Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol
Abstract
1. Introduction
2. Results and Discussion
2.1. Microflotation Experiments
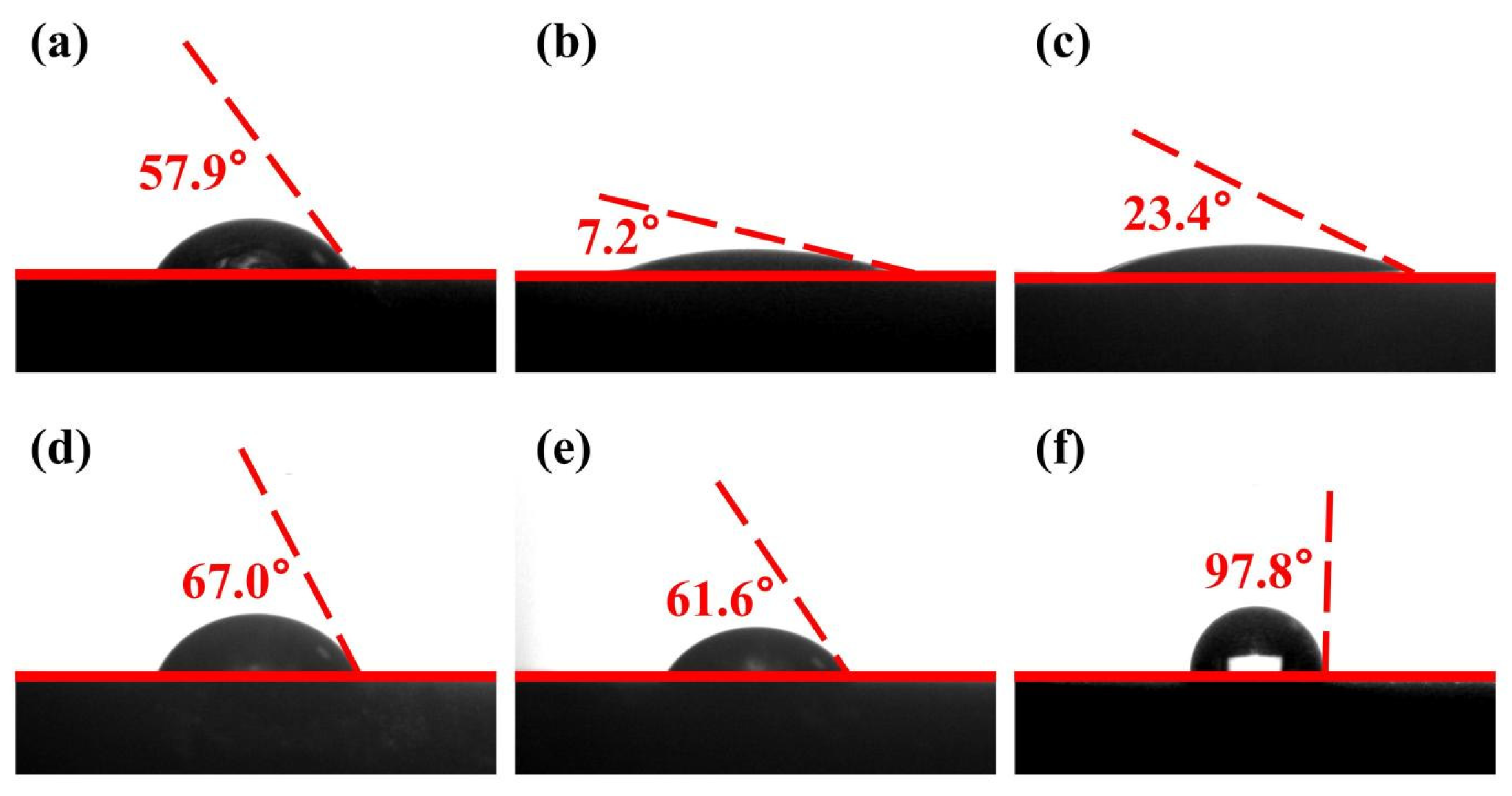
2.2. Contact Angle Measurements
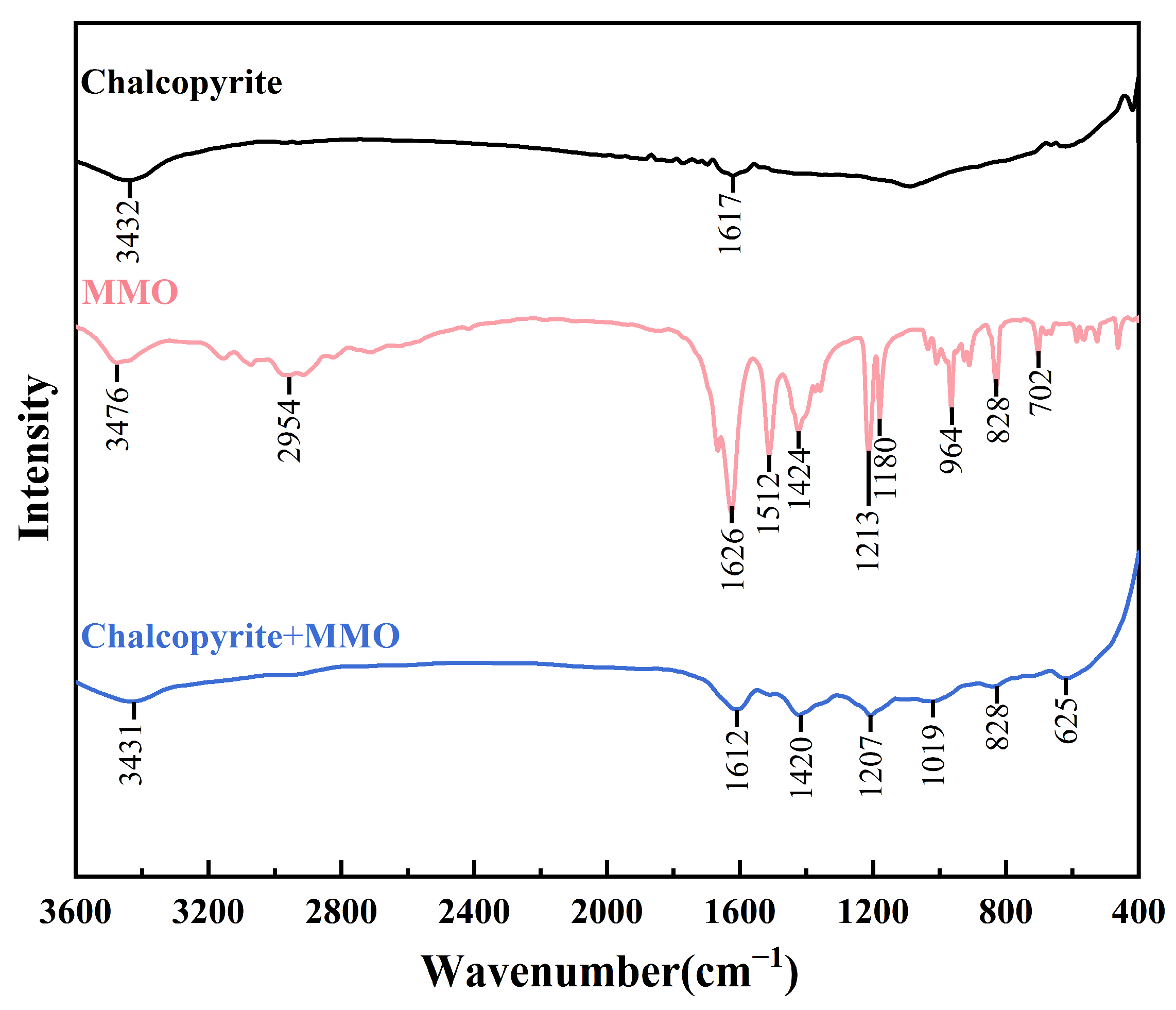
2.3. FT-IR Measurements
2.4. ToF-SIMS Measurement
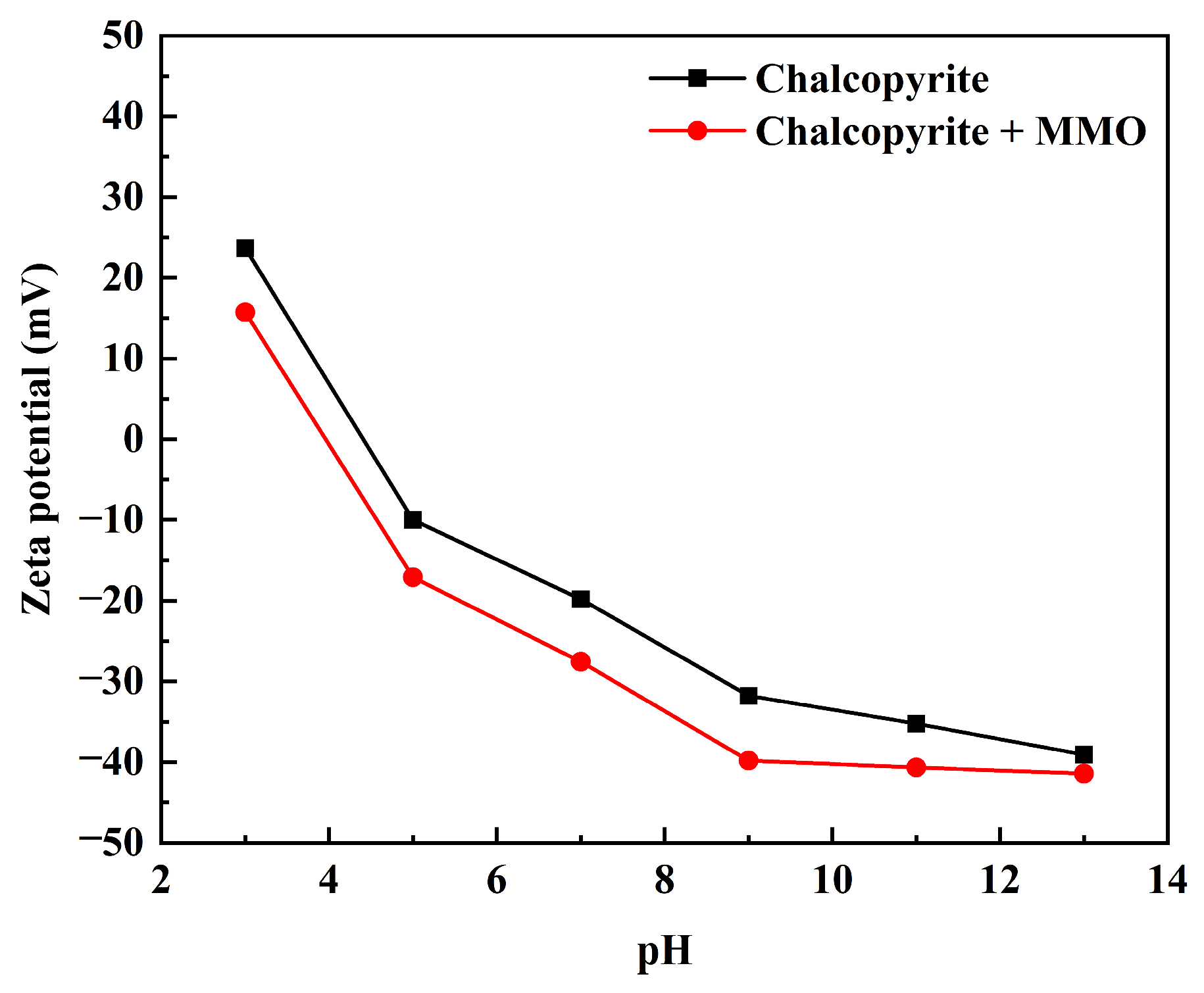
2.5. Zeta Potential Measurement
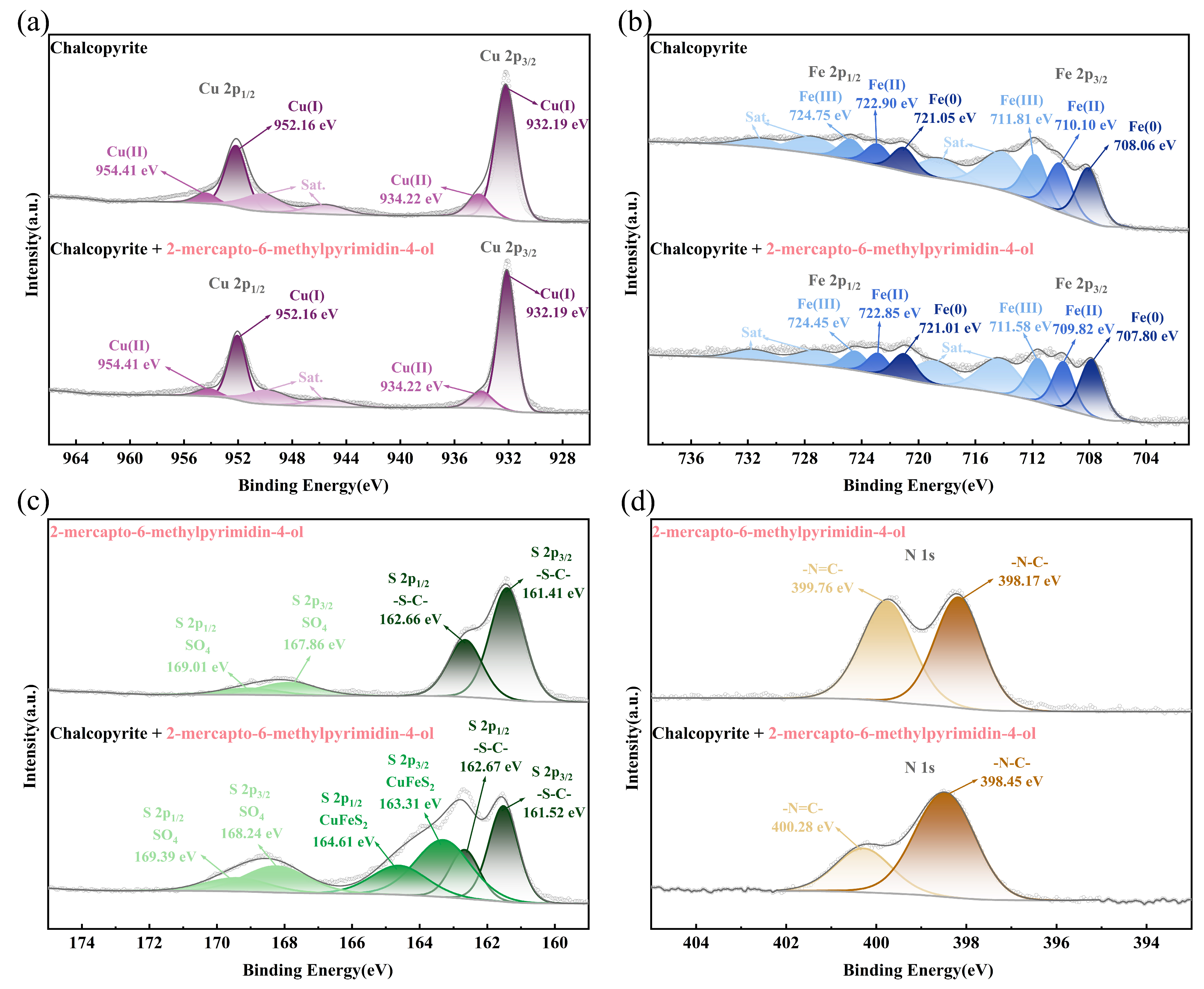
2.6. XPS Measurements
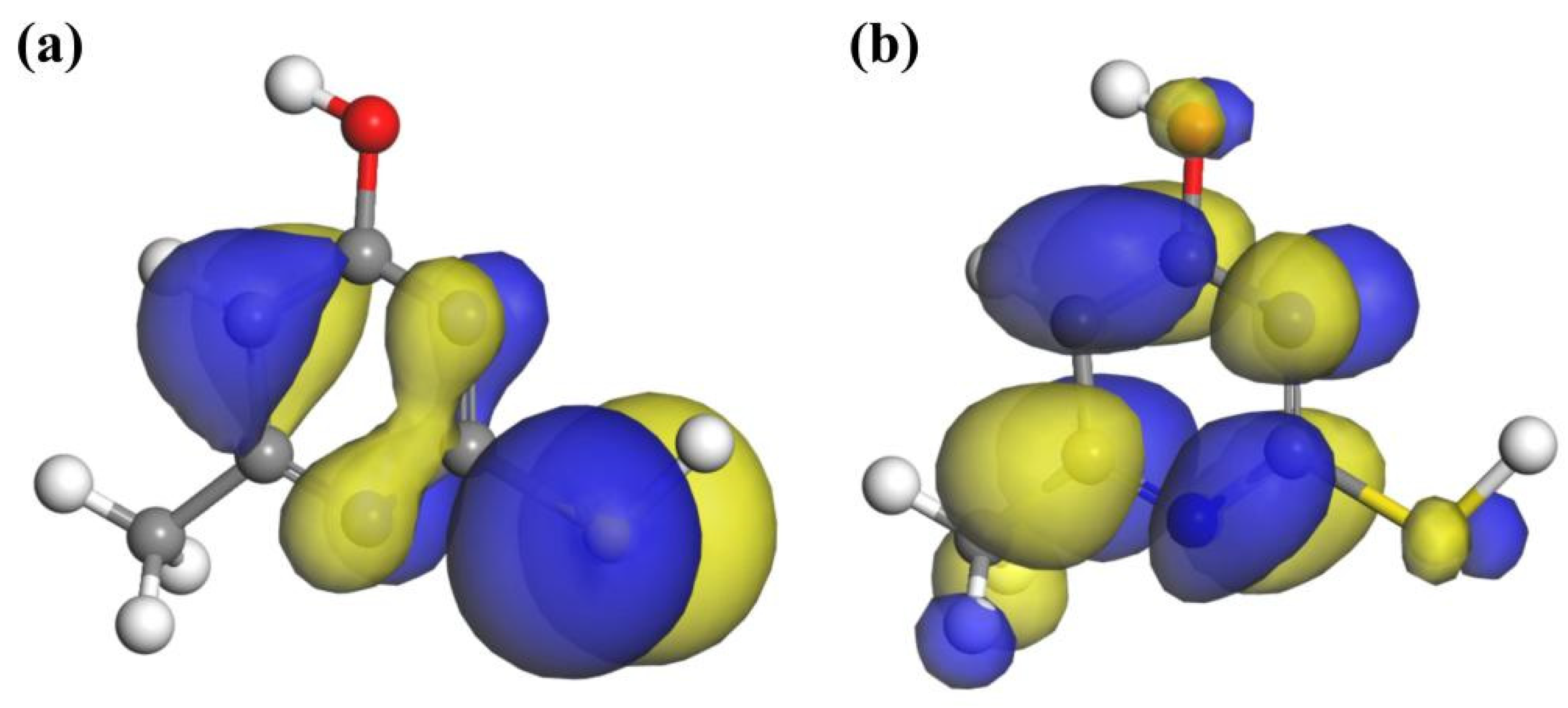
2.7. DFT Simulations
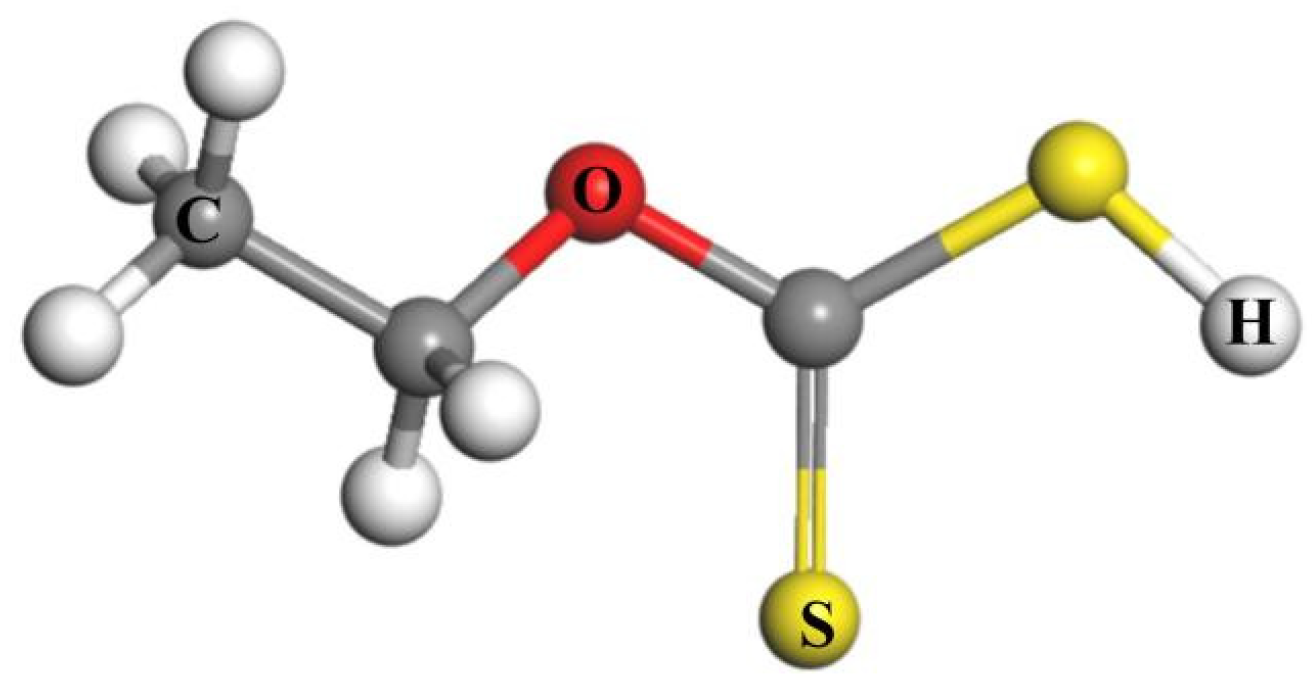
2.7.1. Molecular Structure of Reagents
2.7.2. Adsorption on the Surface of Chalcopyrite
2.8. Actual Ore Flotation Experiments
3. Materials and Methods
3.1. Materials and Reagents
3.2. Microflotation Experiments
3.3. Contact Angle Measurements
3.4. FT-IR Measurements
3.5. TOF-SIMS Measurements
3.6. Zeta Potential Measurements
3.7. XPS Measurements
3.8. DFT Simulations
3.9. Actual Ore Flotation Experiments
4. Conclusions
- The contact angle measurement results indicated that MMO substantially enhanced the hydrophilicity of the chalcopyrite surface while having a minimal effect on molybdenite. The increase in surface hydrophobicity of chalcopyrite treated with ethyl xanthate followed by MMO was not significant; in contrast, molybdenite experienced a considerable improvement in hydrophobicity after being treated with ethyl xanthate followed by MMO, leading to a notable difference in surface wettability. Consequently, MMO could serve as a depressant for chalcopyrite in copper–molybdenum separation flotation. Microflotation experiments corroborated this finding, showing that MMO effectively depressed chalcopyrite across a wide pH range while exerting little influence on the flotation recovery rate of molybdenite.
- FT-IR, TOF-SIMS, and XPS tests and DFT simulation results revealed that MMO could adsorb onto the chalcopyrite surface. During the adsorption process, the N1, N2, and S atoms in the MMO molecule bonded with the Fe1, Fe2, and Cu ions on the chalcopyrite surface, respectively, resulting in the MMO molecule losing electrons while the chalcopyrite surface gained them. This configuration represented triple bond adsorption, forming a stable chelate structure with strong adsorption.
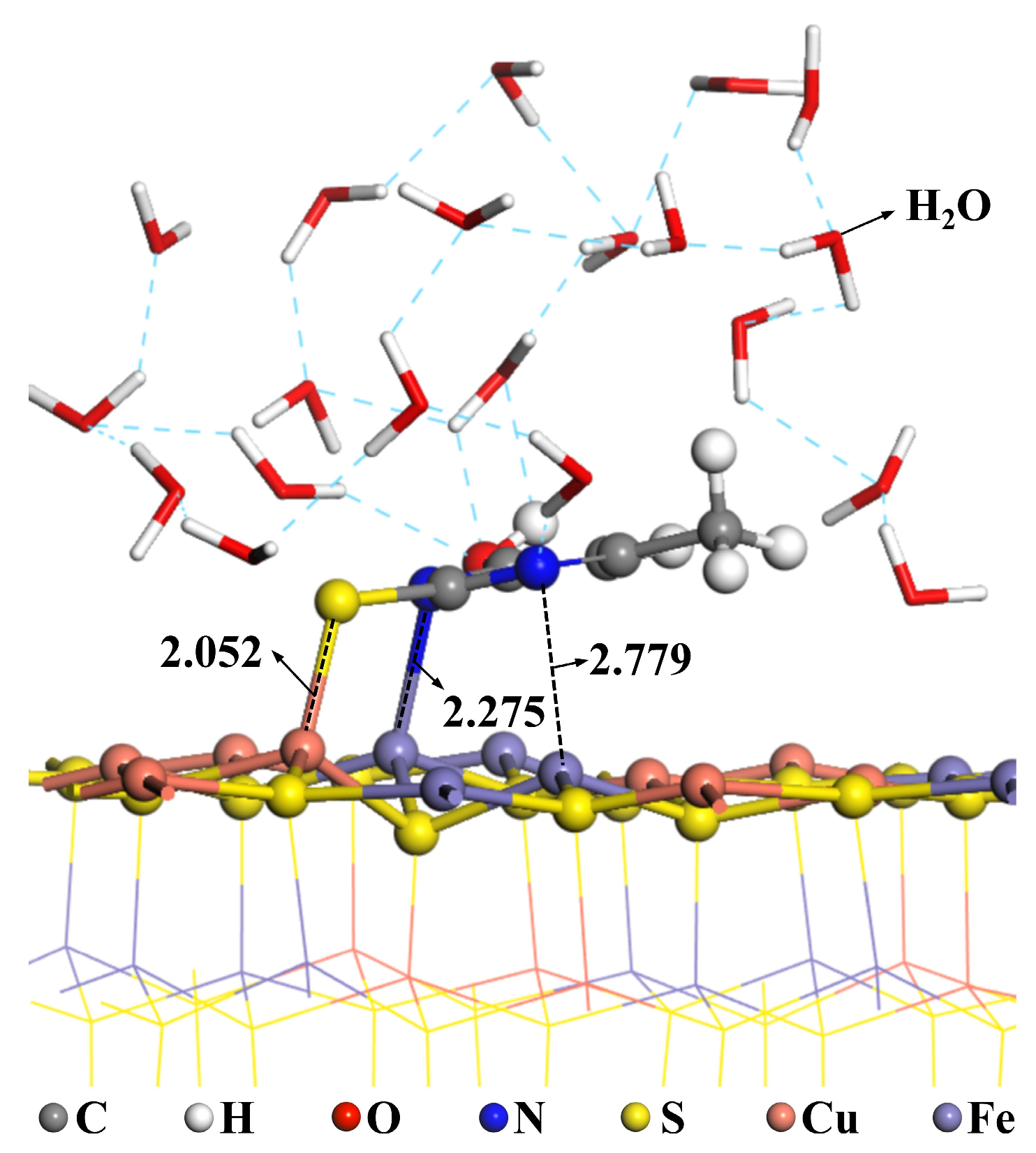
- Dynamic simulations were employed to examine the effect of water molecules on the adsorption of MMO on the chalcopyrite surface. It was found that MMO remains stably adsorbed on the chalcopyrite surface in the presence of water. Additionally, water molecules were distributed close to the chalcopyrite surface and were interconnected by hydrogen bonds, indicating that MMO adsorption enhanced the hydrophilicity of the chalcopyrite surface, thereby depressing its flotation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Kawashima, N.; Li, J.A.; Chandra, P.A.R. Gerson a review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, L.; He, J.; Wu, H.; Fang, S.; Khoso, S.A.; Hu, Y.; Sun, W. Evaluation of l-cysteine as an eco-friendly depressant for the selective separation of MoS2 from PbS by flotation. J. Mol. Liq. 2019, 282, 177–186. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents; Elsevier: Amsterdam, The Netherlands, 2007; pp. 235–293. ISBN 9780444530295. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Yang, B.; Lopez-Mendoza, A. Flotation of molybdenite fines as hydrophobic agglomerates. Sep. Purif. Technol. 2012, 98, 451–455. [Google Scholar] [CrossRef]
- Abdollahi, M.; Bahrami, A.; Mirmohammadi, M.S.; Kazemi, F.; Danesh, A.; Ghorbani, Y. A process mineralogy approach to optimize molybdenite flotation in copper—Molybdenum processing plants. Miner. Eng. 2020, 157, 106557. [Google Scholar] [CrossRef]
- Yi, G.; Macha, E.; Van Dyke, J.; Macha, R.E.; McKay, T.; Free, M.L. Recent progress on research of molybdenite flotation: A review. Adv. Colloid Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective flotation of chalcopyrite and molybdenite with plasma pre-treatment. Miner. Eng. 2014, 66–68, 102–111. [Google Scholar] [CrossRef]
- Bazin, C.; Proulx, M. Distribution of reagents down a flotation bank to improve the recovery of coarse particles. Int. J. Miner. Process. 2001, 61, 1–12. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yu, Y.; Gao, H.; Qin, X.; Bai, X. Depressants for separation of chalcopyrite and molybdenite: Review and prospects. Miner. Eng. 2023, 201, 108209. [Google Scholar] [CrossRef]
- Saim, A.K.; Darteh, F.K. Eco-Friendly and Biodegradable Depressants in Chalcopyrite Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2022, 44, 492–510. [Google Scholar] [CrossRef]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Wang, X.-H.; Forssberg, K.S.E. The solution electrochemistry of sulfide-xanthate-cyanide systems in sulfide mineral flotation. Miner. Eng. 1996, 9, 527–546. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Qingjun, G. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Peng, Y. The effect of sodium hydrosulfide on molybdenite flotation as a depressant of copper sulfides. Miner. Eng. 2020, 148, 106203. [Google Scholar] [CrossRef]
- Huang, W.-X.; Tang, H.-H.; Cao, Y.; Liu, R.-H.; Sun, W. Separation of molybdenite from chalcopyrite with thiolactic acid depressant: Flotation behavior and mechanism. Trans. Nonferrous Met. Soc. China 2023, 33, 3157–3167. [Google Scholar] [CrossRef]
- Poorkani, M.; Banisi, S. Industrial use of nitrogen in flotation of molybdenite at the Sarcheshmeh copper complex. Miner. Eng. 2005, 18, 735–738. [Google Scholar] [CrossRef]
- Huiqing, P.; Wu, D.; Abdalla, M.; Luo, W.; Jiao, W.; Bie, X. Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite. Minerals 2017, 7, 51. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Liu, J.; Hu, Y.; Guan, Q.; Zhang, C.; Tang, H.; Guan, C. Investigation into the flotation response of refractory molybdenum ore to depressant mixtures: A case study. Int. J. Min. Sci. Technol. 2016, 26, 1089–1094. [Google Scholar] [CrossRef]
- Qin, W.; Wu, J.; Jiao, F.; Zeng, J. Mechanism study on flotation separation of molybdenite from chalcocite using thioglycollic acid as depressant. Int. J. Min. Sci. Technol. 2017, 27, 1043–1049. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lan, L.-H.; Liao, X.-J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper–molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Xu, H.; Ye, T.; Zhang, X.; Lu, L.; Xiong, W.; Zhu, Y. Insights into the adsorption mechanism of N-thiourea-maleamic acid on chalcopyrite surface in the flotation separation of Cu-Mo sulfide ores. J. Mol. Liq. 2022, 350, 118554. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wei, D.-Z.; Shen, Y.-B.; Liu, W.-G.; Gao, S.-L.; Liang, G.-Q. Selective depression effect in flotation separation of copper–molybdenum sulfides using 2,3-disulfanylbutanedioic acid. Trans. Nonferrous Met. Soc. China 2015, 25, 3126–3132. [Google Scholar] [CrossRef]
- Ansari, A.; Pawli, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Dai, L.; Feng, B.; Tang, M.; Chen, Y. Effect and mechanism of environmentally friendly depressant 2, 4-Diamino-6-hydroxypyrimidine in separation of chalcopyrite and molybdenite. Process Saf. Environ. Prot. 2024, 187, 993–999. [Google Scholar] [CrossRef]
- Cao, J.; Liao, R.; Wu, D.; Zuo, Q.; Liu, J.; Wen, S. Utilizing phosphonyl carboxylic acid copolymer as an efficient depressant for flotation separation of chalcopyrite from galena: Experimental and DFT study. Sep. Purif. Technol. 2024, 348, 127725. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Y.; Chen, K.; Yi, C.; Nong, Y.; Chen, Z. Adsorption of Polycarboxylate Ether Superplasticizer Incorporating Various Functional Groups on the Kaolin (001) Surface: Theoretical Study and Experimental Testing. MetaResource 2024, 1, 61–73. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, H.; Tong, L.; Jin, R.; Ma, P. Understanding the effect of grinding media on the adsorption mechanism of cyanide to chalcopyrite surface by ToF–SIMS, XPS, contact angle, zeta potential and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128799. [Google Scholar] [CrossRef]
- Meng, J.; Xu, L.; Wang, D.; Xue, K.; Luo, L.; Shi, X. The activation mechanism of metal ions on spodumene flotation from the perspective of in situ ATR-FTIR and ToF-SIMS. Miner. Eng. 2022, 182, 107567. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, M.; Yuan, J.; Yu, A.; Dai, H.; Bai, S. Fenton oxidation modification mechanism of pyrite and its response to Cu-S flotation separation: Experiment, DFT, XPS and ToF-SIMS studies. Appl. Surf. Sci. 2024, 652, 159305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Han, G.; Feng, Q. A novel activation method for regulating surface characteristics and flotation performance of smithsonite: XPS, SEM-EDS, AFM, ToF-SIMS and FT-IR studies. Sep. Purif. Technol. 2025, 352, 128276. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q. Depression mechanism of acid for flotation separation of fluorapatite and dolomite using ToF-SIMS and XPS. J. Mol. Liq. 2024, 394, 123584. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.-L.; Wei, D.-H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Tehrani, M.E.H.N.; Naderi, H.; Rashchi, F. Electrochemical study and XPS analysis of chalcopyrite dissolution in sulfuric acid in the presence of ethylene glycol. Electrochim. Acta 2021, 369, 137663. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Chen, J.; Chen, Y. Effect of content and spin state of iron on electronic properties and floatability of iron-bearing sphalerite: A DFT+U study. Int. J. Min. Sci. Technol. 2023, 33, 1563–1571. [Google Scholar] [CrossRef]
- Luo, A.; Chen, J. Low-temperature collector for smithsonite flotation: Experiments and DFTB+ study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133651. [Google Scholar] [CrossRef]
- Svärd, M.; Rasmuson, Å.C. Force Fields and Point Charges for Crystal Structure Modeling. Ind. Eng. Chem. Res. 2009, 48, 2899–2912. [Google Scholar] [CrossRef]
- Ismail, M.; Abir, A.T.; Mondal, B.K.; Chowdhury, M.A.H.; Hossain, M.; Rahaman, M.M.; Hossain, J. Theoretical insights into narrow bandgap CuFeS2 chalcopyrite for thermophotovoltaic applications. Mater. Today Commun. 2024, 39, 109089. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Luo, A.; Xiong, W.; Chen, J. Interface adsorption of 5-amino-1,3,4-thiadiazole-2-thiol on chalcopyrite surface as flotation depressant in Cu/Mo separation. Appl. Surf. Sci. 2023, 611, 155703. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, Y.; Zhao, C. First-principles study on the co-adsorption of water and oxygen molecules on chalcopyrite (112)-M surface. Int. J. Min. Sci. Technol. 2023, 33, 1055–1063. [Google Scholar] [CrossRef]











| Mineral | Dosage (×10−5 mol/L) | Recovery1 (%) | Recovery2 (%) | Recovery3 (%) | Recovery Average (%) | Standard Deviation | Error Bar |
|---|---|---|---|---|---|---|---|
| Chalcopyrite | 0.5 | 57.26 | 60.31 | 59.78 | 59.12 | 1.63 | 59.12 ± 1.63 |
| 1 | 16.23 | 16.98 | 17.89 | 17.03 | 0.83 | 17.03 ± 0.83 | |
| 1.5 | 9.98 | 9.07 | 9.93 | 9.66 | 0.48 | 9.66 ± 0.48 | |
| 2 | 4.03 | 4.51 | 4.16 | 4.23 | 0.24 | 4.23 ± 0.24 | |
| 2.5 | 0.59 | 0.47 | 0.44 | 0.50 | 0.08 | 0.5 ± 0.08 | |
| Molybdenite | 0.5 | 69.45 | 64.23 | 66.77 | 66.82 | 2.63 | 66.82 ± 2.63 |
| 1 | 61.33 | 61.90 | 63.76 | 62.33 | 1.22 | 62.33 ± 1.22 | |
| 1.5 | 57.84 | 58.35 | 60.10 | 58.76 | 1.14 | 58.76 ± 1.14 | |
| 2 | 57.49 | 55.86 | 53.55 | 55.63 | 1.97 | 55.63 ± 1.97 | |
| 2.5 | 52.43 | 54.26 | 55.95 | 54.21 | 1.76 | 54.21 ± 1.76 |
| Dosage (×10−5 mol/L) | t-Value | Degrees of Freedom (df) | p-Value Range | Significance (α = 0.05) |
|---|---|---|---|---|
| 0.5 | −4.33 | 3.4 | p < 0.05 | Significant |
| 1 | −29.02 | 3.28 | p < 0.001 | Significant |
| 1.5 | −29.8 | 3.78 | p < 0.001 | Significant |
| 2 | −31.72 | 3.35 | p < 0.001 | Significant |
| 2.5 | −33.94 | 3.63 | p < 0.001 | Significant |
| Mineral | Dosage (×10−5 mol/L) | Recovery1 (%) | Recovery2 (%) | Recovery3 (%) | Recovery Average (%) | Standard Deviation | Error Bar |
|---|---|---|---|---|---|---|---|
| Chalcopyrite | 3.5 | 66.86 | 68.33 | 67.32 | 67.5 | 0.75 | 67.5 ± 0.75 |
| 6.5 | 60.3 | 61.36 | 61.69 | 61.12 | 0.7 | 61.12 ± 0.7 | |
| 20 | 44.15 | 48.94 | 46.95 | 46.68 | 2.4 | 46.68 ± 2.4 | |
| 50 | 23.01 | 21.17 | 22 | 22.06 | 0.92 | 22.06 ± 0.92 | |
| 60 | 11.98 | 10.45 | 11.51 | 11.31 | 0.77 | 11.31 ± 0.77 | |
| 80 | 10.63 | 9.96 | 9.02 | 9.87 | 0.83 | 9.87 ± 0.83 | |
| Molybdenite | 3.5 | 89.78 | 86.94 | 88.52 | 88.41 | 1.43 | 88.41 ± 1.43 |
| 6.5 | 84.96 | 85.63 | 85.20 | 85.26 | 0.34 | 85.26 ± 0.34 | |
| 20 | 80.11 | 81.57 | 81.21 | 80.96 | 0.77 | 80.96 ± 0.77 | |
| 50 | 72.46 | 74.65 | 72.64 | 73.25 | 1.12 | 73.25 ± 1.12 | |
| 60 | 70.01 | 70.96 | 71.54 | 70.84 | 0.77 | 70.84 ± 0.77 | |
| 80 | 66.96 | 69.78 | 68.25 | 68.33 | 1.41 | 68.33 ± 1.41 |
| Dosage (×10−5 mol/L) | t-Value | Degrees of Freedom (df) | p-Value Range | Significance (α = 0.05) |
|---|---|---|---|---|
| 3.5 | −22.48 | 2.71 | p < 0.001 | Significant |
| 6.5 | −21.53 | 2.2 | p < 0.001 | Significant |
| 20 | −25.61 | 2.4 | p < 0.001 | Significant |
| 50 | −35.17 | 2.96 | p < 0.001 | Significant |
| 60 | −67.32 | 2.98 | p < 0.001 | Significant |
| 80 | −42.19 | 2.53 | p < 0.001 | Significant |
| Mineral | pH | Recovery1 (%) | Recovery2 (%) | Recovery3 (%) | Recovery Average (%) | Standard Deviation (%) | Error Bar (%) |
|---|---|---|---|---|---|---|---|
| Chalcopyrite | 3 | 95.34 | 96.28 | 97.01 | 96.21 | 0.84 | 96.21 ± 0.84 |
| 5 | 13.88 | 14.56 | 14.06 | 14.17 | 0.34 | 14.17 ± 0.34 | |
| 7 | 11.88 | 11.03 | 11.02 | 11.31 | 0.47 | 11.31 ± 0.47 | |
| 9 | 1.13 | 1.21 | 1.22 | 1.19 | 0.05 | 1.19 ± 0.05 | |
| 11 | 0.66 | 0.78 | 0.69 | 0.71 | 0.06 | 0.71 ± 0.06 | |
| 13 | 0.51 | 0.52 | 0.44 | 0.49 | 0.04 | 0.49 ± 0.04 | |
| Molybdenite | 3 | 90.06 | 91.58 | 90.61 | 90.75 | 0.78 | 90.75 ± 0.78 |
| 5 | 73.49 | 72.75 | 71.69 | 72.64 | 0.90 | 72.64 ± 0.90 | |
| 7 | 69.93 | 70.96 | 71.63 | 70.84 | 0.86 | 70.84 ± 0.86 | |
| 9 | 68.88 | 69.06 | 67.54 | 68.49 | 0.76 | 68.49 ± 0.76 | |
| 11 | 65.46 | 64.91 | 65.03 | 65.13 | 0.28 | 65.13 ± 0.28 | |
| 13 | 61.98 | 62.97 | 60.99 | 61.98 | 1.03 | 61.98 ± 1.03 |
| pH | t-Value | Degrees of Freedom (df) | p-Value Range | Significance (α = 0.05) |
|---|---|---|---|---|
| 3 | 8.27 | 3.8 | p < 0.01 | Significant |
| 5 | −65.34 | 3.98 | p < 0.001 | Significant |
| 7 | −66.01 | 3.97 | p < 0.001 | Significant |
| 9 | −101.5 | 2.03 | p < 0.001 | Significant |
| 11 | −202.8 | 2.1 | p < 0.001 | Significant |
| 13 | −58.45 | 3.57 | p < 0.001 | Significant |
| Mineral | Sample | Contact Angle1 (°) | Contact Angle2 (°) | Contact Angle3 (°) | Recovery Average (°) | Standard Deviation (°) | Error Bar (°) |
|---|---|---|---|---|---|---|---|
| Chalcopyrite | Untreated | 56.7 | 58.6 | 58.4 | 57.9 | 1.04 | 57.9 ± 1.04 |
| Treated by MMO | 7.8 | 7 | 6.8 | 7.2 | 0.5 | 7.2 ± 0.5 | |
| Treated by ethyl xanthate and MMO | 22.9 | 24.1 | 23.2 | 23.4 | 0.62 | 23.4 ± 0.62 | |
| Molybdenite | Untreated | 66.8 | 67.3 | 66.9 | 67 | 0.25 | 67 ± 0.25 |
| Treated by MMO | 61.3 | 61.5 | 61.9 | 61.6 | 0.31 | 61.6 ± 0.31 | |
| Treated by ethyl xanthate and MMO | 97.6 | 97.2 | 98.5 | 97.8 | 0.7 | 97.8 ± 0.7 |
| Sample | t-Value | Degrees of Freedom (df) | p-Value Range | Significance (α = 0.05) |
|---|---|---|---|---|
| Untreated | −14.68 | 2.33 | p < 0.001 | Significant |
| Treated by MMO | −87.6 | 2.03 | p < 0.001 | Significant |
| Treated by ethyl xanthate and MMO | −60.47 | 2.93 | p < 0.001 | Significant |
| Atomic | N1 | N2 | S | O |
|---|---|---|---|---|
| Mulliken charges | −0.594 | −0.558 | −0.42 | −0.793 |
| Fukui | 0.054 | 0.091 | 0.352 | 0.045 |
| Polarizabilities (a.u.) | 7.343 | 7.165 | 18.913 | 4.948 |
| Bond | N1-Fe1 | N2-Fe2 | S-Cu |
|---|---|---|---|
| Mulliken population | 0.24 | 0.28 | 0.47 |
| Distance/Å | 2.062 | 2.193 | 2.228 |
| Depressant | Dosage (g/t) | Concentrate | Tailings | ||||
|---|---|---|---|---|---|---|---|
| Recovery (Mo/%) | Grade (Mo/%) | Grade (Cu/%) | Recovery (Cu/%) | Grade (Cu/%) | Grade (Mo/%) | ||
| Na2S | 1000 | 98.71 | 16.12 | 9.42 | 76.72 | 23.28 | 0.114 |
| NaCN | 25 | 98.74 | 13.04 | 12.51 | 63.03 | 17.91 | 0.14 |
| MMO | 100 | 97.27 | 20.72 | 8.03 | 82.95 | 19.54 | 0.11 |
| Particle Size (mm) | 0.074 | −0.037 | −0.018 | −0.009 | −0.005 | −0.002 | Total |
| Productivity (%) | 8.73 | 37.42 | 46.59 | 4.16 | 1.18 | 1.92 | 100 |
| Composition | Cu | Mo | Fe | Zn | Pb | Mn | S |
| Content (%) | 23.8 | 0.24 | 31.9 | 0.06 | 0.05 | 0.03 | 36.1 |
| Composition | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | Others |
| Content (%) | 0.15 | 0.3 | 0.28 | 2.8 | 0.23 | 0.37 | 3.69 |
| Functional | Lattice Parameters/Å | Δa | Δc | |
|---|---|---|---|---|
| a = b | c | |||
| BLYP | 5.0975 | 9.9868 | −3.62% | −4.18% |
| PW91 | 5.1388 | 10.1350 | −2.84% | −2.76% |
| PBE | 5.1337 | 10.1339 | −2.94% | −2.77% |
| PBESOL | 5.0981 | 9.6995 | −3.61% | −6.94% |
| RPBE | 5.0870 | 10.0561 | −3.82% | −3.52% |
| WC | 5.1037 | 9.7223 | −3.50% | −6.72% |
| Exp. | 5.289 | 10.423 | 0.00% | 0.00% |
| Cutoff Energy (eV) | Lattice Parameters/Å | Δa | Δc | |
|---|---|---|---|---|
| a = b | c | |||
| 280 | 5.1033 | 10.0868 | −3.51% | −3.23% |
| 320 | 5.1388 | 10.135 | −2.84% | −2.76% |
| 360 | 5.1599 | 10.1487 | −2.44% | −2.63% |
| 400 | 5.1783 | 10.1921 | −2.09% | −2.22% |
| 440 | 5.1802 | 10.1955 | −2.06% | −2.18% |
| 480 | 5.1837 | 10.2007 | −1.99% | −2.13% |
| Exp. | 5.289 | 10.423 | 0.00% | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Luo, A.; Tong, X.; Chen, J.; Jian, S. Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol. Molecules 2025, 30, 1396. https://doi.org/10.3390/molecules30061396
Lv X, Luo A, Tong X, Chen J, Jian S. Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol. Molecules. 2025; 30(6):1396. https://doi.org/10.3390/molecules30061396
Chicago/Turabian StyleLv, Xiangwen, Anruo Luo, Xiong Tong, Jianhua Chen, and Sheng Jian. 2025. "Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol" Molecules 30, no. 6: 1396. https://doi.org/10.3390/molecules30061396
APA StyleLv, X., Luo, A., Tong, X., Chen, J., & Jian, S. (2025). Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol. Molecules, 30(6), 1396. https://doi.org/10.3390/molecules30061396






