2.1. Fourier Transform Infrared (FTIR) Spectroscopy
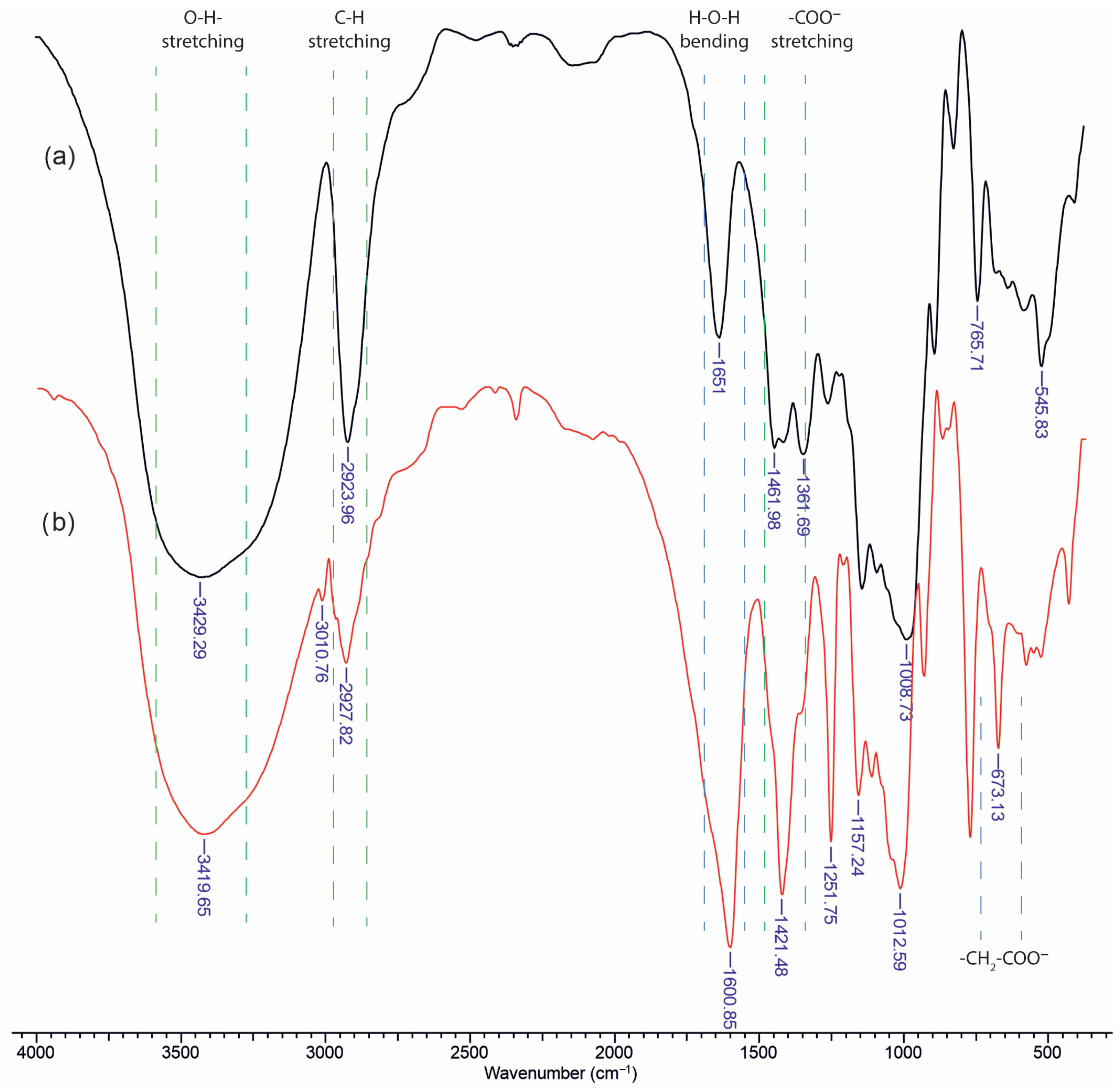
Infrared analysis of unsubstituted dextran (
Figure 1a) reveals several characteristic polysaccharide bands, including a broad O–H stretching vibration in the region of 3400–3500 cm
−1, which is indicative of extensive hydrogen bonding. Polysaccharides also tend to strongly retain water, leading to an H–O–H bending vibration commonly observed in the 1640–1660 cm
−1 region. In the case of unmodified dextran, the band near 1650 cm
−1 arises primarily from bound water rather than from functional groups of the polymer itself. Since there is no intrinsic carbonyl or amide group in dextran, any apparent absorption in that region is generally attributed to retained moisture.
Upon carboxymethylation of dextran (
Figure 1b), new prominent features appear in both the high- and low-wavenumber regions. Notably, additional absorptions emerge near 1600 cm
−1 and 1400 cm
−1, corresponding to the asymmetric and symmetric stretching vibrations of the carboxylate group (–COO
−), respectively. Furthermore, a distinct band becomes visible around 673 cm
−1, which is attributed to an out-of-plane deformation of the glucopyranose ring. This band is only weakly observed or absent in unsubstituted dextran, but the introduction of –CH
2–COO
− substituents modify the ring environment and hydrogen-bonding patterns, thereby enhancing or “activating” this low-frequency mode. Overall, the appearance of these new vibrational signals confirms successful carboxymethylation and provides insights into the altered molecular interactions within the polysaccharide chain.
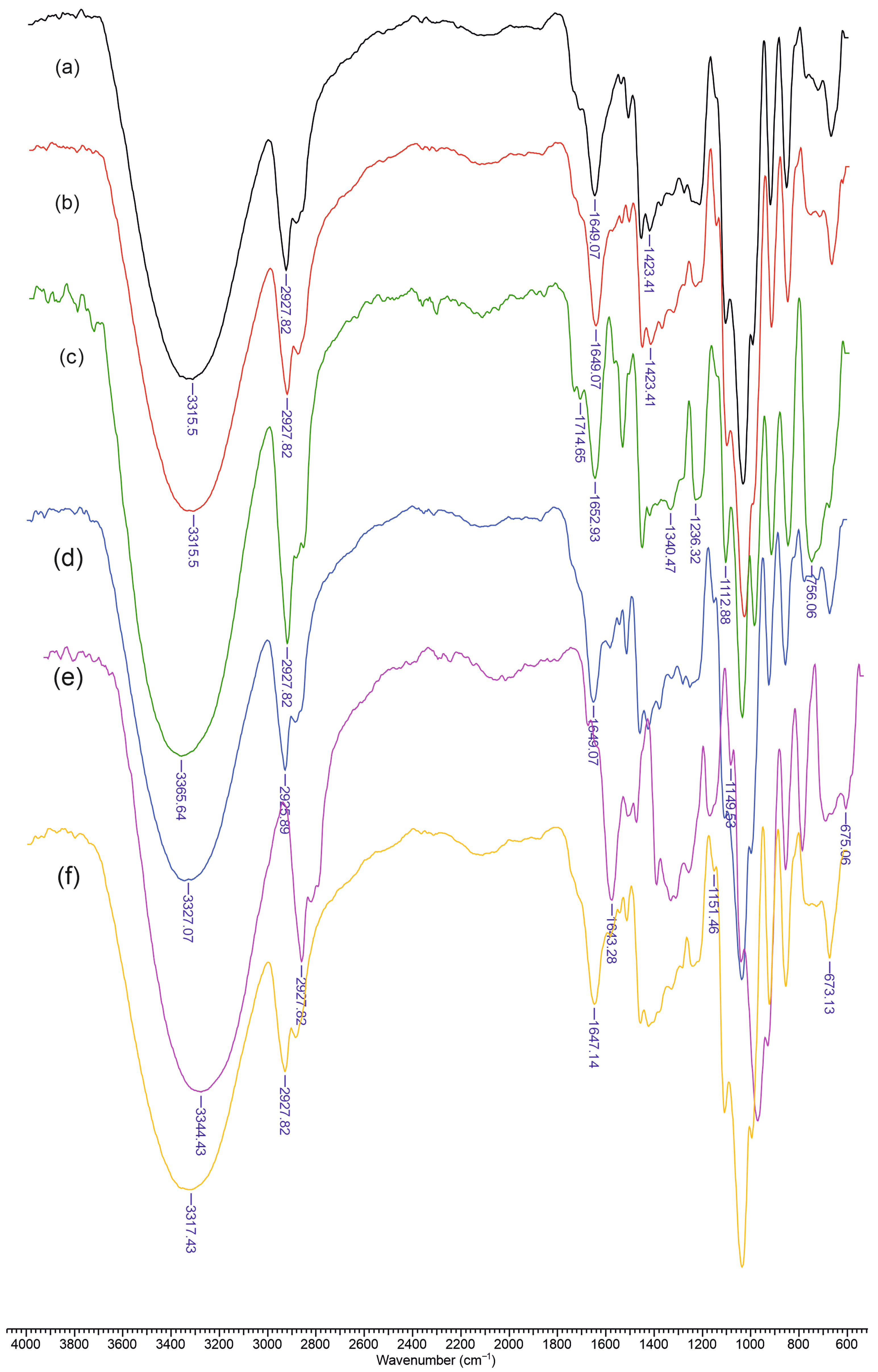
Figure 2 presents the FTIR spectra of the materials containing chitosan (Chi), dextran (Dex) or carboxymethyl dextran (mDex), lignin (L), and curcumin (Cu). In spectrum (a), corresponding to Chi–L, a broad absorption centered around 3300–3400 cm
−1 is evident, reflecting overlapping O–H (from lignin phenolic groups) and N–H (from chitosan) stretching bands. A moderate peak near 2920 cm
−1 is attributed to C–H stretching vibrations, common to polysaccharides and lignin’s aliphatic side chains. The amide I region of chitosan (around 1640–1650 cm
−1) and the aromatic ring vibrations of lignin (approximately 1600 and 1510 cm
−1) overlap slightly, although the lignin band near 1510 cm
−1 remains visible. Chitosan’s saccharide ring vibrations appear in the 1150–1000 cm
−1 region, producing a characteristic polysaccharide fingerprint.
Upon addition of dextran, as shown in spectrum (b) (Chi–Dex–L), the broad hydrogen-bonded region (3300–3400 cm−1) remains prominent, but the 1150–1000 cm−1 region becomes more intense due to the glycosidic ring signals of dextran. The lignin aromatic ring vibrations and chitosan amide band persist near 1600 and 1650 cm−1, respectively. Introducing curcumin into the Chi–Dex–L matrix—spectrum (c) (Chi–Dex–L–Cu)—adds a conjugated C=O stretching band (1620–1630 cm−1) that overlaps with the lignin/chitosan region. Additional aromatic ring modes from curcumin overlap with those of lignin near 1500–1450 cm−1, and the phenolic O–H of curcumin contributes further to broadening in the 3300–3400 cm−1 range.
A similar trend appears in spectrum (d) (Chi–L–Cu), where the absence of dextran simplifies the carbohydrate region, yet the curcumin band near 1620–1630 cm−1 and lignin aromatic absorptions around 1600 and 1510 cm−1 remain clearly visible. In spectrum (e) (Chi–mDex–L), the carboxylate groups in carboxymethyl dextran shift or intensify absorptions near 1600–1610 cm−1. Finally, in spectrum (f) (Chi–mDex–L–Cu), the characteristic signals of all four components—chitosan, mDex, lignin, and curcumin—overlap. The broad band at 3300–3400 cm−1 encompasses O–H and N–H stretching from all components, while the COO− stretch (mDex), the aromatic ring vibrations (lignin and curcumin), and the saccharide skeleton bands (chitosan and mDex) combine, confirming effective integration of each constituent into the material.
2.2. Mechanical Properties of Materials
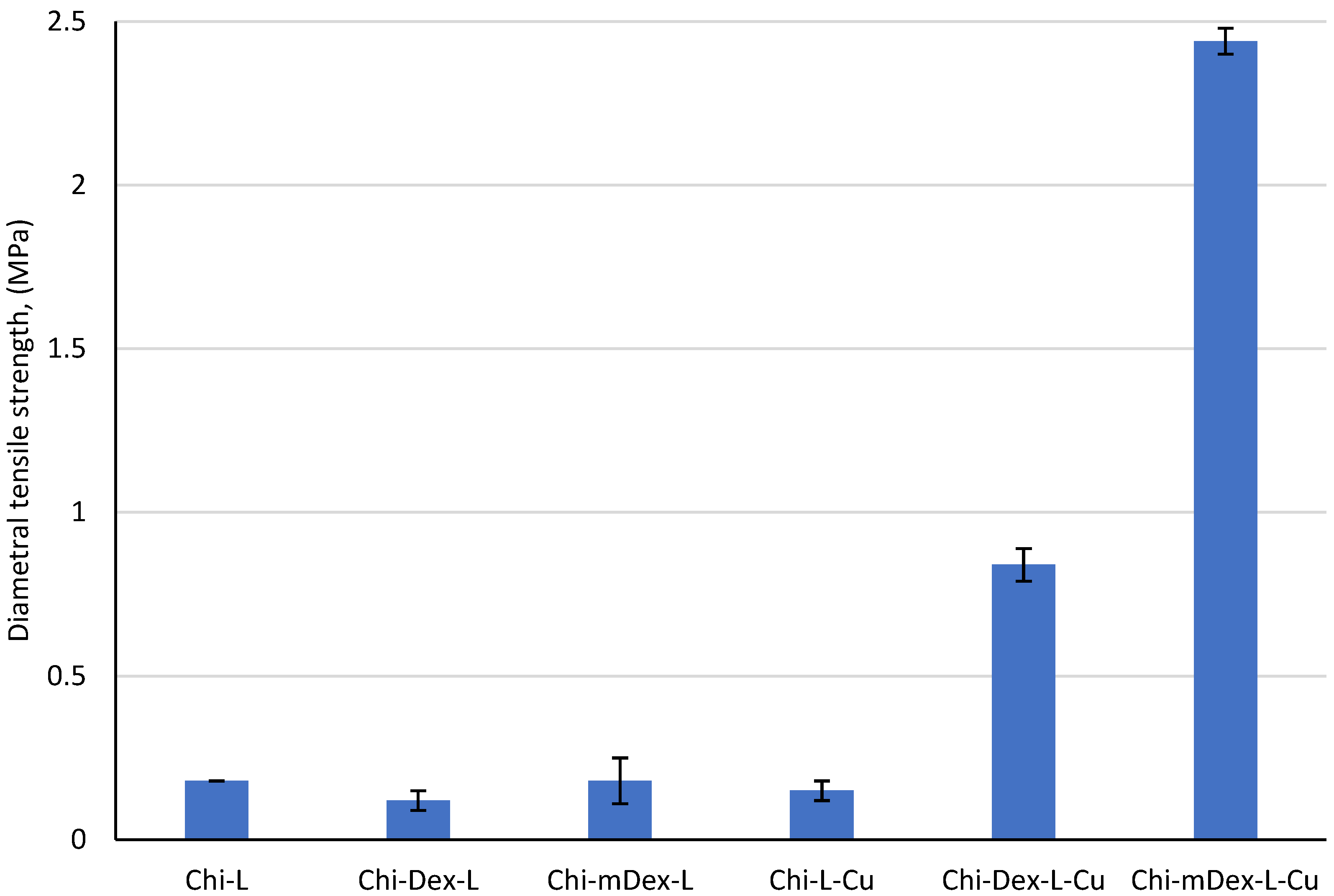
The diametral tensile strength (DTS) data (
Figure 3) reveal clear material’s composition–property correlations within these formulations. Chi–L exhibits a moderate DTS value of 0.18 MPa, reflecting hydrogen bonding and limited electrostatic interactions between chitosan’s amine groups and lignin’s phenolic moieties. Addition of dextran (Chi–Dex–L) diminishes DTS to 0.12 MPa—a 33% decrease—likely because dextran’s relatively linear structure and hydroxyl groups do not contribute enough additional crosslinking sites to strengthen the matrix. In contrast, replacing dextran with carboxymethyl dextran (Chi–mDex–L) recovers the original 0.18 MPa, indicating that the carboxymethyl groups facilitate somewhat stronger hydrogen bonding or ionic interactions that restore the baseline strength.
The introduction of curcumin (Cu) drives dramatic enhancement in the DTS of certain formulations. Although Chi–L–Cu (0.15 MPa) is 17% lower than the original Chi–L, adding curcumin to Chi–Dex–L (Chi–Dex–L–Cu) boosts the DTS to 0.84 MPa—an increase of 600% over its non-curcumin counterpart, and nearly 370% higher than Chi–L. Even more striking is the Chi–mDex–L–Cu, achieving 2.40 MPa: a 1233% increase relative to Chi–mDex–L and 1200% higher than Chi–L. These improvements reflect the multifunctional role of curcumin, whose polyphenolic rings can participate in hydrogen bonding, hydrophobic interactions [
40], and potential electrostatic attractions with both chitosan and lignin. Furthermore, the carboxyl groups of mDex introduce additional binding sites that amplify curcumin’s crosslinking efficacy, leading to highly interconnected, mechanically robust networks. Hence, the synergistic effect of curcumin and mDex produces the most significant rising in DTS, underscoring the importance of complementary functional groups for maximizing material strength.
The diametral tensile strength of the tested materials exhibited significant variations, as confirmed by the ANOVA results (F = 936.1433,
p = 4.09 × 10
−15), indicating that the mechanical properties are strongly influenced by the material composition and the presence of curcumin (
Table S1, Supplementary Materials). The post-hoc Bonferroni test further confirmed significant differences between specific groups, particularly between curcumin-loaded and non-loaded materials.
Among the non-curcumin-loaded materials, Chi-L (0.18 MPa), Chi-Dex-L (0.12 MPa), and Chi-mDex-L (0.18 MPa) exhibited similar tensile strengths, with no statistically significant differences between them (p > 0.05). The introduction of curcumin had a profound impact on the mechanical properties, as evidenced by the significant augmentation in DTS values for Chi-Dex-L-Cu (0.84 MPa) and Chi-mDex-L-Cu (2.44 MPa). The tensile strength of Chi-mDex-L-Cu was significantly higher than all other groups (p < 3.4 × 10−7 when compared to Chi-L, Chi-Dex-L, and Chi-L-Cu).
While Chi-L-Cu (0.15 MPa) did not show a significant rise in strength compared to its non-curcumin counterpart (Chi-L, p = 0.607), the system comprising modified dextran exhibited a distinct mechanical enhancement when curcumin was incorporated. Chi-Dex-L-Cu showed a nearly sevenfold increase in strength compared to Chi-Dex-L (p = 6.31 × 10−5), and Chi-mDex-L-Cu demonstrated an even more pronounced increase (p = 3.23 × 10−7). These results suggest that the interaction between curcumin and Chi-Dex-based matrices contributes to enhanced mechanical reinforcement, likely due to improved structural integrity and polymer cross-linking effects.
The findings demonstrate that Chi-mDex-L-Cu is the most mechanically robust material, exhibiting the highest diametral tensile strength among all tested formulations. This enhanced mechanical performance makes it a promising candidate for applications requiring structural stability and durability, such as biomedical scaffolds or drug delivery systems requiring mechanical resilience. In contrast, Chi-L-Cu exhibited only marginal improvements in mechanical properties, suggesting that the chitosan-lignin system alone does not provide significant reinforcement upon curcumin loading.
Overall, the results emphasize that the mechanical behavior of curcumin-loaded materials depends not only on the presence of curcumin but also on the specific polymeric matrix used. The formulations comprising dextran, particularly Chi-mDex-L-Cu, benefit from a synergistic effect that significantly enhances their tensile strength, making them more suitable for applications requiring both controlled drug release and improved mechanical stability.
2.4. Release Kinetic of Bioactive Principles from Polymeric Matrix
The Weibull model is a versatile mathematical tool widely employed to describe the kinetics of drug release from polymeric matrices. Its flexible form allows for the characterization of various release mechanisms, ranging from diffusion-dominated processes to degradation-controlled systems. The general equation (Equation (1)) of the Weibull model is:
where:
y—cumulative drug release at time
x;
A—maximum cumulative release (asymptotic value);
k—release rate constant, indicating the speed of drug release;
xc—lag time before significant drug release begins;
d—shape parameter, which determines the nature of the release curve.
By fitting experimental data to the Weibull model, the values of A, k, xc, and d provide valuable insights into the drug release profile: A—indicates the total amount of drug that can be released; k—reflects the speed of release, influenced by the matrix’s structure and drug properties; xc—identifies any delay in release, such as a hydration phase or lag due to matrix swelling; d—reveals the dominant release mechanism, distinguishing between diffusion and matrix degradation.
The model accommodates various types of polymeric matrices, including hydrogels, crosslinked networks, and composite materials. Its adaptability allows it to describe systems with single or combined release mechanisms.
The Weibull model is often used to fit experimental release data, enabling the determination of the kinetics and mechanisms of drug release. For example, a low d value (<1) indicates diffusion-dominated release, common in hydrophilic polymeric matrices.
By comparing k and d values across different formulations, researchers can evaluate the influence of polymer composition, crosslinking density, and drug-polymer interactions on release behavior.
The model assists in designing formulations tailored for specific therapeutic needs. For instance, fast-release systems can be designed by optimizing matrices with higher k and A values. Sustained-release systems can be achieved by introducing carboxylic groups or enhancing crosslinking, reducing A and k.
The Weibull model is a cornerstone in the study of drug release kinetics from polymeric matrices. Its flexibility and interpretability make it invaluable for understanding and optimizing release profiles. By using the insights provided by the model, there could be designed advanced drug delivery systems adapted for a wide range of therapeutic applications. Whether for fast-acting formulations or prolonged-release therapies, the Weibull model remains a powerful tool in pharmaceutical research.
The release of curcumin from Chi-L-Cu, Chi-Dex-L-Cu, Chi-mDex-L-Cu, Chi-L-Cu*, Chi-Dex-L-Cu*, and Chi-mDex-L-Cu* materials was assessed using the Weibull model to evidence the release kinetics (
Figure 5). The parameters analyzed include the maximum release (
A) and the rate constant (
k), which are influenced by the composition and chemical structure of the materials components (
Table 1).
The release kinetics of pure curcumin (Cu*) from the chitosan-dextran/carboxymethyl dextran-lignin matrix exhibited a significantly slower and lower cumulative release compared to curcumin extracted from turmeric. This behavior can be attributed to the strong molecular interactions between curcumin and the polymeric components, including hydrogen bonding, electrostatic interactions, and π-π stacking with lignin. These interactions contribute to curcumin entrapment within the polymeric network, limiting its diffusion and prolonging its release. Additionally, the polymeric matrix forms a semi-rigid, hydrophilic environment that restricts the solubility and mobility of curcumin, further reducing the release rate. In contrast, curcumin in turmeric extract exists in a more bioavailable form, likely due to its association with other bioactive compounds that enhance its solubility and diffusion.
Chi-L-Cu exhibited the highest release capacity, with an asymptotic A value of 400 µg. This represents a 3.47% higher release compared to Chi-Dex-L-Cu (A = 386) and a 55.66% higher release as compared to Chi-mDex-L-Cu (A = 177.37). The significantly lower A value for Chi-mDex-L-Cu suggests that the introduction of carboxymethyl dextran into the matrix substantially limits curcumin release. The carboxymethylation process introduces carboxylic (-COOH) groups, which enhance crosslinking density, thereby reducing matrix porosity and curcumin diffusion.
Chi-L-Cu demonstrated the fastest release rate, with k = 0.00456. This was 68.16% higher than Chi-Dex-L-Cu (k = 0.00145) and 35.96% higher than Chi-mDex-L-Cu (k = 0.00292). The slower k recorded for Chi-Dex-L-Cu reflects the impact of dextran incorporation, which increases matrix rigidity, slowing the diffusion of curcumin. Chi-mDex-L-Cu, with its lower k, balances curcumin diffusion and matrix degradation. The presence of carboxylic groups in carboxymethyl dextran enhances ionic interactions with chitosan’s amine groups, creating a compact and highly crosslinked network.
Carboxymethyl dextran contains carboxyl (-COOH) groups that significantly influence the matrix structure. These groups react with the amine (-NH2) groups in chitosan, forming ionic crosslinks that increase matrix density. Hydrogen bonding between carboxylic groups and hydroxyl groups in lignin or dextran further strengthens the network, enhancing mechanical stability. The additional crosslinking reduces water uptake and matrix swelling, thereby limiting the diffusion of curcumin. To conclude, the chemical modification of dextran (carboxymethylation) introduces additional reactive groups, which: increase the potential for crosslinking within the matrix; reduce permeability of the material, leading to lower A and slower k; in Chi-mDex-L-Cu, the enhanced crosslinking results in a highly compact structure, making it ideal for applications requiring prolonged and controlled release.
The differences in A and k between the materials highlight the impact of polymeric composition and chemical modifications on curcumin release: Chi-L-Cu—high A and k, indicating a permeable matrix ideal for rapid and extensive curcumin release; Chi-Dex-L-Cu—moderate A and k, reflecting the effect of dextran incorporation, which slows release while maintaining a high release capacity; Chi-mDex-L-Cu—the lowest A and lowest k, underlining the role of carboxymethyl dextran in creating a denser, more rigid matrix with prolonged release.
These findings suggest that the chemical modifications of dextran, particularly the introduction of carboxylic groups, play a pivotal role in optimizing the release profiles of curcumin, enabling the design of materials for specific drug delivery applications.
The controlled release of curcumin from polymeric matrices was analyzed through key pharmacokinetic parameters, including area under the curve (AUC), maximum drug concentration (Cmax), and time to maximum concentration (Tmax). These parameters provide insight into the extent, rate, and duration of drug release from the tested materials: Chi-L-Cu, Chi-Dex-L-Cu, and Chi-mDex-L-Cu.
The computed AUC values were 43,950.0, 29,425.0, and 20,025.0 µg·min/g material for Chi-L-Cu, Chi-Dex-L-Cu, and Chi-mDex-L-Cu, respectively. The corresponding C
max values were 250.0, 165.0, and 110.0 µg/g material, all occurring at T
max of 270 min (
Table S2, Supplementary Materials). These results indicate distinct drug release behaviors across the three formulations.
Chi-L-Cu exhibited the highest AUC, suggesting the greatest total drug exposure over time. The peak concentration of 250.0 µg/g material at 270 min confirms that this formulation achieved the highest release among the tested materials. The combination of a high AUC and Cmax indicates a rapid and efficient drug release, likely attributed to a porous polymeric matrix that facilitates curcumin diffusion. This system is optimal for applications requiring high initial drug availability, where curcumin must reach therapeutic levels quickly.
Chi-Dex-L-Cu demonstrated an AUC of 29,425.0 µg·min/g material, lower than Chi-L-Cu but still significant. The Cmax of 165.0 µg/g material at 270 min suggests a more controlled release compared to Chi-L-Cu. The presence of dextran within the chitosan matrix likely contributes to a reduction in diffusion rates, promoting sustained release characteristics. This formulation is suitable for applications requiring extended drug delivery, where maintaining moderate curcumin concentrations over time is beneficial.
Chi-mDex-L-Cu exhibited the lowest AUC of 20,025.0 µg·min/g material, indicating the least overall drug exposure. The Cmax of 110.0 µg/g material at 270 min reflects the slowest drug release among the three formulations. The modified dextran component appears to further restrict curcumin diffusion, leading to a gradual and prolonged release profile. This formulation is ideal for long-term drug administration, where maintaining a lower but consistent drug concentration over time is required.
A comparative analysis of the three formulations suggests that material composition significantly influences the release kinetics of curcumin. Chi-L-Cu is best suited for rapid drug delivery, ensuring high curcumin availability early in the release period. Chi-Dex-L-Cu offers a balance between rapid and sustained release, making it an effective controlled-release system. Chi-mDex-L-Cu provides the most prolonged release profile, maintaining curcumin concentrations at lower but steady levels, making it ideal for applications requiring long-term therapeutic effects. These findings highlight the potential for tailoring polymeric drug delivery systems on specific therapeutic requirements.
The delivery of curcumin from the three tested polymeric materials (Chi-L-Cu, Chi-Dex-L-Cu, and Chi-mDex-L-Cu) exhibit significant differences, as shown in the release profile (
Figure 5). The release trends indicate that Chi-L-Cu presents the highest release rate and maximum cumulative release, followed by Chi-Dex-L-Cu, while Chi-mDex-L-Cu shows the lowest release of curcumin over time.
The ANOVA results (
Table S3, Supplementary Materials) confirm a statistically significant difference between the release profiles of the tested materials (F = 1633.561,
p = 6.16 × 10
−9), indicating that the variations observed in curcumin release are not due to random fluctuations but are attributable to differences in materials composition. The post-hoc Bonferroni correction further validates these findings, with all pairwise comparisons being statistically significant (
p < 0.0003 in all cases).
Chi-L-Cu exhibited the highest release rate and maximum cumulative release, making it ideal for fast-release applications, such as immediate drug delivery where rapid bioavailability is required.
Chi-Dex-L-Cu demonstrated a more moderate and sustained release profile, which could be beneficial for controlled-release formulations, where a prolonged therapeutic effect is desired while minimizing drug fluctuations.
Chi-mDex-L-Cu displayed the slowest and lowest release, suggesting its suitability for extended-release or long-term drug delivery systems, where a gradual and sustained drug release is required to reduce dose administration.
These findings emphasize the influence of polymeric modifications on the drug release behavior, highlighting the potential of chitosan-dextran based matrices for adjusting drug delivery to specific therapeutic needs.
2.5. Anti-Inflammatory Activity of Materials
BSA (bovine serum albumin) protein denaturation assay for the in vitro evaluation of anti-inflammatory potential of curcumin circumvented the ethical issues associated with the use of animals, having in mind that these are results of the first screenings.
The curcumin release profiles from the tested materials (
Figure 5) reveal distinct kinetic behaviors that significantly influence their anti-inflammatory efficacy (
Figure 6). Among the formulations, Chi-L-Cu exhibits the fastest curcumin release, followed by Chi-Dex-L-Cu, while Chi-mDex-L-Cu demonstrates the most sustained and controlled release over time. This suggests that the nature of the polymer matrix plays a crucial role in curcumin diffusion, with the carboxymethyl dextran (mDex) component contributing to prolonged retention of curcumin within the polymeric network. The presence of carboxyl groups in mDex likely enhances hydrogen bonding and electrostatic interactions, slowing down the diffusion rate while maintaining a continuous, steady release.
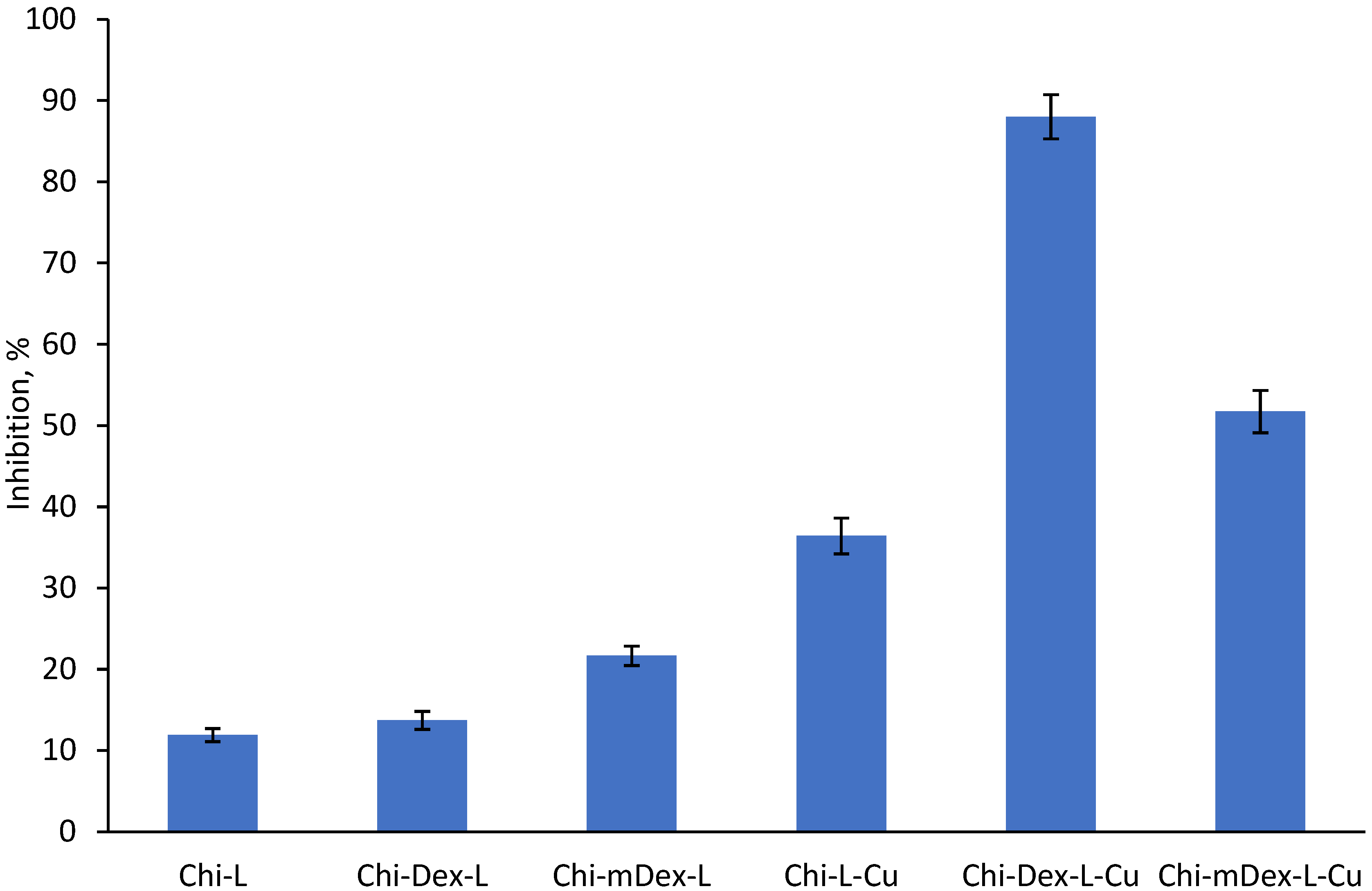
The anti-inflammatory data further support the idea that curcumin bioavailability is strongly linked to its release kinetics. Chi-Dex-L-Cu achieves the highest inhibition (~90%), demonstrating that an intermediate release rate optimally balances bioavailability with sustained action. Notably, Chi-mDex-L-Cu exhibits substantial anti-inflammatory activity (~70%) despite slower curcumin release, indicating that its controlled release mechanism may offer prolonged therapeutic effects, potentially reducing the need for frequent dosing. This is a key advantage in biomedical applications where sustained drug release is desirable for long-term anti-inflammatory effects.
While Chi-L-Cu releases curcumin more rapidly, leading to an initial strong inhibition effect, its faster depletion may limit prolonged bioactivity. In contrast, Chi-mDex-L-Cu, with its extended-release profile, could be of particular interest for applications requiring prolonged curcumin action, such as chronic inflammatory conditions. The ability of mDex to modulate curcumin release while still achieving significant inhibition underscores its potential as a strategic polymeric component in sustained-release formulations.
Overall, these results highlight that mDex-containing formulations not only ensure extended curcumin availability but also retain significant anti-inflammatory efficacy, making them highly attractive for long-term therapeutic applications. Future studies should explore further optimization of mDex-based matrices to fine-tune release rates and to enhance sustained bioactivity for applications in osteoarthritis treatment, or gastrointestinal inflammatory disorders.
The release kinetics of curcumin from the tested materials exhibit distinct profiles, which directly impact their anti-inflammatory effectiveness. The release profile demonstrated that Chi-L-Cu exhibited the highest curcumin release rate and cumulative release over time, followed by Chi-Dex-L-Cu, while Chi-mDex-L-Cu displayed the slowest and lowest curcumin release. The statistical analysis of anti-inflammatory capacity (
Table S4, Supplementary Materials) further confirmed significant differences among the tested materials, as indicated by the ANOVA results (F = 456.308,
p = 2.99 × 10
−13). The post-hoc Bonferroni test revealed that nearly all compared pairwise were statistically significant (
p < 0.0003 in most cases), reinforcing the impact of dextran modifications on curcumin bioavailability and therapeutic efficiency.
Chi-L-Cu exhibited an average anti-inflammatory activity of 36.42%, correlating with its rapid curcumin release, suggesting that materials with high initial release provide quick bioavailability and an early anti-inflammatory effect. Chi-Dex-L-Cu showed the highest overall anti-inflammatory activity, with an average of 88.01%, aligning with its more controlled release profile. This suggests that a moderate and sustained release enhances prolonged bioactivity, preventing the rapid depletion of curcumin and thereby maintaining an extended therapeutic effect. Chi-mDex-L-Cu, while exhibiting the lowest release profile, displayed significant anti-inflammatory activity with an average of 51.73%, higher than Chi-L-Cu but lower than Chi-Dex-L-Cu. This indicates that while the slow release profile may delay maximum effectiveness, it still provides prolonged anti-inflammatory action.
These findings demonstrate a strong correlation between curcumin release kinetics and its biological activity. Fast-releasing materials such as Chi-L-Cu are suitable for applications requiring immediate anti-inflammatory action, whereas controlled-release systems like Chi-Dex-L-Cu are more effective for prolonged therapeutic effects. Slow-release systems, such as Chi-mDex-L-Cu, may be advantageous for extended treatments where a sustained, lower-dose effect is required. The results highlight the importance of dextran modifications in optimizing curcumin delivery for enhanced bioactivity and suggest that carefully tuning release kinetics is essential for maximizing therapeutic benefits in drug delivery applications.
2.6. Antioxidant Activity of Materials
The antioxidant activity of the tested materials, as evaluated using the ABTS assay (
Figure 7), highlights the influence of both the polymeric matrix and curcumin release kinetics on radical scavenging capacity. When fillers were incorporated into the matrix, the antioxidant properties were modified, with Chi-Dex-L showing a similar inhibition rate (~72%), while the inclusion of carboxymethyl dextran (mDex) slightly reduced the antioxidant activity to ~68%.
Curcumin, known for its potent inhibitory capacity, plays a crucial role in modulating the antioxidant activity of these materials. Its C=C bonds, β-diketo group, and phenyl rings contribute significantly to radical scavenging, acting as H-atom donors in antioxidant mechanisms [
41]. This effect is evident in the Chi-L-Cu (~80% inhibition) and Chi-Dex-L-Cu (~65% inhibition) samples, which exhibited the highest antioxidant activity due to the presence of phenolic hydroxyl groups in both curcumin and lignin, capable of donating hydrogen protons to neutralize free radicals [
42]. However, the most intriguing finding is that while Chi-mDex-L-Cu demonstrated slightly lower radical scavenging capacity (~60%), its extended curcumin release profile (
Figure 5) suggests a prolonged antioxidant effect over time.
Unlike Chi-L-Cu, which releases curcumin rapidly and reaches higher radical scavenging activity in the short term, Chi-mDex-L-Cu ensures a more sustained release of curcumin, maintaining antioxidant potential over an extended period. This suggests that while its immediate inhibition rate is ~25% lower than Chi-L-Cu, it may offer prolonged antioxidant protection, making it a lead candidate for applications where sustained activity is required, such as food packaging, biomedical coatings, or pharmaceutical formulations for chronic oxidative stress conditions.
Overall, these results emphasize the strategic role of mDex-based matrices in modulating curcumin release and prolonging its bioactivity. Future research should explore optimizing these formulations to fine-tune the balance between initial radical scavenging capacity and sustained curcumin availability, positioning Chi-mDex-L-Cu as a promising material for long-term antioxidant applications.
The relationship between curcumin release and antioxidant activity plays a crucial role in evaluating the effectiveness of polymers carriers for bioactive compound delivery. The release kinetics demonstrated that Chi-Dex-L-Cu and Chi-mDex-L-Cu exhibited a more controlled release profile compared to Chi-L-Cu, which showed the highest and fastest curcumin release. This controlled release behavior is particularly relevant when considering the antioxidant efficiency over time, as the antioxidant activity is directly dependent on the amount of curcumin available at a given time (t).
The statistical analysis of antioxidant capacity confirmed significant differences among the tested materials, with ANOVA results (
Table S5, Supplementary Materials) indicating a highly significant effect (F = 73.01071,
p = 1.46 × 10
−8). Post-hoc Bonferroni analysis showed that while Chi-L-Cu demonstrated the highest initial antioxidant capacity, Chi-Dex-L-Cu and Chi-mDex-L-Cu exhibited a more sustained antioxidant effect, attributed to the controlled curcumin release. The average antioxidant activity for Chi-Dex-L-Cu (57.74%) and Chi-mDex-L-Cu (60.00%) was lower than that of Chi-L-Cu (76.23%); however, this difference must be interpreted in the context of release kinetics. At a given time point, the antioxidant capacity is proportional to the cumulative amount of curcumin released, meaning that a slower but continuous release, as observed in Chi-Dex-L-Cu and Chi-mDex-L-Cu, ensures prolonged bioactivity.
A key observation is that while Chi-L-Cu provides an initial burst of antioxidant activity due to rapid curcumin release, its efficiency may decline over time as the available curcumin is depleted. In contrast, Chi-Dex-L-Cu and Chi-mDex-L-Cu maintain a more stable release profile, ensuring that curcumin remains bioavailable over extended periods, thus sustaining antioxidant activity at later time points. This controlled-release mechanism is particularly advantageous in applications where long-term free radical scavenging is required rather than an immediate but transient effect. The statistical significance of the differences between materials further supports this finding, with Chi-Dex-L-Cu and Chi-mDex-L-Cu demonstrating distinct antioxidant behaviors compared to Chi-L-Cu (p < 0.0006 in most cases).
These results highlight that Chi-Dex-L-Cu and Chi-mDex-L-Cu are particularly valuable for applications requiring sustained antioxidant activity, such as chronic oxidative stress management or prolonged therapeutic delivery. Their ability to regulate curcumin availability over time enhances their potential for biomedical applications, where maintaining a steady antioxidant effect is more beneficial than a rapid but short-lived response. The findings emphasize the importance of selecting matrices components to optimize curcumin bioavailability and maximize its therapeutic potential over extended durations.
2.7. In Vitro Biocompatibility of Samples
The proliferative effect of the developed materials was evaluated using the MTS (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay against gingival fibroblasts. The method is a widely used, reliable colorimetric method to assess cell viability and cytotoxicity, based on the ability of mitochondrial dehydrogenases in metabolically active cells to reduce MTS into formazan crystals. The amount of formazan produced is directly proportional to the number of viable cells, providing a quantitative measure of cell survival and proliferation.
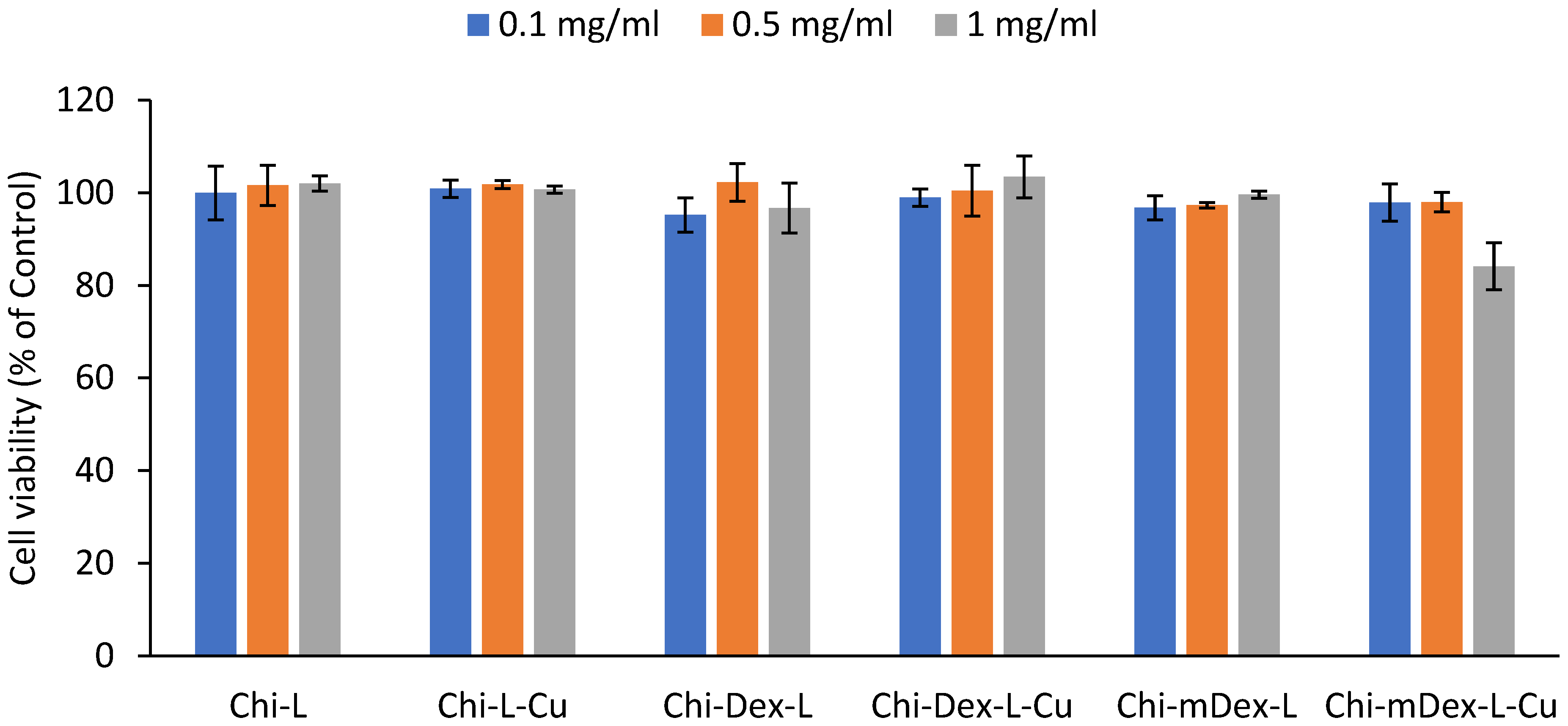
After 24 h of incubation, all tested formulations exhibited high cell viability across the three concentrations (0.1 mg/mL, 0.5 mg/mL, and 1 mg/mL), confirming their excellent biocompatibility (
Figure 8). The cell survival rates remained above 85% in all cases, indicating low cytotoxicity and minimal interference with cellular metabolic activity.
Among the tested materials, Chi-L and Chi-L-Cu demonstrated the highest cell viability (~95–100%), suggesting that the chitosan-lignin does not induce adverse effects on fibroblast proliferation. Similarly, Chi-Dex-L and Chi-Dex-L-Cu maintained high biocompatibility, with cell viability exceeding 90% across all tested concentrations, indicating that the incorporation of dextran and curcumin does not negatively impact cell survival and further supports their potential as safe therapeutic materials.
Chi-mDex-L and Chi-mDex-L-Cu also displayed excellent biocompatibility, although a slight decrease in viability (~10% reduction at 1 mg/mL for Chi-mDex-L-Cu) was observed compared to other formulations. This reduction is likely attributed to the increased interaction between carboxymethyl dextran (mDex) and the fibroblast membrane at higher concentrations. However, even at the highest tested dose, cell viability remained above 80%, reinforcing the safety profile of mDex-containing formulations.
For an MTS assay, cell viability above 80% is generally considered non-cytotoxic, while viability between 60–80% suggests moderate biocompatibility and below 60% may indicate cytotoxic effects. In this study, all materials met the biocompatibility criteria, confirming their potential for biomedical applications.
Given their biocompatibility and potential for sustained curcumin release, mDex-based formulations stand out as lead candidates for in vivo evaluation, where their long-term therapeutic efficacy and biological interactions will be further assessed.