Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation
Abstract
1. Introduction
2. Results and Discussion
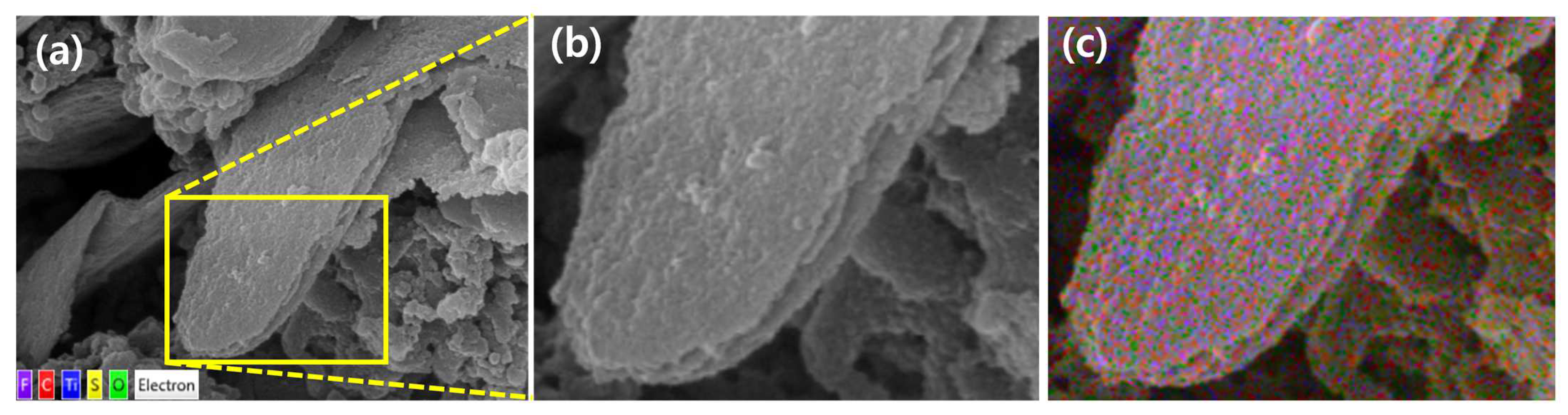
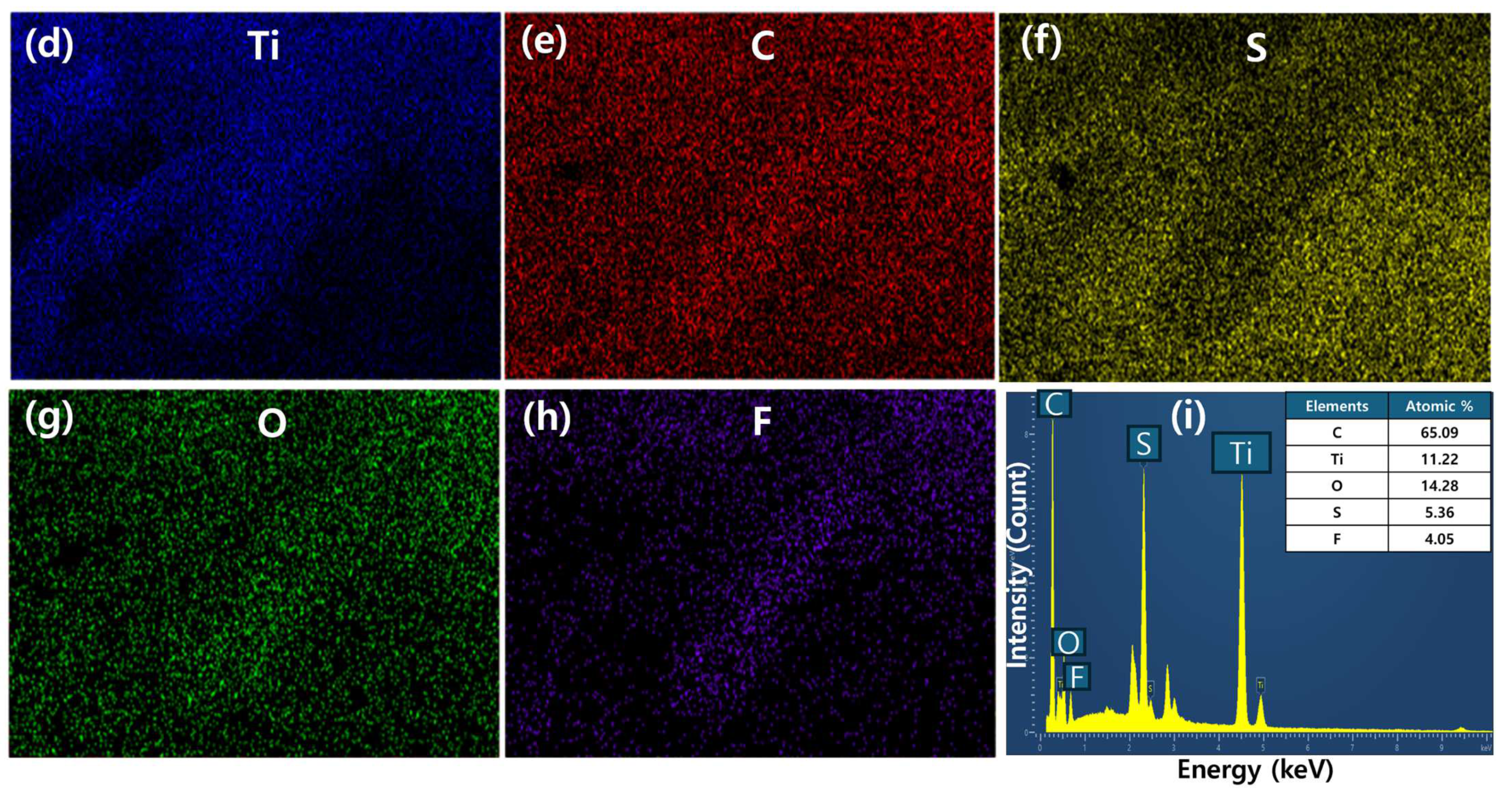
2.1. Morphologies and Compositions
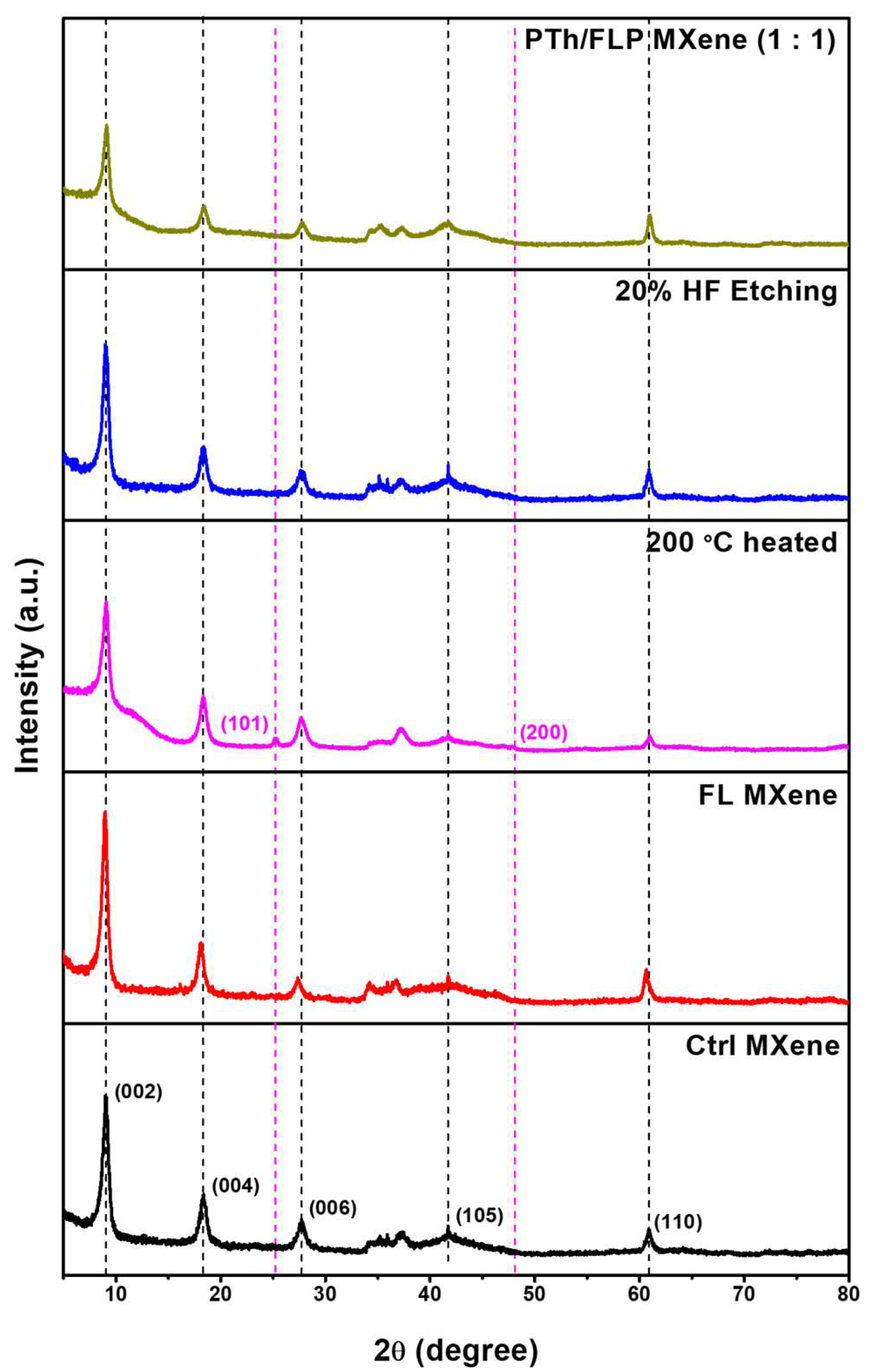
2.2. Crystalline and Bonding Characteristics
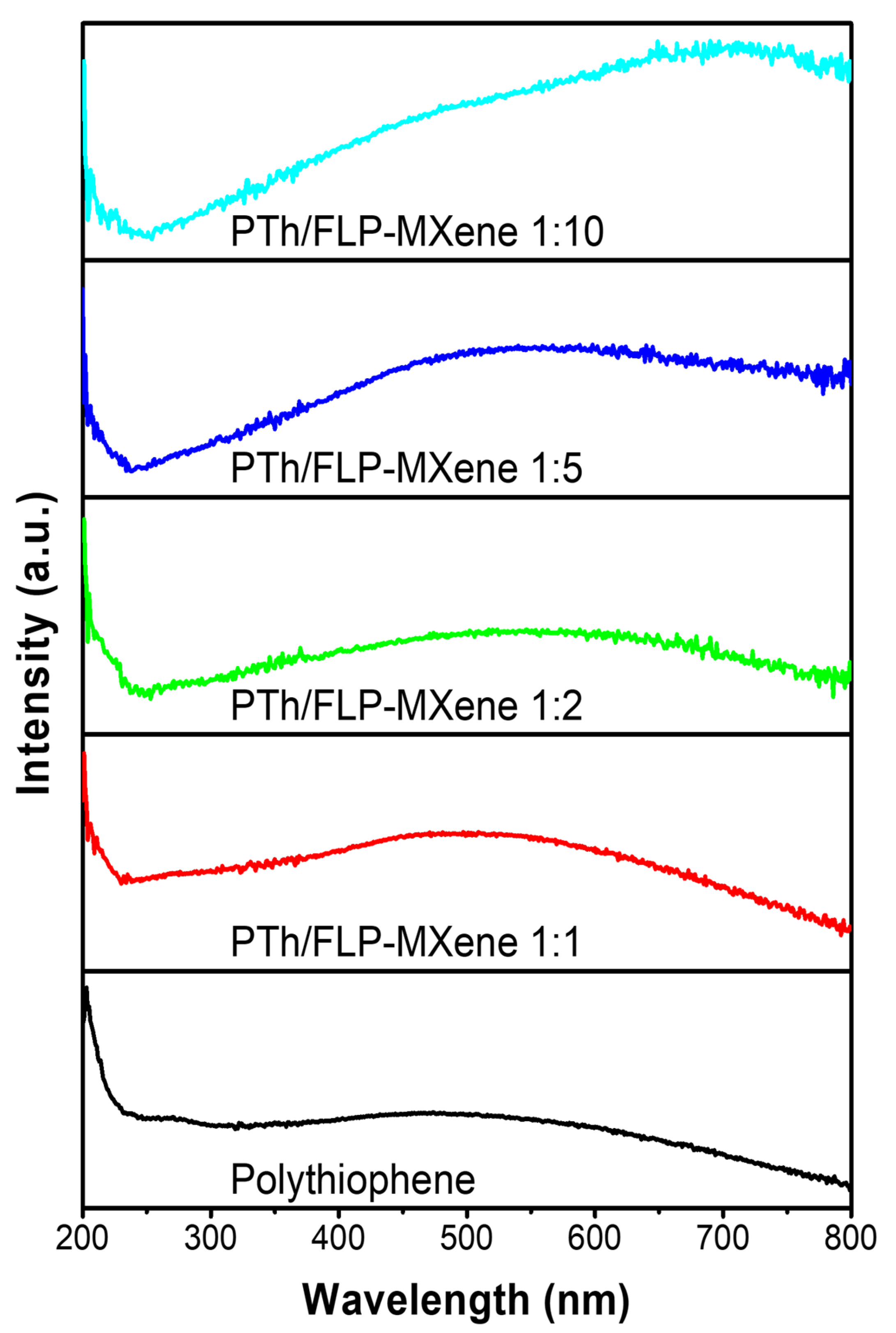
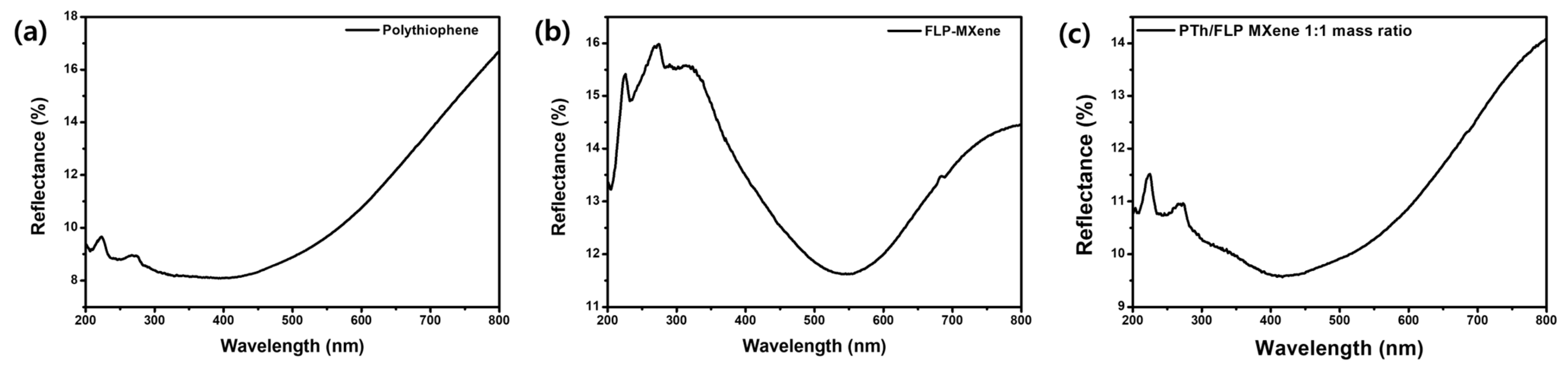
2.3. Optical Characteristics
2.4. BET Analysis
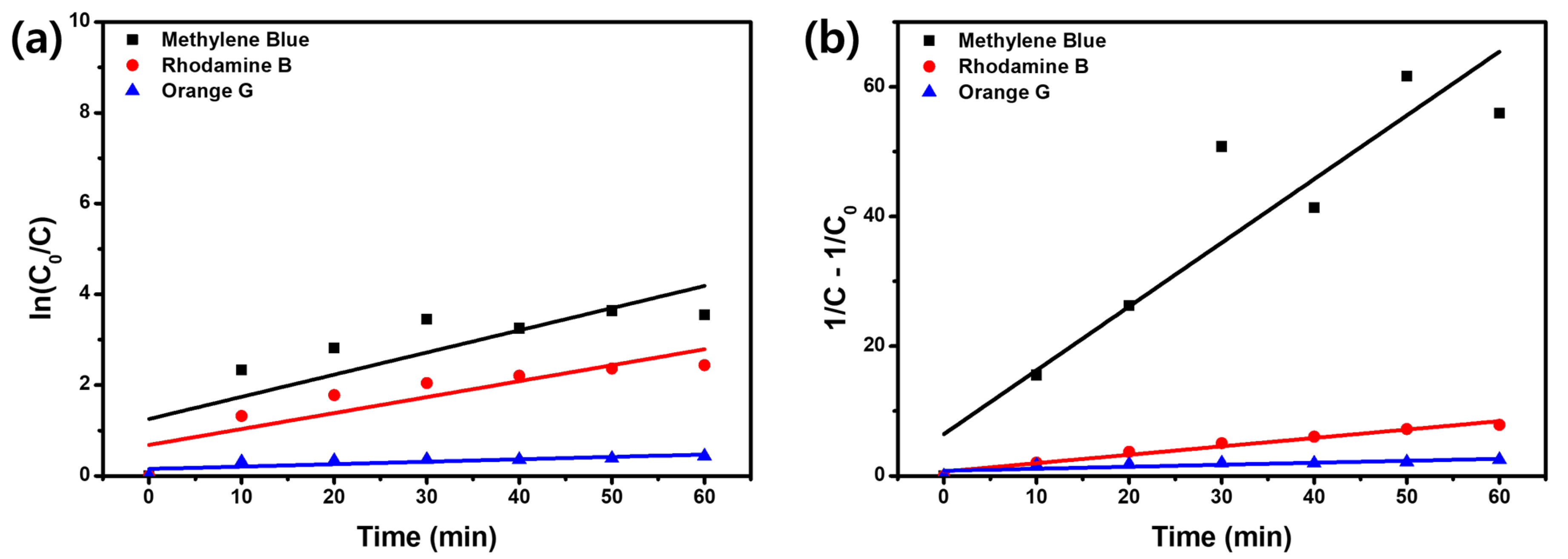
2.5. Photodegradation and Adsorption of Organic Dyes
2.6. Mechanisms for Dye Removal
2.7. Recyclability, Radical Scavenging, and Expandible Degradation Tests
3. Materials and Methods
3.1. Materials and Reagents
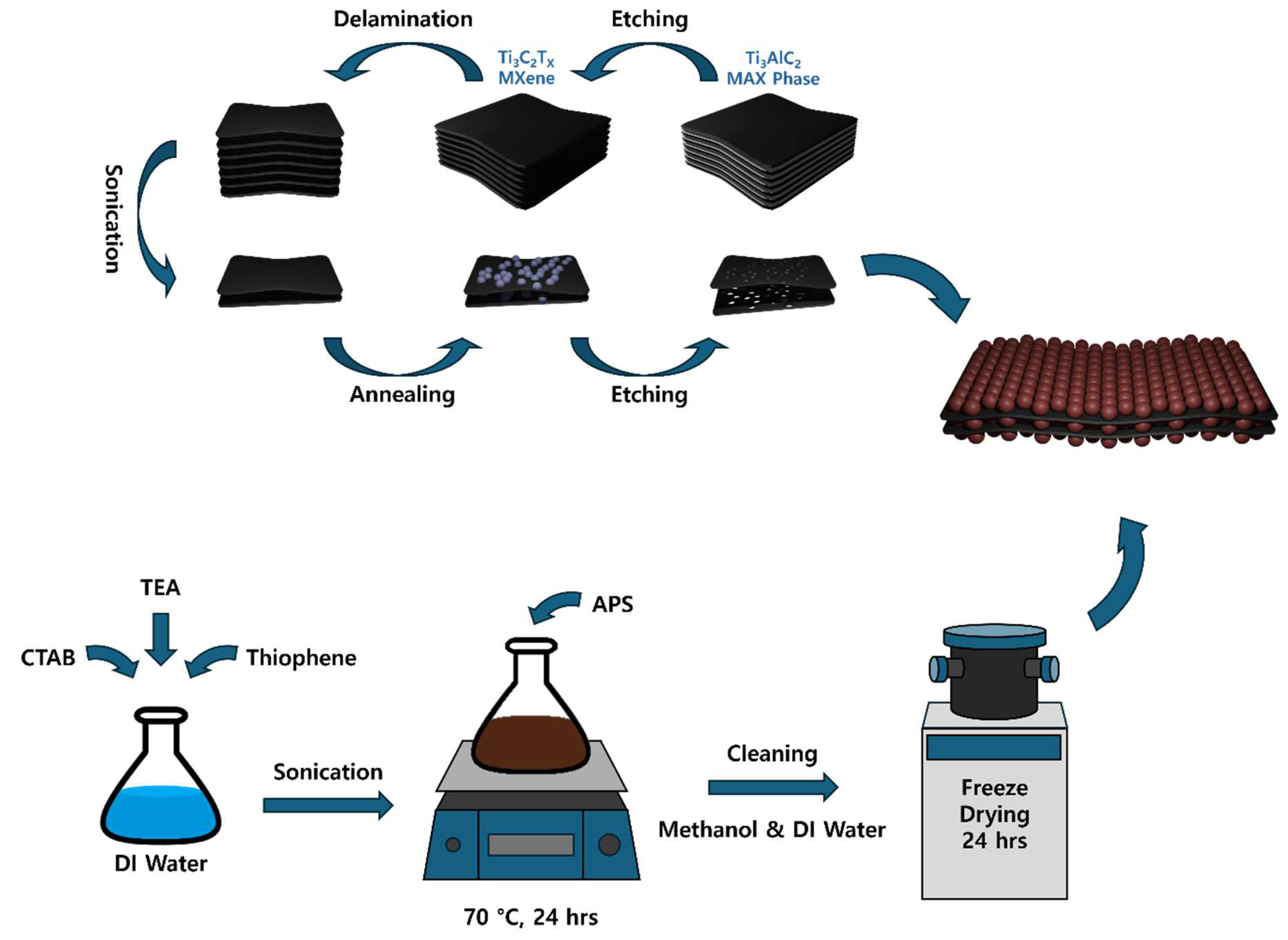
3.2. Synthesis of Standard Ti3C2TX MXene
3.3. Synthesis of Few Layered Porous Ti3C2TX MXene
3.4. Synthesis of Polythiophene
3.5. Synthesis of Composites
3.6. Material Characterizations
3.7. Photocatalytic and Physical Adsorption Dye Degradation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zia, J.; Fatima, F.; Riaz, U. A comprehensive review on the photocatalytic activity of polythiophene-based nanocomposites against degradation of organic pollutants. Catal. Sci. Technol. 2021, 11, 6630–6648. [Google Scholar]
- Dutta, K.; Rana, D. Polythiophenes: An emerging class of promising water purifying materials. Eur. Polym. J. 2019, 119, 370–385. [Google Scholar]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- You, Z.; Liao, Y.; Li, X.; Fan, J.; Xiang, Q. State-of-the-art recent progress in MXene-based photocatalysts: A comprehensive review. Nanoscale 2021, 13, 9463–9504. [Google Scholar]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2TX) in photocatalysis: A review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar]
- Yu, H.; Jiang, H.; Zhang, S.; Feng, X.; Yin, S.; Zhao, W. Review of Two-Dimensional MXenes (Ti3C2TX) Materials in Photocatalytic Applications. Processes 2023, 11, 1413. [Google Scholar] [CrossRef]
- Hao, W.; Ren, S.; Wu, X.; Shen, X.; Cui, S. Recent advance in the construction of 3D porous structure Ti3C2TX MXene and their multi-functional applications. J. Alloys Compd. 2023, 968, 172219. [Google Scholar]
- Kumar, G.; Ahlawat, A.; Bhardwaj, H.; Sahu, G.K.; Rana, P.S.; Solanki, P.R. Ultrasonication-assisted synthesis of transition metal carbide of MXene: An efficient and promising material for photocatalytic organic dyes degradation of rhodamine B and methylene blue in wastewater. Environ. Sci. Pollut. Res. 2024, 31, 38232–38250. [Google Scholar]
- Kalyani, R.; Gurunathan, K. Metal ions doped and polythiophene coated nanophotocatalysts: Synthesis and spectroscopic characterization for H2 production and dye degradation. Optik 2016, 127, 4741–4745. [Google Scholar]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Chandra, M.R.; Rao, T.S.; Pammi, S.V.N.; Sreedhar, B. An enhanced visible light active rutile titania-copper/polythiophene nanohybrid material for the degradation of rhodamine B dye. Mater. Sci. Semicond. Process. 2015, 30, 672–681. [Google Scholar]
- Mustafa, M.; Bashir, S.; Moosvi, S.K.; Najar, M.H.; Masoodi, M.H.; Rizvi, M.A. Hybrid Polymer Composite of Prussian Red Doped Polythiophene for Adsorptive Wastewater Treatment Application. Acta Chim. Slov. 2022, 69, 848–862. [Google Scholar]
- Ramirez, J.H.; Maldonaldo-Hodar, F.J.; Perez-Cadenas, A.F.; Moreno-Casilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B-Environ. Energy 2007, 75, 312–323. [Google Scholar]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional Nanocrystals Produced by Exfloliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar]
- Hemanth, N.R.; Kim, T.; Kim, B.; Jadhav, A.H.; Lee, K.; Chaudhari, N.K. Transition metal dichalcogenide-decorated MXenes: Promising hybrid electrodes for energy storage and conversion applications. Mater. Chem. Front. 2021, 5, 3298–3321. [Google Scholar]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Tran, N.M.; Ta, Q.T.H.; Sreedhar, A.; Noh, J.S. Ti3C2TX MXene playing as a strong methylene blue adsorbent in wastewater. Appl. Surf. Sci. 2021, 537, 148006. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, F.; Zhang, Y.; Xu, H.; Li, M.; Qi, J.; Wang, H.; Wang, Z.; Hu, Y.; Fan, X.; et al. Few-layered Ti3C2TX MXene synthesized via water-free etching toward high-performance supercapacitors. J. Colloid. Interface Sci. 2023, 632, 216–222. [Google Scholar]
- Yang, H.; Zheng, Q.; Zhang, P.; Nie, G.; Ali, T.; Raza, S. Fabrication of MXene (Ti2C3Tx) based conducting polymer materials and their applications as anticancer and metal ions removal from wastewater. Surf. Interfaces 2023, 36, 102493. [Google Scholar]
- Bassaid, S.; Benhaoua, C.; Taleb, M.; Sahli, M.; Dehbi, A. Physical and Chemical Properties of Composites Based on Polythiophene and Titanium Dioxide Nanoparticles for Photocatalysis. Polym. Sci. Ser. B 2021, 63, 291–303. [Google Scholar]
- Ta, Q.T.H.; Sreedhar, A.; Tri, N.N.; Noh, J.S. In Situ Growth of TiO2 on Ti3C2Tx MXene for Improved Gas-Sensing Performances. Ceram. Int. 2024, 50, 27227–27236. [Google Scholar]
- Natarajan, S.K.; Cano, A.M.; Partridge, J.L.; George, S.M.; Elliott, S.D. Prediction and Validation of the Process Window for Atomic Layer Etching: HF Exposure on TiO2. J. Phys. Chem. C 2021, 125, 25589–25599. [Google Scholar]
- Wang, S.; Liu, Y.; Liu, Y.; Hu, W. Effect of HF etching on titanium carbide (Ti3C2Tx) microstructure and its capacitive properties. Chem. Eng. J. 2023, 452, 139512. [Google Scholar]
- Shan, D.; He, J.; Deng, L.; Yan, S.; Luo, H.; Huang, S.; Xu, Y. The underlying mechanisms of enhanced microwave absorption performance for the NiFe2O4-decorated Ti3C2Tx MXene. Results Phys. 2019, 15, 102750. [Google Scholar]
- Parker, T.; Zhang, D.; Bugallo, D.; Shevchunk, K.; Downes, M.; Valurouthu, G.; Inman, A.; Chacon, B.; Zhang, T.; Shunk, C.E.; et al. Fourier-Transform Infrared Spectral Library of MXenes. Chem. Mater. 2024, 36, 8437–8446. [Google Scholar]
- Liu, R.; Liu, Z. Polythiophene: Synthesis in aqueous medium and controllable morphology. Sci. Bull. 2009, 54, 2028–2032. [Google Scholar]
- Bouzzine, S.M.; Morán, G.S.; Hamidi, M.; Bouachrine, M.; Pacheco, A.G.; Mitnik, D.G. DFT Study of Polythiophene Energy Band Gap and Substitution Effects. J. Chem. 2015, 2015, 296386. [Google Scholar]
- Springborg, M. The electronic properties of polythiophene. J. Phys. Condens. Matter. 1992, 4, 101. [Google Scholar]
- Ma, J.; Li, S.; Jiang, Y. A Time-Dependent DFT Study on Band Gaps and Effective Conjugation Lengths of Polyacetylene, Polyphenylene, Polypentafulvene, Polycyclopentadiene, Polypyrrole, Polyfuran, Polysilole, Polyphosphole, and Polythiophene. Macromolecules 2001, 35, 1109–1115. [Google Scholar]
- Hu, C.; Du, Z.; Wei, Z.; Li, L.; Shen, G. Functionalized Ti3C2Tx MXene with layer-dependent band gap for flexible NIR photodetectors. Appl. Phys. Rev. 2023, 10, 021402. [Google Scholar]
- Schultz, T.; Bärmann, P.; Longhi, E.; Meena, R.; Geerts, Y.; Gogotsi, Y.; Barlow, S.; Marder, S.R.; Petit, T.; Koch, N. Work function and energy level alignment tuning at Ti3C2Tx MXene surfaces and interfaces using (metal-)organic donor/acceptor molecules. Phys. Rev. Mater. 2023, 7, 045002. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Thakur, D.; Noh, J.S. Enhanced Gas Sensing Performance of ZnO/Ti3C2Tx MXene Nanocomposite. Micromachines 2022, 13, 1710. [Google Scholar] [CrossRef]
- Vinodhini, S.P.; Xavier, J.R. Electrochemical evaluation and structural characterization of polythiophene surfaces modified with PbO/PbS for energy storage applications. Mater. Chem. Phys. 2024, 318, 129233. [Google Scholar] [CrossRef]
- Dutta, M.; De, S. Polythiophene incorporated polysulfone blend membranes for effective removal of zinc and iron from electroplating effluent: Experimental studies and performance modelling. Chem. Eng. Sci. 2024, 285, 119581. [Google Scholar] [CrossRef]
- Fierro, X.J.; Cuenca, G. Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite. Int. J. Mol. Sci. 2024, 25, 4367. [Google Scholar] [CrossRef]
- Farghaly, A.; Maher, E.; Gad, A.; Bery, H.E. Synergistic photocatalytic degradation of methylene blue using TiO2 composites with activated carbon and reduced graphene oxide: A kinetic and mechanistic study. Appl. Water Sci. 2024, 14, 228. [Google Scholar] [CrossRef]
- Jin, L.; Tanzeel, Q.; Arif, U.; Ali, F.; Ali, N.; Haotian, C.; Mehmood, S.; Akbar, Y.; Raziq, F. Efficient photodegradation of organic dyes from industrial wastewater using novel Ni-decorated g-C3N4-TiO2 photocatalysts. Colloid Polym. Sci. 2024, 302, 487–502. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugum, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of Efficient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Gaur, J.; Kumar, S.; Pal, M.; Kaur, H.; Supreet; Badru, R.; Momoh, J.; Pal, R.; Kumar, S. Bio-engineered, phyto-decorated, multi-form P. betle/ZnO as a potential photocatalytic agent. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 035014. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Pseudo-first-order kinetic studies of the sorption of acid dyes onto chitosan. J. Appl. Polym. Sci. 2004, 92, 1633–1645. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar]





| Photocatalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Polythiophene | 29.05 | 0.147 | 20.19 |
| FLP MXene | 9.73 | 0.0321 | 13.18 |
| PTh/FLP MXene composite | 32.01 | 0.0616 | 7.70 |
| Organic Dye | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|
| K1 | R2 | K2 | R2 | |
| MB | 0.048 | 0.61 | 0.98 | 0.84 |
| RhB | 0.035 | 0.74 | 0.13 | 0.97 |
| OG | 0.0053 | 0.56 | 0.03 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, Y.-H.; Hong, S.; Noh, J.-S. Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation. Molecules 2025, 30, 1393. https://doi.org/10.3390/molecules30061393
Bae Y-H, Hong S, Noh J-S. Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation. Molecules. 2025; 30(6):1393. https://doi.org/10.3390/molecules30061393
Chicago/Turabian StyleBae, Young-Hwan, Seongin Hong, and Jin-Seo Noh. 2025. "Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation" Molecules 30, no. 6: 1393. https://doi.org/10.3390/molecules30061393
APA StyleBae, Y.-H., Hong, S., & Noh, J.-S. (2025). Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation. Molecules, 30(6), 1393. https://doi.org/10.3390/molecules30061393






