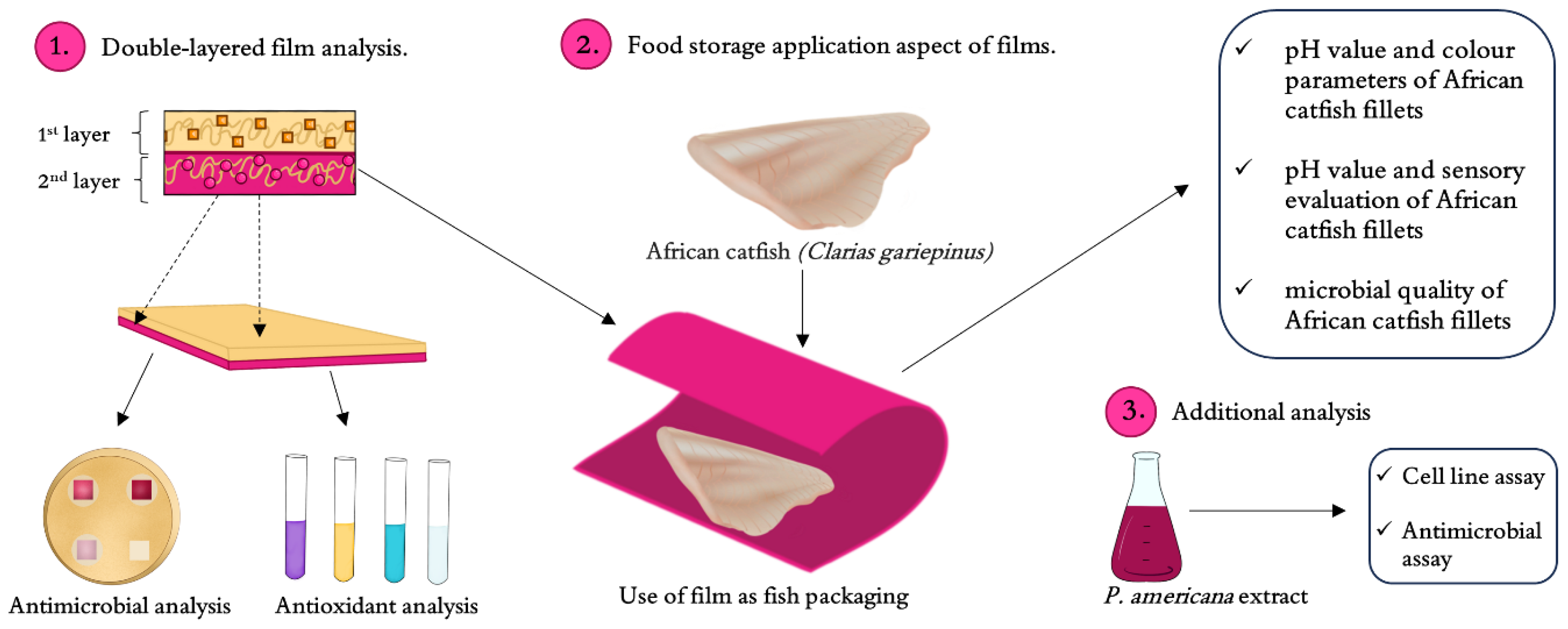
Storage Properties of Double-Layer Films Enriched with Phytolacca americana L. Extract as Active Packaging for African Catfish, with a New Approach to Antioxidant Film Assay and Additional Analysis of P. americana Extract Toxicity on Human Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. The Impact of Phytolacca americana Extract on Cell Cultures
2.2. Antimicrobial Properties of Films and Phytolacca americana Extracts
2.3. Antioxidant Properties of Films
2.3.1. Iron Ion Reduction Ability
2.3.2. Free Radical Scavenging
2.3.3. Total Reducing Power
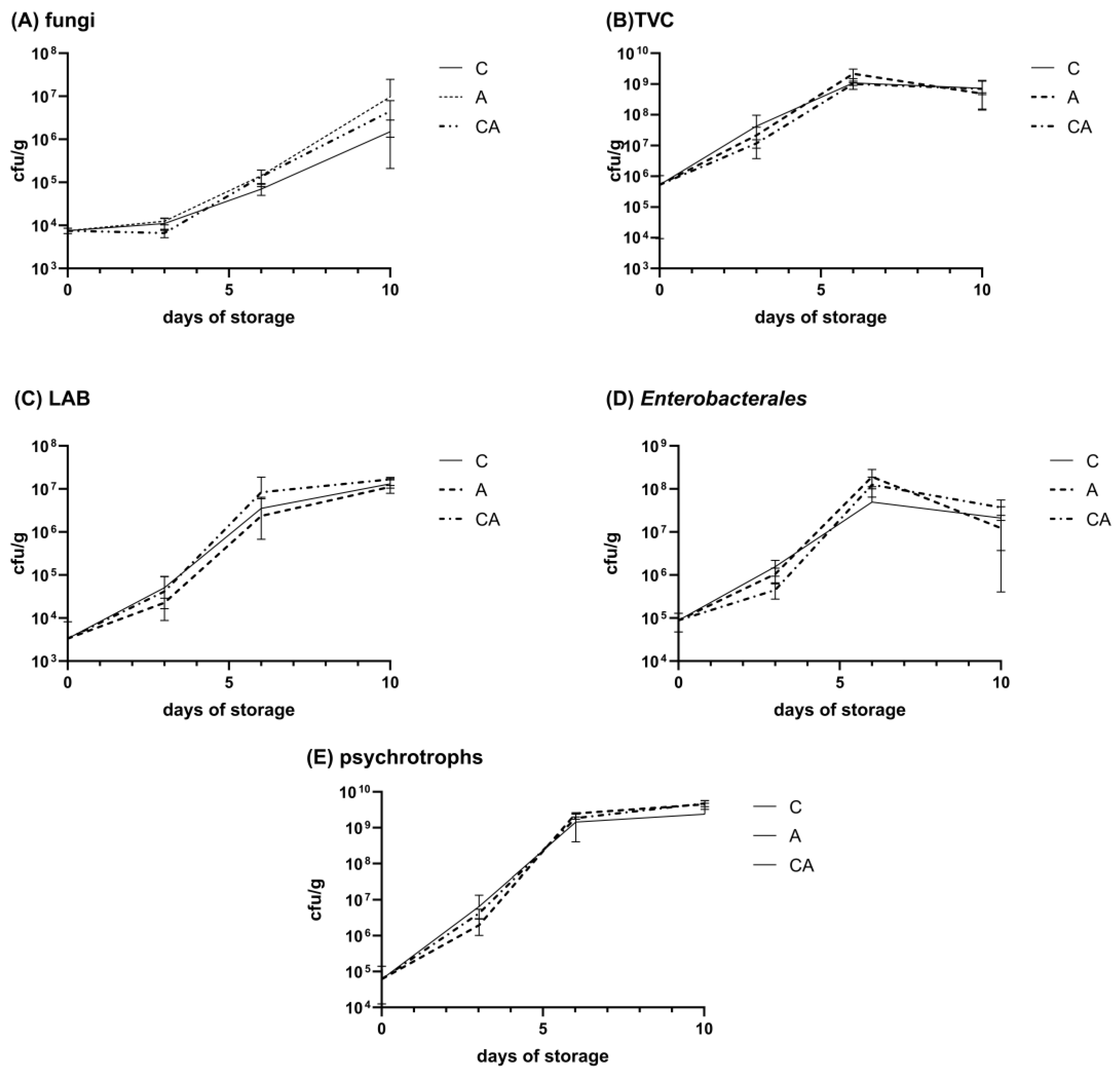
2.4. African Catfish Quality During Storage
2.4.1. pH Value and Color Parameters of African Catfish Fillets
2.4.2. Sensory Evaluation of African Catfish Fillets
2.4.3. Microbial Quality of African Catfish Fillets
3. Materials and Methods
3.1. Films
3.2. Impact of Phytolacca americana Extract on Cell Cultures
3.2.1. Cell Cultures
3.2.2. Cell Viability Assay
3.3. Antimicrobial Properties of Films and Phytolacca americana Extract—In Vitro Analyses
3.3.1. Films Assays
3.3.2. Extract Assay
3.4. Antioxidant Properties of Films
3.4.1. Ion Reduction Ability
3.4.2. Free Radical Scavenging Activity
3.4.3. Folin-Ciocâlteu Method
3.5. Determination Impact of the Film on African Catfish Quality During Storage
3.5.1. Preparation of the African Catfish Samples
3.5.2. Determination of pH Value and Color Parameters
3.5.3. Sensory Evaluation
3.5.4. Microbial Quality
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romero, J.; Cruz, R.M.S.; Díez-Méndez, A.; Albertos, I. Valorization of berries’ agro-industrial waste in the development of biodegradable pectin-based films for fresh salmon (Salmo salar) shelf-life monitoring. Int. J. Mol. Sci. 2022, 23, 8970. [Google Scholar] [CrossRef] [PubMed]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural polymeric materials: A solution to plastic pollution from the agro-food sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, M.; Kim, Y.J. Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high-density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment. Environ. Sci. Pollut. Res. 2022, 29, 17580–17590. [Google Scholar]
- Degnan, T.; Shinde, S.L. Waste-plastic processing provides global challenges and opportunities. MRS Bull. 2019, 44, 436–437. [Google Scholar]
- Wahyuningtiyas, N.E.; Suryanto, H. Analysis of biodegradation of bioplastics made of cassava starch. J. Mech. Eng. Sci. Technol. (JMEST) 2017, 1, 24–31. [Google Scholar]
- Suksankraisorn, K.; Patumsawad, S.; Vallikul, P.; Fungtammasan, B.; Accary, A. Co-combustion of municipal solid waste and Thai lignite in a fluidized bed. Energy Convers. Manag. 2004, 45, 947–962. [Google Scholar]
- Abelti, A.L.; Teka, T.A. Development and Characterization of Biodegradable Polymers for Fish Packaging Applications. J. Packag. Technol. Res. 2022, 6, 149–166. [Google Scholar]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar]
- Birania, S.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Rohilla, P.; Kumar, R. Advances in development of biodegradable food packaging material from agricultural and agro-industry waste. J. Food Process Eng. 2022, 45, e13930. [Google Scholar]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar]
- Srinivasa, P.; Baskaran, R.; Ramesh, M.; Harish Prashanth, K.; Tharanathan, R. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 2002, 215, 504–508. [Google Scholar]
- Balogh, L.; Juhász, M. American and Chinese pokeweed. Phytolacca Am. 2008, 35–46. [Google Scholar]
- Chmura, D. Is phytolacca americana (Phytolaccaceae) a new antropophyte in Polish flora? Fragm. Florist. Geobot. Pol. 2016, 23, 172–174. [Google Scholar]
- Ravikiran, G.; Raju, A.B.; Venugopal, Y. Phytolacca americana: A review. Int. J. Res. Pharma. Biomed. Sci. 2011, 2, 942–946. [Google Scholar]
- Marinas, I.C.; Oprea, E.; Geana, E.-I.; Luntraru, C.M.; Gird, C.E.; Chifiriuc, M.-C. Chemical composition, antimicrobial and antioxidant activity of Phytolacca americana L. fruits and leaves extracts. Farmacia 2021, 69, 883–889. [Google Scholar]
- Wang, L.; Bai, L.; Nagasawa, T.; Hasegawa, T.; Yang, X.; Sakai, J.-i.; Bai, Y.; Kataoka, T.; Oka, S.; Hirose, K. Bioactive triterpene saponins from the roots of Phytolacca americana. J. Nat. Prod. 2008, 71, 35–40. [Google Scholar]
- Yokoyama, K.; Yano, O.; Terao, T.; Osawa, T. Purification and biological activities of pokeweed (Phytolacca americana) mitogens. Biochim. Et Biophys. Acta (BBA) Protein Struct. 1976, 427, 443–452. [Google Scholar]
- Ola-Oladimeji, F.A.; Awodiran, M.O.; Olaleye, V.F.; Awopetu, J.I. Genetic Characterization Based on RAPD-PCR in Cultured Strains of Clarias gariepinus (Siluriformes: Clariidae). Genet. Aquat. Org. 2020, 4, 81–88. [Google Scholar]
- Turan, F.; Turan, C. Natural and non-natural distribution of African catfish Clarias gariepinus (Burchell, 1822) in Turkey. J. Limnol. Freshw. Fish. Res. 2016, 2, 173–177. [Google Scholar]
- Chwastowska-Siwiecka, I.; Skiepko, N.; Pomianowski, J.F.; Kondratowicz, J. Pomiary morfometryczne i ocena jakości mięsa suma afrykańskiego. Med. Weter. 2016, 72, 102–109. [Google Scholar]
- Adewumi, A.A.; Olaleye, V.F. Catfish culture in Nigeria: Progress, prospects and problems. Afr. J. Agric. Res. 2011, 6, 1281–1285. [Google Scholar]
- Otolowo, D.T. Assessment of storage stability of parts of dehydrated catfish (Clarias gariepinus) as influenced by treatments and packaging materials. J. Agric. Food Res. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Jasińska, J.M.; Michalska, K.; Szuwarzyński, M.; Mazur, T.; Cholewa-Wójcik, A.; Kopeć, M.; Juszczak, L.; Kamińska, I.; Nowak, N.; Jamróz, E. Phytolacca americana extract as a quality-enhancing factor for biodegradable double-layered films based on furcellaran and gelatin—Property assessment. Int. J. Biol. Macromol. 2024, 279, 135155. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of in vitro 3D cell model developed from human hepatocellular carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Boo, H.-O.; Park, J.-H.; Woo, S.-H.; Park, H.-Y. Antimicrobial effect, antioxidant and tyrosinase inhibitory activity of the extract from different parts of Phytolacca americana L. Korean J. Crop Sci. 2015, 60, 366–373. [Google Scholar] [CrossRef]
- Prasetyoputri, A.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A. The eagle effect and antibiotic-induced persistence: Two sides of the same coin? Trends Microbiol. 2019, 27, 339–354. [Google Scholar] [CrossRef]
- Stover, K.R.; Cleary, J.D. The eagle-like effect of the echinocandins: Is it relevant for clinical decisions? Curr. Fungal Infect. Rep. 2015, 9, 88–93. [Google Scholar]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef]
- Giménez, B.; De Lacey, A.L.; Pérez-Santín, E.; López-Caballero, M.; Montero, P. Release of active compounds from agar and agar–gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.Z. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacogn. Mag. 2013, 9, 109. [Google Scholar] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Hamissou, M.; Kurdmongkoltham, P. Molecular and cytotoxicity investigations of Phytolacca americana (L.) root, leaf, and berry extracts. Int. J. Pharmacol. Toxicol. 2014, 3, 11. [Google Scholar] [CrossRef]
- DemİRkan, E.; ErtÜRk, E.; Yildiz, G.; Sevgİ, T.; Aybey, A. In Vitro Evaluations of Antioxidant, Antimicrobial and Anticancer Potential of Phytolacca americana L. (Pokeweed) Seed Extract. Trak. Univ. J. Nat. Sci. 2022, 23, 135–143. [Google Scholar]
- Lai, W.-F. Design of polymeric films for antioxidant active food packaging. Int. J. Mol. Sci. 2021, 23, 12. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Jerz, G.; Skotzki, T.; Fiege, K.; Winterhalter, P.; Wybraniec, S. Separation of betalains from berries of Phytolacca americana by ion-pair high-speed counter-current chromatography. J. Chromatogr. A 2008, 1190, 63–73. [Google Scholar]
- Marinas, I.C.; Gradisteanu Pircalabioru, G.; Oprea, E.; Geana, E.-I.; Zgura, I.; Romanitan, C.; Matei, E.; Angheloiu, M.; Brincoveanu, O.; Georgescu, M. Physico-chemical and pro-wound healing properties of microporous cellulosic sponge from Gleditsia triacanthos pods functionalized with Phytolacca americana fruit extract. Cellulose 2023, 30, 10313–10339. [Google Scholar]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Wilms, L.C.; Beetstra, S.A.J.N.; Heijnen, C.G.M.; Voss, H.-P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar]
- Von Staszewski, M.; Pilosof, A.M.R.; Jagus, R.J. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011, 125, 186–192. [Google Scholar]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar]
- Almajano, M.P.; Delgado, M.E.; Gordon, M.H. Albumin causes a synergistic increase in the antioxidant activity of green tea catechins in oil-in-water emulsions. Food Chem. 2007, 102, 1375–1382. [Google Scholar]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar]
- Otálora González, C.M.; Bonifazi, E.L.; Fissore, E.N.; Basanta, M.F.; Gerschenson, L.N. Thermal stability of betalains in by-products of the blanching and cutting of Beta vulgaris L. var conditiva. Pol. J. Food Nutr. Sci. 2020, 70, 15–24. [Google Scholar]
- Khan, M.I.; Giridhar, P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L. berry juice. LWT—Food Sci. Technol. 2014, 58, 649–657. [Google Scholar]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and characterization of fish myofibrillar protein/chitosan/rosemary extract composite edible films and the improvement of lipid oxidation stability during the grass carp fillets storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [PubMed]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT—Food Sci. Technol. 2003, 36, 625–631. [Google Scholar]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar]
- Derbew Gedif, H.; Tkaczewska, J.; Jamróz, E.; Zając, M.; Kasprzak, M.; Pająk, P.; Grzebieniarz, W.; Nowak, N. Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products. Foods 2022, 12, 26. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldana, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Junior, E.E.R.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar]
- Özvural, E.B.; Huang, Q.; Chikindas, M.L. The comparison of quality and microbiological characteristic of hamburger patties enriched with green tea extract using three techniques: Direct addition, edible coating and encapsulation. LWT—Food Sci. Technol. 2016, 68, 385–390. [Google Scholar]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar]
- Galanty, A.; Zagrodzki, P.; Miret, M.; Paśko, P. Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells. Molecules 2022, 27, 9030. [Google Scholar] [CrossRef]
- Jasińska, J.M.; Kamińska, I.; Chmiel, M.J.; Jamróz, E. Biological potential of polysaccharides extracted from Nostoc colonies for film production–Physical and biological properties. Biotechnol. J. 2023, 18, 2200455. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N. American Meat Science Association guidelines for meat color measurement. Meat Muscle Biol. 2023, 6, 12473. [Google Scholar]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar]
| Incubation Time: 24 h | ||||
|---|---|---|---|---|
| Concentration [µg/mL] | Viable Cells ± SD [% of Control] | |||
| PNT2 | HepG2 | HaCaT | Nty-hori 3-1 | |
| 25 | 89.2 a ± 3.1 | 89.0 a ± 1.2 | 95.0 a ± 3.3 | 98.5 a ± 5.8 |
| 50 | 92.1 a ± 2.4 | 83.6 a,b ± 2.1 | 89.7 a,b ± 1.9 | 92.7 a,b ± 5.6 |
| 100 | 91.2 a ± 2.8 | 84.0 a,b ± 3.7 | 88.2 a,b ± 2.6 | 90.7 a,b,c ± 1.6 |
| 200 | 87.6 a ± 4.3 | 82.9 a,b ± 4.2 | 82.9 b,c ± 2.7 | 85.3 b,c,d ± 3.2 |
| 300 | 88.7 a ± 2.9 | 80.0 a,b ± 5.1 | 81.9 b,c ± 3.2 | 80.9 c,d ± 1.2 |
| 400 | 69.9 b ± 5.2 | 75.4 b,c ± 2.0 | 77.8 c,d ± 3.0 | 79.4 c,d ± 2.7 |
| 500 | 66.0 b ± 4.6 | 69.6 c ± 2.6 | 72.7 d ± 3.8 | 81.2 d ± 4.6 |
| IC50 | >500 | >500 | >500 | >500 |
| Incubation Time: 48 h | ||||
| Concentration [µg/mL] | Viable Cells ± SD [% of Control] | |||
| PNT2 | HepG2 | HaCaT | Nty-hori 3-1 | |
| 25 | 97.4 a ± 3.4 | 91.9 a,b ± 4.0 | 96.5 a ± 4.5 | 83.0 a ± 3.5 |
| 50 | 98.4 a ± 2.7 | 98.3 a ± 5.9 | 88.3 a,b ± 3.8 | 73.6 a,b ± 4.1 |
| 100 | 88.2 b ± 2.3 | 90.5 a,b ± 4.3 | 82.2 b,c ± 1.8 | 74.6 a,b ± 6.4 |
| 200 | 75.5 c ± 2.2 | 84.9 a,b ± 2.3 | 75.3 c,d ± 3.3 | 74.5 a,b± 1.9 |
| 300 | 68.2 d ± 1.2 | 74.6 c ± 1.7 | 70.6 d,e ± 2.5 | 72.3 a,b,c ± 5.0 |
| 400 | 56.3 e ± 2.4 | 61.1 d ± 2.4 | 62.9 e,f ± 2.3 | 69.7 b,c ± 1.8 |
| 500 | 54.0 e ± 1.1 | 57.4 d ± 3.2 | 58.9 f ± 3.2 | 62.2 c ± 1.2 |
| IC50 | >500 | >500 | >500 | >500 |
| Antimicrobial Assay According to A1 Methodology | ||||
|---|---|---|---|---|
| Film | ||||
| FUR/FUR + GEL | FUR + 2% PA/FUR + GEL | FUR + 4% PA/FUR + GEL | FUR + 6% PA/FUR + GEL | |
| Microorganism | ||||
| S. aureus ATCC 6538 |  |  | 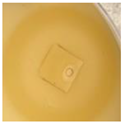 |  |
| Effect resembling “eagle effect” | Effect resembling “eagle effect” | Effect resembling “eagle effect” | Effect resembling “eagle effect” | |
| E. coli ATCC 8739 |  |  |  |  |
| No effect | No effect | No effect | No effect | |
| S. cerevisiae |  |  |  |  |
| No effect | No effect | No effect | No effect | |
| Antioxidant assays | ||||
| Method | ||||
| FRAP [mM TE·g−1] | 0.0 a ± 0.0 | 2.07 a,b ± 0.12 | 5.19 b ± 0.15 | 11.0 c ± 2.4 |
| CUPRAC Cupric Reducing Antioxidant Power [mM TE·g−1] | 20.0 a ± 1.5 | 8.3 c ± 1.1 | 3.8 b ± 0.9 | 4.5 b ± 1.9 |
| DPPH radical scavenging activity [%] | 5.4 a ± 0.7 | 7.9 a ± 1.7 | 7.0 a ± 2.3 | 5.9 a ± 2.0 |
| ABTS radical scavenging activity [%] | 30 a ± 4 | 61.2 b ± 2.8 | 72.8 c ± 2.4 | 71 c ± 5 |
| Folin-Ciocâlteu [mg GAE/g] | 5.02 a ± 0.05 | 21.7 b ± 1.8 | 29.5 c ± 2.8 | 35.8 d ± 1.7 |
| Attribute | Time (T, days) | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Film (F) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 10 | F | T | F × T | |
| pH | C | 6.6 ± 0.2 | 6.34 ± 0.05 | 6.19 ± 0.03 | 6.56 ± 0.06 | 6.44 ± 0.05 | 6.33 ± 0.08 | 6.45 ± 0.1 | 6.13 ± 0.08 | NS | * | NS |
| CA | - | 6.26 ± 0.11 | 6.49 ± 0.10 | 6.35 ± 0.07 | 6.29 ± 0.05 | 6.34 ± 0.07 | 6.52 ± 0.05 | 6.51 ± 0.09 | ||||
| A | - | 6.35 ± 0.12 | 6.35 ± 0.04 | 6.49 ± 0.08 | 6.38 ± 0.16 | 6.40 ± 0.09 | 6.35 ± 0.07 | 6.53 ± 0.03 | ||||
| L* | C | 40.6 ± 1.3 | 40.6 ± 0.6 | 39.7 ± 0.5 | 40.4 ± 0.7 | 41.3 ± 1.2 | 42.9 ± 0.7 | 38.4 ± 0.9 | 39.7 ± 0.4 | *** | *** | *** |
| CA | - | 35.5 ± 0.3 | 37.2 ± 0.6 | 37.7 ± 0.5 | 36.2 ± 1.1 | 31.5 ± 0.8 | 32.4 ± 0.8 | 30.1 ± 0.4 | ||||
| A | - | 27.13 ± 0.24 | 26.7 ± 0.4 | 27.1 ± 0.5 | 27.5 ± 0.8 | 27.2 ± 0.5 | 26.2 ± 0.8 | 26.5 ± 0.5 | ||||
| a* | C | 6.6 ± 0.3 | 4.1 ± 0.4 | 4.2 ± 0.4 | 4.4 ± 0.4 | 4.1 ± 0.6 | 5.4 ± 0.6 | 4.0 ± 0.6 | 7.2 ± 0.6 | *** | *** | *** |
| CA | - | 4.6 ± 0.2 | 5.0 ± 0.6 | 3.5 ± 0.4 | 3.8 ± 0.6 | 4.9 ± 0.4 | 5.0 ± 0.4 | 6.6 ± 0.4 | ||||
| A | - | 18.7 ± 0.5 | 18.2 ± 0.9 | 15.4 ± 0.5 | 14.5 ± 0.6 | 11.1 ± 0.5 | 12.5 ± 0.6 | 10.32 ± 0.3 | ||||
| b* | C | 4.9 ± 0.3 | 2.93 ± 0.5 | 3.8 ± 0.3 | 4.9 ± 0.3 | 4.2 ± 0.6 | 4.7 ± 0.4 | 3.8 ± 0.5 | 4.5 ± 0.3 | *** | *** | ** |
| CA | - | 2.82 ± 0.14 | 3.1 ± 0.4 | 3.85 ± 0.20 | 4.1 ± 0.6 | 2.85 ± 0.18 | 3.18 ± 0.16 | 3.71 ± 0.17 | ||||
| A | - | 1.55 ± 0.13 | −1.7 ± 0.20 | −1.53 ± 0.1 | 0.79 ± 0.10 | 1.17 ± 0.19 | 1.27 ± 0.2 | 1.59 ± 0.16 | ||||
| C* | C | 7.8 ± 0.4 | 5.1 ± 0.6 | 5.8 ± 0.5 | 6.6 ± 0.5 | 6.0 ± 0.7 | 7.2 ± 0.7 | 5.7 ± 0.5 | 4.5 ± 0.3 | *** | *** | *** |
| CA | - | 5.41 ± 0.27 | 5.9 ± 0.6 | 5.2 ± 0.4 | 5.8 ± 0.6 | 5.7 ± 0.4 | 6.0 ± 0.4 | 7.6 ± 0.6 | ||||
| A | - | 18.8 ± 0.5 | 18.3 ± 0.9 | 15.5 ± 0.5 | 14.5 ± 0.6 | 11.2 ± 0.6 | 12.6 ± 0.6 | 10.4 ± 0.26 | ||||
| h° | C | 33.0 ± 1.0 | 34 ± 3 | 42.0 ± 2.7 | 48.6 ± 2.0 | 47 ± 5 | 41.5 ± 2.6 | 44 ± 5 | 32.1 ± 1.8 | *** | *** | *** |
| CA | - | 31.6 ± 1.0 | 32 ± 3 | 49.4 ± 2.7 | 50 ± 6 | 30.4 ± 1.1 | 32.8 ± 1.5 | 30.6 ± 2.7 | ||||
| A | - | 4.8 ± 0.5 | 354.3 ± 1.0 | 354.3 ± 0.5 | 3.2 ± 0.5 | 5.9 ± 0.8 | 5.9 ± 1.1 | 8.7 ± 0.8 | ||||
| |RI| | C | 1.55 ± 0.1 | 1.42 ± 0.10 | 1.13 ± 0.14 | 0.90 ± 0.08 | 0.97 ± 0.09 | 1.14 ± 0.15 | 1.04 ± 0.15 | 1.63 ± 0.16 | *** | *** | *** |
| CA | - | 1.64 ± 0.07 | 1.59 ± 0.08 | 0.90 ± 0.12 | 0.93 ± 0.09 | 1.72 ± 0.06 | 1.59 ± 0.14 | 1.78 ± 0.25 | ||||
| A | - | 12.2 ± 1.0 | 10.8 ± 0.6 | 10.3 ± 0.9 | 19 ± 3 | 9.5 ± 0.6 | 10.0 ± 0.8 | 6.6 ± 0.6 | ||||
| ΔE | C | - | 3.4 ± 0.5 | 3.1 ± 0.4 | 3.14 ± 0.27 | 3.5 ± 0.06 | 3.3 ± 1.7 | 3.7 ± 0.6 | 3.1 ± 1.1 | *** | * | ** |
| appreciable | ||||||||||||
| CA | - | 5.7 ± 1.2 | 4.2 ± 1.2 | 4.6 ± 1.1 | 5.5 ± 1.6 | 9.4 ± 0.8 | 8.5 ± 0.4 | 10.6 ± 1.6 | ||||
| appreciable | much | |||||||||||
| A | - | 18.4 ± 1.0 | 19.2 ± 0.8 | 17.3 ± 0.9 | 15.8 ± 0.9 | 14.6 ± 1.0 | 15.9 ± 1.2 | 15.7 ± 1.8 | ||||
| very much | ||||||||||||
| Attribute | Time (T, days) | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Film (F) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | F | T | F × T | ||
| Color scores | dark and gray | C | 5.0 ± 0.0 | 4.17 ± 0.09 | 3.67 ± 0.18 | 3.71 ± 0.14 | 3.72 ± 0.14 | 3.95 ± 0.19 | 3.62 ± 0.16 | NS | * | NS |
| CA | - | 5.0 ± 0.0 | 4.00 ± 0.20 | 3.19 ± 0.19 | 3.86 ± 0.21 | 3.33 ± 0.14 | 2.86 ± 0.19 | |||||
| A | - | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.00 ± 0.24 | 3.72 ± 0.23 | 4.29 ± 0.12 | 2.9 ± 0.14 | |||||
| dark and brown | C | 5.0 ± 0.0 | 5.0 ± 0.0 | 3.67 ± 0.11 | 4.71 ± 0.10 | 3.78 ± 0.15 | 3.91 ± 0.10 | 3.00 ± 0.10 | *** | *** | *** | |
| CA | - | 4.11 ± 0.08 | 3.67 ± 0.13 | 3.67 ± 0.13 | 3.38 ± 0.18 | 3.14 ± 0.13 | 2.86 ± 0.19 | |||||
| A | - | 5.0 ± 0.0 | 4.22 ± 0.31 | 3.00 ± 0.18 | 3.44 ± 0.25 | 4.29 ± 0.12 | 2.43 ± 0.16 | |||||
| purple | C | 5.0 ± 0.0 | 4.3 ± 0.4 | 5.0 ± 0.0 | 4.95 ± 0.05 | 4.83 ± 0.09 | 4.81 ± 0.19 | 4.95 ± 0.05 | *** | *** | *** | |
| CA | - | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.71 ± 0.10 | 5.0 ± 0.0 | |||||
| A | - | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.05 ± 0.05 | 1.9 ± 0.4 | 1.48 ± 0.16 | 1.19 ± 0.15 | |||||
| Odor scores | C | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.71 ± 0.10 | 4.28 ± 0.16 | 2.6 ± 0.3 | 1.38 ± 0.13 | *** | *** | ** | |
| CA | - | 5.0 ± 0.0 | 4.68 ± 0.11 | 4.62 ± 0.15 | 4.29 ± 0.12 | 3.00 ± 0.24 | 2.10 ± 0.15 | |||||
| A | - | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.71 ± 0.10 | 4.50 ± 0.12 | 2.86 ± 0.16 | 3.1 ± 0.21 | |||||
| Surface | C | 4.22 ± 0.10 | 4.06 ± 0.06 | 4.0 ± 0.0 | 4.14 ± 0.19 | 4.11 ± 0.18 | 4.0 ± 0.15 | 3.91 ± 0.12 | *** | *** | *** | |
| CA | - | 4.94 ± 0.06 | 4.33 ± 0.11 | 4.33 ± 0.19 | 4.29 ± 0.17 | 4.62 ± 0.11 | 3.95 ± 0.16 | |||||
| A | - | 4.67 ± 0.11 | 4.94 ± 0.06 | 3.95 ± 0.23 | 4.50 ± 0.15 | 4.33 ± 0.13 | 4.43 ± 0.16 | |||||
| Overall acceptance | C | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.22 ± 0.13 | 4.10 ± 0.10 | 3.39 ± 0.28 | 2.24 ± 0.19 | 1.38 ± 0.11 | *** | *** | *** | |
| CA | - | 4.17 ± 0.09 | 4.0 ± 0.0 | 3.76 ± 0.14 | 3.14 ± 0.20 | 2.33 ± 0.19 | 2.00 ± 0.12 | |||||
| A | - | 4.67 ± 0.11 | 3.67 ± 0.11 | 3.57 ± 0.20 | 3.00 ± 0.23 | 2.24 ± 0.17 | 2.57 ± 0.20 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, J.M.; Michalska, K.; Tkaczewska, J.; Tkacz, K.; Zakrzewski, A.; Galanty, A.; Kamińska, I.; Chmiel, M.J.; Jamróz, E. Storage Properties of Double-Layer Films Enriched with Phytolacca americana L. Extract as Active Packaging for African Catfish, with a New Approach to Antioxidant Film Assay and Additional Analysis of P. americana Extract Toxicity on Human Cell Lines. Molecules 2025, 30, 1447. https://doi.org/10.3390/molecules30071447
Jasińska JM, Michalska K, Tkaczewska J, Tkacz K, Zakrzewski A, Galanty A, Kamińska I, Chmiel MJ, Jamróz E. Storage Properties of Double-Layer Films Enriched with Phytolacca americana L. Extract as Active Packaging for African Catfish, with a New Approach to Antioxidant Film Assay and Additional Analysis of P. americana Extract Toxicity on Human Cell Lines. Molecules. 2025; 30(7):1447. https://doi.org/10.3390/molecules30071447
Chicago/Turabian StyleJasińska, Joanna Maria, Klaudia Michalska, Joanna Tkaczewska, Katarzyna Tkacz, Arkadiusz Zakrzewski, Agnieszka Galanty, Iwona Kamińska, Maria J. Chmiel, and Ewelina Jamróz. 2025. "Storage Properties of Double-Layer Films Enriched with Phytolacca americana L. Extract as Active Packaging for African Catfish, with a New Approach to Antioxidant Film Assay and Additional Analysis of P. americana Extract Toxicity on Human Cell Lines" Molecules 30, no. 7: 1447. https://doi.org/10.3390/molecules30071447
APA StyleJasińska, J. M., Michalska, K., Tkaczewska, J., Tkacz, K., Zakrzewski, A., Galanty, A., Kamińska, I., Chmiel, M. J., & Jamróz, E. (2025). Storage Properties of Double-Layer Films Enriched with Phytolacca americana L. Extract as Active Packaging for African Catfish, with a New Approach to Antioxidant Film Assay and Additional Analysis of P. americana Extract Toxicity on Human Cell Lines. Molecules, 30(7), 1447. https://doi.org/10.3390/molecules30071447










