2021, 3, 2025–2034. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Wu, C. SupEnzyme: Combining enzymes with supramolecules for recyclable catalysis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131719. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Zhou, P.-P.; Wang, Y. Catalysis with Supramolecular Carbon-Bonding Interactions. Angew. Chem. Int. Ed. 2021, 60, 22717–22721. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chu, D.; Tang, X.; Liu, Y.; Xuan, W.; Cui, Y. Supramolecular Coordination Cages for Asymmetric Catalysis. Chem. A Eur. J. 2019, 25, 662–672. [Google Scholar] [CrossRef]
- Eiji, Y.; Katsuhiro, M.; Hiroki, I.; Yoshio, F.; Kanji, N.; Nagai, K. Helical Polymers: Synthesis, Structures, and Functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar]
- Nagata, Y.; Nishikawa, T.; Suginome, M. Poly(quinoxaline-2,3-diyl)s bearing (S)-3-octyloxymethyl side chains as an efficient amplifier of alkane solvent effect leading to switch of main-chain helical chirality. J. Am. Chem. Soc. 2014, 136, 15901–15904. [Google Scholar] [CrossRef]
- Mayte, A.M.; Yan, L.; Nicolas, V.; Laurent, B.; Raynal, M. Dissecting the Role of the Sergeants in Supramolecular Helical Catalysts: From Chain Capping to Intercalation. Angew. Chem. Int. Ed. 2021, 60, 4183. [Google Scholar]
- Zhang, L.; Wang, H.X.; Li, S.; Liu, M.H. Supramolecular chiroptical switches. Chem. Soc. Rev. 2020, 49, 9095–9120. [Google Scholar]
- Jha, S.K.; Cheon, K.S.; Green, M.M.; Selinger, J.V. Chiral optical properties of a helical polymer synthesized from nearly racemic chiral monomers highly diluted with achiral monomers. J. Am. Chem. Soc. 1999, 121, 1665–1673. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Schenning, A.P.H.J.; Meijer, E.W. Insight into the mechanisms of cooperative self-assembly: The “Sergeants-and-Soldiers” principle of chiral and achiral C3-Symmetrical discotic triamides. J. Am. Chem. Soc. 2008, 130, 606–611. [Google Scholar]
- Kang, J.; Miyajima, D.; Mori, T.; Inoue, Y.; Itoh, Y.; Aida, T. A rational strategy for the realization of chain-growth supramolecular polymerization. Science 2015, 347, 646–651. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, W.S.; Fukushima, T.; Mori, T.; Aida, T. Helix Sense-Selective Supramolecular Polymerization Seeded by a One-Handed Helical Polymeric Assembly. J. Am. Chem. Soc. 2015, 137, 13792–13795. [Google Scholar] [CrossRef]
- Rodríguez, R.; Ignés-Mullol, J.; Sagués, F.; Quiñoá, E.; Riguera, R.; Freire, F. Helical sense selective domains and enantiomeric superhelices generated by Langmuir-Schaefer deposition of an axially racemic chiral helical polymer. Nanoscale 2016, 8, 3362–3367. [Google Scholar] [PubMed]
- Greenfield, J.L.; Evans, E.W.; Di Nuzzo, D.; Di Antonio, M.; Friend, R.H.; Nitschke, J.R. Unraveling Mechanisms of Chiral Induction in Double-Helical Metallopolymers. J. Am. Chem. Soc. 2018, 140, 10344–10353. [Google Scholar] [CrossRef] [PubMed]
- Luk’yanchuk, B.; Zheludev, N.I.; Maier, S.A.; Halas, N.J.; Nordlander, P.; Giessen, H.; Chong, C.T. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 2010, 9, 707–715. [Google Scholar]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar]
- Wu, J.-R.; Wu, G.; Yang, Y.-W. Pillararene-Inspired Macrocycles: From Extended Pillar[n]arenes to Geminiarenes. Accounts Chem. Res. 2022, 55, 3191–3204. [Google Scholar]
- Wang, L.; Li, G.; Yang, L.; Qu, H.N.; Cheng, J.; Abdallah, M.; Barakat, D.A.; Li, H.B. Highly Improved Chiral-Selective Resolution in Pillar[5]arene-Functionalized Molecularly Imprinted Membranes. ACS Appl. Polym. Mater. 2022, 4, 6723–6730. [Google Scholar] [CrossRef]
- Kato, K.; Kaneda, T.; Ohtani, S.; Ogoshi, T. Per-Arylation of Pillar[n]arenes: An Effective Tool to Modify the Properties of Macrocycles. J. Am. Chem. Soc. 2023, 145, 6905–6913. [Google Scholar] [CrossRef]
- Khalil-Cruz, L.E.; Liu, P.; Huang, F.; Khashab, N.M. Multifunctional Pillar[n]arene-Based Smart Nanomaterials. ACS Appl. Mater. Interfaces 2021, 13, 31337–31354. [Google Scholar] [CrossRef]
- Liu, L.Z.; Ma, C.G.; He, Q.; Huang, Y.; Duan, W.G. Effective enantiomeric identification of aromatic amines by tyrosine-modified pillar[5]arenes as chiral NMR solvating agents. Org. Chem. Front. 2021, 8, 4144–4152. [Google Scholar] [CrossRef]
- Lin, C.; Shen, Y.; Guo, X.J.; Duan, W.G.; Huang, Y.; Huang, G.B.; Liu, L.Z. Construction of a pillar[5]arene-based supramolecular chiral polymer linked to aminophosphine salt for chiral recognition of enantiomers of mandelic acid. RSC Adv. 2024, 14, 16278–16283. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Li, G.; Qu, H.N.; Zhang, H.F.; Ma, C.G.; He, Q.; Cheng, J.; Li, H.B. Enantioselective Separation of Agricultural Fungicides Based on Chiral Hybrid Nanochannel Membranes. Chem. Mater. 2024, 36, 1975–1981. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C. 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Zhan, H.; Li, F.; Gao, P.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Methanol synthesis from CO2 hydrogenation over La–M–Cu–Zn–O (M = Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors. J. Power Sources 2014, 251, 113–121. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Xiao, F.; Zhao, N.; Sun, N.; Wei, W.; Zhong, L.; Sun, Y. Preparation and activity of Cu/Zn/Al/Zr catalysts via hydrotalcite-containing precursors for methanol synthesis from CO2 hydrogenation. Catal. Sci. Technol. 2012, 2, 1447–1454. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Huang, J.J.; Wu, X.W.; Yuan, D.Q.; Liu, Y.; Cui, Y. Chiral induction in covalent organic frameworks. Nat. Commun. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Maji, A.; Hazra, A.; Maiti, D. Direct synthesis of a-trifluoromethyl ketone from (hetero)arylacetylene: Design, intermediate trapping, and mechanistic investigations. Org. Lett. 2014, 16, 4524–4527. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, J.; Wang, S.N.; Gu, Z.N.; Acena, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluor. Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Zhu, R.; Buchwald, S.L. Enantioselective Functionalization of Radical Intermediates in Redox Catalysis: Copper-Catalyzed Asymmetric Oxytrifluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2013, 52, 12655–12658. [Google Scholar] [CrossRef]
- Zhu, R.; Buchwald, S.L. Versatile Enantioselective Synthesis of Functionalized Lactones via Copper-Catalyzed Radical Oxyfunctionalization of Alkenes. J. Am. Chem. Soc. 2015, 137, 8069–8077. [Google Scholar] [CrossRef]
- Lin, J.S.; Dong, X.Y.; Li, T.T.; Jiang, N.C.; Tan, B.; Liu, X.Y. A Dual-Catalytic Strategy to Direct Asymmetric Radical Aminotrifluoromethylation of Alkenes. J. Am. Chem. Soc. 2016, 138, 9357–9360. [Google Scholar] [CrossRef]
- Wang, F.; Wang, D.H.; Wan, X.L.; Wu, L.Q.; Chen, P.H.; Liu, G.S. Enantioselective Copper-Catalyzed Intermolecular Cyanotrifluoromethylation of Alkenes via Radical Process. J. Am. Chem. Soc. 2016, 138, 15547–15550. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Bittelli, M.; Tuller, M.; Arthur, E. Estimating specific surface area: Incorporating the effect ofsurface roughness and probing molecule size. Soil Sci. Soc. Am. J. 2021, 85, 534–545. [Google Scholar] [CrossRef]
- Su, Y.L.; Tram, L.; Wherritt, D.; Arman, H.; Griffith, W.P.; Doyle, M.P. Doyle α-Amino Radical-Mediated Diverse Difunctionalization of Alkenes:Construction of C−C, C−N, and C−S Bonds. ACS Catal. 2020, 10, 13682–13687. [Google Scholar] [CrossRef]
- Liu, L.Z.; Cao, D.R.; Jin, Y.; Tao, H.Q.; Kou, Y.H.; Meier, H. Efficient synthesis of copillar[5]arenes and their host-guest properties with dibromoalkanes. Org. Biomol. Chem. 2011, 9, 7007–7010. [Google Scholar] [PubMed]
 | |||
|---|---|---|---|
| Entry | Supramolecular Catalyst | Yield (%) | ee(%): (R − S)/(R + S) |
| 1 | (P)-Helix1 | 39 | 85 (R) |
| 2 | (M)-Helix1 | 84 | 12 (S) |
| 3 | (P)-Helix2 | 35 | 28 (R) |
| 4 | (M)-Helix2 | 89 | 3 (S) |
| 5 | (P)-Helix3 | 20 | 21 (R) |
| 6 | (M)-Helix3 | 70 | 59 (S) |
| 7 | Rac-1 | 85 | <2% (R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Jia, X.; He, Q.; Duan, W.; Zhang, Y.; Huang, Y.; Liu, L. Supramolecular Double-Helical Polymers: Supramolecular Chiral Induction and Asymmetric Catalysis. Molecules 2025, 30, 1517. https://doi.org/10.3390/molecules30071517
Guo X, Jia X, He Q, Duan W, Zhang Y, Huang Y, Liu L. Supramolecular Double-Helical Polymers: Supramolecular Chiral Induction and Asymmetric Catalysis. Molecules. 2025; 30(7):1517. https://doi.org/10.3390/molecules30071517
Chicago/Turabian StyleGuo, Xiaojun, Xinyu Jia, Qin He, Wengui Duan, Yanjun Zhang, Yan Huang, and Luzhi Liu. 2025. "Supramolecular Double-Helical Polymers: Supramolecular Chiral Induction and Asymmetric Catalysis" Molecules 30, no. 7: 1517. https://doi.org/10.3390/molecules30071517
APA StyleGuo, X., Jia, X., He, Q., Duan, W., Zhang, Y., Huang, Y., & Liu, L. (2025). Supramolecular Double-Helical Polymers: Supramolecular Chiral Induction and Asymmetric Catalysis. Molecules, 30(7), 1517. https://doi.org/10.3390/molecules30071517





