Antioxidant Capacity and Thermal Stability of Arthrospira platensis Extract Encapsulated in Starch Sodium Octenyl Succinate with Freeze-, Spray-, and Nanospray-Drying
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation of A. platensis Extract in Starch Sodium Octenyl Succinate
2.2. Characterisation of Encapsulated A. platensis Extract
2.2.1. Morphology of Encapsulated A. platensis Extract
2.2.2. Colour Characteristics of Encapsulated A. platensis Extract
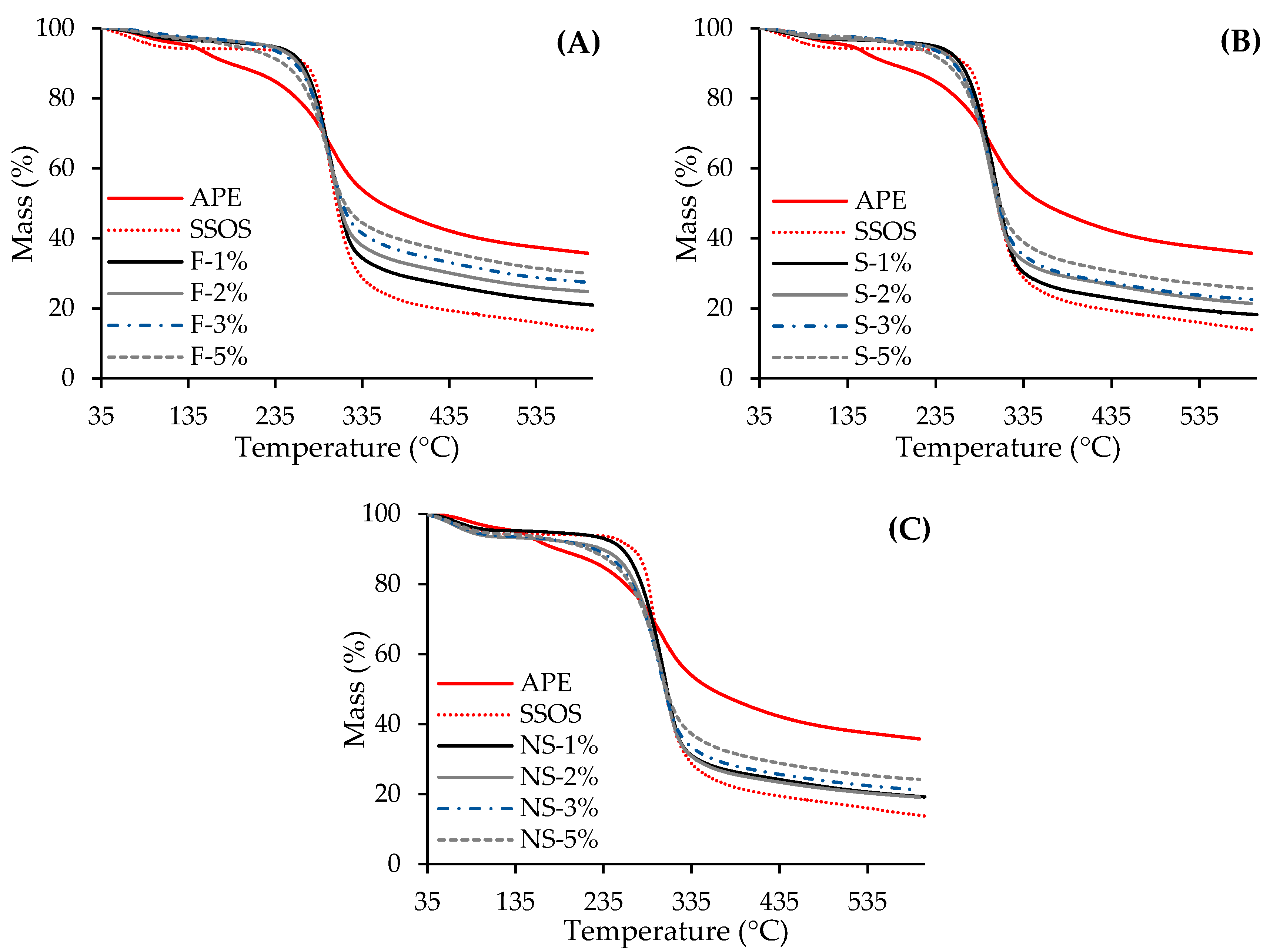
2.2.3. Thermogravimetric (TG) Analysis
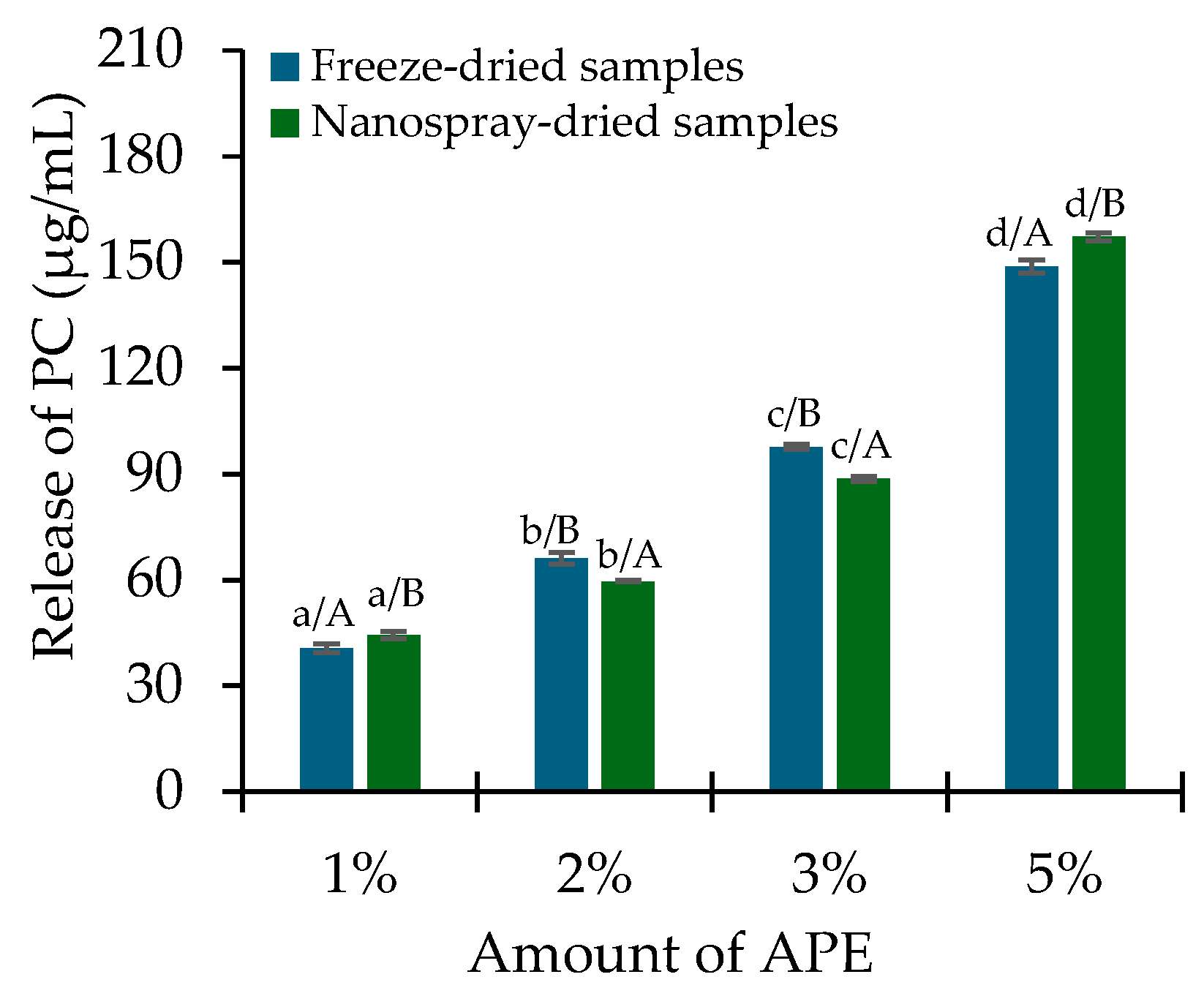
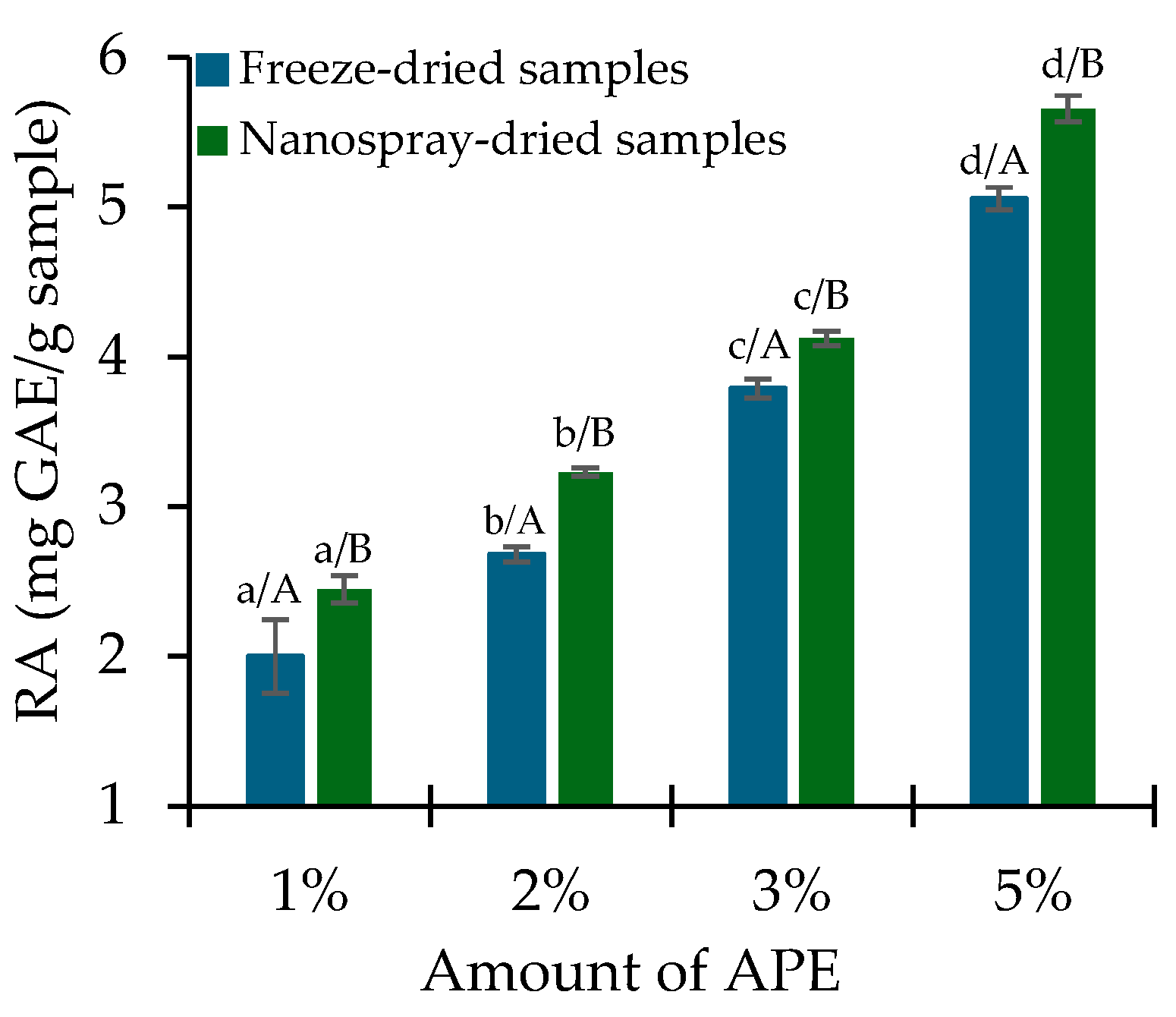
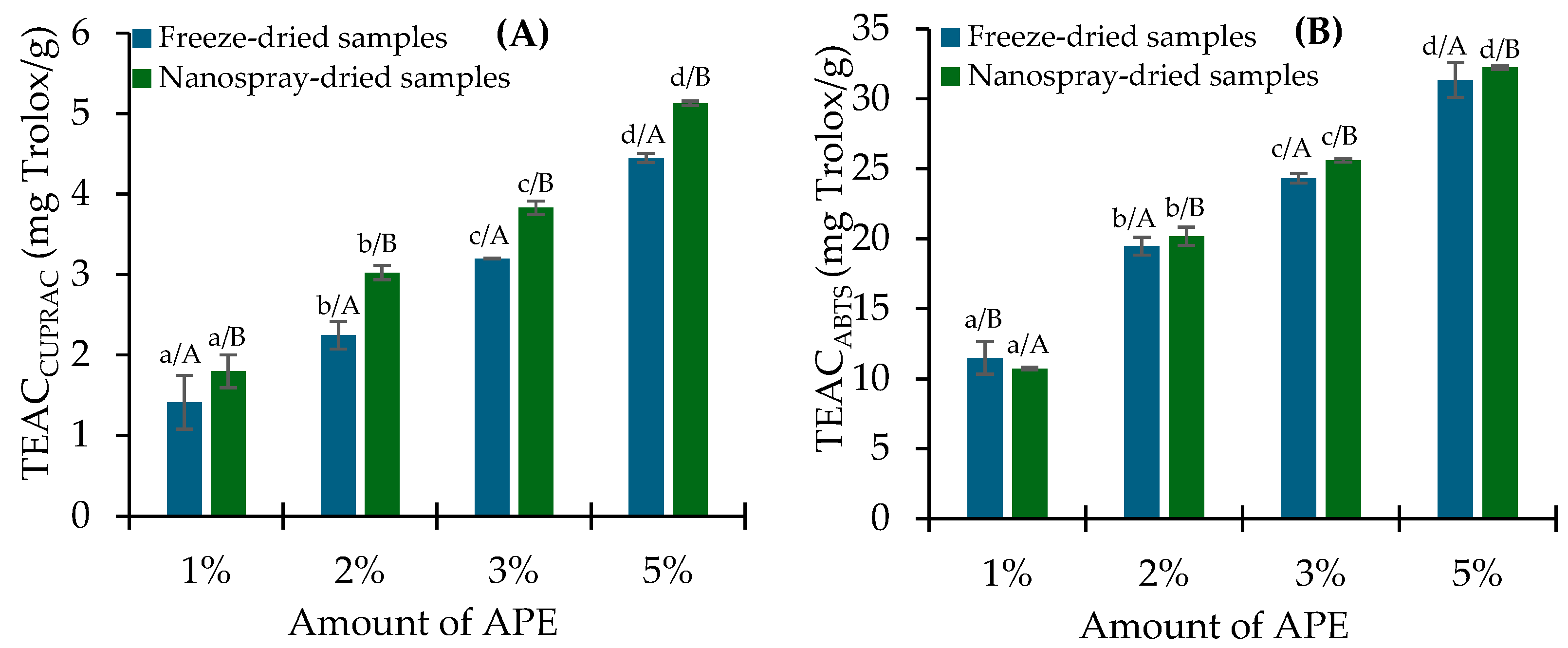
2.3. PC Release and In Vitro Antioxidant Activity Assessment
3. Materials and Methods
3.1. Materials
3.2. Preparation of A. platensis Extract
3.3. Characterisation of the Aqueous A. platensis Extract
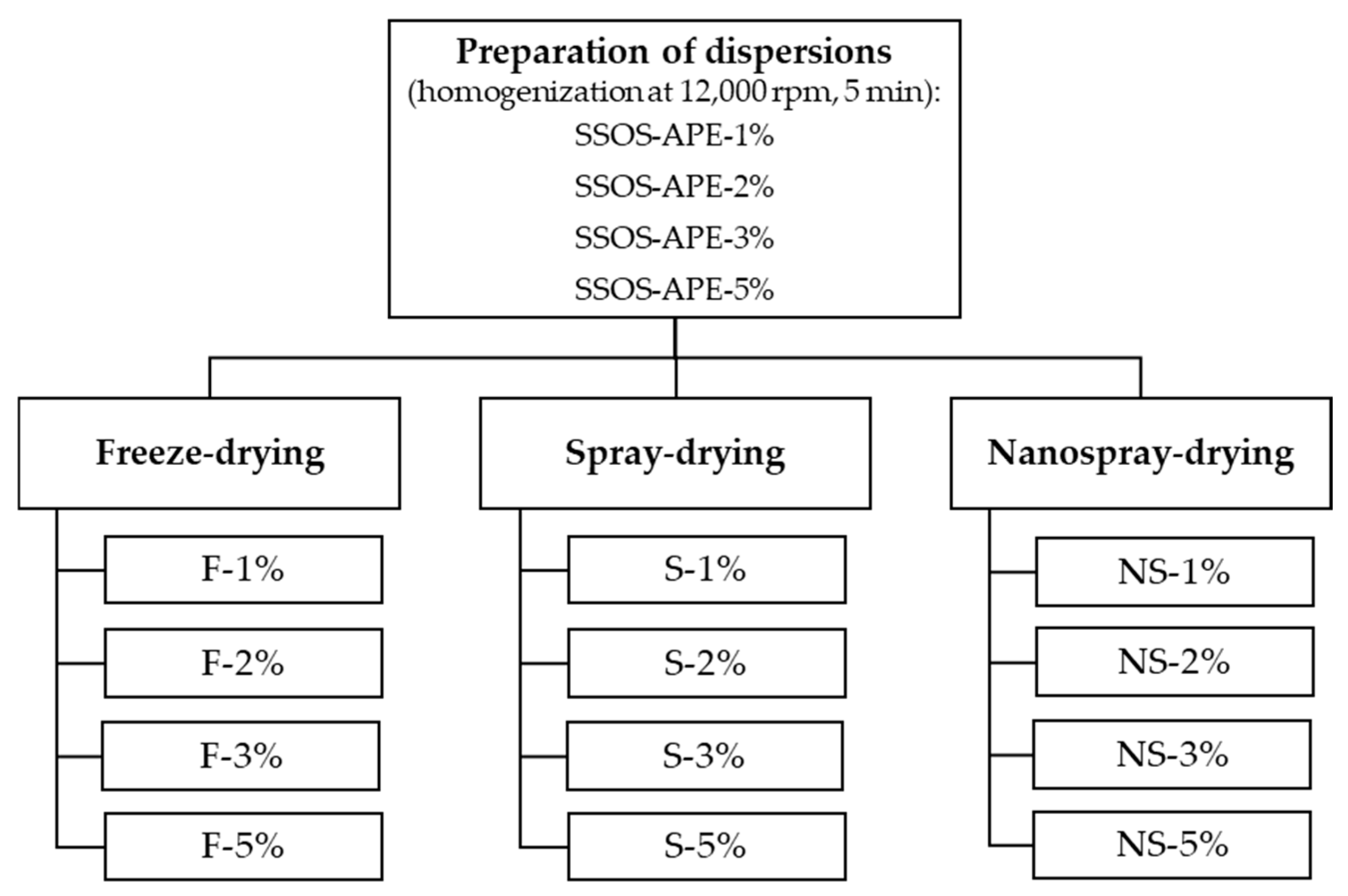
3.4. Preparation of Dispersions Containing A. platensis Extract
3.5. Encapsulation of A. platensis Extract Using Freeze-Drying and Spray-Drying Techniques
3.6. Analyses of Encapsulated A. platensis Extract Powder
3.6.1. SEM Analyses
3.6.2. Colour Measurements
3.6.3. FT-IR Spectroscopic Analysis
3.6.4. Thermogravimetric Analysis
3.6.5. Release Studies in Artificial Saliva
3.6.6. In Vitro Antioxidant Activity Assessment
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PC | Phycocyanin |
| EE | Encapsulation efficiency |
| SSOS | Starch sodium octenyl succinate |
| APE | Arthrospira platensis extract |
| TEAC | Trolox equivalent antioxidant capacity |
| TGA | Thermogravimetric analysis |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
References
- Bortolini, D.G.; Maciel, G.M.; de Andrade Arruda Fernandes, I.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional Properties of Bioactive Compounds from Spirulina spp.: Current Status and Future Trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Venskutonis, P. Algae for the Production of Bio-Based Products. In Biobased Products and Industries; Galanakis, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; p. 243. ISBN 978-0-12-820153-4. [Google Scholar]
- Athiyappan, K.D.; Routray, W.; Paramasivan, B. Phycocyanin from Spirulina: A Comprehensive Review on Cultivation, Extraction, Purification, and Its Application in Food and Allied Industries. Food Humanit. 2024, 2, 100235. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of Phycocyanin—A Natural Blue Colorant from Dried Spirulina Biomass: Influence of Processing Parameters and Extraction Techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Spirulina in Exercise and Sport: A Systematic Review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef] [PubMed]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a Super Functional Ingredient from Algae; Properties, Purification Characterization, and Applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef]
- Naeini, F.; Zarezadeh, M.; Mohiti, S.; Tutunchi, H.; Ebrahimi Mamaghani, M.; Ostadrahimi, A. Spirulina Supplementation as an Adjuvant Therapy in Enhancement of Antioxidant Capacity: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Int. J. Clin. Pract. 2021, 75, e14618. [Google Scholar] [CrossRef]
- Kulkarni, R.; Kalaskar, A.; Kalaskar, R.; Gupta, N. Efficacy of spirulina in management of oral submucous fibrosis—A systematic review. J. Oral. Med. Oral. Surg. 2022, 28, 8. [Google Scholar] [CrossRef]
- Maniyar, R.; Umashankar, G.K. Effectivenes of spirulina mouthwash on the reduction of dental plaque and gingivitis: A clinical study. Int. J. Pharm. Pharm. Sci. 2017, 9, 136–139. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Muthu, J.; John, L.; Romanos, G.E. Clinical Effects of Subgingivally Delivered Spirulina Gel in Chronic Periodontitis Cases: A Placebo Controlled Clinical Trial. J. Clin. Diagn. Res. 2013, 7, 2330–2333. [Google Scholar] [CrossRef]
- Kaipa, V.R.K.; Asif, S.M.; Assiri, K.I.; Saquib, S.A.; Arem, S.A.; Sree, S.; Yassin, S.M.; Ibrahim, M.; Shariff, M.; Shamsudeen, S.M.; et al. Antioxidant Effect of Spirulina in Chronic Periodontitis. Medicine 2022, 101, e31521. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Moon, J.-H.; Park, S.C.; Jang, Y.P.; Choung, S.Y. Spirulina Maxima Reduces Inflammation and Alveolar Bone Loss in Porphyromonas Gingivalis-Induced Periodontitis. Phytomedicine 2021, 81, 153420. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A Review of Recent Strategies to Improve the Physical Stability of Phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liang, Z.-P.; Chang, X.-Y.; Li, F.-T.; Wang, X.-Q.; Lian, X.-J. Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Moreira, A.F.; Miguel, S.P.; Coutinho, P. Recent Advances in Microalgae Encapsulation Techniques for Biomedical Applications. Adv. Colloid Interface Sci. 2024, 333, 103297. [Google Scholar] [CrossRef]
- ElFar, O.A.; Billa, N.; Lim, H.R.; Chew, K.W.; Cheah, W.Y.; Munawaroh, H.S.H.; Balakrishnan, D.; Show, P.L. Advances in Delivery Methods of Arthrospira platensis (Spirulina) for Enhanced Therapeutic Outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles. Polim. Med. 2022, 52, 101–111. [Google Scholar] [CrossRef]
- Demarco, M.; Moraes, J.O.D.; Ferrari, M.C.; Neves, F.D.F.; Laurindo, J.B.; Tribuzi, G. Production of Spirulina (Arthrospira platensis) powder byinnovative and traditional drying techniques. J. Food. Process. Eng. 2022, 45, e13919. [Google Scholar] [CrossRef]
- Minić, S.; Gligorijević, N.; Veličković, L.; Nikolić, M. Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins. Int. J. Mol. Sci. 2024, 25, 7187. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and Characterization of C-Phycocyanin Coated with STMP/STPP Cross-Linked Starches from Different Botanical Sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Enhanced Microencapsulation of C-Phycocyanin from Arthrospira by Freeze-Drying with Different Wall Materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef]
- İlter, I.; Koç, M.; Demirel, Z.; Conk Dalay, M.; Kaymak Ertekin, F. Improving the Stability of Phycocyanin by Spray Dried Microencapsulation. J. Food Process. Preserv. 2021, 45, e15646. [Google Scholar] [CrossRef]
- Faieta, M.; Corradini, M.G.; Di Michele, A.; Ludescher, R.D.; Pittia, P. Effect of Encapsulation Process on Technological Functionality and Stability of Spirulina Platensis Extract. Food Biophys. 2020, 15, 50–63. [Google Scholar] [CrossRef]
- Apostolidis, E.; Stoforos, N.G.; Mandala, I. Starch physical treatment, emulsion formation, stability, and their applications. Carbohydr. Polym. 2023, 305, 120554. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of Phycocyanin Extracted from Spirulina sp.: Influence of Temperature, pH and Preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Athiyappan, K.D.; Chaudhuri, R.; Paramasivan, B. Enhancing Phycocyanin Stability through Encapsulation: Optimization of Maltodextrin and Sodium Alginate Ratios. J. Food Meas. Charact. 2025, 19, 1112–1123. [Google Scholar] [CrossRef]
- Soliman, T.N.; Negm El-Dein, A.; Abd Al-Daim, S.; Allayeh, A.; Awad, H.; Flefil, N.S. Characterization of C-Phycocyanin Antioxidant, Anti-Inflammatory, Anti-Tumour, and Anti-HCoV-229E Activities and Encapsulation for Implementation in an Innovative Functional Yogurt. Heliyon 2024, 10, e31642. [Google Scholar] [CrossRef]
- Seyed Yagoubi, A.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, Characterization and Evaluation of Physicochemical Properties of Phycocyanin-Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Nair, M.S.; Rajarathinam, R.; Velmurugan, S.; Devakumar, J.; Karthikayan, J.; Saravanakumar, S.P. Phycocyanin-Conjugated Chitosan-Coated Iron Oxide Nanoparticles for the Separation of Escherichia Coli Cells. Biomass Convers. Biorefinery 2023, 1–12. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of Octenylsuccinylation on Physicochemical, Thermal, Morphological and Stability of Octenyl Succinic Anhydride (OSA) Modified Sago Starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Dolores, F.-P.M.; Consuelo, C.-M.A.; Rafael, C.-R.; Rita, P.-S.; Luisa, M.-L.A.; Elizabeth, C.-M.; Dolores, F.-P.M.; Consuelo, C.-M.A.; Rafael, C.-R.; Rita, P.-S.; et al. Influence of Arabinoxylan and Crosslinked Arabinoxylan Consumption on Blood Serum Lipids and Glucose Levels of Wistar Rats. AIMS Bioeng. 2021, 8, 208–220. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Bougatef, H.; Fatma Hadrich, F.; Gazbar, M.; Sila, A.; Chamkha, M.; Bougatef, A. Development of a novel method for the extraction of phycocyanin pigment from Spirulina platensis and assessment of its antioxidant, antimicrobial, and anticancer activities. Biomass Convers. Biorefin. 2025, 15, 8001–8013. [Google Scholar] [CrossRef]
- İlter, I.; Akyil, S.; Demirel, M.; Koç, Z.; Conk Dalay, M.; Kaymak Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Holden, G.; Wang, B.; Adhikari, B. Maillard Reaction-Based Conjugation of Spirulina Protein with Maltodextrin Using Wet-Heating Route and Characterisation of Conjugates. Food Chem. 2023, 406, 134931. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Suzery, M.; Sutanto, H.; Nilamsari, A.M.; Yunanda, A. Effects of Carrageenan and Chitosan as Coating Materials on the Thermal Degradation of Microencapsulated Phycocyanin from Spirulina sp. Int. J. Food Eng. 2019, 15, 20180290. [Google Scholar] [CrossRef]
- Shateri, F.; Rahaie, M.; Jalili, H. Chemical, Functional and Therapeutic Properties of Encapsulated Black Cumin Extract in Spirulina Platensis. J. Food Process. Preserv. 2022, 46, e16956. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Xiong, H.; Zhao, Q. Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira Platensis. Foods 2022, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Romero, F.; Suárez, L.; Ríos, R.; Alemán, M.; Venuleo, M.; Ortega, Z. Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding. Polymers 2024, 16, 1807. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and Anti-Inflammatory Properties of C-Phycocyanin from Blue-Green Algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef]
- Stunda-Zujeva, A.; Berele, M.; Lece, A.; Šķesters, A. Comparison of Antioxidant Activity in Various Spirulina Containing Products and Factors Affecting It. Sci. Rep. 2023, 13, 4529. [Google Scholar] [CrossRef]
- Shang, J.; Liu, H.; Zheng, Y.; Zhang, Z. Role of Oxidative Stress in the Relationship between Periodontitis and Systemic Diseases. Front. Physiol. 2023, 14, 1210449. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-Inflammatory Effect and Mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaite, J.; Ramanauskien, K.; Karosiene, J.; Majien, D.; Basinskiene, L.; Venskutonis, P. Ultrasound-Assisted Extraction and Assessment of Biological Activity of Phycobiliprotein-Rich Aqueous Extracts from Wild Cyanobacteria (Aphanizomenon Flos-Aquae). J. Agric. Food Chem. 2020, 68, 1896–1909. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A New Method for Rapid Determination of Carbohydrate and Total Carbon Concentrations Using UV Spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaite, J.; Paskauskas, R.; Basinskiene, L.; Venskutonis, P. Recovery of Lipophilic Products from Wild Cyanobacteria (Aphanizomenon Flos-Aquae) Isolated from the Curonian Lagoon by Means of Supercritical Carbon Dioxide Extraction. Algal. Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Ding, S.; Ye, M.; Jiang, L.; Wang, R. Changes in Free Amino Acids of Fermented Minced Peppers during Natural and Inoculated Fermentation Process Based on HPLC-MS/MS. J. Food Sci. 2020, 85, 2803–2811. [Google Scholar] [CrossRef]
- Kuley, E.; Uslu, L.; Durmus, M.; Sakarya, Y.; Özyurt, G. Enhancement of Spirulina platensis Bioactivity by Probiotic Fer-mentation and Encapsulation by Spray-Drying. Int. J. Food Sci. Technol. 2023, 58, 6015–6024. [Google Scholar] [CrossRef]
- Pistone, S.; Goycoolea, F.M.; Young, A.; Smistad, G.; Hiorth, M. Formulation of Polysaccharide-Based Nanoparticles for Local Administration into the Oral Cavity. Eur. J. Pharm. Sci. 2017, 96, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Subbarayadu, K.; Kitryte, V.; Venskutonis, P. High-Pressure Extraction of Antioxidant-Rich Fractions from Shrubby Cinquefoil (Dasiphora fruticose L. Rydb.) Leaves: Process Optimization and Extract Characterization. Antioxidants 2020, 9, 457. [Google Scholar] [CrossRef]
- Bytautaitė, M.; Kitrytė-Syrpa, V.; Jonutė, P.; Endriulaitytė, U.; Petrikaitė, V.; Syrpas, M. Short-Chain Fructooligosaccharides Synthesis Using a Commercial Enzyme Complex from Aspergillus sp. and Anti-Cancer Activity on HCT116 and HT-29 Cell Lines. Food Biosci. 2024, 61, 104995. [Google Scholar] [CrossRef]
- Nagybákay, N.; Sarapinaite, L.; Syrpas, M.; Venskutonis, P.; Kitryte-Syrpa, V. Optimization of Pressurized Ethanol Extraction for Efficient Recovery of Hyperoside and Other Valuable Polar Antioxidant-Rich Extracts from Betula Pendula Roth Leaves. Ind. Crops Prod. 2023, 205, 117565. [Google Scholar] [CrossRef]
- Pérez-Alva, A.; MacIntosh, A.J.; Baigts-Allende, D.K.; García-Torres, R.; Ramírez-Rodrigues, M.M. Fermentation of Algae to Enhance Their Bioactive Activity: A Review. Algal Res. 2022, 64, 102684. [Google Scholar] [CrossRef]

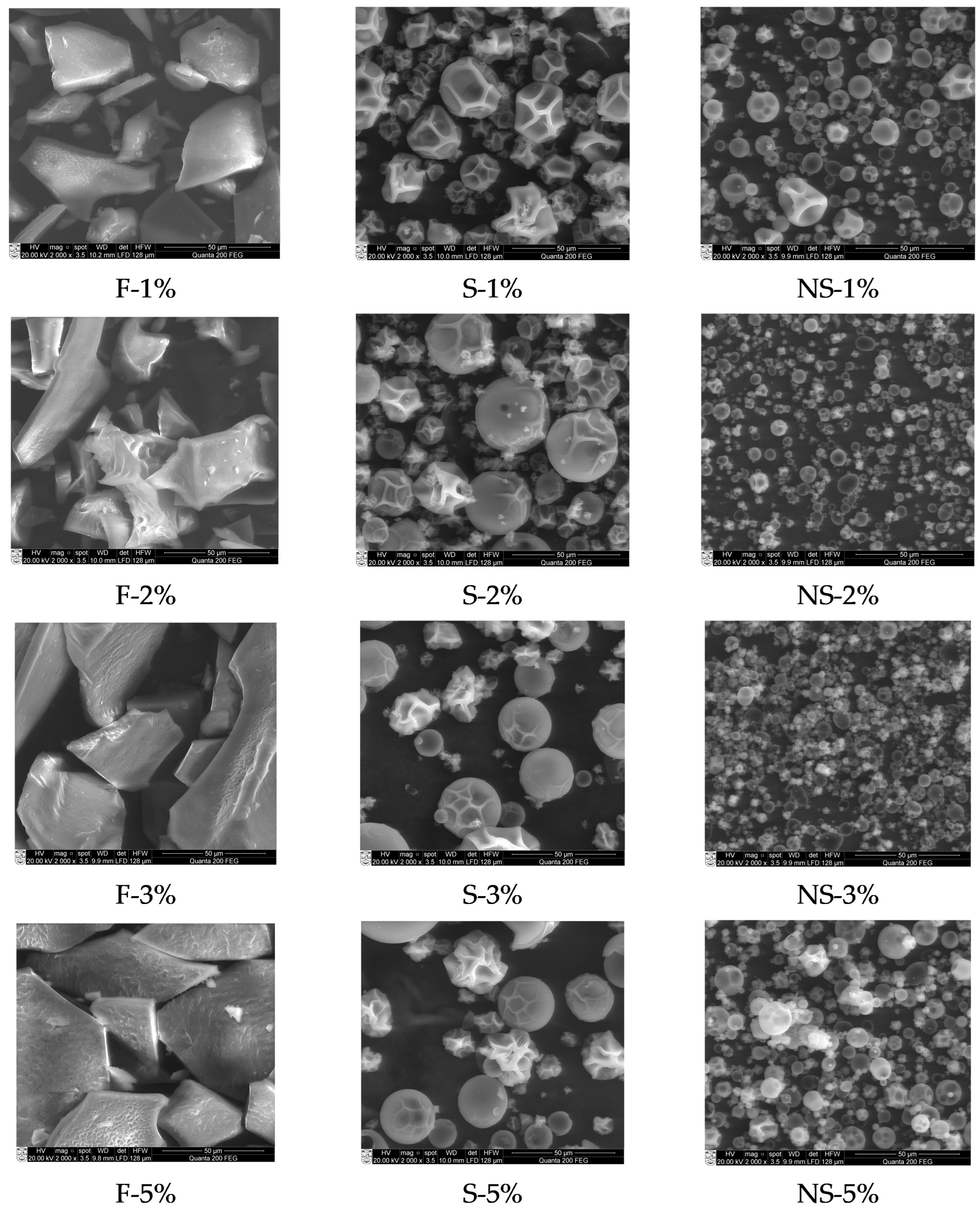
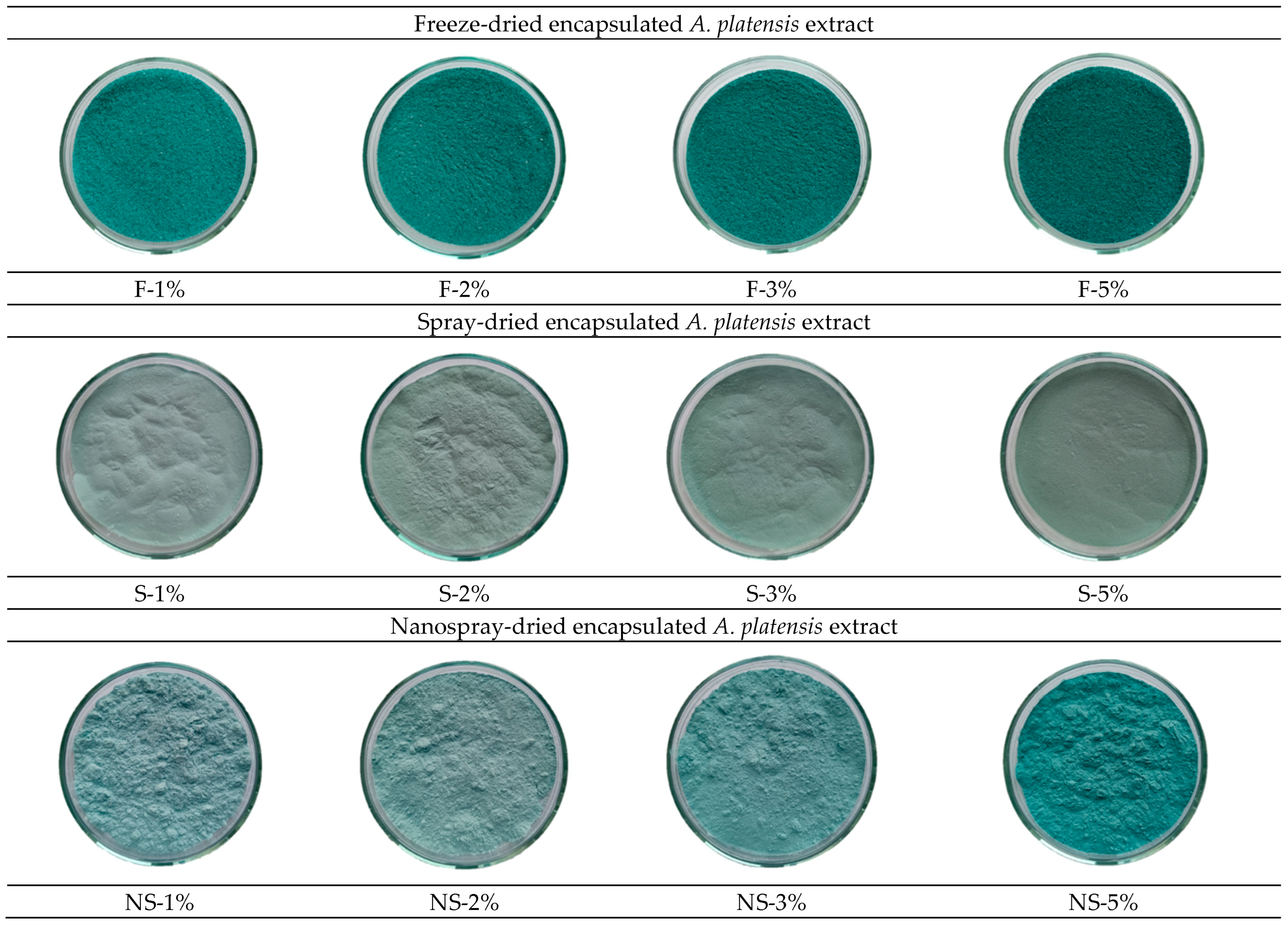
| Sample * | Solids Content in Dispersion, wt.% | Particle Size (nm) | PI | |
|---|---|---|---|---|
| SSOS | APE | |||
| SSOS-APE-1% | 10 | 1 | 396 ± 21 a | 0.32 ± 0.01 ab |
| SSOS-APE-2% | 10 | 2 | 423 ± 25 ab | 0.34 ± 0.05 ab |
| SSOS-APE-3% | 10 | 3 | 466 ± 23 b | 0.34 ± 0.03 b |
| SSOS-APE-5% | 10 | 5 | 542 ± 20 c | 0.34 ± 0.04 ab |
| Sample * | Total Content of PC (mg/g) | Encapsulation Efficiency of PC (%) |
|---|---|---|
| F-1% | 4.3 ± 0.1 a/C | 67.1 ± 1.0 abc/C |
| F-2% | 7.4 ± 0.0 b/C | 63.6 ± 0.1 a/C |
| F-3% | 11.3 ± 0.2 c/C | 69.8 ± 1.1 bc/C |
| F-5% | 16.6 ± 0.4 d/B | 71.2 ± 1.8 c/B |
| S-1% | 2.4 ± 0.2 a/A | 38.4 ± 3.8 ab/A |
| S-2% | 5.1 ± 0.2 b/A | 43.9 ± 1.4 a/A |
| S-3% | 6.3 ± 0.0 c/A | 39.8 ± 0.2 ab/A |
| S-5% | 9.7 ± 0.1 d/A | 41.6 ± 0.5 ab/A |
| NS-1% | 4.5 ± 0.1 a/C | 70.2 ± 2.1 b/C |
| NS-2% | 6.7 ± 0.1 b/C | 57.8 ± 0.6 a/C |
| NS-3% | 11.2 ± 0.0 c/C | 69.0 ± 0.1 b/C |
| NS-5% | 18.3 ± 0.0 d/C | 78.3 ± 0.0 c/C |
| Sample * | Colour Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| F-1% | 61.80 ± 0.36 d/A | −16.93 ± 0.17 a/A | −5.18 ± 0.05 b/B |
| F-2% | 55.37 ± 0.10 c/A | −15.36 ± 0.12 c/A | −4.47 ± 0.03 c/A |
| F-3% | 51.48 ± 0.29 b/A | −16.22 ± 0.04 b/A | −5.19 ± 0.02 b/B |
| F-5% | 44.60 ± 0.35 a/A | −16.17 ± 0.09 b/B | −5.36 ± 0.10 a/B |
| S-1% | 75.48 ± 0.43 d/B | −10.53 ± 0.08 a/C | −2.88 ± 0.08 a/C |
| S-2% | 69.59 ± 0.21 c/B | −10.24 ± 0.05 b/C | −1.50 ± 0.02 b/B |
| S-3% | 63.95 ± 0.26 b/B | −10.01 ± 0.09 c/C | −1.22 ± 0.02 c/C |
| S-5% | 59.01 ± 0.35 a/B | −9.47 ± 0.06 d/C | −0.16 ± 0.03 d/C |
| NS-1% | 77.88 ± 0.54 d/C | −12.17 ± 0.03 c/B | −5.54 ± 0.07 c/A |
| NS-2% | 74.89 ± 0.01 c/C | −12.09 ± 0.22 c/B | −4.65 ± 0.16 d/A |
| NS-3% | 72.17 ± 0.09 b/C | −15.14 ± 0.08 d/B | −6.78 ± 0.09 b/A |
| NS-5% | 60.02 ± 0.01 a/C | −20.87 ± 0.17 a/A | −9.03 ± 0.04 a/A |
| Sample * | I Step | II Step | III Step | Ash Residue at 600 °C (%) | |||
|---|---|---|---|---|---|---|---|
| Δw (%) | To (°C) | Δw (%) | To (°C) | Δw (%) | Tmax (°C) | ||
| APE | 4.3 ± 0.3 | 140.1 ± 0.2 | 5.6 ± 0.2 | 257.3 ± 0.3 | 54.4 ± 0.2 | 299.0 ± 0.2 | 35.8 ± 0.3 |
| SSOS | 5.7 ± 0.1 | – | – | 278.2 ± 0.2 | 80.6 ± 0.1 | 297.1 ± 0.2 | 13.8 ± 0.1 |
| F-1% | 3.2 ± 0.3 | 163.4 ± 0.2 | 0.8 ± 0.2 | 267.2 ± 0.1 | 75.1 ± 0.3 | 300.1 ± 0.1 | 20.9 ± 0.3 |
| F-2% | 2.5 ± 0.3 | 162.0 ± 0.1 | 1.0 ± 0.1 | 268.1 ± 0.2 | 71.7 ± 0.1 | 297.0 ± 0.2 | 24.8 ± 0.2 |
| F-3% | 2.2 ± 0.2 | 163.3 ± 0.1 | 1.3 ± 0.1 | 266.0 ± 0.1 | 69.1 ± 0.1 | 296.2 ± 0.2 | 27.5 ± 0.1 |
| F-5% | 2.8 ± 0.2 | 162.1 ± 0.3 | 1.9 ± 0.1 | 261.0 ± 0.2 | 65.2 ± 0.2 | 294.1 ± 0.1 | 30.1 ± 0.2 |
| S-1% | 3.2 ± 0.2 | 148.0 ± 0.1 | 0.5 ± 0.2 | 269.0 ± 0.2 | 78.1 ± 0.1 | 303.1 ± 0.2 | 18.2 ± 0.3 |
| S-2% | 2.9 ± 0.3 | 149.3 ± 0.3 | 0.8 ± 0.1 | 264.3 ± 0.2 | 74.9 ± 0.2 | 298.0 ± 0.1 | 21.0 ± 0.1 |
| S-3% | 2.2 ± 0.3 | 150.1 ± 0.2 | 1.1 ± 0.2 | 264.1 ± 0.1 | 74.1 ± 0.3 | 298.3 ± 0.2 | 22.6 ± 0.2 |
| S-5% | 2.3 ± 0.1 | 151.3 ± 0.2 | 1.5 ± 0.1 | 261.1 ± 0.1 | 70.6 ± 0.2 | 296.3 ± 0.1 | 25.6 ± 0.2 |
| NS-1% | 4.8 ± 0.3 | 154.4 ± 0.3 | 0.6 ± 0.2 | 267.2 ± 0.2 | 75.5 ± 0.2 | 303.0 ± 0.1 | 19.2 ± 0.1 |
| NS-2% | 6.7 ± 0.3 | 153.1 ± 0.2 | 1.0 ± 0.1 | 265.3 ± 0.2 | 73.2 ± 0.3 | 299.2 ± 0.1 | 19.1 ± 0.2 |
| NS-3% | 6.3 ± 0.2 | 155.1 ± 0.3 | 1.2 ± 0.2 | 260.0 ± 0.1 | 71.3 ± 0.3 | 298.1 ± 0.2 | 21.2 ± 0.3 |
| NS-5% | 5.7 ± 0.3 | 151.3 ± 0.4 | 1.7 ± 0.3 | 259.2 ± 0.2 | 68.4 ± 0.2 | 296.3 ± 0.1 | 24.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navikaitė-Šnipaitienė, V.; Liudvinavičiūtė, D.; Rutkaitė, R.; Kitrytė-Syrpa, V.; Syrpas, M. Antioxidant Capacity and Thermal Stability of Arthrospira platensis Extract Encapsulated in Starch Sodium Octenyl Succinate with Freeze-, Spray-, and Nanospray-Drying. Molecules 2025, 30, 1303. https://doi.org/10.3390/molecules30061303
Navikaitė-Šnipaitienė V, Liudvinavičiūtė D, Rutkaitė R, Kitrytė-Syrpa V, Syrpas M. Antioxidant Capacity and Thermal Stability of Arthrospira platensis Extract Encapsulated in Starch Sodium Octenyl Succinate with Freeze-, Spray-, and Nanospray-Drying. Molecules. 2025; 30(6):1303. https://doi.org/10.3390/molecules30061303
Chicago/Turabian StyleNavikaitė-Šnipaitienė, Vesta, Dovilė Liudvinavičiūtė, Ramunė Rutkaitė, Vaida Kitrytė-Syrpa, and Michail Syrpas. 2025. "Antioxidant Capacity and Thermal Stability of Arthrospira platensis Extract Encapsulated in Starch Sodium Octenyl Succinate with Freeze-, Spray-, and Nanospray-Drying" Molecules 30, no. 6: 1303. https://doi.org/10.3390/molecules30061303
APA StyleNavikaitė-Šnipaitienė, V., Liudvinavičiūtė, D., Rutkaitė, R., Kitrytė-Syrpa, V., & Syrpas, M. (2025). Antioxidant Capacity and Thermal Stability of Arthrospira platensis Extract Encapsulated in Starch Sodium Octenyl Succinate with Freeze-, Spray-, and Nanospray-Drying. Molecules, 30(6), 1303. https://doi.org/10.3390/molecules30061303







