Potential of Bio-Sourced Oligogalacturonides in Crop Protection
Abstract
1. Introduction
2. From Plant Cell Wall to Oligogalacturonides (OGs)
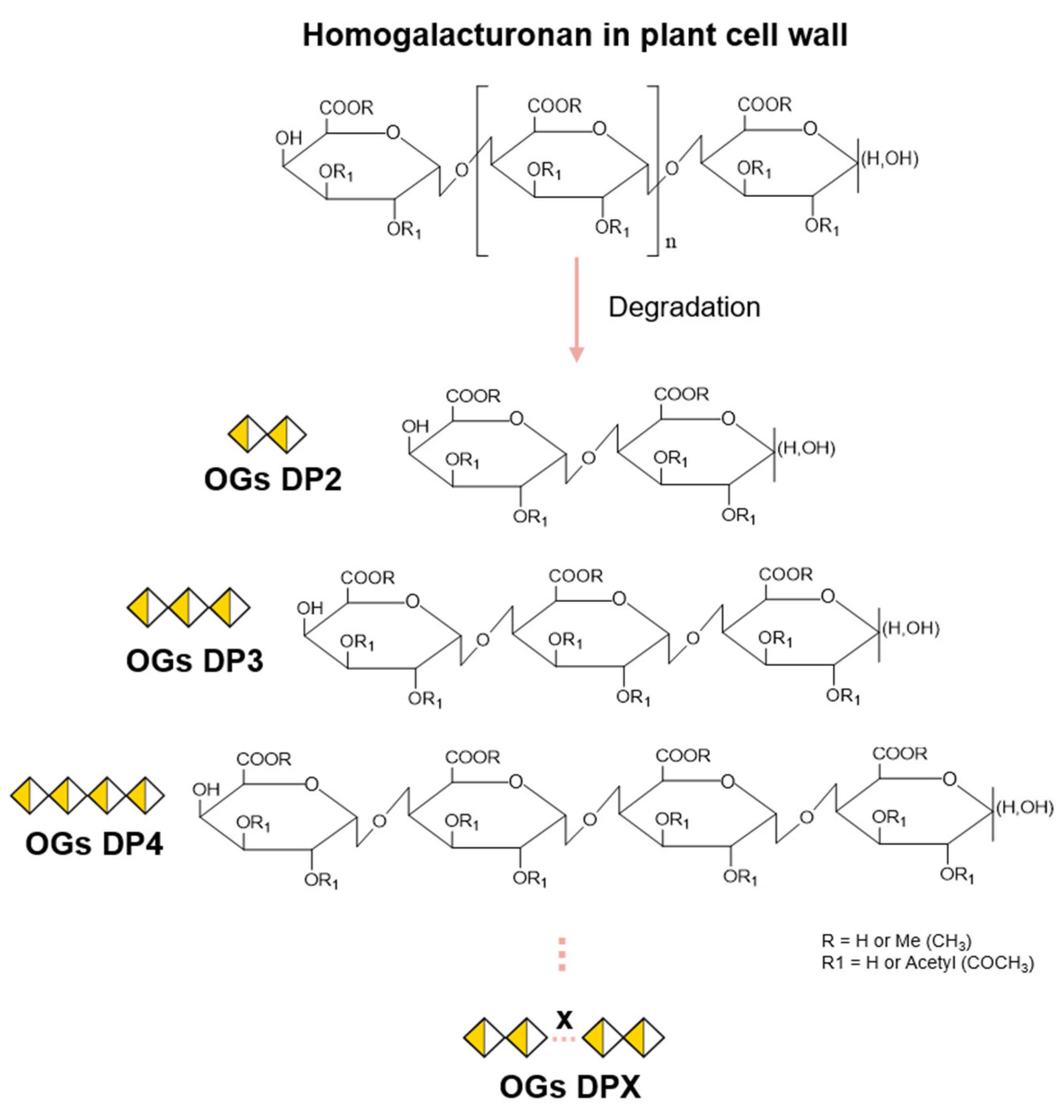
2.1. OGs’ Chemical Structure
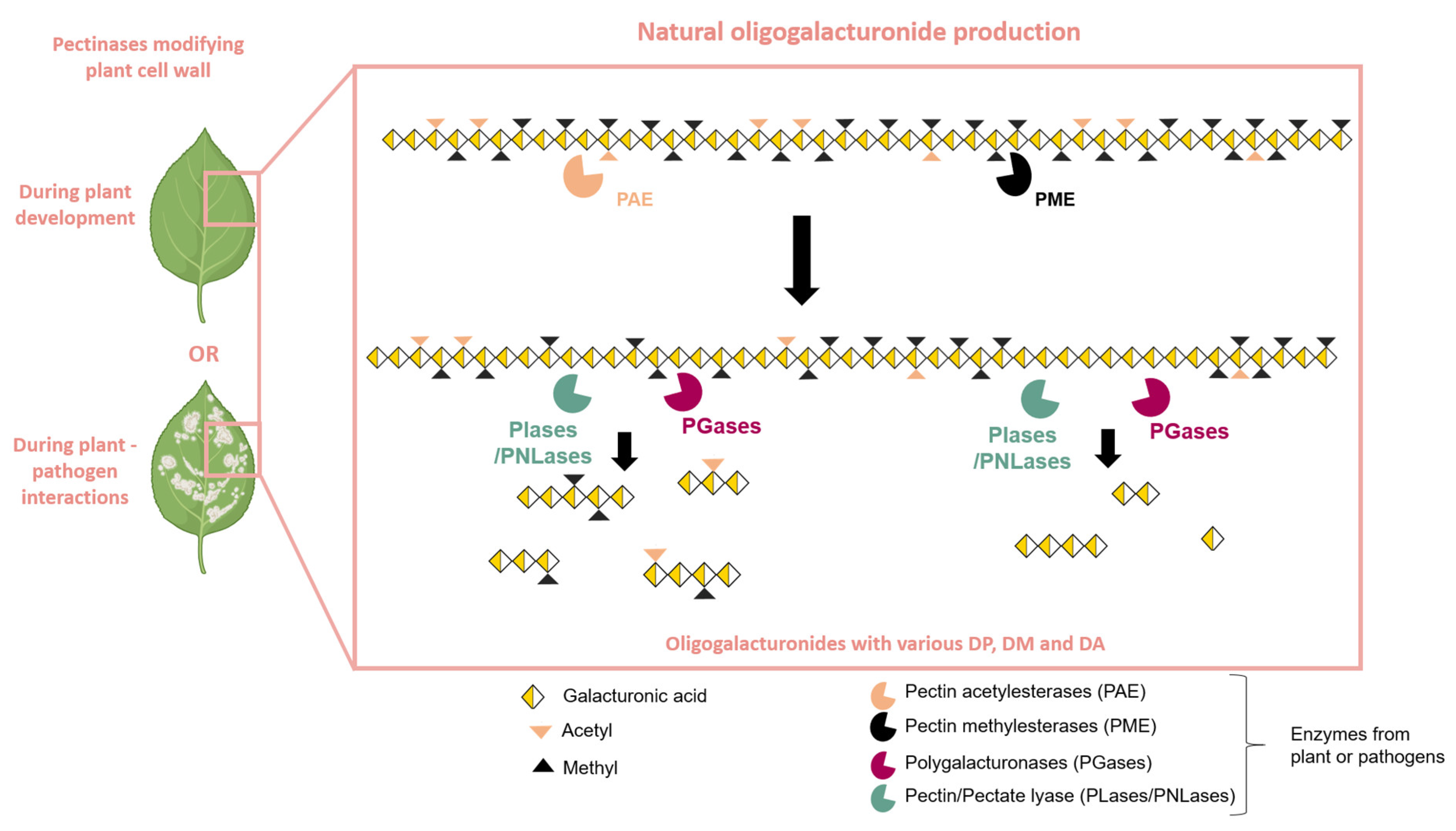
2.2. OG Production Methods
2.2.1. Chemical Approach
2.2.2. Thermal Approach
2.2.3. Enzymatic Approach
3. Role of Oligogalacturonides (OGs) in the Plant’s Immune System Activation
3.1. OGs’ Perception
3.2. Stimulation of Plant Defense Responses by OGs
3.2.1. Plant Defense Mechanisms Induced by OGs
3.2.2. Protection Induced by OGs Against Plant Diseases
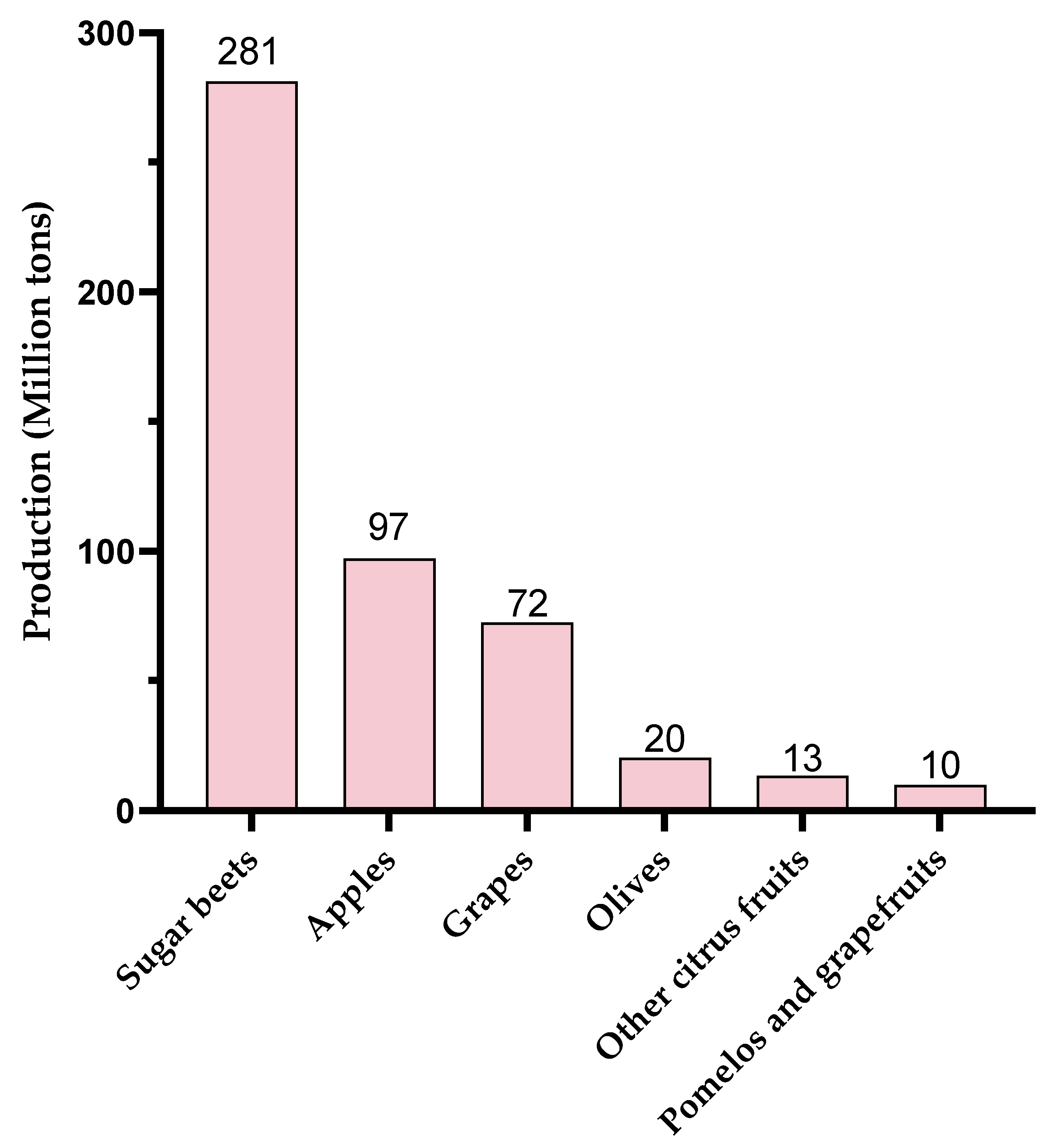
4. Applications, Limitations, and Sustainable Production Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holka, M. Environmental Impact Assessment of Chemical Plant Protection in Intensive Crop Production. J. Cent. Eur. Agric. 2017, 18, 529–541. [Google Scholar] [CrossRef]
- Marina, I.; Grujić Vučkovski, B.; Todorović, M.J. Impact of Intensive Agricultural Production on the Environment; Institute of Agricultural Economics: Beograd, Serbia, 2024. [Google Scholar]
- Farm to Fork Farm to Fork: New Rules to Reduce the Risk and Use of Pesticides in the EU. Available online: https://ec.europa.eu/commission/presscorner/detail/en/qanda_22_3694 (accessed on 8 January 2025).
- Moreno-Pérez, A.; Martínez-Ferri, F.; Pliego-Alfaro, F.; Pliego, C. Elicitors and Plant Defence. JOJ Hortic. Arboric. 2020, 2, 555600. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–407. [Google Scholar] [CrossRef]
- Henry, G.; Thonart, P.; Ongena, M. PAMPs, MAMPs, DAMPs and Others: An Update on the Diversity of Plant Immunity Elicitors. Biotechnol. Agron. Soc. Environ. 2012, 16, 257–268. [Google Scholar]
- Zipfel, C. Plant Pattern-Recognition Receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar]
- Couto, D.; Zipfel, C. Regulation of Pattern Recognition Receptor Signalling in Plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant Damage-Associated Molecular Patterns and Regulators of Growth and Development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef]
- Benedetti, M.; Mattei, B.; Pontiggia, D.; Salvi, G.; Savatin, D.V.; Ferrari, S. Methods of Isolation and Characterization of Oligogalacturonide Elicitors. Methods Mol. Biol. 2017, 1578, 25–38. [Google Scholar]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant Cell Wall-Mediated Immunity: Cell Wall Changes Trigger Disease Resistance Responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and Effects of Cytosolic Free Calcium Increases in Response to Elicitors in Nicotiana Plumbaginifolia Cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Navazio, L.; Moscatiello, R.; Bellincampi, D.; Baldan, B.; Meggio, F.; Brini, M.; Bowler, C.; Mariani, P. The Role of Calcium in Oligogalacturonide-Activated Signalling in Soybean Cells. Planta 2002, 215, 596–605. [Google Scholar] [CrossRef]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short Oligogalacturonides Induce Pathogen Resistance-Associated Gene Expression in Arabidopsis Thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial Disease Resistance in Arabidopsis through Flagellin Perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of Defense Response Pathways by OGs and Flg22 Elicitors in Arabidopsis Seedlings. Mol. Plant 2008, 1, 423–445. [Google Scholar] [CrossRef]
- Galletti, R.; Ferrari, S.; de Lorenzo, G. Arabidopsis MPK3 and MPK6 Play Different Roles in Basal and Oligogalacturonide-or Flagellin-Induced Resistance against Botrytis Cinerea. Plant Physiol. 2011, 157, 804–814. [Google Scholar] [CrossRef]
- Camejo, D.; Martí, M.C.; Olmos, E.; Torres, W.; Sevilla, F.; Jiménez, A. Oligogalacturonides Stimulate Antioxidant System in Alfalfa Roots. Biol. Plant 2012, 56, 537–544. [Google Scholar] [CrossRef]
- Randoux, B.; Renard-Merlier, D.; Mulard, G.; Rossard, S.; Duyme, F.; Sanssené, J.; Courtois, J.; Durand, R.; Reignault, P. Distinct Defenses Induced in Wheat against Powdery Mildew by Acetylated and Nonacetylated Oligogalacturonides. Phytopathology 2010, 100, 1352–1363. [Google Scholar] [CrossRef]
- Martínez, M.; Yáñez, R.; Alonsó, J.L.; Parajó, J.C. Chemical Production of Pectic Oligosaccharides from Orange Peel Wastes. Ind. Eng. Chem. Res. 2010, 49, 8470–8476. [Google Scholar] [CrossRef]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic Induction of the Defensin and Phytoalexin Pisatin Pathways in Pea (Pisum Sativum) against Aphanomyces Euteiches by Acetylated and Nonacetylated Oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, C.; Li, K.; Kennedy, J.F.; Wisniewski, M.; Gao, L.; Han, C.; Liu, J.; Yin, H.; Wu, X. Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root. J. Fungi 2022, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Production of Pectin-Derived Oligosaccharides from Lemon Peels by Extraction, Enzymatic Hydrolysis and Membrane Filtration. J. Chem. Technol. Biotechnol. 2016, 91, 234–247. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Zavala, D.; Moraga, F.; Herrera-Vásquez, A.; Blanco-Herrera, F. Oligogalacturonides Enhance Resistance against Aphids through Pattern-Triggered Immunity and Activation of Salicylic Acid Signaling. Int. J. Mol. Sci. 2022, 23, 9753. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, S.; Fernández-Bautista, M.; Rivas, S.; Yáñez, R.; Alonso, J.L. Recent Advances in the Production of Oligogalacturonides and Their Biological Properties. Food Funct. 2023, 14, 4507–4521. [Google Scholar] [CrossRef]
- Keegstra, K. Plant Cell Walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Höfte, H.; Voxeur, A. Plant Cell Walls. Curr. Biol. 2017, 27, R865–R870. [Google Scholar] [CrossRef]
- Bédouet, L.; Denys, E.; Courtois, B.; Courtois, J. Changes in esterified pectins during development in the flax stems and leaves. Carbohydrate Polymers 2006, 65, 165–173. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights into Pectin O-Acetylation in the Plant Cell Wall: Structure, Synthesis, and Modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, Biosynthesis, and Oligogalacturonide-Related Signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent Advances in Understanding the Roles of Pectin as an Active Participant in Plant Signaling Networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Sakuragi, Y.; Zhu, X.; Burrell, A.J.; Mohanty, S.S.; Atwood, J.A.; Orlando, R.; Scheller, H.V.; Mohnen, D. Galacturonosyltransferase (GAUT)1 and GAUT7 Are the Core of a Plant Cell Wall Pectin Biosynthetic Homogalacturonan:Galacturonosyltransferase Complex. Proc. Natl. Acad. Sci. USA 2011, 108, 20225–20230. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.-J.W.M.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.I.E.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the Degree and Pattern of Methyl-Esterification of Pectic Homogalacturonan in Plant Cell Walls. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-Modifying Enzymes: Structure, Expression, and Roles in Plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef]
- Philippe, F.; Pelloux, J.; Rayon, C. Plant Pectin Acetylesterase Structure and Function: New Insights from Bioinformatic Analysis. BMC Genom. 2017, 18, 456. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Liang, Y.; Anderson, C.T.; Cao, J. A Profusion of Molecular Scissors for Pectins: Classification, Expression, and Functions of Plant Polygalacturonases. Front. Plant Sci. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Hocq, L.; Guinand, S.; Habrylo, O.; Voxeur, A.; Tabi, W.; Safran, J.; Fournet, F.; Domon, J.M.; Mollet, J.C.; Pilard, S.; et al. The Exogenous Application of AtPGLR, an Endo-Polygalacturonase, Triggers Pollen Tube Burst and Repair. Plant J. 2020, 103, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Zheng, Y.; Zhang, H.; Chen, J.; Yan, L.; Ding, T.; Linhardt, R.J.; Orfila, C.; Liu, D.; et al. Fast Preparation of Rhamnogalacturonan I Enriched Low Molecular Weight Pectic Polysaccharide by Ultrasonically Accelerated Metal-Free Fenton Reaction. Food Hydrocoll. 2019, 95, 551–561. [Google Scholar] [CrossRef]
- Coenen, G.J.; Kabel, M.A.; Schols, H.A.; Voragen, A.G.J. CE-MSn of Complex Pectin-Derived Oligomers. Electrophoresis 2008, 29, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In Vitro Determination of Prebiotic Properties of Oligosaccharides Derived from an Orange Juice Manufacturing By-Product Stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef]
- Valdivieso Ramirez, C.S.; Sanchez Gallego, J.E.; Gänzle, M.; Temelli, F.; Saldaña, M.D.A. Carboxylic Acid-Catalysed Hydrolysis of Polygalacturonic Acid in Subcritical Water Media. J. Supercrit. Fluids 2021, 169, 105103. [Google Scholar] [CrossRef]
- Elboutachfaiti, R.; Delattre, C.; Michaud, P.; Courtois, B.; Courtois, J. Oligogalacturonans Production by Free Radical Depolymerization of Polygalacturonan. Int. J. Biol. Macromol. 2008, 43, 257–261. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P.; Rungsardthong, V. Production of Pectic-Oligosaccharides from Pomelo Peel Pectin by Oxidative Degradation with Hydrogen Peroxide. Food Chem. 2021, 348, 129078. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kastner, H.; Fatouros, A.; Krähmer, A.; Kroh, L.W.; Drusch, S. Thermal Degradation of Citrus Pectin in Low-Moisture Environment—Investigation of Backbone Depolymerisation. Food Hydrocoll. 2020, 107, 105937. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic Oligosacharides from Lemon Peel Wastes: Production, Purification, and Chemical Characterization. J. Agric. Food Chem. 2013, 61, 10043–10053. [Google Scholar] [CrossRef]
- Quemener, B.; Desire, C.; Debrauwer, L.; Rathahao, E. Structural Characterization by Both Positive and Negative Electrospray Ion Trap Mass Spectrometry of Oligogalacturonates Purified by High-Performance Anion-Exchange Chromatography. J. Chromatogr. A 2003, 984, 185–194. [Google Scholar] [CrossRef]
- Miyazawa, T.; Ohtsu, S.; Funazukuri, T. Hydrothermal Degradation of Polysaccharides in a Semi-Batch Reactor: Product Distribution as a Function of Severity Parameter. J. Mater. Sci. 2008, 43, 2447–2451. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.C.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of Low Molecular Weight Pectins via Electron Beam Irradiation and Their Potential Prebiotic Functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Salvati, A.; Sciubba, F.; Diomaiuti, A.; Leone, G.P.; Pizzichini, D.; Bellincampi, D.; Pontiggia, D. Olive Mill Wastewater as a Source of Defense-Promoting by-Products against Microbial Pathogens. Plant Stress 2024, 14, 100623. [Google Scholar] [CrossRef]
- Safran, J.; Tabi, W.; Ung, V.; Lemaire, A.; Habrylo, O.; Bouckaert, J.; Rouffle, M.; Voxeur, A.; Pongrac, P.; Bassard, S.; et al. Plant Polygalacturonase Structures Specify Enzyme Dynamics and Processivities to Fine-Tune Cell Wall Pectins. Plant Cell 2023, 35, 3073–3091. [Google Scholar] [CrossRef]
- Safran, J.; Ung, V.; Bouckaert, J.; Habrylo, O.; Molini, R.; Fontaine, J.; Lemaire, A.; Voxeur, A.; Pilard, S.; Pau-roblot, C.; et al. The Specificity of Pectate Lyase VdPelB from Verticilium Dahliae Is Highlighted by Structural, Dynamical and Biochemical Characterizations. Int. J. Biol. Macromol. 2023, 231, 123137. [Google Scholar] [CrossRef]
- Gamir, J.; Minchev, Z.; Berrio, E.; García, J.M.; De Lorenzo, G.; Pozo, M.J. Roots Drive Oligogalacturonide-Induced Systemic Immunity in Tomato. Plant Cell Environ. 2021, 44, 275–289. [Google Scholar] [CrossRef]
- Bigini, V.; Sillo, F.; Giulietti, S.; Pontiggia, D.; Giovannini, L.; Balestrini, R.; Savatin, D.V. Oligogalacturonide Application Increases Resistance to Fusarium Head Blight in Durum Wheat. J. Exp. Bot. 2024, 75, 3070–3091. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Tan, H.; Li, K.; Chen, W.; Yin, H. A Highly Efficient Biocatalytic Conversion of Renewable Pectic Polysaccharide Biomass into Bioactive Oligogalacturonides. ACS Food Sci. Technol. 2021, 1, 338–346. [Google Scholar] [CrossRef]
- Zimin, Y.S.; Borisova, N.S.; Kutlugildina, G.G.; Mudarisova, R.K.; Borisov, I.M.; Mustafin, A.G. Oxidation and Destruction of Arabinogalactan and Pectins under the Action of Hydrogen Peroxide and Ozone-Oxygen Mixture. React. Kinet. Mech. Catal. 2017, 120, 673–690. [Google Scholar] [CrossRef]
- Pheulpin, P.; Rossart, S.; Barbier, M.; Kovensky, J.; Courtois, B.; Courtois, J. Method for Producing Families of Galacturonic Acid Oligomers and Use Thereof 2009. Available online: https://patents.google.com/patent/WO2016146941A1/fr (accessed on 10 January 2025).
- Downs, C.G.; Brady, C.J.; Gooley, A. Exopolygalacturonase Protein Accumulates Late in Peach Fruit Ripening. Physiol. Plant 1992, 85, 133–140. [Google Scholar] [CrossRef]
- Downs, C.G.; Brady, C.J. Two Forms of Exopolygalacturonase Increase as Peach Fruits Ripen. Plant Cell Environ. 1990, 13, 523–530. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Sun, D.; Wang, J.; Wang, N.; Qiao, L.; Guo, Q.; Wang, C. Fungus Polygalacturonase-Generated Oligogalacturonide Restrains Fruit Softening in Ripening Tomato. J. Agric. Food Chem. 2022, 70, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Babu, Y.; Bayer, M. Plant Polygalacturonases Involved in Cell Elongation and Separation—The Same but Different? Plants 2014, 3, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant Cell Wall Dynamics and Wall-Related Susceptibility in Plant-Pathogen Interactions. Front. Plant Sci. 2014, 5, 228. [Google Scholar] [CrossRef]
- Voxeur, A.; Habrylo, O.; Guénin, S.; Miart, F.; Soulié, M.C.; Rihouey, C.; Pau-Roblot, C.; Domon, J.M.; Gutierrez, L.; Pelloux, J.; et al. Oligogalacturonide Production upon Arabidopsis Thaliana-Botrytis Cinerea Interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 19743–19752. [Google Scholar] [CrossRef]
- Albersheim, P.; Darvill, A.G.; Hcneil, M.; Valent, B.S.; Sharp, J.K.; Nothnagel, E.A.; Davis, K.R.; Yamazaki, N.; Gollin, D.J.; York, W.S.; et al. Oligosaccharins: Naturally Occurring Carbohydrates with Biological Regulatory Functions. Struct. Funct. Plant Genomes 1983, 293–312. [Google Scholar] [CrossRef]
- Bishop, P.D.; Makus, D.J.; Pearce, G.; Ryan, C.A. Proteinase Inhibitor-Inducing Factor Activity in Tomato Leaves Resides in Oligosaccharides Enzymically Released from Cell Walls. Proc. Natl. Acad. Sci. USA 1981, 78, 3536–3540. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Osorio, S. Signaling Role of Oligogalacturonides Derived during Cell Wall Degradation. Plant Signal Behav. 2012, 7, 1447–1449. [Google Scholar] [CrossRef]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent Advances in PAMP-Triggered Immunity against Bacteria: Pattern Recognition Receptors Watch over and Raise the Alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Kohorn, B.D.; Kohorn, S.L. The Cell Wall-Associated Kinases, WAKs, as Pectin Receptors. Front. Plant Sci. 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Wagner, T.A.; Perret, M.; He, Z.-H.; He, D.; Kohorn, B.D. WAKs: Cell Wall-Associated Kinases Linking the Cytoplasm to the Extracellular Matrix. Plant Mol. Biol. 2001, 47, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Verica, J.A.; Chae, L.; Tong, H.; Ingmire, P.; He, Z.H. Tissue-Specific and Developmentally Regulated Expression of a Cluster of Tandemly Arrayed Cell Wall-Associated Kinase-Like Kinase Genes in Arabidopsis. Plant Physiol. 2003, 133, 1732–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, C.; Li, L.; Meng, L.; Singh, J.; Jiang, N.; Deng, X.W.; He, Z.H.; Lemaux, P.G. Evolutionary Expansion, Gene Structure, and Expression of the Rice Wall-Associated Kinase Gene Family. Plant Physiol. 2005, 139, 1107–1124. [Google Scholar] [CrossRef]
- Herold, L.; Ordon, J.; Hua, C.; Kohorn, B.D.; Nürnberger, T.; DeFalco, T.A.; Zipfel, C. Arabidopsis WALL-ASSOCIATED KINASES Are Not Required for Oligogalacturonide-Induced Signaling and Immunity. Plant Cell 2024, 37, koae317. [Google Scholar] [CrossRef]
- Kohorn, B.D. Cell Wall-Associated Kinases and Pectin Perception. J. Exp. Bot. 2016, 67, 489–494. [Google Scholar] [CrossRef]
- Huerta, A.I.; Sancho-Andrés, G.; Montesinos, J.C.; Silva-Navas, J.; Bassard, S.; Pau-Roblot, C.; Kesten, C.; Schlechter, R.; Dora, S.; Ayupov, T.; et al. The WAK-like Protein RFO1 Acts as a Sensor of the Pectin Methylation Status in Arabidopsis Cell Walls to Modulate Root Growth and Defense. Mol. Plant 2023, 16, 865–881. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Humphries, J.; Ratcliffe, J.; Bacic, A.; Johnson, K.L.; Qu, G. WALL-ASSOCIATED KINASE Like 14 Regulates Vascular Tissue Development in Arabidopsis and Tomato. Plant Sci. 2024, 341, 112013. [Google Scholar] [CrossRef]
- Rasul, S.; Dubreuil-Maurizi, C.; Lamotte, O.; Koen, E.; Poinssot, B.; Alcaraz, G.; Wendehenne, D.; Jeandroz, S. Nitric Oxide Production Mediates Oligogalacturonide-Triggered Immunity and Resistance to Botrytis Cinerea in Arabidopsis Thaliana. Plant Cell Environ. 2012, 35, 1483–1499. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in Plant Defence-Signalling Pathways: Tansley Review. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis Cinerea Induced in Arabidopsis by Elicitors Is Independent of Salicylic Acid, Ethylene, or Jasmonate Signaling but Requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Galletti, R.; Denoux, C.; Gambetta, S.; Dewdney, J.; Ausubel, F.M.; De Lorenzo, G.; Ferrari, S. The AtrbohD-Mediated Oxidative Burst Elicited by Oligogalacturonides in Arabidopsis Is Dispensable for the Activation of Defense Responses Effective against Botrytis Cinerea. Plant Physiol. 2008, 148, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Castillejo, C.; Quesada, M.A.; Medina-Escobar, N.; Brownsey, G.J.; Suau, R.; Heredia, A.; Botella, M.A.; Valpuesta, V. Partial Demethylation of Oligogalacturonides by Pectin Methyl Esterase 1 Is Required for Eliciting Defence Responses in Wild Strawberry (Fragaria Vesca). Plant J. 2008, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Heyraud, A.; Lambert, B. Oligogalacturonide Signal Transduction, Induction of Defense-Related Responses and Protection of Grapevine against Botrytis Cinerea. Planta 2004, 218, 767–774. [Google Scholar] [CrossRef]
- Aziz, A.; Gauthier, A.; Bézier, A.; Poinssot, B.; Joubert, J.M.; Pugin, A.; Heyraud, A.; Baillieul, F. Elicitor and Resistance-Inducing Activities of β-1,4 Cellodextrins in Grapevine, Comparison with β-1,3 Glucans and α-1,4 Oligogalacturonides. J. Exp. Bot. 2007, 58, 1463–1472. [Google Scholar] [CrossRef]
- Greco, M.; Kouzounis, D.; Fuertes-Rabanal, M.; Gentile, M.; Agresti, S.; Schols, H.A.; Mélida, H.; Lionetti, V. Upcycling Olive Pomace into Pectic Elicitors for Plant Immunity and Disease Protection. Plant Physiol. Biochem. 2024, 217, 109213. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Influence of Extraction Conditions on the Yield and Physico-Chemical Parameters of Pectin from Grape Pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Structural, Functional and Physicochemical Properties of Pectin from Grape Pomace as Affected by Different Extraction Techniques. Int. J. Biol. Macromol. 2023, 224, 739–753. [Google Scholar] [CrossRef]
- Joanna, B.; Michal, B.; Piotr, D.; Agnieszka, W.; Dorota, K.; Izabela, W. Sugar Beet Pulp as a Source of Valuable Biotechnological Products. In Advances in Biotechnology for Food Industry: Volume 14; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–392. ISBN 9780128114438. [Google Scholar]

| Methods | Pectin Source | Treatment Conditions | Product | References |
|---|---|---|---|---|
| Chemical | Apple pulp | HCl (48 h, 80 °C) + TFA (6 h, 100 °C) | DP 1–10 | [45] |
| Orange by-product | Nitric acid (pH 1.5, 120 °C, 0.5 h) | DP 2–30 | [46] | |
| PGA | Citric and malic acid (pH 2.6, 125–135 °C, 10 min to 1 h, 100 bar) | DP 2–7 | [47] | |
| PGA | H2O2, cupric acetate (1 h, 60 °C) | DP 2–6 | [48] | |
| Pomelo peel pectin | H2O2 + microwave (0 to 0.15 h) | DP 2–6 | [49] | |
| Thermal | Citrus pectin | 60 °C for 4 weeks, controlled humidity conditions (40% or 80%) | nd | [50] |
| Orange peel wastes | Hydrothermal treatment at 140–200 °C | nd | [22] | |
| Lemon peel wastes | Hydrothermal treatment at 160 °C | DP 2–8 | [51] | |
| Orange polygalacturonic acid | 100 °C—48 h | DP 4–6 | [52] | |
| PGA | pH 4–5, 121 °C, 1 atm, and 40 min | DP 2–25 | [21] | |
| PGA | Hydrothermal, range of 160 °C to 240 °C | DP 1–10 | [53] | |
| Pomelo pectin | Electron beam irradiation (125 kGy) | DP 9 | [54] | |
| Enzymatic | Olive by-product | Endo-PGase from Aspergillus aculeatus, 30 °C, 3 or 24 h | DP 7–15 | [55] |
| Pectin DM20–34% | Endo-PGases: AtPGLR and AtADPG2 from Arabidopsis thaliana, overnight, 25 °C | DP 1–10 or DP 1–9, esterified | [56] | |
| Pectins DM 24–30% and sugar beet pectins DM 42% DA 31% | PLase from Verticillium dahliae (VdPeIB), overnight, 35 °C | DP 2–7, esterified | [57] | |
| PGA | Endo-PGase Kluyveromyces fragilis, 16 h at 37 °C | DP 1–2 | [45] | |
| PGA | Endo-PGase Aspergillus niger, 3 h at 30° C | DP 10–15 | [58] | |
| PGA | Endo-PGase Aspergillus niger, 3 h | DP 3–18 | [59] | |
| PGA | Endo-PGase AnPG28A from Aspergillus niger | DP 2–10 | [60] | |
| Sunflower pectin | Endo-PGase AnPG28A from Aspergillus niger, 36 h, 30 °C | DP 2–7, esterified | [24] |
| OG Production | DP | DA | DM | Target Pathogens | Host Plant | Plant Responses/ Protective Effect | References |
|---|---|---|---|---|---|---|---|
| PGA | 11 | 0% | 0% | Botrytis cinerea | Grapevine | Production of reactive oxygen species, induction of defense genes (chitinases, phenylalanine ammonia-lyase, glucanasesreduction of lesions | [87] |
| 3–10 | 0% | 0% | [88] | ||||
| Endo-PGase A. niger + PGA | 10–15 | 0% | 0% | Botrytis cinerea | Tomato | Synthesis of abscisic acid, salicylic acid, jasmonic acid | [58] |
| Trigalacturonic acid (Sigma T7407, France) | 3 | 0% | 0% | Pectobacterium carotovor | Arabidopsis thaliana | Synthesis of salicylic acid, jasmonic acid, reduction of lesions | [16] |
| OGs (Elicityl, France) | 10–15 | 0% | 0% | ||||
| Endo-PGase (AnPG28A) + sunflower pectin | 2–7 | nd | nd | Rhizoctonia solani | Sugar beet | Overexpression of defense genes encoding peroxidases and superoxide dismutase; reduction of symptoms | [24] |
| Endo-PGase A. niger + PGA | 3–18 | 0% | 0% | Fusarium graminearum | Wheat | Expression of defense genes (PR1, thaumatin, ethylene biosynthesis); reduction of symptoms | [59] |
| PGA thermal degradation and chemical acetylation | 2–25 | 30% | 0% | Blumeria graminis | Wheat | Reduction of symptoms and induction of enzymes activities (POX, LOX and OXO) | [21] |
| 2–25 | 30% | 0% | Aphanomyces euteiches | Pea | Up-regulation of defense genes (PR proteins, catalase); reduction of symptoms | [23] | |
| OGs (Elicityl, France) | 10–15 | 0% | 0% | Botrytis cinerea | Arabidopsis thaliana | Reduction of lesions | [78] |
| Olive by-product + PGase from Aspergillus aculeatus | 7–15 | nd | nd | Botrytis cinerea | Arabidopsis thaliana | Induction of Ca2+ release and defense genes | [55] |
| Olive pomace | 1–30 | nd | nd | Botrytis cinerea | Arabidopsis thaliana | Expression of defense genes (PAD, WRKY); partial reduction of lesions | [89] |
| Pseudomonas syringae | Arabidopsis thaliana and tomato | Expression of defense genes; reduced bacterial growth (20% on A. thaliana and 10% on tomato) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carton, C.; Magnin-Robert, M.; Randoux, B.; Pau-Roblot, C.; Lounès-Hadj Sahraoui, A. Potential of Bio-Sourced Oligogalacturonides in Crop Protection. Molecules 2025, 30, 1392. https://doi.org/10.3390/molecules30061392
Carton C, Magnin-Robert M, Randoux B, Pau-Roblot C, Lounès-Hadj Sahraoui A. Potential of Bio-Sourced Oligogalacturonides in Crop Protection. Molecules. 2025; 30(6):1392. https://doi.org/10.3390/molecules30061392
Chicago/Turabian StyleCarton, Camille, Maryline Magnin-Robert, Béatrice Randoux, Corinne Pau-Roblot, and Anissa Lounès-Hadj Sahraoui. 2025. "Potential of Bio-Sourced Oligogalacturonides in Crop Protection" Molecules 30, no. 6: 1392. https://doi.org/10.3390/molecules30061392
APA StyleCarton, C., Magnin-Robert, M., Randoux, B., Pau-Roblot, C., & Lounès-Hadj Sahraoui, A. (2025). Potential of Bio-Sourced Oligogalacturonides in Crop Protection. Molecules, 30(6), 1392. https://doi.org/10.3390/molecules30061392








