Bioethanol Steam Reforming for Hydrogen Production over Ni-Cr/SBA 15: Influence of Metal Loading and Ni/Cr Ratio
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
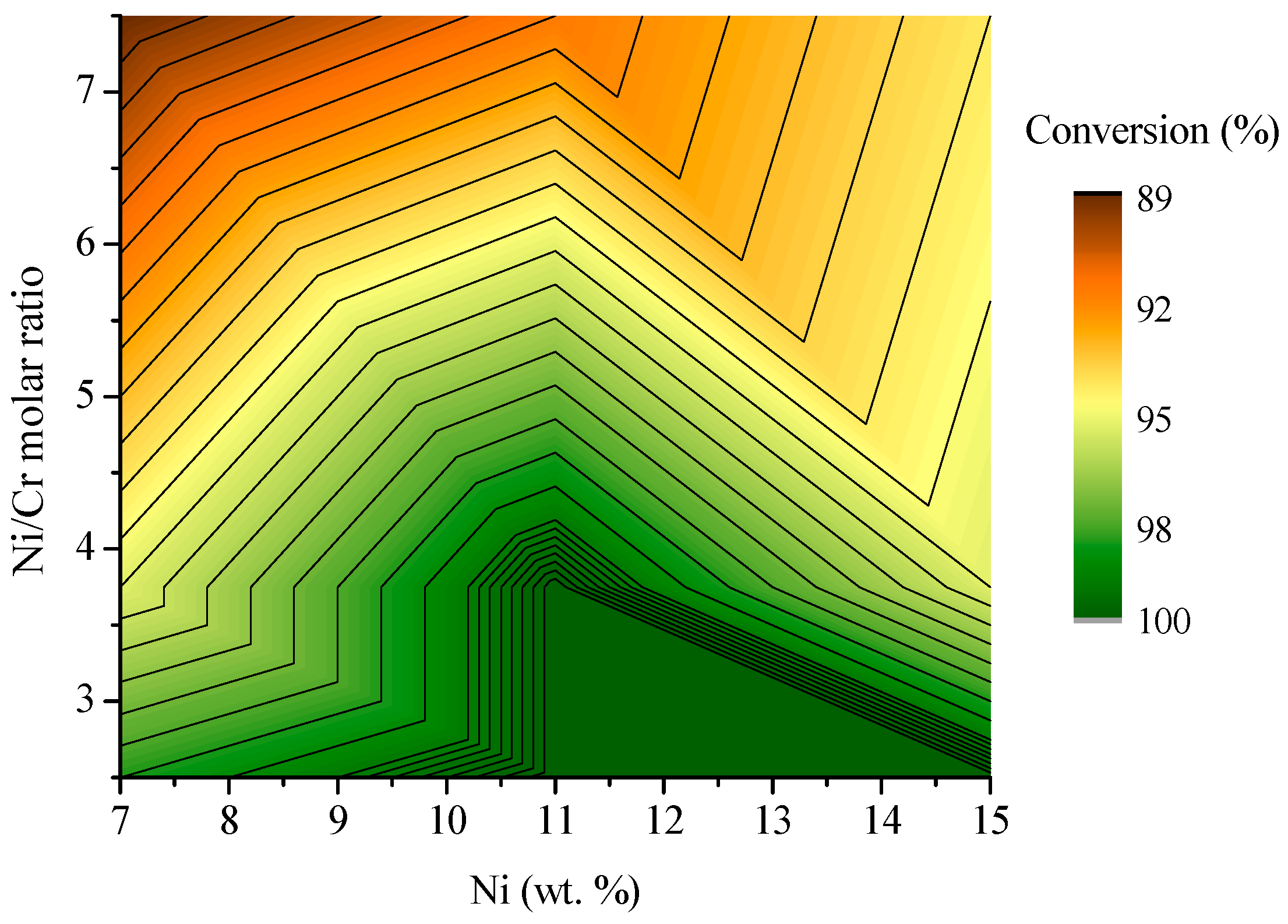
2.2. Ethanol Steam Reforming Tests
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2023—Analysis—IEA. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 18 January 2024).
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: Summary of recent techno-economic analyses. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jewaratnam, J.; Ganesan, P. Prospect of hydrogen production from oil palm biomass by thermochemical process—A review. Int. J. Hydrogen Energy 2016, 41, 16637–16655. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Li, B.; He, Y.; Liu, H. Performance of ethanol steam reforming in a membrane-assisted packed bed reactor using multiscale modelling. Fuel 2020, 274, 117829. [Google Scholar] [CrossRef]
- Graschinsky, C.; Labore, M.; Amadeo, N.; Le Valant, A.; Bion, N.; Epron, F.; Duprez, D. Ethanol Steam Reforming over Rh(1%)MgAl2O4/Al2O3: A Kinetic Study. Ind. Eng. Chem. Res. 2010, 49, 12383–12389. [Google Scholar] [CrossRef]
- Montero, C.; Oar-Arteta, L.; Remiro, A.; Arandia, A.; Bilbao, J.; Gayubo, A.G. Thermodynamic comparison between bio-oil and ethanol steam reforming. Int. J. Hydrogen Energy 2015, 40, 15963–15971. [Google Scholar] [CrossRef]
- Lindo, M.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Ethanol steam reforming on Ni/Al-SBA-15 catalysts: Effect of the aluminium content. Int. J. Hydrogen Energy 2010, 35, 5895–5901. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hao, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B 2019, 258, 117940. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts-A review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Megía, P.J.; Morales, A.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Oxidative steam reforming of acetic acid on Ni catalysts: Influence of the La promotion on mesostructured supports. Int. J. Hydrogen Energy 2024, 52, 1136–1145. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Montero, C.; Ochoa, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming in a fluidized bed. J. Catal. 2015, 331, 181–192. [Google Scholar] [CrossRef]
- Claude, V.; Mahy, J.G.; Tilkin, R.G.; Lambert, S.D. Enhancement of the catalytic performances and lifetime of Ni/γ-Al2O3 catalysts for the steam toluene reforming via the combination of dopants: Inspection of Cu, Co, Fe, Mn, and Mo species addition. Mater. Today Chem. 2020, 15, 100229. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu,Co,Cr) catalysts supported on SBA-15 silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L.; Megía, P.J. Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts. Int. J. Mol. Sci. 2019, 20, 512. [Google Scholar] [CrossRef]
- Bui, Q.T.P.; Kim, Y.; Yool, S.P.; Han, J.; Ham, H.C.; Nam, S.K.; Yoon, C.W. Steam reforming of simulated biogas over plate NiCr catalysts: Influence of pre-oxidation on catalytic activity. Appl. Catal. B 2015, 166–167, 335–344. [Google Scholar] [CrossRef]
- Casanovas, A.; Roig, M.; de Leitenburg, C.; Trovarelli, A.; Llorca, J. Ethanol steam reforming and water gas shift over Co/ZnO catalytic honeycombs doped with Fe, Ni, Cu, Cr and Na. Int. J. Hydrogen Energy 2010, 35, 7690–7698. [Google Scholar] [CrossRef]
- Megía, P.J.; Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen Production from Steam Reforming of Acetic Acid as a Model Compound of the Aqueous Fraction of Microalgae HTL Using Co-M/SBA-15 (M: Cu, Ag, Ce, Cr) Catalysts. Catalysts 2019, 9, 1013. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Zheng, C.; Zhao, H. CrOx/Ce1−xZrxO2 for chemical looping propane oxidative dehydrogenation: The redox interaction between CrOx and the support. Chem. Eng. Sci. 2023, 274, 118697. [Google Scholar] [CrossRef]
- Rouibah, K.; Barama, A.; Benrabaa, R.; Guerrero-Caballero, J.; Kane, T.; Vannier, R.N.; Rubbens, A.; Löfberg, A. Dry reforming of methane on nickel-chrome, nickel-cobalt and nickel-manganese catalysts. Int. J. Hydrogen Energy 2017, 42, 29725–29734. [Google Scholar] [CrossRef]
- Ramantani, T.; Bampos, G.; Kaponi, K.; Kalamaris, E.; Kondarides, D.I. Propane steam reforming over La0.8Sr0.2Ni1-yMyO3 (M = Cr, Mn, Fe, Co) perovskite-type oxides. Appl. Catal. B Environ. Energy 2024, 358, 124391. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Ntow, E.B.; Jennings, T.; Stevens-Boyd, R.; Hossain, T.; Swain, J.; Bepari, S.; Hassan, S.; Mohammad, N.; Kuila, D. Cr and CeO2 promoted Ni/SBA-15 framework for hydrogen production by steam reforming of glycerol. Environ. Sci. Pollut. Res. Int. 2023, 30, 120945–120962. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.G.E.; Assaf, P.G.M.; Carvalho, H.W.P.; Assaf, E.M. Catalytic steam reforming of acetic acid as a model compound of bio-oil. Appl. Catal. B 2014, 160–161, 188–199. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yang, Z.; Liang, T.; Liu, S.; Zhou, Z.; Li, X. Bimetallic Ni-M (M = Co, Cu and Zn) supported on attapulgite as catalysts for hydrogen production from glycerol steam reforming. Appl. Catal. A Gen. 2018, 550, 214–227. [Google Scholar] [CrossRef]
- Anh, A.N.; Nguyen, H.H.; Pham, T.P.T.; Pham, L.K.H.; Phuong, D.H.L.; Nguyen, N.A.; Vo, D.N.V.; Pham, P.T.H. Insight into the role of material basicity in the coke formation and performance of Ni/Al2O3 catalyst for the simulated- biogas dry reforming. J. Energy Inst. 2023, 108, 101252. [Google Scholar] [CrossRef]
- Puron, H.; Pinilla, J.L.; Saraev, A.A.; Kaichev, V.V.; Millan, M. Hydroprocessing of Maya vacuum residue using a NiMo catalyst supported on Cr-doped alumina. Fuel 2020, 263, 116717. [Google Scholar] [CrossRef]
- Yun, D.; Baek, J.; Choi, Y.; Kim, W.; Lee, H.J.; Yi, J. Promotional Effect of Ni on a CrOx Catalyst Supported on Silica in the Oxidative Dehydrogenation of Propane with CO2. ChemCatChem 2012, 4, 1952–1959. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Brito, J.L.F.; Dieguez, L.C. Characterization of chromium species in catalysts for dehydrogenation and polymerization. J. Mol. Catal. A Chem. 2003, 203, 251–266. [Google Scholar] [CrossRef]
- Gholami, S.; Alavi, S.M.; Rezaei, M. Preparation of highly active and stable nanostructured Ni-Cr2O3 catalysts for hydrogen purification via CO2 methanation reaction. J. Energy Inst. 2021, 95, 132–142. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Neuberg, S.; Hessel, V.; Kolb, G. Effect of ceria and zirconia promotors on Ni/SBA-15 catalysts for coking and sintering resistant steam reforming of propylene glycol in microreactors. Appl. Catal. B 2017, 203, 859–869. [Google Scholar] [CrossRef]
- Xie, T.; Zhao, X.; Zhang, J.; Shi, L.; Zhang, D. Ni nanoparticles immobilized Ce-modified mesoporous silica via a novel sublimation-deposition strategy for catalytic reforming of methane with carbon dioxide. Int. J. Hydrogen Energy 2015, 40, 9685–9695. [Google Scholar] [CrossRef]
- Nakayama, O.; Ikenaga, N.; Miyake, T.; Yagasaki, E.; Suzuki, T. Production of synthesis gas from methane using lattice oxygen of NiO-Cr2O3-MgO complex oxide. Ind. Eng. Chem. Res. 2010, 49, 526–534. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Cheng, L.; Shin, E.W.; Men, Y. Three-dimensionally ordered macroporous spinel-type MCr2O4 (M = Co, Ni, Zn, Mn) catalysts with highly enhanced catalytic performance for soot combustion. Catal. Sci. Technol. 2015, 5, 4594–4601. [Google Scholar] [CrossRef]
- Xiao, Z.; Xiu, J.; Wang, X.; Zhang, B.; Williams, C.T.; Su, D.; Liang, C. Controlled preparation and characterization of supported CuCr2O4 catalysts for hydrogenolysis of highly concentrated glycerol. Catal. Sci. Technol. 2013, 3, 1108–1115. [Google Scholar] [CrossRef]
- Da Silva, A.L.M.; Den Breejen, J.P.; Mattos, L.V.; Bitter, J.H.; De Jong, K.P.; Noronha, F.B. Cobalt particle size effects on catalytic performance for ethanol steam reforming—Smaller is better. J. Catal. 2014, 318, 67–74. [Google Scholar] [CrossRef]
- Gupta, S.; Deo, G. Effect of metal amount on the catalytic performance of Ni–Al2O3 catalyst for the Tri-reforming of methane. Int. J. Hydrogen Energy 2023, 48, 5478–5492. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Sandupatla, A.; Deo, G.; Bal, R. Synthesis and catalytic activity of a Pd doped Ni–MgO catalyst for dry reforming of methane. J. Mater. Chem. A Mater. 2017, 5, 15688–15699. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Provendier, H.; Mirodatos, C. Ethanol oxidative steam reforming over Ni-based catalysts. J. Power Sources 2005, 145, 659–666. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Ruiz-Abad, M.; Calles, J.A.; Carrero, A. Coke evolution in simulated bio-oil aqueous fraction steam reforming using Co/SBA-15. Catal. Today 2021, 367, 145–152. [Google Scholar] [CrossRef]
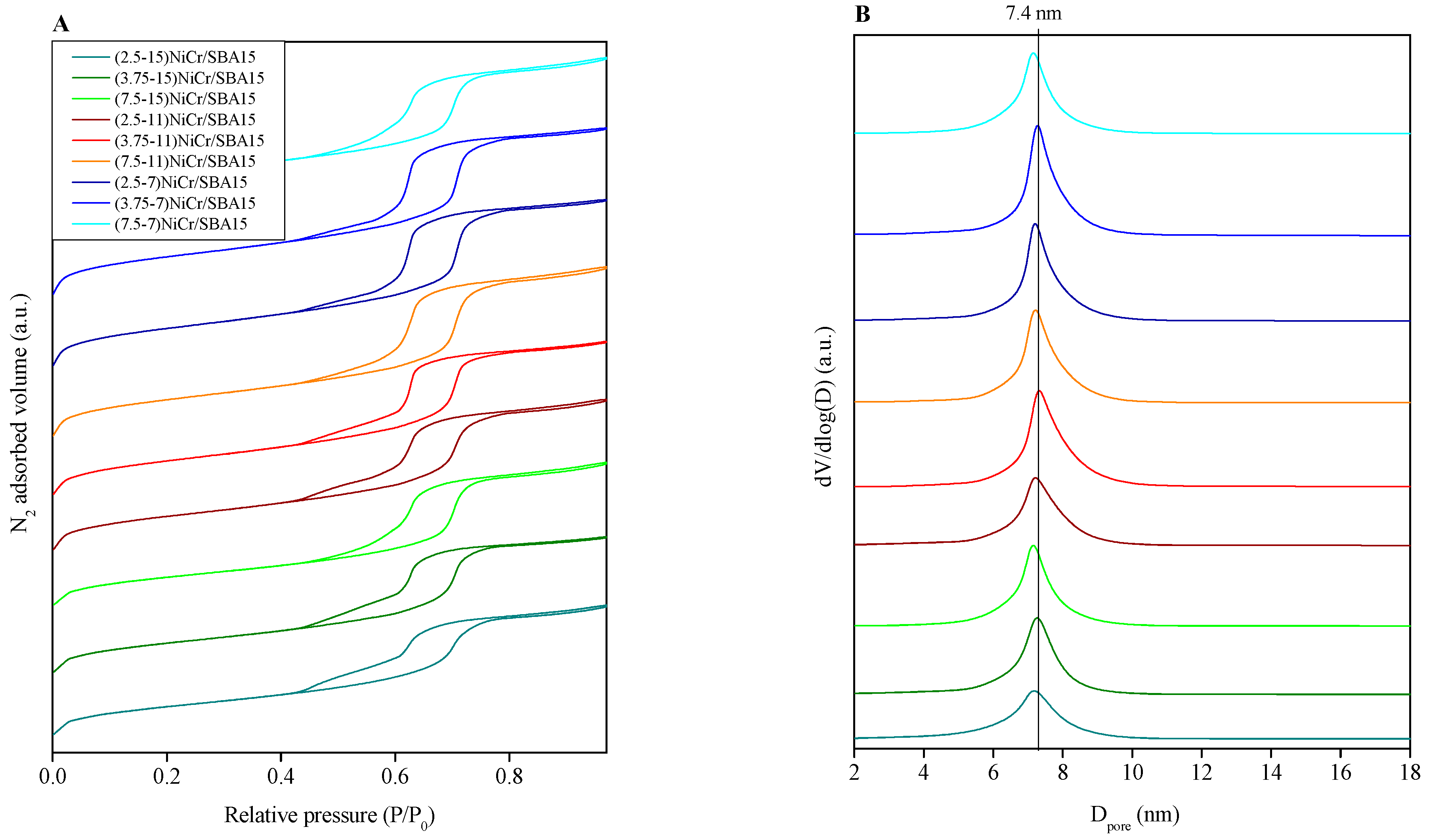
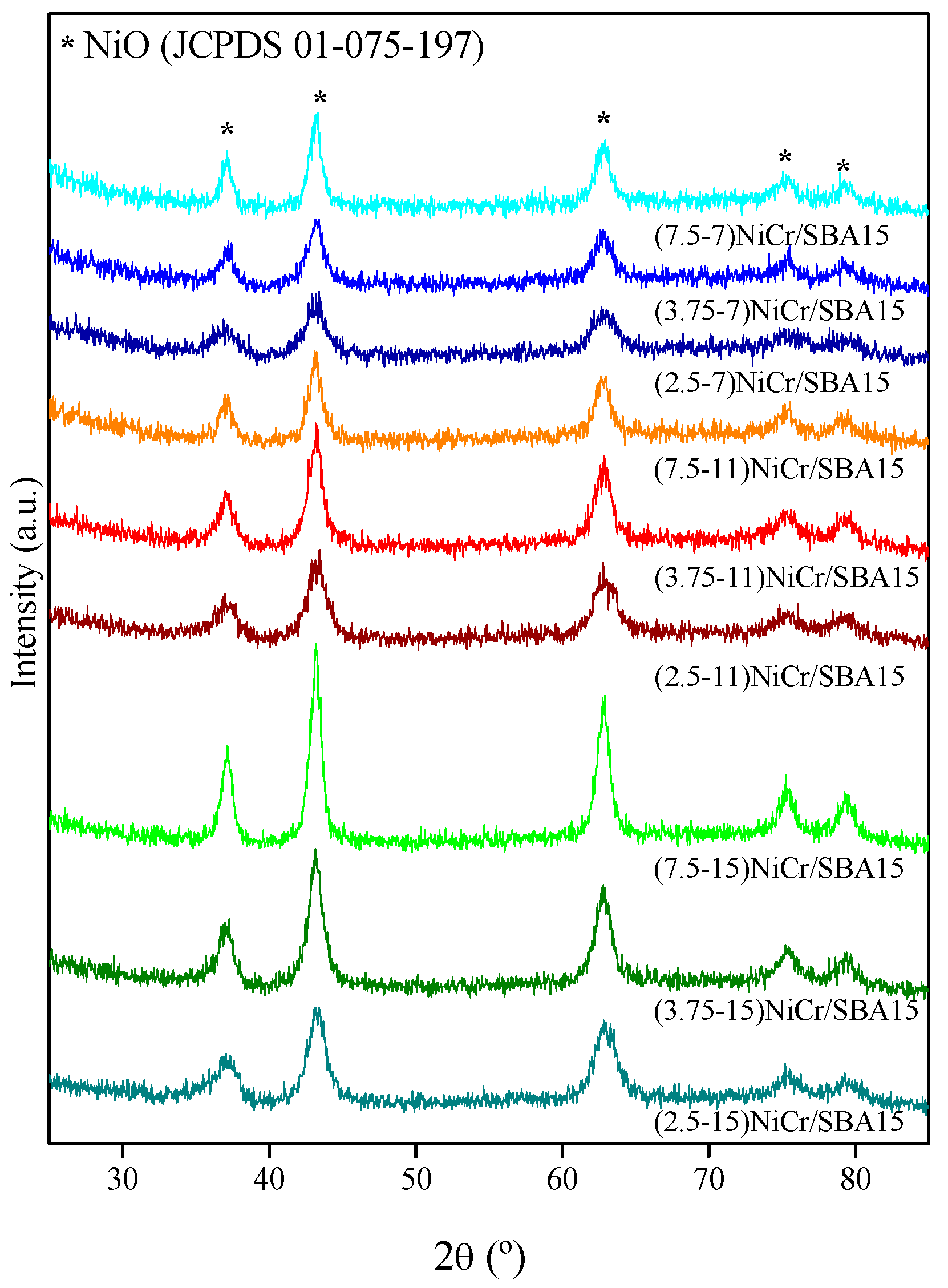
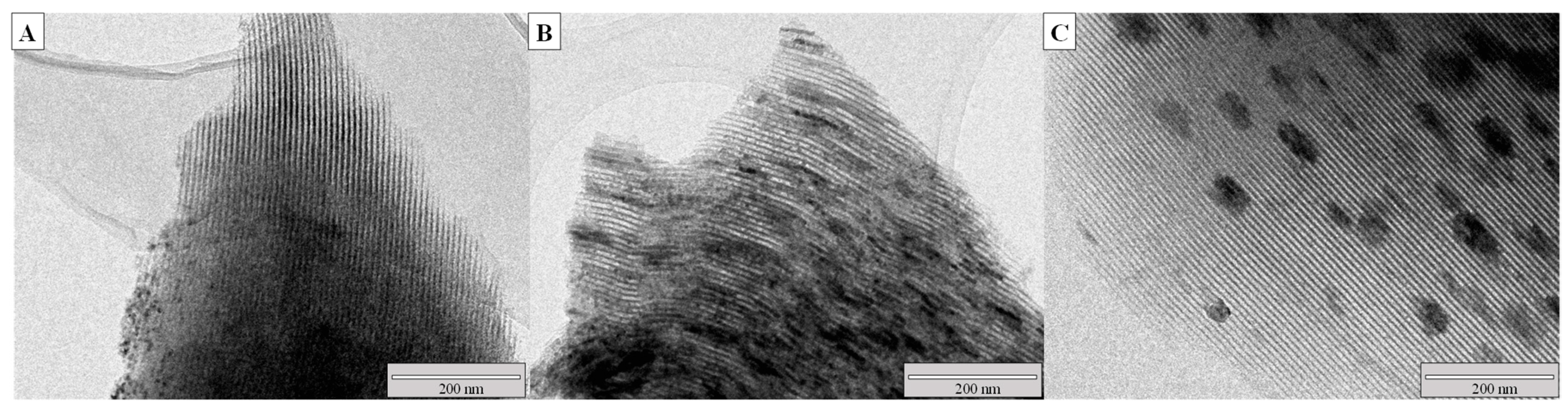
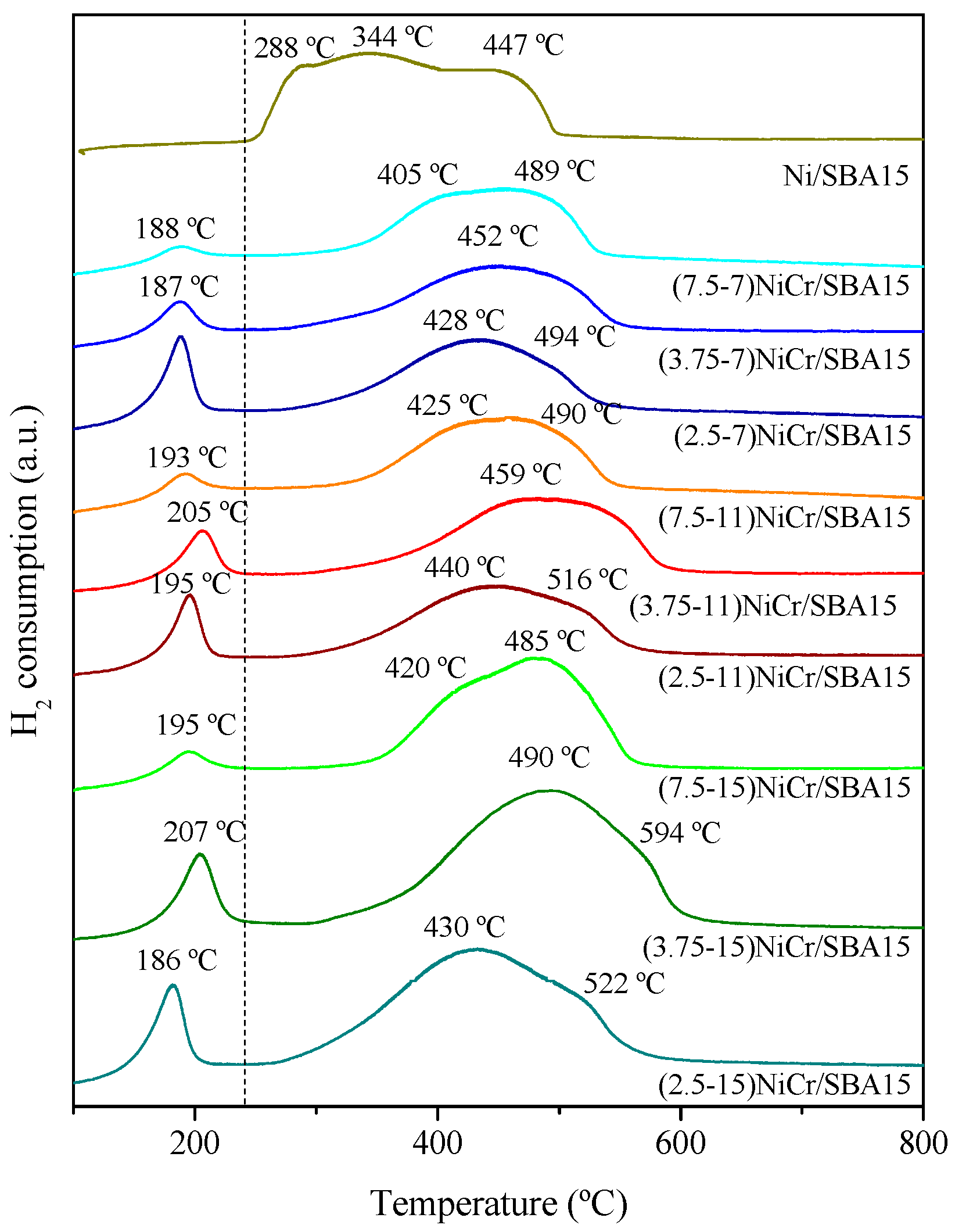


| Catalyst | Ni (wt.%) a | Cr (wt.%) a | SBET (m2/g) | Vpore (cm3/g) b | Dpore (nm) c | d NiO (nm) d | d Ni (nm) e | Ni Dispersion (%) f |
|---|---|---|---|---|---|---|---|---|
| (7.5-7)NiCr/SBA15 | 6.4 | 1.0 | 534 | 0.82 | 7.1 | 7.8 | 7.3 | 13.8 |
| (3.75-7)NiCr/SBA15 | 7.0 | 1.8 | 539 | 0.80 | 7.3 | 7.6 | 6.2 | 16.3 |
| (2.5-7)NiCr/SBA15 | 7.1 | 2.7 | 527 | 0.79 | 7.2 | 5.0 | 4.5 | 22.4 |
| (7.5-11)NiCr/SBA15 | 10.8 | 1.1 | 527 | 0.81 | 7.2 | 7.9 | 7.6 | 13.3 |
| (3.75-11)NiCr/SBA15 | 11.0 | 2.7 | 513 | 0.74 | 7.3 | 7.3 | 6.8 | 16.2 |
| (2.5-11)NiCr/SBA15 | 10.6 | 4.1 | 496 | 0.73 | 7.2 | 5.3 | 4.6 | 22.0 |
| (7.5-15)NiCr/SBA15 | 14.4 | 1.8 | 461 | 0.70 | 7.2 | 9.5 | 8.9 | 11.3 |
| (3.75-15)NiCr/SBA15 | 14.8 | 3.8 | 463 | 0.67 | 7.3 | 7.0 | 6.2 | 16.3 |
| (2.5-15)NiCr/SBA15 | 14.7 | 5.7 | 444 | 0.64 | 7.2 | 5.7 | 4.9 | 20.6 |
| Catalyst | H2 Consumption (μmol H2) | |
|---|---|---|
| Theoretical | Experimental | |
| (7.5-7)NiCr/SBA15 | 109.0 | 102.3 |
| (3.75-7)NiCr/SBA15 | 105.6 | 99.3 |
| (2.5-7)NiCr/SBA15 | 121.0 | 114.3 |
| (7.5-11)NiCr/SBA15 | 184.0 | 175.8 |
| (3.75-11)NiCr/SBA15 | 187.4 | 178.2 |
| (2.5-11)NiCr/SBA15 | 180.6 | 171.2 |
| (7.5-15)NiCr/SBA15 | 245.3 | 236.5 |
| (3.75-15)NiCr/SBA15 | 252.2 | 249.3 |
| (2.5-15)NiCr/SBA15 | 250.5 | 246.4 |
| Catalyst | S H2 (%) | Product Distribution (mol %) | Coke (gcoke/gcat·h) | TDTG, max (°C) | d Ni a (nm) | ||
|---|---|---|---|---|---|---|---|
| CO2 | CO | CH4 | |||||
| (7.5-7)NiCr/SBA15 | 52.2 | 20.4 | 13.8 | 4.0 | 0.008 | 531 | 7.6 |
| (3.75-7)NiCr/SBA15 | 55.3 | 20.3 | 14.3 | 5.3 | 0.008 | 526 | 6.7 |
| (2.5-7)NiCr/SBA15 | 57.8 | 20.8 | 15.6 | 3.4 | 0.006 | 512 | 4.8 |
| (7.5-11)NiCr/SBA15 | 61.1 | 21.3 | 13.1 | 2.9 | 0.012 | 538 | 8.3 |
| (3.75-11)NiCr/SBA15 | 63.1 | 21.2 | 12.3 | 6.1 | 0.013 | 532 | 7.1 |
| (2.5-11)NiCr/SBA15 | 58.9 | 20.6 | 15.1 | 5.5 | 0.003 | 518 | 4.9 |
| (7.5-15)NiCr/SBA15 | 59.0 | 21.1 | 14.9 | 3.2 | 0.018 | 540 | 9.7 |
| (3.75-15)NiCr/SBA15 | 62.9 | 21.8 | 12.7 | 5.4 | 0.014 | 519 | 6.2 |
| (2.5-15)NiCr/SBA15 | 63.8 | 21.9 | 14.1 | 2.6 | 0.003 | 511 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megía, P.J.; García-Moreno, L.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Bioethanol Steam Reforming for Hydrogen Production over Ni-Cr/SBA 15: Influence of Metal Loading and Ni/Cr Ratio. Molecules 2025, 30, 1206. https://doi.org/10.3390/molecules30061206
Megía PJ, García-Moreno L, Vizcaíno AJ, Calles JA, Carrero A. Bioethanol Steam Reforming for Hydrogen Production over Ni-Cr/SBA 15: Influence of Metal Loading and Ni/Cr Ratio. Molecules. 2025; 30(6):1206. https://doi.org/10.3390/molecules30061206
Chicago/Turabian StyleMegía, Pedro J., Lourdes García-Moreno, Arturo J. Vizcaíno, José A. Calles, and Alicia Carrero. 2025. "Bioethanol Steam Reforming for Hydrogen Production over Ni-Cr/SBA 15: Influence of Metal Loading and Ni/Cr Ratio" Molecules 30, no. 6: 1206. https://doi.org/10.3390/molecules30061206
APA StyleMegía, P. J., García-Moreno, L., Vizcaíno, A. J., Calles, J. A., & Carrero, A. (2025). Bioethanol Steam Reforming for Hydrogen Production over Ni-Cr/SBA 15: Influence of Metal Loading and Ni/Cr Ratio. Molecules, 30(6), 1206. https://doi.org/10.3390/molecules30061206









