Effects of Crude Shea Butters and Their Polar Extracts on Singlet Oxygen Quenching and Against Rose Bengal-Induced HaCaT Cell Phototoxicity
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content of Shea Butter and Kariten Samples
2.2. Antioxidant Activities of Crude Shea Butters and Their Polar Fractions
2.2.1. Choice of Solvent to Solubilize Crude Shea Butter
2.2.2. Antioxidant Activities
2.3. Photoprotection Effect of Raw Shea Butters and Their Polar Extracts on Singlet Oxygen
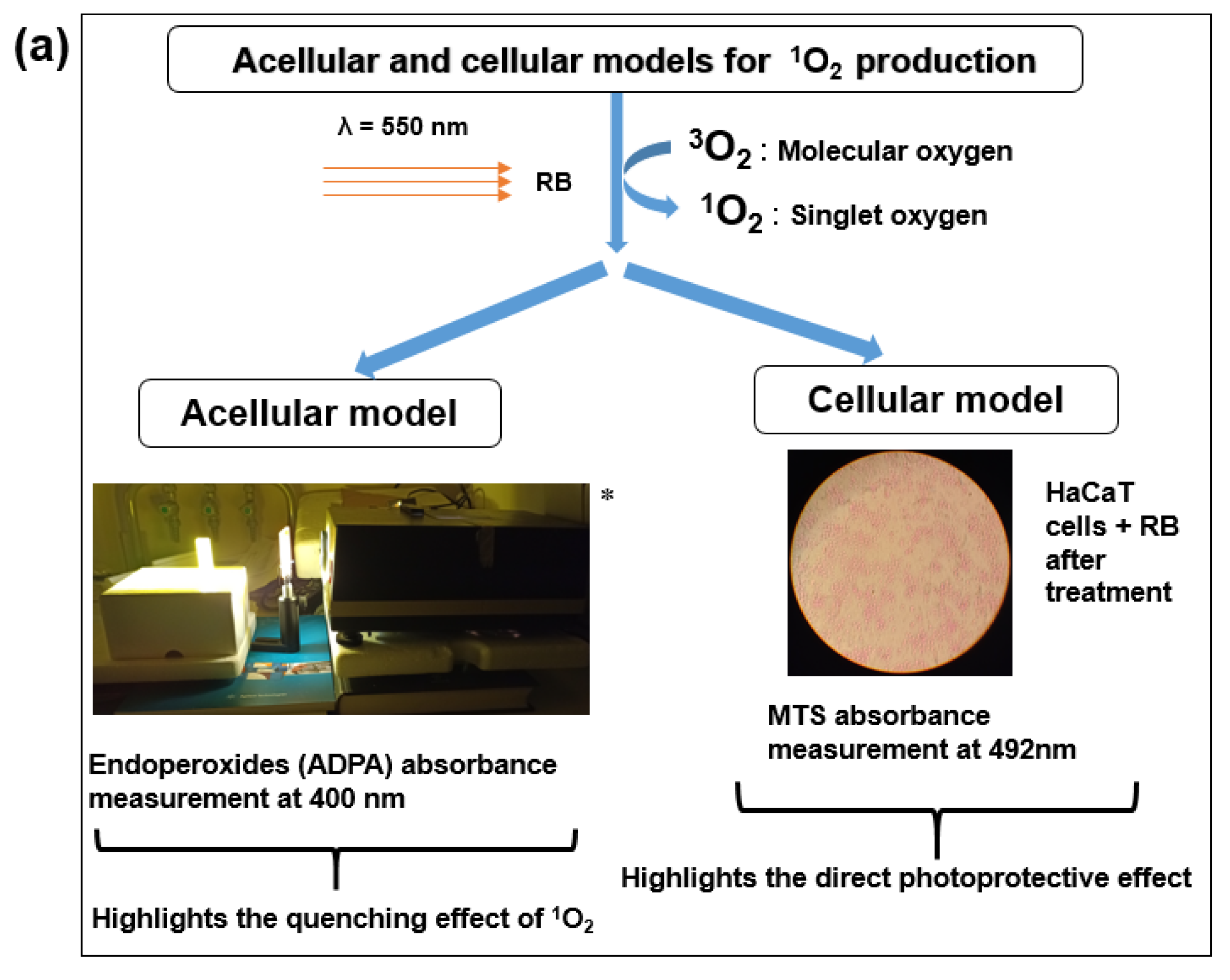
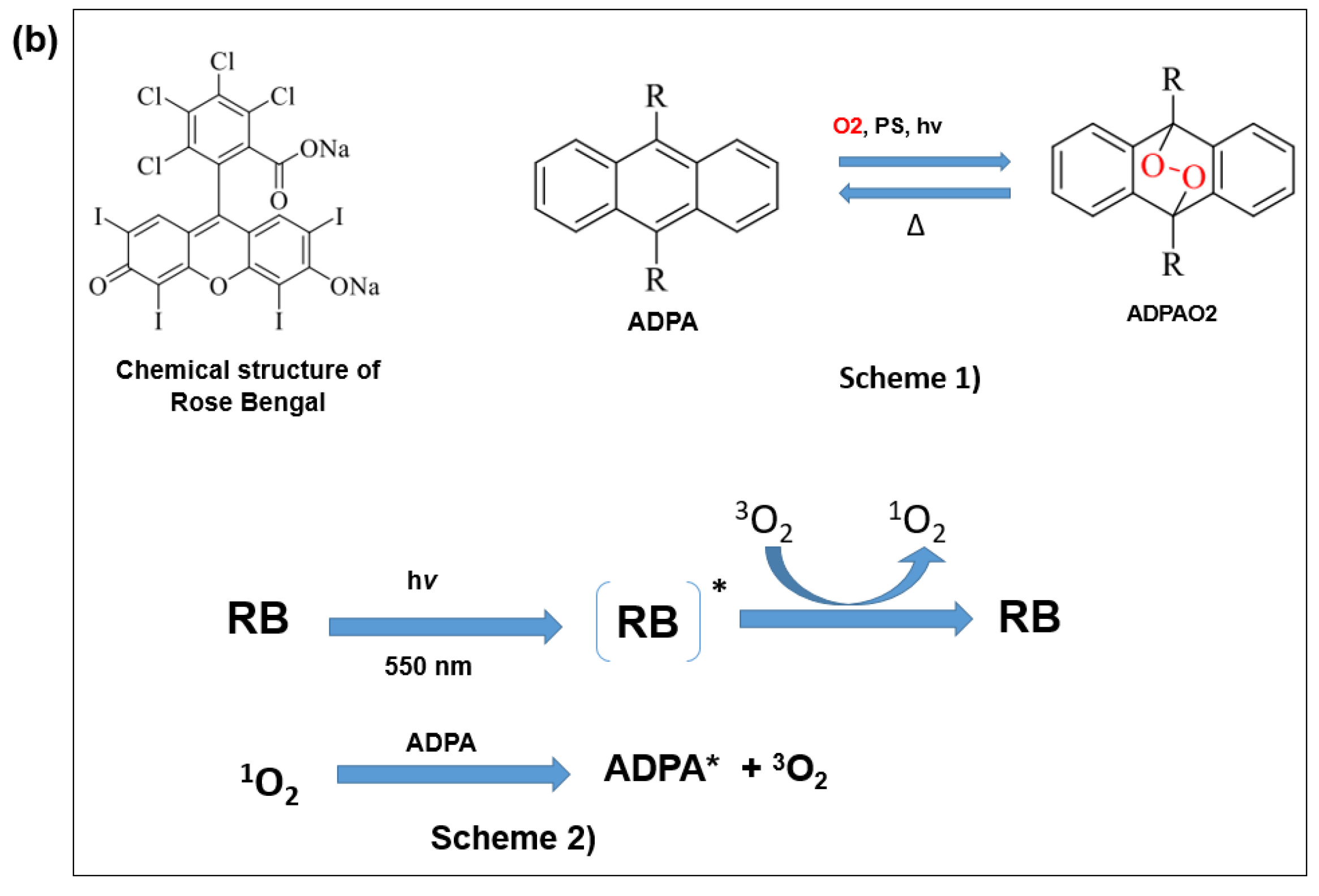
2.3.1. Effect of Crude Shea Butters and Their Polar Fractions on Singlet Oxygen by Photosensitization of Rose Bengal (Acellular Model)
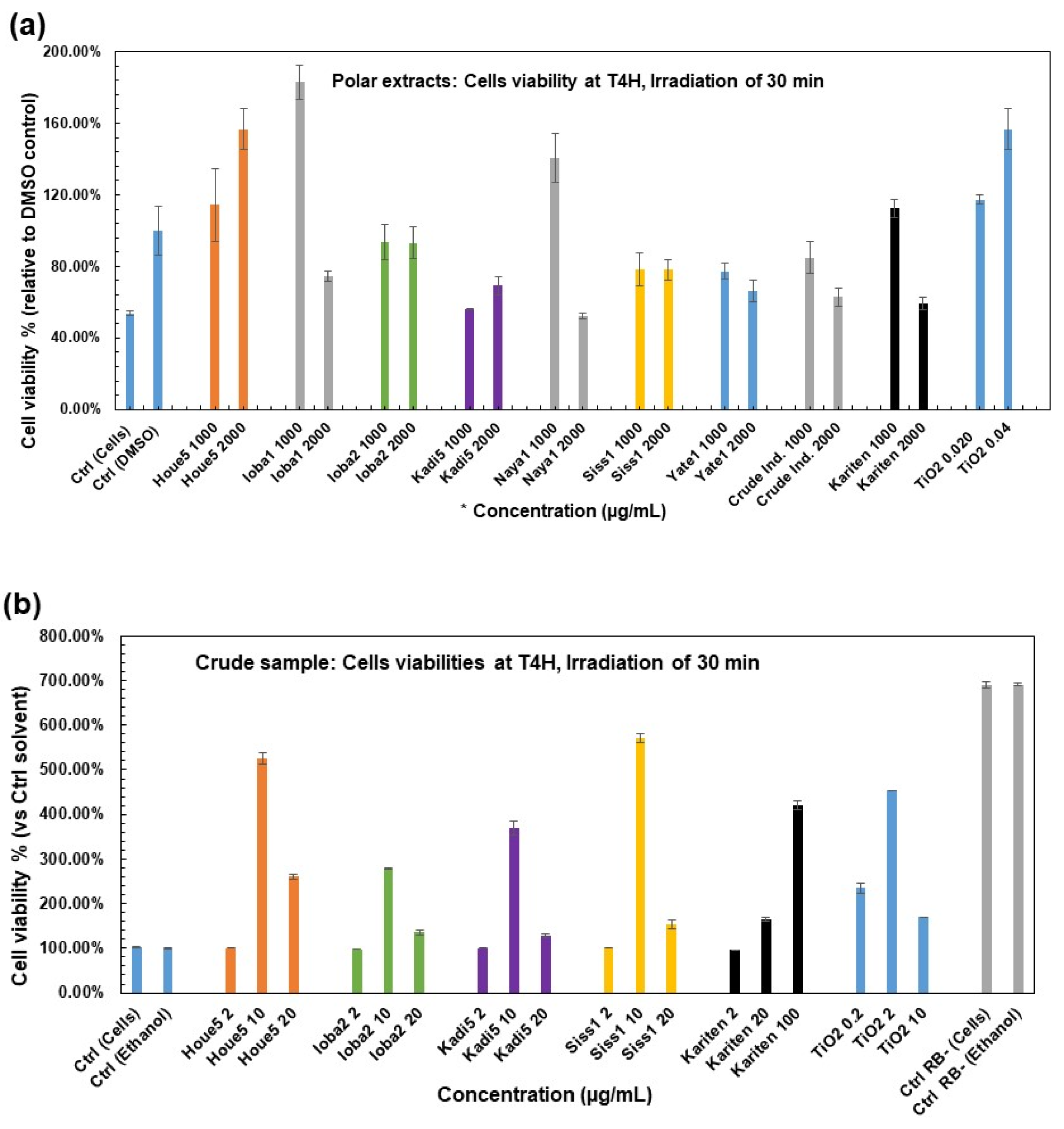
2.3.2. Photoprotective Effects of Crude Shea Butter and Their Polar Extracts on Cells’ Viability by Photosensitization of Rose Bengal (Cellular Model)
- Inherent toxicity of crude shea butter on HaCaT cells
- Protective effects of raw shea samples and their polar extracts against 1O2 production
2.4. LC Analyses of Shea Butter Polyphenols Content
- Analyses of polar compounds in polar fractions and quantification by RP-HPLC
3. Materials and Methods
3.1. Materials
3.1.1. Materials for Total Phenol Content
3.1.2. Materials for Antioxidant Test
3.1.3. Materials for Photoprotection Test
3.1.4. Materials for Polyphenol Identification and Assay
3.1.5. Cell Culture and Media
3.1.6. Chemicals
3.2. Methods
- Sample preparation for photoprotective and antioxidant investigation (solution A)
- Polar fraction preparation (solution B)
3.2.1. Methods for Total Phenol Content
3.2.2. Methods for Antioxidant Test
- DPPH radical-scavenging activity
- ABTS radical-scavenging activity
3.2.3. Methods for Photoprotection Test
- Designing experimental devices
- In vitro experimental design to investigate 1O2 quenching capacity
- Crude Shea Butter and Its Polar Fraction Assay on HaCaT Cell Viability
3.2.4. Methods for Polyphenols Identification and Assay
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 3-ethylbenzothiazoline 6-sulfonic acid |
| ADPA | 9,10-Anthracenedipropanoic acid |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EtOH | Ethanol |
| FC | Folin–Ciocalteu |
| GAE | Gallic acid equivalent |
| HaCaT | Human keratinocyte cell line |
| LOQ | Limit of quantification |
| MeOH | Methanol |
| MTS | 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| PGE | Pyrogallol equivalent |
| RB | Rose Bengal |
| RP-HPLC/LC | Reverse phase-High performance liquid chromatography |
| ROS | Reactive Oxygen Species |
| Rt | Retention time |
| SB | Shea butter |
| TiO2 | Titanium dioxide |
| TPC | Total Polyphenol Content |
| UV | Ultraviolet |
| UVR | Ultraviolet Radiation |
| 1O2 | Singlet oxygen |
References
- Huang, C.C.; Wu, W.B.; Fang, J.Y.; Chiang, H.S.; Chen, S.K.; Chen, B.H.; Chen, Y.T.; Hung, C.F. (-)-Epicatechin-3-Gallate, a Green Tea Polyphenol Is a Potent Agent against UVB-Induced Damage in HaCaT Keratinocytes. Molecules 2007, 12, 1845–1858. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular Photoprotection of Human Keratinocytes in Vitro by the Naturally Occurring Mycosporine-like Amino Acid Palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.K.; Srivastav, A.K.; Dubey, D.; Chopra, D.; Singh, J.; Mujtaba, S.F. Sunscreen-Induced Expression and Identification of Photosensitive Marker Proteins in Human Keratinocytes under UV Radiation. Toxicol. Ind. Health 2019, 35, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Izutsu, Y.; Yahagi, S.; Okano, Y. Reactive Oxygen Species in HaCaT Keratinocytes after UVB Irradiation Are Triggered by Intracellular Ca2+ Levels. J. Investig. Dermatol. Symp. Proc. 2009, 14, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 1–33. [Google Scholar] [CrossRef]
- Bogusz, K.; Tehei, M.; Lerch, M.; Dou, S.X.; Liu, H.K.; Konstantinov, K. TiO2/(BiO)2CO3 Nanocomposites for Ultraviolet Filtration with Reduced Photocatalytic Activity. J. Mater. Chem. C 2018, 6, 5639–5650. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2020, 1, 1–21. [Google Scholar] [CrossRef]
- Daimon, T.; Nosaka, Y. Formation and Behavior of Singlet Molecular Oxygen in TiO2 Photocatalysis Studied by Detection of Near-Infrared Phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar] [CrossRef]
- Hirakawa, K.; Hirano, T. Singlet Oxygen Generation Photocatalyzed by TiO2 Particles and Its Contribution to Biomolecule Damage. Chem. Lett. 2006, 35, 832–833. [Google Scholar] [CrossRef]
- Wright, C.; Iyer, A.K.V.; Wang, L.; Wu, N.; Yakisich, J.S.; Rojanasakul, Y.; Azad, N. Effects of Titanium Dioxide Nanoparticles on Human Keratinocytes. Drug Chem. Toxicol. 2017, 40, 90–100. [Google Scholar] [CrossRef]
- Yin, J.J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of Nano Titanium Dioxides in HaCaT Keratinocytes-Generation of Reactive Oxygen Species and Cell Damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 119, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Saussey, M. Le Barattage Des Savoirs. Circulations Des Ressources et Apprentissages Des Artisanes Burkinabè Dans Un Contexte de Mondialisation. Rev. d’anthropologie des Connaissances 2011, 5, 551–572. [Google Scholar] [CrossRef]
- Goumbri, B.W.F.; da Silva, T.L.T.; Marini, R.D.; Semdé, R.; Somé, T.I.; Danthine, S. African Shea Butter Properties Related to Common Extraction Technologies: A Review. Food Bioprocess Technol. 2021, 15, 231–248. [Google Scholar] [CrossRef]
- Fernandez, X.; Merck, F.; Kerdudo, A. Conservateurs Pour Cosmétiques-Antioxydants et Anti-UV. Tech. l’Ingénieur 2012, J2285 V1, 1–25. [Google Scholar] [CrossRef]
- Lovett, P. The Shea Butter Value Chain: Production, Transformation and Marketing in West Africa; USAID: Washington, DC, USA, 2004. [Google Scholar]
- Megnanou, R.-M.; Zoue, L.T.; Niamke, S. Marketed and Original Shea Butters of Côte d’Ivoire: Physicochemical and Biochemical Characterization and Evaluation of the Potential Utilizations. Sustain. Agric. Res. 2014, 3, 50. [Google Scholar] [CrossRef]
- Nahm, H.S.; Juliani, H.R.; Simon, J.E. Quality Characteristics of Shea Butter, Vitellaria Paradoxa. In African Natural Plant Products Volume II: Discoveries and Challenges in Chemistry, Health, and Nutrition; American Chemical Society: Washington, DC, USA, 2013; pp. 167–184. ISBN 9780841228047. [Google Scholar]
- Israel, M.O. Effects of Topical and Dietary Use of Shea Butter on Animals. Am. J. Life Sci. 2014, 2, 303. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z.; Garti, N. Phenolic Constituents of Shea (Vitellaria Paradoxa) Kernels. J. Agric. Food Chem. 2003, 51, 6268–6273. [Google Scholar] [CrossRef]
- Alander, J. Shea Butter a Multifunctional Ingredient for Food and Cosmetics. Lipid Technol. 2004, 16, 202–205. [Google Scholar]
- Packer, L.; Valacchi, G. Antioxidants and the Response of Skin to Oxidative Stress: Vitamin E as a Key Indicator. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Padda, M.S.; Picha, D.H. Antioxidant Activity and Phenolic Composition in “beauregard” Sweetpotato Are Affected by Root Size and Leaf Age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of Carotenoids and Vitamin E in Selected Oilseeds, Press Cakes and Oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- Goumbri, B.W.F.; Kouassi, A.K.; Djang’eing’a, R.M.; Semdé, R.; Mouithys-Mickalad, A.; Sakira, A.K.; Josias Yaméogo, G.B.; Somé, T.I.; Danthine, S. Quality Characteristics and Thermal Behavior Diversity of Traditional Crude Shea (Vitellaria paradoxa Gaertn) Butter from Burkina Faso. Food Biophys. 2024, 19, 609–626. [Google Scholar] [CrossRef]
- McKim, J.M.; Keller, D.J.; Gorski, J.R. An in Vitro Method for Detecting Chemical Sensitization Using Human Reconstructed Skin Models and Its Applicability to Cosmetic, Pharmaceutical, and Medical Device Safety Testing. Cutan. Ocul. Toxicol. 2012, 31, 292–305. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.F.; Zhou, Z.Q. Phenolic and Flavonoid Contents of Mandarin (Citrus Reticulata Blanco) Fruit Tissues and Their Antioxidant Capacity as Evaluated by DPPH and ABTS Methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Honfo, F.; Akissoe, N.; Linnemann, A.R.; Soumanou, M.; Van Boekel, M.A.J.S. Nutritional Composition of Shea Products and Chemical Properties of Shea Butter: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 673–686. [Google Scholar] [CrossRef]
- Nahm, H.S. Quality Characteristics of West African Shea Butter (Vitellaria Paradoxa) and Approaches to Extend Shelf-Life; Graduate School-New Brunswick Rutgers: New Brunswick, NJ, USA, 2011; Volume 2. [Google Scholar]
- Allal, F.; Piombo, G.; Kelly, B.A.; Okullo, J.B.L.; Thiam, M.; Diallo, O.B.; Nyarko, G.; Davrieux, F.; Lovett, P.N.; Bouvet, J.M. Fatty Acid and Tocopherol Patterns of Variation within the Natural Range of the Shea Tree (Vitellaria Paradoxa). Agrofor. Syst. 2013, 87, 1065–1082. [Google Scholar] [CrossRef]
- Moloney, F.J.; Collins, S.; Murphy, G.M. Sunscreens: Safety, Efficacy and Appropriate Use. Am. J. Clin. Dermatol. 2002, 3, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Konaka, R.; Kasahara, E.; Dunlap, W.C.; Yamamoto, Y.; Chien, K.C.; Inoue, M. Irradiation of Titanium Dioxide Generates Both Singlet Oxygen and Superoxide Anion. Free Radic. Biol. Med. 1999, 27, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef] [PubMed]
- Thioune, O.; Koueimel Fall, A.B.; Dieng, S.; Diop, M. Focus on the Use of Shea Butter as Excipient For Ointment. Am. J. PharmTech Res. 2019, 9, 254–266. [Google Scholar] [CrossRef]
- Akihisa, T.; Kojima, N.; Kikuchi, T.; Yasukawa, K.; Tokuda, H.; Masters, E.T.; Manosroi, A.; Manosroi, J. Anti-Inflammatory and Chemopreventive Effects of Triterpene Cinnamates and Acetates from Shea Fat. J. Oleo Sci. 2010, 59, 273–280. [Google Scholar] [CrossRef]
- Akihisa, T.; Kojima, N.; Katoh, N.; Kikuchi, T.; Fukatsu, M.; Shimizu, N.; Masters, E.T. Triacylglycerol and Triterpene Ester Composition of Shea Nuts from Seven African Countries. J. Oleo Sci. 2011, 60, 385–391. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Butyrospermum Parkii (Shea)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2024, 43, 82S–95S. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv. Pharmacol. Sci. 2018, 2018, 5341487. [Google Scholar] [CrossRef]
- Gilaberte, Y.; González, S. Update on Photoprotection. Actas Dermosifiliogr. 2010, 101, 659–672. [Google Scholar] [CrossRef]
- de José, M.T.A.F.; Pedrita, A.S.; Emanuella, C.V.P.; de Raimundo, G.O.J.; Fabrício, S.S.; da Jackson, R.G.S.A.; Larissa, A.R.; Xirley, P.N.; da Edigênia, C.C.A. Flavonoids as Photoprotective Agents: A Systematic Review. J. Med. Plants Res. 2016, 10, 848–864. [Google Scholar] [CrossRef]
- Ghazi, S. Do the Polyphenolic Compounds from Natural Products Can Protect the Skin from Ultraviolet Rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural Products as Photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.D.; O’Donoghue, M.N. Update on Photoprotection. Dermatol. Ther. 2007, 20, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Kim, Y.M.; Chin, Y.W.; Chae, S. Protective Effects of Compounds from Garcinia mangostana L. (Mangosteen) against UVB Damage in HaCaT Cells and Hairless Mice. Int. J. Mol. Med. 2017, 40, 1941–1949. [Google Scholar] [CrossRef][Green Version]
- Cui, X.; Ma, Y.; Wang, H.; Huang, J.; Li, L.; Tang, J.; Cheng, B. The Anti-Photoaging Effects of Pre- and Post-Treatment of Platelet-Rich Plasma on UVB-Damaged HaCaT Keratinocytes. Photochem. Photobiol. 2021, 97, 589–599. [Google Scholar] [CrossRef]
- Etsè, K.S.; Etsè, K.D.; Nyssen, P.; Mouithys-Mickalad, A. Assessment of Anti-Inflammatory-like, Antioxidant Activities and Molecular Docking of Three Alkynyl-Substituted 3-Ylidene-Dihydrobenzo[d]Isothiazole 1,1-Dioxide Derivatives. Chem. Biol. Interact. 2021, 344, 109513. [Google Scholar] [CrossRef]
- Degotte, G.; Frederich, M.; Francotte, P.; Franck, T.; Colson, T.; Serteyn, D.; Mouithys-Mickalad, A. Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules 2023, 28, 4516. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Deby-Dupont, G.; Deby, C.; Mouithys-Mickalad, A.; Hoebeke, M.; Mathy-Hartert, M.; Jadoul, L.; Vandenberghe, A.; Lamy, M. The Antibiotic Ceftazidime Is a Singlet Oxygen Quencher as Demonstrated by Ultra-Weak Chemiluminescence and by Inhibition of AAP Consumption. Biochim. Biophys. Acta Gen. Subj. 1998, 1379, 61–68. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB Damage Onset and Progression 24 h Post Exposure in Human-Derived Skin Cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Lan, F.; Liu, W.; Yang, X.; Zeng, F.; Xu, H. Nano Titanium Dioxide Induces the Generation of ROS and Potential Damage in HaCaT Cells under UVA Irradiation. J. Nanosci. Nanotechnol. 2010, 10, 8500–8507. [Google Scholar] [CrossRef]
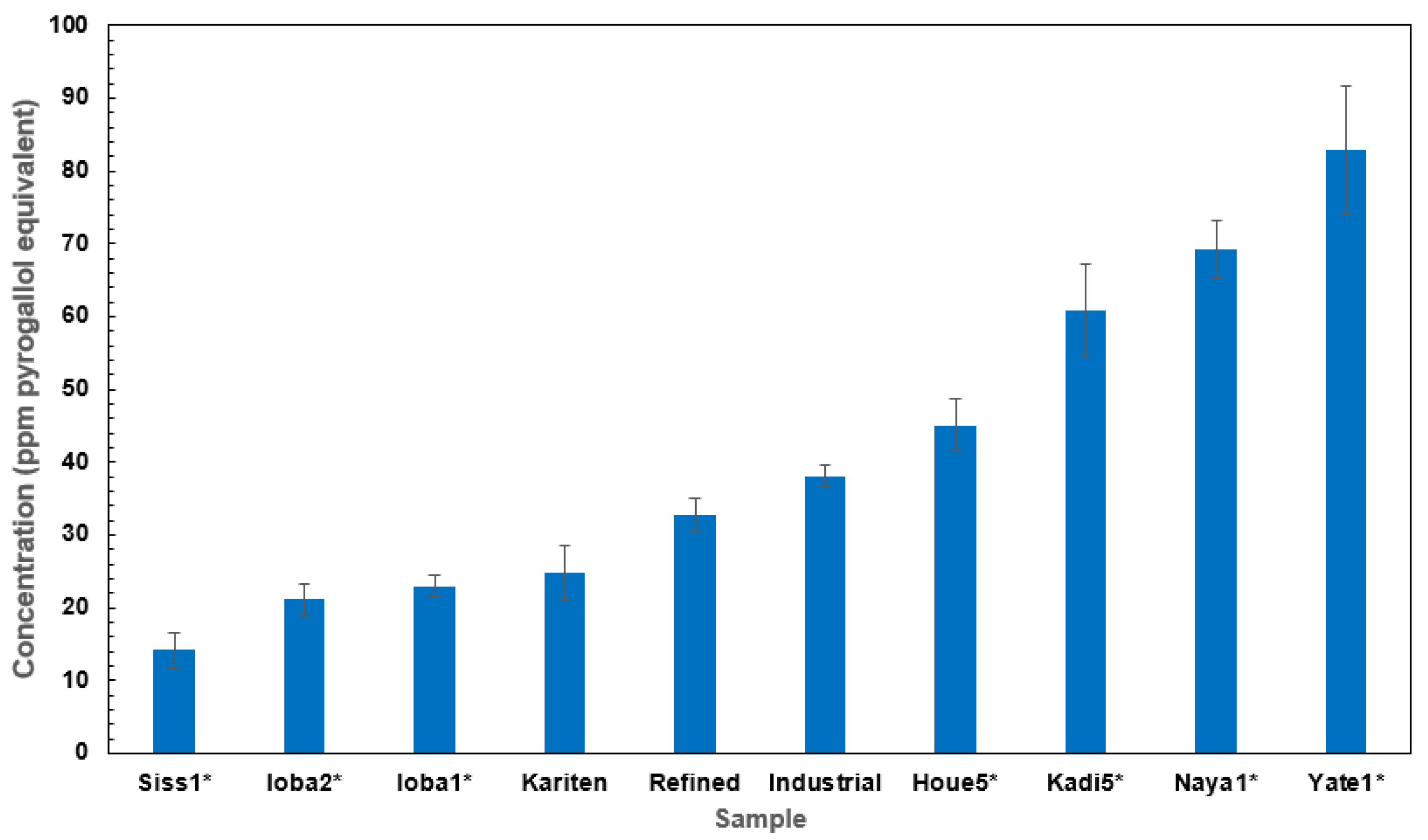
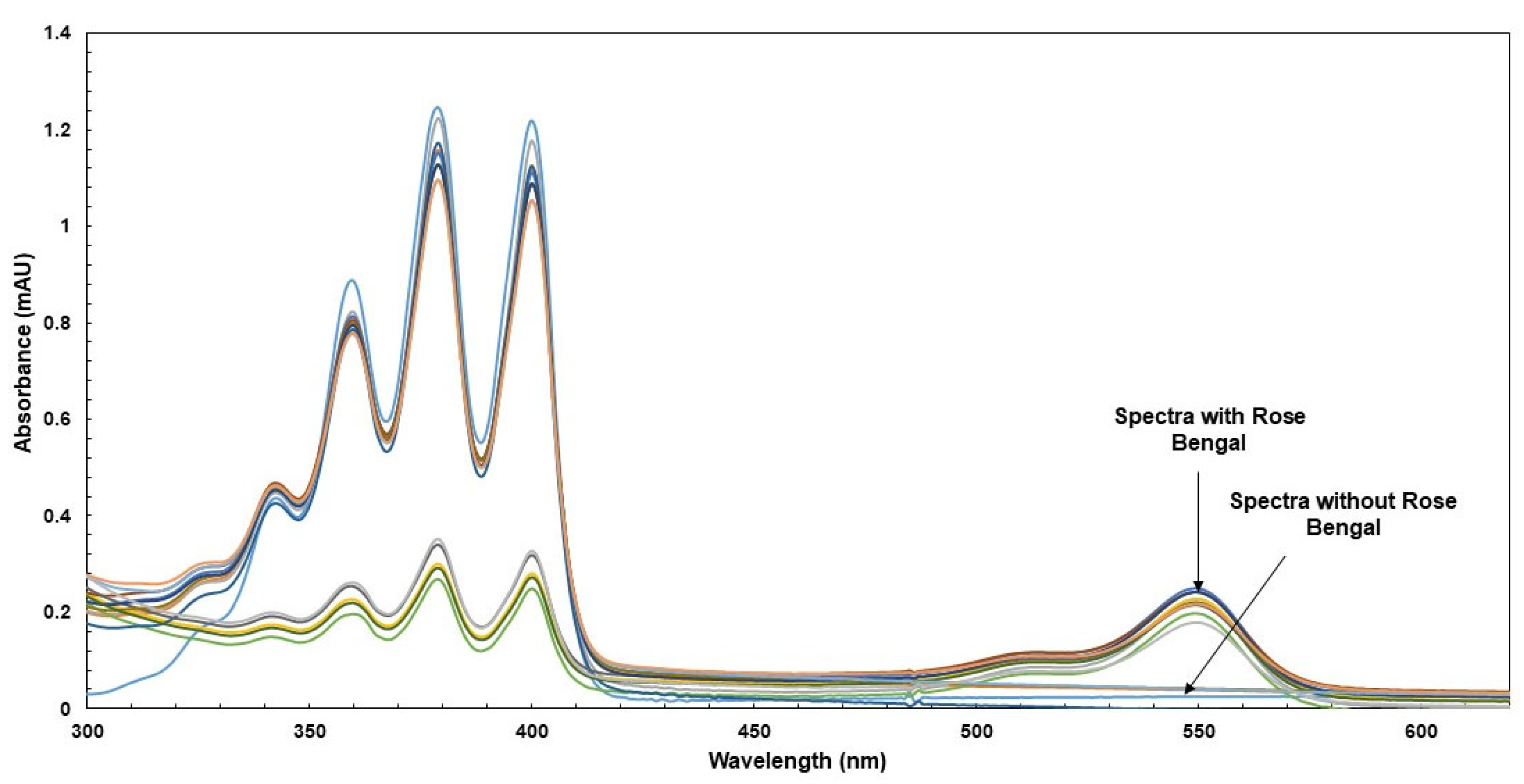
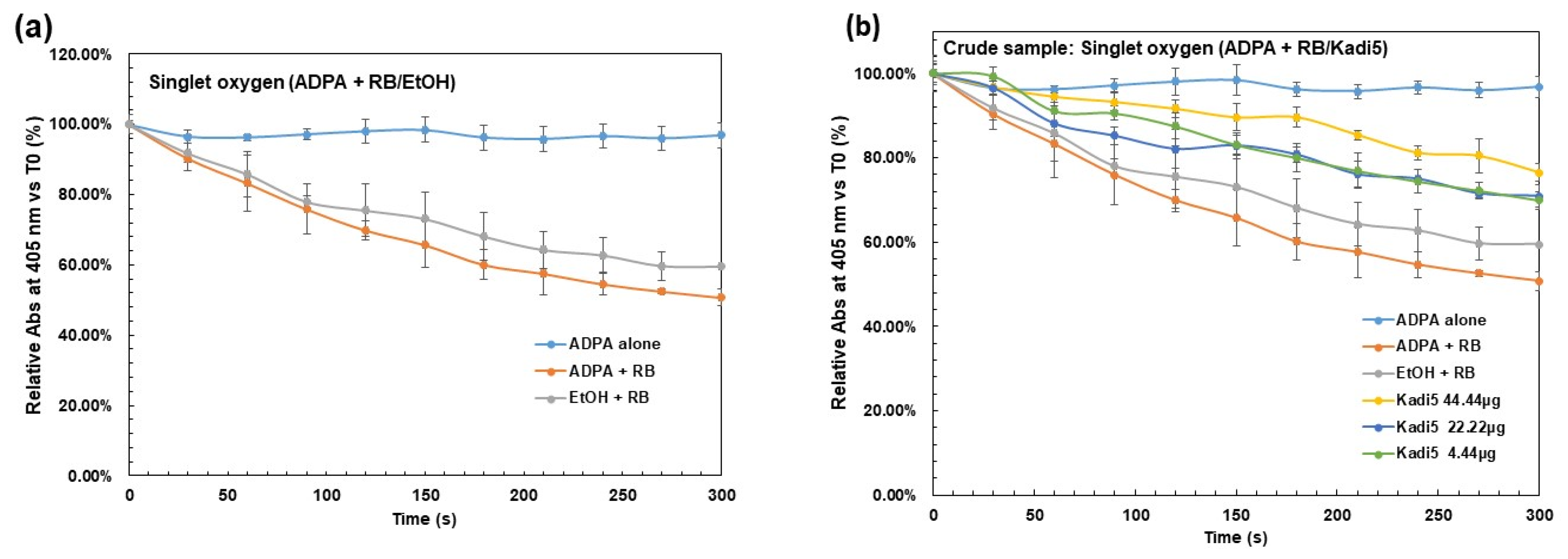
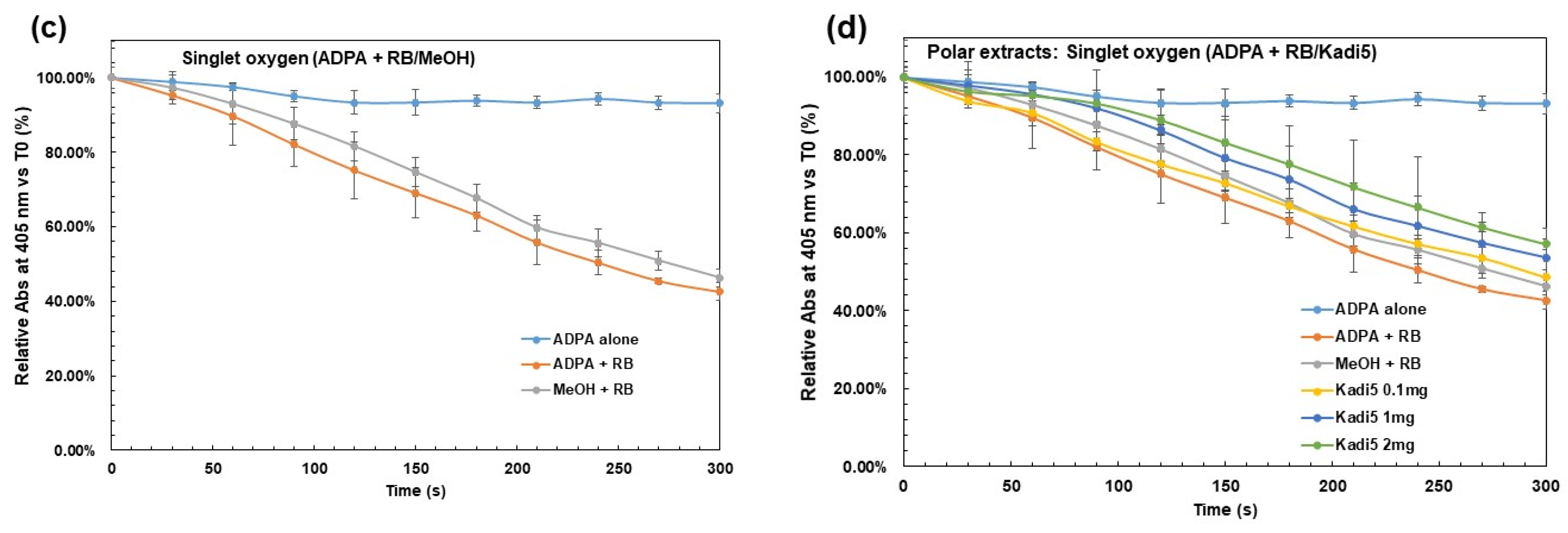
| Sample | Concentration (mg/mL) | DPPH: I (%) at 60 min | ABTS: I (%) at 60 min |
|---|---|---|---|
| Crude Industrial | 0.05 | 5.07 ± 0.17 | 7.85 ± 0.31 |
| Crude Industrial | 0.5 | 3.85 ± 0.12 | 13.60 ± 0.32 |
| Crude Industrial | 1 | 6.82 ± 0.21 | 21.15 ± 0.46 |
| Crude Industrial | 2 | 15.44 ± 0.28 | 27.29 ± 0.49 |
| Crude Industrial | 3 | - | 82.01 ± 0.15 |
| Refined Industrial | 0.05 | 4.40 ± 1.06 | 10.42 ± 0.96 |
| Refined Industrial | 0.5 | 7.17 ± 0.26 | 8.16 ± 0.50 |
| Refined Industrial | 1 | 5.24 ± 0.06 | 9.82 ± 0.46 |
| Refined Industrial | 2 | 10.84 ± 0.72 | 5.18 ± 0.44 |
| Refined Industrial | 3 | - | 56.12 ± 0.75 |
| Kariten | 0.05 | 8.39 ± 1.18 | 6.19 ± 1.22 |
| Kariten | 0.5 | 4.55 ± 0.56 | 7.85 ± 0.61 |
| Kariten | 1 | 7.34 ± 0.49 | 16.01 ± 0.40 |
| Kariten | 2 | 25.13 ± 0.71 | 39.02 ± 0.14 |
| Kariten | 3 | - | 81.29 ± 0.25 |
| Houe5 | 0.05 | 2.62 ± 1.17 | 2.11 ± 0.98 |
| Houe5 | 0.5 | 2.80 ± 0.21 | 3.63 ± 0.29 |
| Houe5 | 1 | 6.64 ± 0.26 | 11.33 ± 0.35 |
| Houe5 | 2 | 17.86 ± 0.92 | 27.59 ± 0.21 |
| Houe5 | 3 | - | 73.81 ± 0.15 |
| Ioba1 | 0.05 | 5.24 ± 1.54 | 2.11 ± 0.60 |
| Ioba1 | 0.5 | 3.67 ± 0.40 | 4.68 ± 0.61 |
| Ioba1 | 1 | 6.47 ± 0.74 | 7.10 ± 0.79 |
| Ioba1 | 2 | 6.28 ± 0.85 | 12.35 ± 0.25 |
| Ioba1 | 3 | - | 66.33 ± 0.26 |
| Ioba2 | 0.05 | 0.70 ± 0.25 | 1.21 ± 0.36 |
| Ioba2 | 0.5 | 3.67 ± 0.67 | 4.23 ± 0.90 |
| Ioba2 | 1 | 11.19 ± 1.46 | 12.08 ± 1.35 |
| Ioba2 | 2 | 15.17 ± 0.21 | 16.46 ± 0.34 |
| Ioba2 | 3 | - | 55.54 ± 0.30 |
| Kadi5 | 0.05 | 4.55 ± 0.10 | 11.78 ± 1.11 |
| Kadi5 | 0.5 | 12.06 ± 0.47 | 19.64 ± 0.57 |
| Kadi5 | 1 | 19.06 ± 0.38 | 35.05 ± 1.32 |
| Kadi5 | 2 | 26.48 ± 0.49 | 46.8 ± 0.56 |
| Kadi5 | 3 | - | 69.21 ± 0.21 |
| Naya1 | 0.05 | 7.34 ± 0.58 | 21.00 ± 2.72 |
| Naya1 | 0.5 | 13.99 ± 0.36 | 28.55 ± 1.01 |
| Naya1 | 1 | 21.68 ± 0.25 | 44.71 ± 0.75 |
| Naya1 | 2 | 35.64 ± 1.06 | 67.99 ± 0.31 |
| Naya1 | 3 | - | 82.88 ± 0.06 |
| Siss1 | 0.05 | 8.92 ± 1.81 | 5.14 ± 0.81 |
| Siss1 | 0.5 | 4.90 ± 0.70 | 3.32 ± 0.55 |
| Siss1 | 1 | 4.20 ± 0.76 | 4.68 ± 1.10 |
| Siss1 | 2 | 5.48 ± 0.78 | 2.44 ± 0.29 |
| Siss1 | 3 | - | 45.18 ± 0.56 |
| Yate1 | 0.05 | 0.35 ± 0.20 | 2.72 ± 0.40 |
| Yate1 | 0.5 | 3.32 ± 0.25 | 4.53 ± 0.49 |
| Yate1 | 1 | 6.82 ± 0.06 | 13.90 ± 0.35 |
| Yate1 | 2 | 17.06 ± 0.42 | 32.16 ± 0.32 |
| Yate1 | 3 | 69.21 ± 0.12 |
| DPPH * | ABTS * | TPC | |
|---|---|---|---|
| DPPH | 1 | 0.924 | 0.508 |
| ABTS | 0.924 | 1 | 0.642 |
| TPC | 0.508 | 0.642 | 1 |
| Sample | Polar Extracts | Crude Sample | ||
|---|---|---|---|---|
| Concentration (ppm) | Relative Abs at 300 s (%) | Concentration (µg/mL) | Relative Abs at 300 s (%) | |
| ADPA Alone Ctrl (ADPA + RB) Ctrl (MeOH) | 93.22 ± 2.56 | ADPA Alone | 96.83 ± 2.56 | |
| 42.59 ± 2.31 | Ctrl (ADPA + RB) | 50.75 ± 2.31 | ||
| 46.27 ± 2.27 | Ctrl (EtOH) | 59.48 ± 1.06 | ||
| Houe5 | 0.010 | 50.82 ± 1.11 | 4.44 | 67.01 ± 2.49 |
| Houe5 | 0.100 | 53.54 ± 2.07 | 22.22 | 70.98 ± 1.06 |
| Houe5 | 0.201 | 55.39 ± 0.23 | 44.44 | 76.02 ± 2.25 |
| Ioba1 | 0.005 | 50.13 ± 2.77 | 4.44 | 66.23 ± 1.01 |
| Ioba1 | 0.051 | 49.00 ± 4.98 | 22.22 | 73.23 ± 2.1 |
| Ioba1 | 0.102 | 50.34 ± 1.91 | 44.44 | 78.13 ± 1.04 ° |
| Ioba2 | 0.005 | 48.43 ± 1.05 | 4.44 | 67.07 ± 1.05 |
| Ioba2 | 0.047 | 51.79 ± 1.86 | 22.22 | 71.03 ± 2.80 |
| Ioba2 | 0.094 | 57.59 ± 2.61 *** | 44.44 | 74.00 ± 3.01 |
| Kadi5 | 0.013 | 48.60 ± 1.90 | 4.44 | 69.84 ± 2.1 |
| Kadi5 | 0.135 | 53.57 ± 7.70 | 22.22 | 70.90 ± 2.64 |
| Kadi5 | 0.270 | 57.15 ± 1.35 ** | 44.44 | 76.46 ± 2.02 |
| Naya1 | 0.015 | 49.19 ± 6.60 | 4.44 | 68.28 ± 3.07 |
| Naya1 | 0.154 | 49.50 ± 7.60 | 22.22 | 74.26 ± 2.07 |
| Naya1 | 0.308 | 55.79 ± 3.84 | 44.44 | 79.30 ± 0.62 °° |
| Siss1 | 0.003 | 50.73 ± 3.06 | 4.44 | 69.72 ± 1.01 |
| Siss1 | 0.031 | 53.37 ± 0.02 | 22.22 | 70.94 ± 2.04 |
| Siss1 | 0.063 | 53.76 ± 0.42 | 44.44 | 70.64 ± 0.02 |
| Yate1 | 0.018 | 53.47 ± 0.48 | 4.44 | 64.50 ± 0.60 |
| Yate1 | 0.184 | 55.15 ± 0.49 | 22.22 | 71.60 ± 0.93 |
| Yate1 | 0.369 | 56.48 ± 2.27 * | 44.44 | 80.32 ± 0.92 °°° |
| Crude Industrial | 0.008 | 48.73 ± 8.37 | 4.44 | 66.60 ± 2.26 |
| Crude Industrial | 0.085 | 50.78 ± 7.45 | 22.22 | 70.41 ± 2.02 |
| Crude Industrial | 0.170 | 55.12 ± 0.86 | 44.44 | 72.79 ± 1.63 |
| Refined Industrial | 0.007 | 53.17 ± 1.64 | 4.44 | 68.02 ± 2.08 |
| Refined Industrial | 0.073 | 53.07 ± 0.17 | 22.22 | 72.42 ± 2.09 |
| Refined Industrial | 0.146 | 54.14 ± 0.65 | 44.44 | 77.13 ± 0.92 |
| Kariten | 0.006 | 46.44 ± 6.33 | 4.44 | 65.75 ± 0.02 |
| Kariten | 0.055 | 50.82 ± 3.40 | 22.22 | 68.80 ± 4.02 |
| Kariten | 0.110 | 54.61 ± 3.42 | 44.44 | 69.94 ± 0.11 |
| TiO2 | - | - | 0.44 | 62.76 ± 0.79 |
| TiO2 | - | - | 4.44 | 62.88 ± 6.53 |
| TiO2 | - | - | 22.22 | 64.98 ± 3.28 |
| Sample | (1) Gallic Acid (ppm GAE) | (2) Protocatechuic Acid (ppm GAE) | (C3) Rt = 19 min (ppm GAE) | (4) Cinnamic Acid (ppm GAE) | (5) Quercetin (ppm GAE) | (C6) Rt = 54 min (ppm GAE) | (C7) Rt = 61 min (ppm GAE) | (C8) Rt = 68 min (ppm GAE) | (C9) Rt = 68 min (ppm GAE) | (C10) Rt = 69 min (ppm GAE) | (C8/C9) | (C8/C10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Houe5 | nd | <LOQ | 0.928 | 9.438 | nd | 0.165 | <LOQ | 43.718 | 40.587 | 92.709 | 1.1 | 0.5 |
| Ioba1 | nd | <LOQ | nd | 1.134 | nd | 0.015 | <LOQ | 27.046 | 23.646 | 66.189 | 1.1 | 0.4 |
| Ioba2 | nd | nd | nd | <LOQ | nd | 1.486 | <LOQ | 5.226 | 4.001 | 13.184 | 1.3 | 0.4 |
| Kadi5 | nd | nd | nd | 0.321 | nd | 1.549 | <LOQ | 40.714 | 35.617 | 82.284 | 1.1 | 0.5 |
| Naya1 | nd | nd | 1.940 | 12.237 | <LOQ | 1.681 | <LOQ | 15.369 | 13.866 | 35.367 | 1.1 | 0.4 |
| Siss1 | nd | nd | <LOQ | 0.972 | nd | nd | <LOQ | 22.174 | 19.995 | 46.600 | 1.1 | 0.5 |
| Yate1 | <LOQ | nd | 0.149 | 2.338 | <LOQ | 2.014 | 5.282 | 59.585 | 62.168 | 119.037 | 1.0 | 0.5 |
| Crude Industrial | nd | nd | 0.132 | 46.261 | nd | 0.495 | nd | 80.292 | 72.885 | 162.707 | 1.1 | 0.5 |
| Refined | nd | nd | nd | nd | nd | 6.400 | <LOQ | 73.018 | 63.263 | 153.764 | 1.2 | 0.5 |
| Kariten | 3.374 | nd | nd | 0.961 | nd | <LOD | <LOQ | 12.088 | 10.104 | 29.941 | 1.2 | 0.4 |
| Sample | Origin |
|---|---|
| Houe5 | Bobo-Dioulasso (Upper-Bassin) * |
| Ioba1 | Dano (South West) * |
| Ioba2 | Dano (South West) * |
| Kadi5 | Ouagadougou (Center) * |
| Naya1 | Toma (Mouhoun Loop) * |
| Siss1 | Boura (Midwest) * |
| Yate1 | Ouahigouya (North) * |
| Crude Industrial | IOI Loders Croklaan, The Netherlands |
| Refined Industrial | IOI Loders Croklaan, The Netherlands |
| Kariten-rich extract | Belgium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goumbri, B.W.F.; Jansen, O.; Djang’eing’a, R.M.; Frederich, M.; Semdé, R.; Somé, T.I.; Danthine, S.; Mouithys-Mickalad, A. Effects of Crude Shea Butters and Their Polar Extracts on Singlet Oxygen Quenching and Against Rose Bengal-Induced HaCaT Cell Phototoxicity. Molecules 2025, 30, 1360. https://doi.org/10.3390/molecules30061360
Goumbri BWF, Jansen O, Djang’eing’a RM, Frederich M, Semdé R, Somé TI, Danthine S, Mouithys-Mickalad A. Effects of Crude Shea Butters and Their Polar Extracts on Singlet Oxygen Quenching and Against Rose Bengal-Induced HaCaT Cell Phototoxicity. Molecules. 2025; 30(6):1360. https://doi.org/10.3390/molecules30061360
Chicago/Turabian StyleGoumbri, Bertrand W. F., Olivia Jansen, Roland Marini Djang’eing’a, Michel Frederich, Rasmané Semdé, Touridomon Issa Somé, Sabine Danthine, and Ange Mouithys-Mickalad. 2025. "Effects of Crude Shea Butters and Their Polar Extracts on Singlet Oxygen Quenching and Against Rose Bengal-Induced HaCaT Cell Phototoxicity" Molecules 30, no. 6: 1360. https://doi.org/10.3390/molecules30061360
APA StyleGoumbri, B. W. F., Jansen, O., Djang’eing’a, R. M., Frederich, M., Semdé, R., Somé, T. I., Danthine, S., & Mouithys-Mickalad, A. (2025). Effects of Crude Shea Butters and Their Polar Extracts on Singlet Oxygen Quenching and Against Rose Bengal-Induced HaCaT Cell Phototoxicity. Molecules, 30(6), 1360. https://doi.org/10.3390/molecules30061360








