Large Enhancement of the Luminescence Properties of an Eu(III) Dye upon Association with the DNA-CTMA Matrix
Abstract
1. Introduction
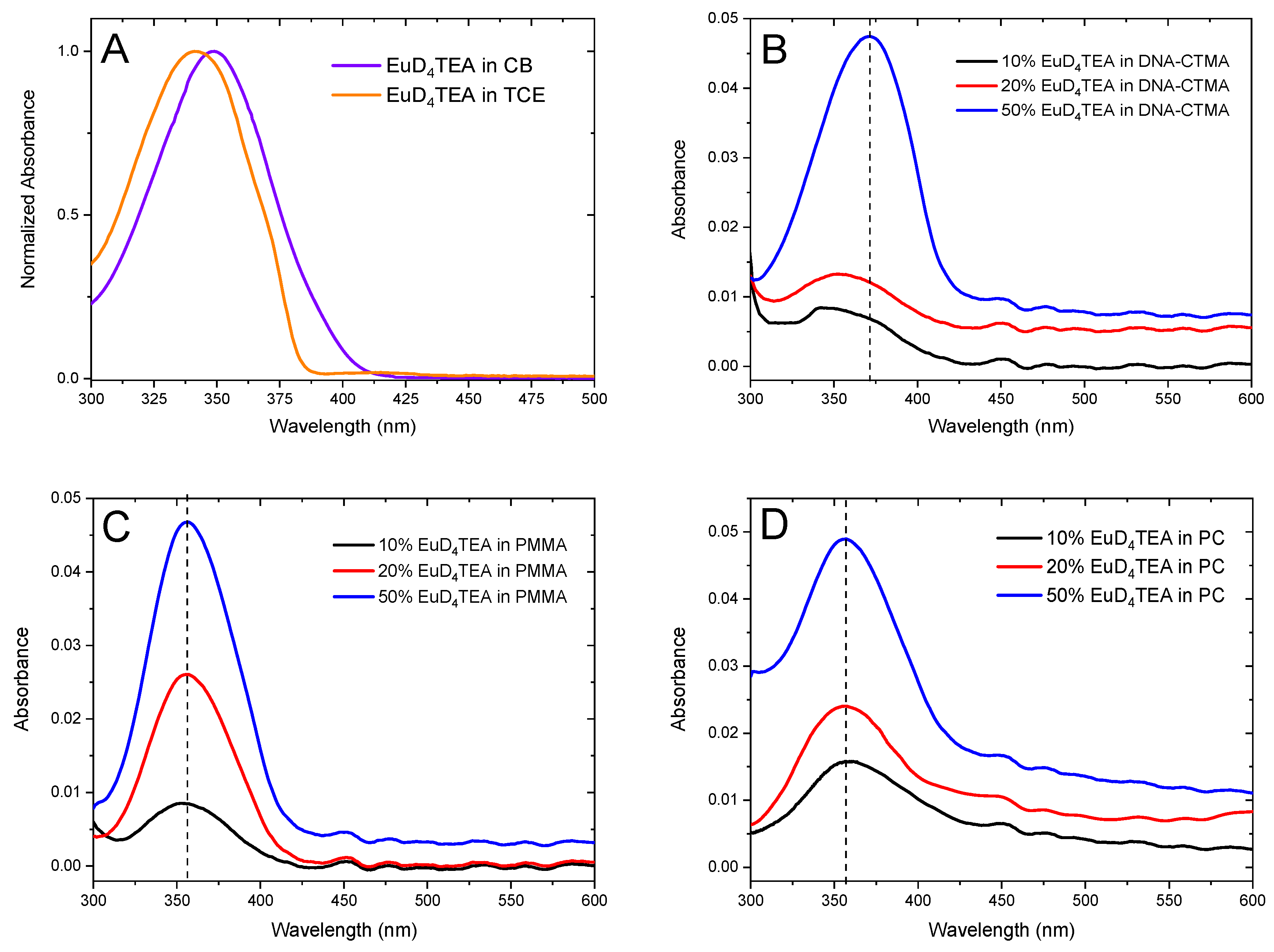
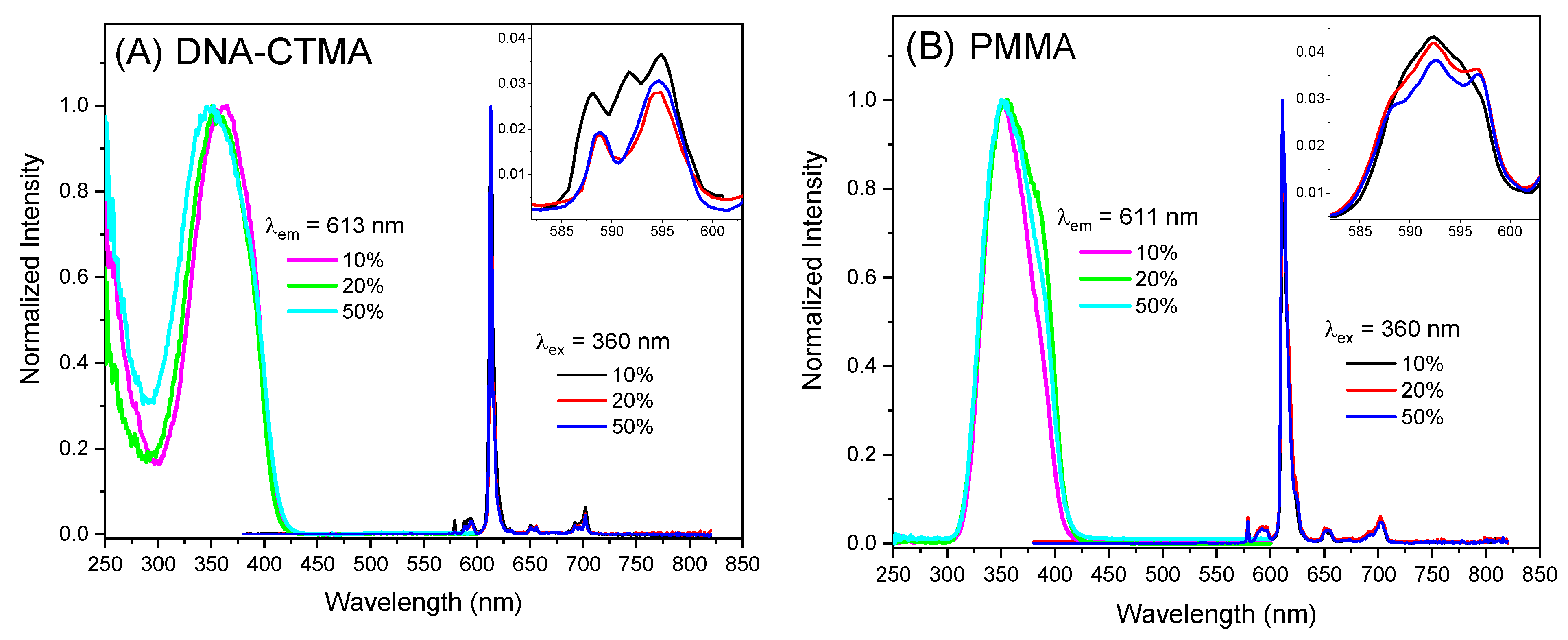
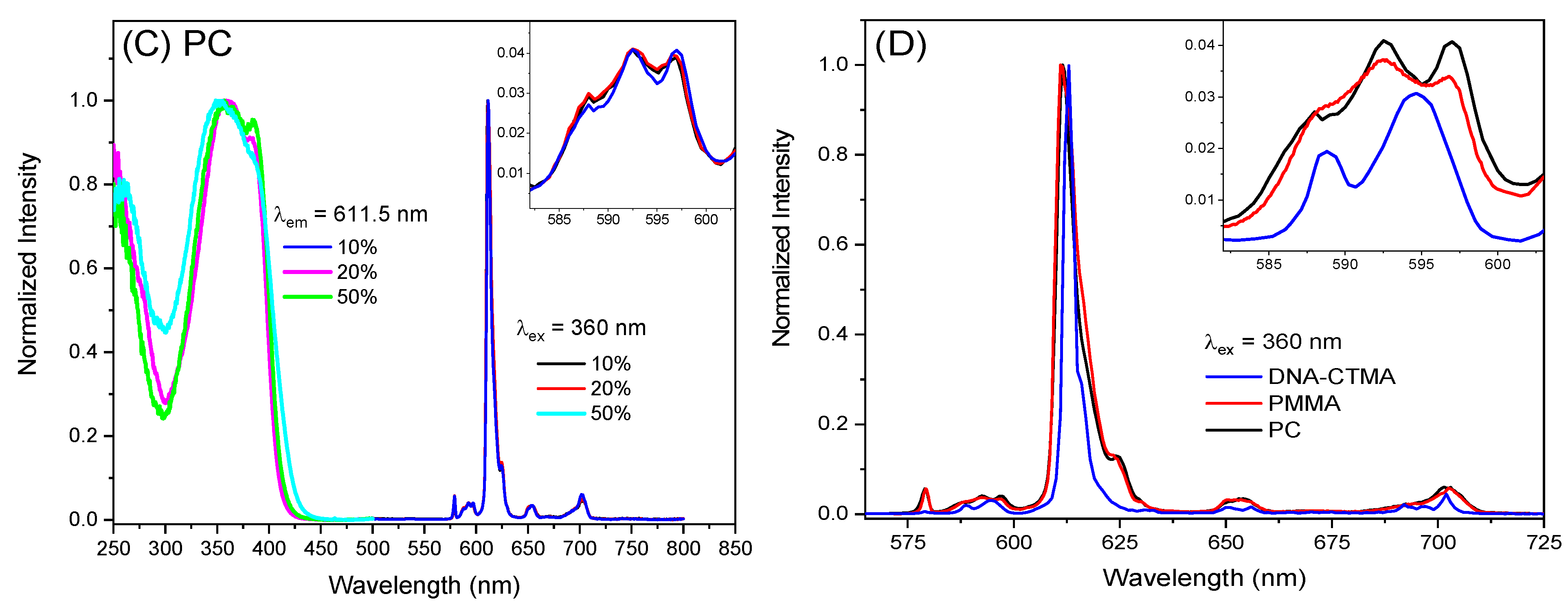
2. Results and Discussion
3. Materials and Methods
3.1. Materials Preparation
3.2. Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCE | 1,1,2-trichloroethane |
| CB | Chloroform–butanol (1:0.7 v/v) |
| EuD4TEA | europium tetrakis(dibenzoylmethide) triethylammonium |
| DNA | deoxyribonucleic acid |
| CTMA | hexadecyltrimethylammonium chloride |
| PMMA | poly(methyl methacrylate) |
| PC | polycarbonate |
References
- Kaplan, D.L. (Ed.) Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Sun, H.S.; Chiu, Y.C.; Chen, W.C. Renewable polymeric materials for electronic applications. Polym. J. 2017, 49, 61–73. [Google Scholar] [CrossRef]
- Steckl, A. DNA—A new material for photonics? Nat. Photon. 2007, 1, 3–5. [Google Scholar] [CrossRef]
- Fukuzaki, A.; Kawai, H.; Sano, T.; Takehara, K.; Nagamura, T. Efficient Upconversion by Highly Water-Soluble Cationic Sensitizer and Emitter in Aqueous Solutions with DNA. ACS Biomater. Sci. Eng. 2017, 3, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Long, E.C.; Barton, J.K. On demonstrating DNA intercalation. Acc. Chem. Res. 1990, 23, 271–273. [Google Scholar] [CrossRef]
- Wang, L.; Yoshida, J.; Ogata, N.; Sasaki, S.; Kajiyama, T. Self-Assembled Supramolecular Films Derived from Marine Deoxyribonucleic Acid (DNA)-Cationic Surfactant Complexes: Large-Scale Preparation and Optical and Thermal Properties. Chem. Mater. 2001, 13, 1273–1281. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, L.; Ma, C.; Göstl, R.; Herrmann, A. DNA–surfactant complexes: Self-assembly properties and applications. Chem. Soc. Rev. 2017, 46, 5147–5172. [Google Scholar] [CrossRef]
- Hagen, J.A.; Li, W.; Steckl, A.J.; Grote, J.G. Enhanced emission efficiency in organic light-emitting diodes using deoxyribonucleic acid complex as an electron blocking layer. Appl. Phys. Lett. 2006, 88, 171109. [Google Scholar] [CrossRef]
- Steckl, A.J.; Spaeth, H.; You, H.; Gomez, E.; Grote, J. DNA as an Optical Material. Opt. Photon. News 2011, 22, 34–39. [Google Scholar] [CrossRef]
- Mysliwiec, J.; Sznitko, L.; Sobolewska, A.; Bartkiewicz, S.; Miniewicz, A. Lasing effect in a hybrid dye-doped biopolymer and photochromic polymer system. Appl. Phys. Lett. 2010, 96, 94–97. [Google Scholar] [CrossRef]
- Kawabe, Y.; Wang, L.; Horinouchi, S.; Ogata, N. Amplified spontaneous emission from fluorescent-dye-doped DNA-surfactant complex films. Adv. Mater. 2000, 12, 1281–1283. [Google Scholar] [CrossRef]
- Massin, J.; Parola, S.; Andraud, C.; Kajzar, F.; Rau, I. Enhanced fluorescence of isophorone derivatives in DNA based materials. Opt. Mater. 2013, 35, 1810–1816. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawabe, Y. Light amplification in DNA-surfactant complex films stained by hemicyanine dye with immersion method. Opt. Mater. Express 2014, 4, 1411. [Google Scholar] [CrossRef]
- Yukimoto, T.; Uemura, S.; Kamata, T.; Nakamura, K.; Kobayashi, N. Non-volatile transistor memory fabricated using DNA and eliminating influence of mobile ions on electric properties. J. Mater. Chem. 2011, 21, 15575–15579. [Google Scholar] [CrossRef]
- Yumusak, C.; Singh, T.B.; Sariciftci, N.S.; Grote, J.G. Bio-organic field effect transistors based on crosslinked deoxyribonucleic acid (DNA) gate dielectric. Appl. Phys. Lett. 2009, 95, 263304. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, S.; Yan, H. Interfacing DNA nanotechnology and biomimetic photonic complexes: Advances and prospects in energy and biomedicine. J. Nanobiotechnol. 2022, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.E.; Elfick, A. Harnessing DNA nanotechnology and chemistry for applications in photonics and electronics. Bioconjug. Chem. 2023, 34, 97–104. [Google Scholar] [CrossRef]
- Petris, A.; Gheorge, P.; Rau, I. DNA-CTMA matrix influence on rhodamine 610 light emission in thin films. Polymers 2023, 15, 3105. [Google Scholar] [CrossRef]
- Grote, J.G.; Hagen, J.A.; Zetts, J.S.; Nelson, R.L.; Diggs, D.E.; Stone, M.O.; Yaney, P.P.; Heckman, E.; Zhang, C.; Steier, W.H.; et al. Investigation of Polymers and Marine-Derived DNA in Optoelectronics. J. Phys. Chem. B 2004, 108, 8584–8591. [Google Scholar] [CrossRef]
- Lazar, C.A.; Kajzar, F.; Rau, I.; Puntus, L.; Manea, A.M. Synthesis, linear and nonlinear optical properties of DNA-CTMA/europium (III) complex. Synth. Met. 2016, 221, 120–126. [Google Scholar] [CrossRef]
- Minami, H.; Itamoto, N.; Watanabe, W.; Li, Z.; Nakamura, K.; Kobayashi, N. Chiroptical property enhancement of chiral Eu(III) complex upon association with DNA-CTMA. Sci. Rep. 2020, 10, 18917. [Google Scholar] [CrossRef]
- Rau, I.; Czaplicki, R.; Derkowska, B.; Grote, J.G.; Kajzar, F.; Krupka, O.; Sahraoui, B. Nonlinear optical properties of functionalized DNA-CTMA complexes. Nonl. Opt. Quant. Opt. 2011, 43, 283–323. [Google Scholar] [CrossRef]
- Lee, J.E.; Do, E.D.; Lee, U.R.; Cho, M.J.; Kim, K.H.; Jin, J.I.; Shin, D.H.; Choi, S.H.; Choi, D.H. Effect of binding mode on the photoluminescence of CTMA–DNA doped with (E)-2-(2-(4-(diethylamino)styryl)-4H-pyran-4-ylidene)malononitrile. Polymer 2008, 49, 5417–5423. [Google Scholar] [CrossRef]
- Grote, J.G.; Diggs, D.E.; Nelson, R.L.; Zetts, J.S.; Hopkins, F.K.; Ogata, N.; Hagen, J.A.; Heckman, E.; Yaney, P.P.; Stone, M.O.; et al. DNA Photonics [Deoxyribonucleic Acid]. Mol. Cryst. Liq. Cryst. 2005, 426, 3–17. [Google Scholar] [CrossRef]
- Nakamura, K.; Minami, H.; Sagara, A.; Itamoto, N.; Kobayashi, N. Enhanced red emissions of europium(III) chelates in DNA–CTMA complexes. J. Mater. Chem. C 2018, 6, 4516–4522. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Hollerman, W.A.; Bhat, K.N.; Aggarwal, M.D. Luminescent properties of lanthanide dibenzoylmethide triethylammonium compounds. J. Theor. Appl. Phys. 2013, 7, 30. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Bhat, K.N.; Hollerman, W.A.; Aggarwal, M.D.; Nguyen, K.M. Comparison of the triboluminescent yield and decay time for europium dibenzoylmethide triethylammonium synthesized using different solvents. Cryst. Eng. Comm. 2012, 14, 1382–1386. [Google Scholar] [CrossRef]
- Griffini, G.; Bella, F.; Nisic, F.; Dragonetti, C.; Roberto, D.; Levi, M.; Bongiovanni, R.; Turri, S. Multifunctional Luminescent Down-Shifting Fluoropolymer Coatings: A Straightforward Strategy to Improve the UV-Light Harvesting Ability and Long-Term Outdoor Stability of Organic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5, 1401312. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Feng, J.; Song, S.Y.; Xing, Y.; Zhang, H.J.; Li, Z.F.; Sun, L.N.; Guo, X.M.; Fan, W.Q. Synthesis, characterization, and near-infrared luminescent properties of the ternary thulium complex covalently bonded to mesoporous MCM-41. J. Solid State Chem. 2009, 182, 435–441. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; He, P.; Shi, J.; Gong, M. An efficient bonding-type Eu-containing copolymer as red phosphor applied in LED. Inorg. Chem. Commun. 2011, 14, 1065–1068. [Google Scholar] [CrossRef]
- Aslanoglu, M. Electrochemical and spectroscopic studies of the interaction of proflavine with DNA. Anal. Sci. 2006, 22, 439–443. [Google Scholar] [CrossRef]
- Annaraj, J.; Srinivasan, S.; Ponvel, K.M.; Athappan, P.R. Mixed ligand copper(II) complexes of phenanthroline/bipyridyl and curcumin diketimines as DNA intercalators and their electrochemical behavior under Nafion® and clay modified electrodes. J. Inorg. Biochem. 2005, 99, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Saleh, E.E.; Aboelnga, M.M.; Toson, E.A. Experimental and computational studies of silver(I) dibenzoylmethane-based complexes, interaction with DNA/RNA/BSA biomolecules, and in vitro cytotoxic activity. J. Inorg. Biochem. 2023, 241, 112132. [Google Scholar] [CrossRef]
- Basu, B.B.J.; Vasantharajan, N. Temperature dependence of the luminescence lifetime of a europium complex immobilized in different polymer matrices. J. Lumin. 2008, 128, 1701–1708. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Eliseeva, S.V. Basics of lanthanide photophysics. In Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 1–45. [Google Scholar]
- Tanner, P.A. Some misconceptions concerning the electronic spectra of tri-positive europium and cerium. Chem. Soc. Rev. 2013, 42, 5090–5101. [Google Scholar] [CrossRef]
- Chauvin, A.; Gumy, F.; Imbert, D.; Bünzli, J.C.G. Europium and Terbium tris(Dipicolinates) as Secondary Standards for Quantum Yield Determination. Spectrosc. Lett. 2004, 37, 517–532. [Google Scholar] [CrossRef]
- Jung, W.; Jun, H.; Hong, S.; Paulson, B.; Nam, Y.S.; Oh, K. Cationic lipid binding control in DNA based biopolymer and its impacts on optical and thermo-optic properties of thin solid films. Opt. Mater. Express 2017, 7, 3796. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, J.W.; Suh, K.D. Poly(methyl methacrylate) toughening with refractive index-controlled core-shell composite particles. J. Appl. Polym. Sci. 1998, 71, 1607–1614. [Google Scholar] [CrossRef]
- Nakamura, H.; Sato, N.; Takahashi, S.; Shirakawa, Y.; Kitamura, H. Undoped polycarbonate for detection of environmental radiation. Jpn. J. Health Phys. 2014, 49, 98–101. [Google Scholar] [CrossRef]
- Ouyang, X.; Wang, M.; Guo, L.; Cui, C.; Liu, T.; Ren, Y.; Zhao, Y.; Ge, Z.; Guo, X.; Xie, G.; et al. DNA Nanoribbon-Templated Self-Assembly of Ultrasmall Fluorescent Copper Nanoclusters with Enhanced Luminescence. Angew. Chem. Int. Ed. Engl. 2020, 59, 11836–11844. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]

| Sample | Eu–Polymer | R | τav/μs | kr/s−1 | knr/s−1 | ΦLn/% | ηsens/% | Φtot/% |
|---|---|---|---|---|---|---|---|---|
| PC/Eu | 1:2 (50% w/w) | 17.6 | 226.55 | 1223 | 3191 | 27.7 | 5.4 | 1.5 |
| 1:5 (20% w/w) | 15.7 | 300.38 | 1106 | 2223 | 33.2 | 3.3 | 1.1 | |
| 1:10 (10% w/w) | 15.9 | 323.74 | 1111 | 1978 | 35.9 | 3.1 | 1.1 | |
| PMMA/Eu | 1:2 (50% w/w) | 20.2 | 367.28 | 1159 | 1564 | 42.6 | 28.7 | 12.2 |
| 1:5 (20% w/w) | 17.9 | 412.52 | 1041 | 1384 | 42.9 | 26.1 | 11.2 | |
| 1:10 (10% w/w) | 16.9 | 487.91 | 984 | 1066 | 48.0 | 19.2 | 9.2 | |
| DNA-CTMA/Eu | 1:2 (50% w/w) | 19.8 | 268.63 | 1180 | 2542 | 31.7 | 79.2 | 25.1 |
| 1:5 (20% w/w) | 20.5 | 246.20 | 1230 | 2831 | 30.3 | 78.2 | 23.7 | |
| 1:10 (10% w/w) | 15.0 | 318.68 | 937 | 2208 | 29.8 | 58.1 | 17.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinotto, D.; Marin, C.A.; Rau, I.; Colombo, A.; Fagnani, F.; Roberto, D.; Dragonetti, C. Large Enhancement of the Luminescence Properties of an Eu(III) Dye upon Association with the DNA-CTMA Matrix. Molecules 2025, 30, 1395. https://doi.org/10.3390/molecules30061395
Marinotto D, Marin CA, Rau I, Colombo A, Fagnani F, Roberto D, Dragonetti C. Large Enhancement of the Luminescence Properties of an Eu(III) Dye upon Association with the DNA-CTMA Matrix. Molecules. 2025; 30(6):1395. https://doi.org/10.3390/molecules30061395
Chicago/Turabian StyleMarinotto, Daniele, Cosmina Andreea Marin, Ileana Rau, Alessia Colombo, Francesco Fagnani, Dominique Roberto, and Claudia Dragonetti. 2025. "Large Enhancement of the Luminescence Properties of an Eu(III) Dye upon Association with the DNA-CTMA Matrix" Molecules 30, no. 6: 1395. https://doi.org/10.3390/molecules30061395
APA StyleMarinotto, D., Marin, C. A., Rau, I., Colombo, A., Fagnani, F., Roberto, D., & Dragonetti, C. (2025). Large Enhancement of the Luminescence Properties of an Eu(III) Dye upon Association with the DNA-CTMA Matrix. Molecules, 30(6), 1395. https://doi.org/10.3390/molecules30061395












