The Double-Edge Sword of Natural Phenanthrenes in the Landscape of Tumorigenesis
Abstract
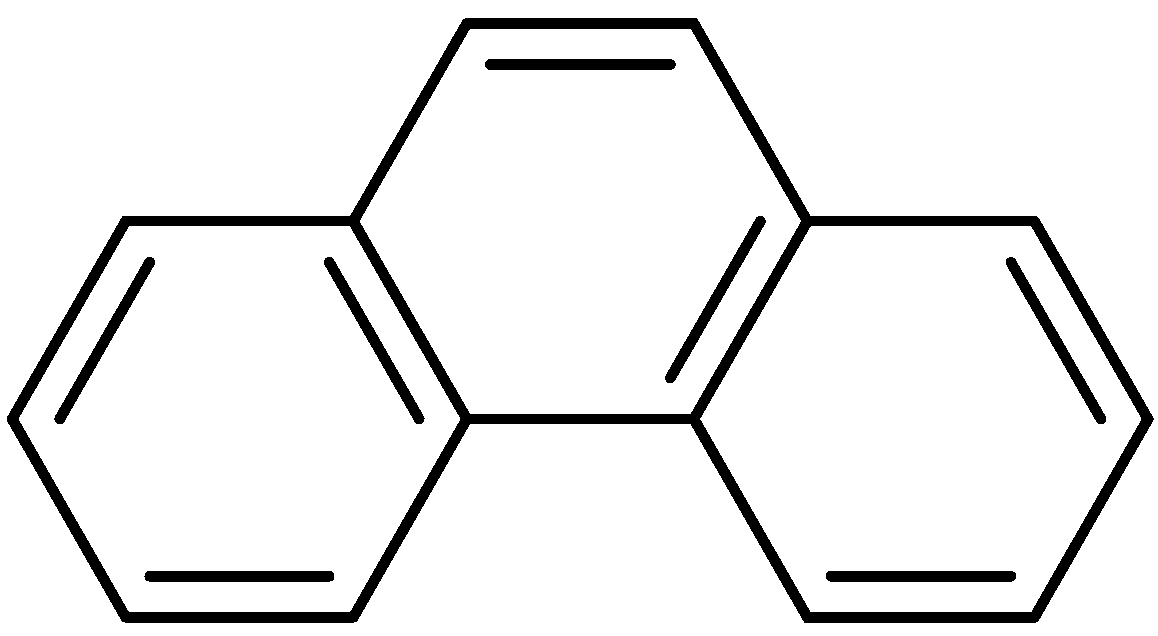
1. Introduction
2. Antitumor Activities of Phenanthrene Alkaloids from Natural Products
2.1. Antitumor Phenanthrenes
2.2. Antitumor 9,10-Dihydrophenanthrene
2.3. Antitumor 9,10-Dihydrophenanthrene Dimer Compounds
2.4. Other Derivatives of Antitumor Phenanthrenes
3. Toxicological Effects of Natural Phenanthrenes
3.1. Hepatotoxicity
3.2. Nephrotoxicity
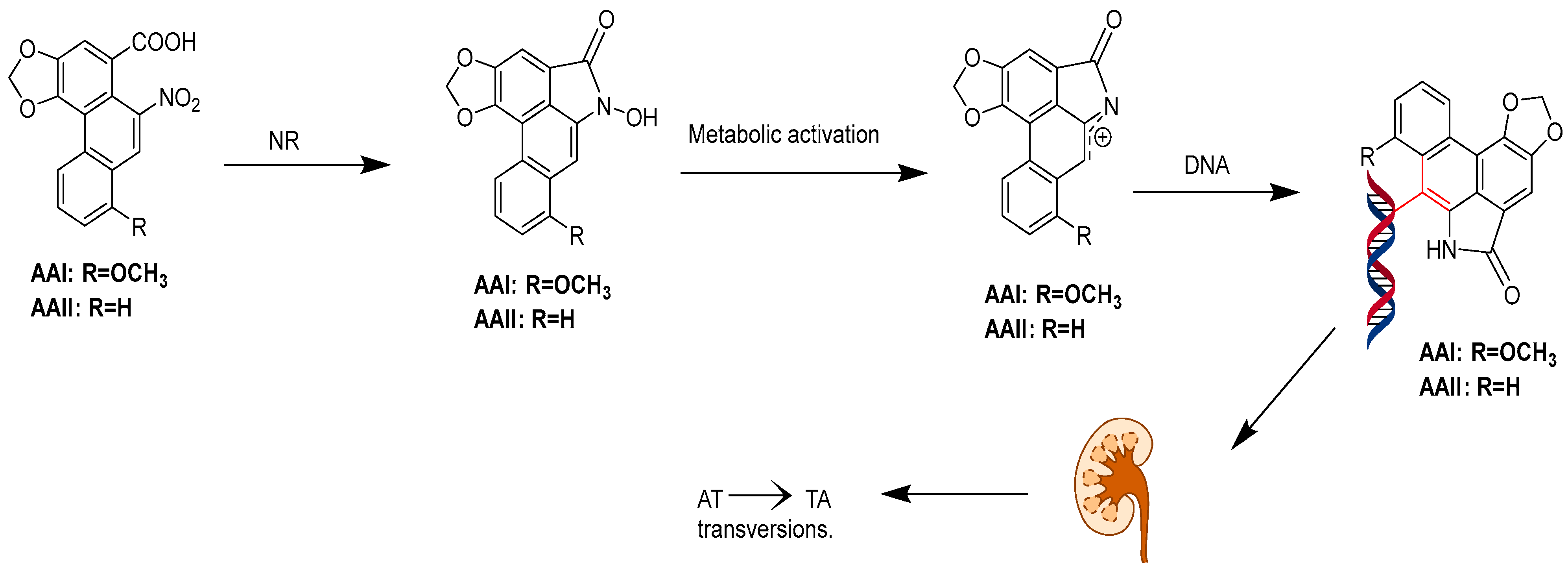
3.3. Carcinogenicity
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovács, A.; Vasas, A.; Hohmann, J. Natural Phenanthrenes and Their Biological Activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, J.J.; Allen, R.W.; Gribble, G.W. A Simple Synthesis of Phenanthrene. Org. Prep. Proced. Int. 2020, 52, 166–169. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.; Carmella, S.G.; Hochalter, J.B.; Rauch, D.; Oliver, A.; Jensen, J.; Hatsukami, D.K.; Upadhyaya, P.; Zimmerman, C.; et al. Metabolism of [D10]Phenanthrene to Tetraols in Smokers for Potential Lung Cancer Susceptibility Assessment: Comparison of Oral and Inhalation Routes of Administration. J. Pharmacol. Exp. Ther. 2011, 338, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.A.; Perwaiz, S. Aristolochic Acids. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 298–301. [Google Scholar]
- Das, S.; Thakur, S.; Korenjak, M.; Sidorenko, V.S.; Chung, F.F.; Zavadil, J. Aristolochic acid-associated cancers: A public health risk in need of global action. Nat. Rev. Cancer 2022, 22, 576–591. [Google Scholar] [CrossRef]
- Thomas, P.C.; Charlie, X.; Kelvin, C.; Chun, G.L. Aristolochic Acids Detected in Some Raw Chinese Medicinal Herbs and Manufactured Herbal Products—A Consequence of Inappropriate Nomenclature and ImpreciseLabelling? Clin. Toxicol. 2006, 44, 371–378. [Google Scholar]
- Sborchia, M.; De Prez, E.G.; Antoine, M.H.; Bienfait, L.; Indra, R.; Valbuena, G.; Phillips, D.H.; Nortier, J.L.; Stiborová, M.; Keun, H.C.; et al. The Impact of P53 on Aristolochic Acid I-Induced Nephrotoxicity and DNA Damage In Vivo and In Vitro. Arch. Toxicol. 2019, 93, 3345–3366. [Google Scholar] [CrossRef]
- Sborchia, M.; Keun, H.C.; Phillips, D.H.; Arlt, V.M. The Impact of P53 on Aristolochic Acid I-Induced Gene Expression In Vivo. Int. J. Mol. Sci. 2019, 20, 6155. [Google Scholar] [CrossRef]
- Zhou, Q.; Pei, J.; Poon, J.; Lau, A.Y.; Zhang, L.; Wang, Y.; Liu, C.; Huang, L. Worldwide Research Trends on Aristolochic Acids (1957–2017): Suggestions for Researchers. PLoS ONE 2019, 14, e0216135. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhao, X.H.; Sun, Z.H.; Li, G.C.; Liu, G.C.; Sun, L.R.; Hou, J.Q.; Zhou, W. Recognition of the Toxicity of Aristolochic Acid. J. Clin. Pharm. Ther. 2019, 44, 157–162. [Google Scholar] [CrossRef]
- Moretti, C.; Rideau, M.; Chénieux, J.; Viel, C. Isolement de l’acide Aristolochique de Deux Aristoloches Malgaches. Détermination de Sa Cytotoxicité Sur Cellules Végétales. Comparaison Avec Les Cellules Animales. Planta Med. 1979, 35, 360–365. [Google Scholar] [CrossRef]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A Promising Group of Plant Secondary Metabolites. J. Nat. Prod. 2018, 81, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New Phenanthrene and 9, 10-Dihydrophenanthrene Derivatives from the Stems of Dendrobium Officinale with Their Cytotoxic Activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Han, M.; Zhang, B.; Sun, L.; Jin, X.; Li, T. Chrysotoxene Induces Apoptosis of Human Hepatoblastoma HepG2 Cells in vitro and in vivo via Activation of the Mitochondria-Mediated Apoptotic Signaling Pathway. Oncol. Lett. 2018, 15, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Nonpanya, N.; Prakhongcheep, O.; Petsri, K.; Jitjaicham, C.; Tungsukruthai, S.; Sritularak, B.; Chanvorachote, P. Ephemeranthol A Suppresses Epithelial to Mesenchymal Transition and FAK-Akt Signaling in Lung Cancer Cells. Anticancer Res. 2020, 40, 4989–4999. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Xin, W.; Liu, N.; Zhang, H. Nudol, a Phenanthrene Derivative from Dendrobium nobile, Induces Cell Cycle Arrest and Apoptosis and Inhibits Migration in Osteosarcoma Cells. Drug Des. Devel. Ther. 2019, 13, 2591–2601. [Google Scholar] [CrossRef]
- Yang, C.R.; Shih, K.S.; Liou, J.P.; Wu, Y.W.; Hsieh, I.N.; Lee, H.Y.; Lin, T.C.; Wang, J.H. Denbinobin Upregulates miR-146a Expression and Attenuates IL-1β-Induced Upregulation of ICAM-1 and VCAM-1 Expressions in Osteoarthritis Fibroblast-like Synoviocytes. J. Mol. Med. 2014, 92, 1147–1158. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.Y.; Han, S.-B.; Uddin, G.M.; Kim, C.Y.; Lee, J.K. Anti-Inflammatory Effects of Dendrobium nobile Derived Phenanthrenes in LPS-Stimulated Murine Macrophages. Arch. Pharm. Res. 2015, 38, 1117–1126. [Google Scholar] [CrossRef]
- Li, C.B.; Wang, C.; Fan, W.W.; Dong, F.W.; Xu, F.Q.; Wan, Q.L.; Luo, H.R.; Liu, Y.Q.; Hu, J.M.; Zhou, J. Chemical Components of Dendrobium crepidatum and Their Neurite Outgrowth Enhancing Activities. Nat. Prod. Bioprospect. 2013, 3, 70–73. [Google Scholar] [CrossRef]
- Réthy, B.; Kovács, A.; Zupkó, I.; Forgo, P.; Vasas, A.; Falkay, G.; Hohmann, J. Cytotoxic Phenanthrenes from the Rhizomes of Tamus Communis. Planta Med. 2006, 72, 767–770. [Google Scholar] [CrossRef]
- Fan, C.; Sun, X.; Wang, X.; Yu, H. Therapeutic Potential of the Chemical Composition of Dendrobium nobile Lindl. Front. Pharmacol. 2023, 14, 1163830. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.J.; Yang, L.; Yuan, M.Y.; Li, J.Y.; Hou, B.; Li, H.M.; Yang, X.Z.; Ding, C.C.; Hu, J.M. Sesquiterpene Amino Ether and Cytotoxic Phenols from Dendrobium Wardianum Warner. Fitoterapia 2017, 122, 76–79. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, C.; Zhuo, Y.; Gong, B.; Xu, W.; Zhang, G. 1,5,6-Trimethoxy-2,7-Dihydroxy-phenanthrene from Dendrobium Officinale Exhibited Antitumor Activities for HeLa Cells. Int. J. Mol. Sci. 2023, 24, 15375. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Monaco, P.; Previtera, L.; Zarrelli, A. Phenanthrenoids from the Wetland Juncus acutus. Phytochemistry 2002, 60, 633–638. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Ding, Y.Y.; Liu, F.; Li, N. Cytotoxic and Anti-Inflammatory Activities of Phenanthrenes from the Medullae of Juncus effusus L. Arch. Pharm. Res. 2016, 39, 154–160. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Kosuge, Y.; Hamagami, H.; Ozaki, M.; Ishige, K.; Ito, Y.; Kitanaka, S. Chemical Constituents Isolated from Juncus effusus Induce Cytotoxicity in HT22 Cells. J. Nat. Med. 2015, 69, 421–426. [Google Scholar] [CrossRef]
- Miles, D.H.; Bhattacharyya, J.; Mody, N.V.; Atwood, J.L.; Black, S.; Hedin, P.A. The Structure of Juncusol. A Novel Cytotoxic Dihydrophenanthrene from the Estuarine Marsh Plant Juncus Roemerianus. J. Am. Chem. Soc. 1977, 99, 618–620. [Google Scholar] [CrossRef]
- Gainche, M.; Ripoche, I.; Senejoux, F.; Cholet, J.; Ogeron, C.; Decombat, C.; Danton, O.; Delort, L.; Vareille-Delarbre, M.; Berry, A.; et al. Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica. Molecules 2020, 25, 2372. [Google Scholar] [CrossRef]
- Liu, W.; Meng, M.; Zhang, B.; Du, L.; Pan, Y.; Yang, P.; Gu, Z.; Zhou, Q.; Cao, Z. Dehydroeffusol Effectively Inhibits Human Gastric Cancer Cell-Mediated Vasculogenic Mimicry with Low Toxicity. Toxicol. Appl. Pharmacol. 2015, 287, 98–110. [Google Scholar] [CrossRef]
- Zhang, B.; Han, H.; Fu, S.; Yang, P.; Gu, Z.; Zhou, Q.; Cao, Z. Dehydroeffusol Inhibits Gastric Cancer Cell Growth and Tumorigenicity by Selectively Inducing Tumor-Suppressive Endoplasmic Reticulum Stress and a Moderate Apoptosis. Biochem. Pharmacol. 2016, 104, 8–18. [Google Scholar] [CrossRef]
- Yang, H.; Lee, P.; Jeong, E.J.; Kim, H.P.; Kim, Y.C. Selective Apoptosis in Hepatic Stellate Cells Mediates the Antifibrotic Effect of Phenanthrenes from Dendrobium nobile. Phytother. Res. 2012, 26, 974–980. [Google Scholar] [CrossRef]
- Nam, B.; Ryu, S.M.; Lee, D.; Jung, C.H.; Jin, C.H.; Kim, J.B.; Lee, I.S.; Han, A.R. Identification of Two New Phenanthrenes from Dendrobii Herba and Their Cytotoxicity towards Human Hypopharynx Squamous Carcinoma Cell (FaDu). Molecules 2019, 24, 2339. [Google Scholar] [CrossRef]
- Rabi, I.I.; Zacharias, J.R.; Millman, S.; Kusch, P. A New Method of Measuring Nuclear Magnetic Moment. Phys. Rev. 1938, 53, 318. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.G.; Sacco, A. Classification of Olive Oils According to Geographical Origin by Using 1H NMR Fingerprinting Combined with Multivariate Analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Maniara, G.; Rajamoorthi, K.; Rajan, S.; Stockton, G.W. Method Performance and Validation for Quantitative Analysis by 1H and 31P NMR Spectroscopy. Applications to Analytical Standards and Agricultural Chemicals. Anal. Chem. 1998, 70, 4921–4928. [Google Scholar] [CrossRef]
- Pauli, G.F. qNMR ? A Versatile Concept for the Validation of Natural Product Reference Compounds. Phytochem. Anal. 2001, 12, 28–42. [Google Scholar] [CrossRef]
- Wells, R.J.; Hook, J.M.; Al-Deen, T.S.; Hibbert, D.B. Quantitative Nuclear Magnetic Resonance (QNMR) Spectroscopy for Assessing the Purity of Technical Grade Agrochemicals: 2,4-Dichlorophenoxyacetic Acid (2,4-D) and Sodium 2,2-Dichloropropionate (Dalapon Sodium). J. Agric. Food Chem. 2002, 50, 3366–3374. [Google Scholar] [CrossRef]
- Deen, T.S.A.; Hibbert, D.B.; Hook, J.M.; Wells, R.J. Quantitative Nuclear Magnetic Resonance Spectrometry II. Purity of Phosphorus-Based Agrochemicals Glyphosate (N-(Phosphonomethyl)-Glycine) and Profenofos (O-(4-Bromo-2-Chlorophenyl) O-Ethyl S-Propyl Phosphorothioate) Measured by 1H and 31P QNMR Spectrometry. Anal. Chim. Acta 2002, 474, 125–135. [Google Scholar]
- Della Greca, M.; Fiorentino, A.; Molinaro, A.; Monaco, P.; Previtera, L. A Bioactive Dihydrodibenzoxepin from Juncus effusus. Phytochemistry 1993, 34, 1182–1184. [Google Scholar] [CrossRef]
- Chapatwala, K.D. Antimicrobial activity of Juncusol, a novel 9-10-dihydrophenanthrene from the marsh plant Juncus roemerianus. Life Sci. 1981, 29, 1997–2001. [Google Scholar] [CrossRef]
- Bús, C.; Kúsz, N.; Kincses, A.; Szemerédi, N.; Spengler, G.; Bakacsy, L.; Purger, D.; Berkecz, R.; Hohmann, J.; Hunyadi, A.; et al. Antiproliferative Phenanthrenes from Juncus Tenuis: Isolation and Diversity-Oriented Semisynthetic Modification. Molecules 2020, 25, 5983. [Google Scholar] [CrossRef]
- Su, X.H.; Yuan, Z.P.; Li, C.Y.; Zhong, Y.J.; Du, H.J.; Wen, Y.Y.; Li, Y.F.; Liang, B. Phenanthrenes from Juncus effusus. Planta Med. 2013, 79, 1447–1452. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Beak, N.; Kim, S.; Ahn, B. In Vitro and In Vivo Antitumoral Phenanthrenes from the Aerial Parts of Dendrobium nobile. Planta Med. 1995, 61, 178–180. [Google Scholar] [CrossRef]
- Kovács, A.; Forgo, P.; Zupkó, I.; Réthy, B.; Falkay, G.; Szabó, P.; Hohmann, J. Phenanthrenes and a Dihydrophenanthrene from Tamus Communis and Their Cytotoxic Activity. Phytochemistry 2007, 68, 687–691. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.K.; Luo, X.; Zhang, X.W. Bioactivity-Guided Isolation of Cytotoxic Phenanthrenes from Spiranthes sinensis. J. Agric. Food Chem. 2019, 67, 7274–7280. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 280, 63. [Google Scholar] [CrossRef]
- Bisoli, E.; Freire, T.V.; Yoshida, N.C.; Garcez, W.S.; Queiróz, L.M.M.; Matos, M.d.F.C.; Perdomo, R.T.; Garcez, F.R. Cytotoxic Phenanthrene, Dihydrophenanthrene, and Dihydrostilbene Derivatives and Other Aromatic Compounds from Combretum laxum. Molecules 2020, 25, 3154. [Google Scholar] [CrossRef]
- Li, B.; Ali, Z.; Chan, M.; Li, J.; Wang, M.; Abe, N.; Wu, C.R.; Khan, I.A.; Wang, W.; Li, S.X. Chemical Constituents of Pholidota cantonensis. Phytochemistry 2017, 137, 132–138. [Google Scholar] [CrossRef]
- Fei, Y.; Liu, Z.-X. Isolation and Characterization of the PISTILLATA Ortholog Gene from Cymbidium faberi Rolfe. Agronomy 2019, 9, 425. [Google Scholar] [CrossRef]
- Lv, S.; Fu, Y.; Chen, J.; Jiao, Y.; Chen, S. Six Phenanthrenes from the Roots of Cymbidium faberi Rolfe. and Their Biological Activities. Nat. Prod. Res. 2022, 36, 1170–1181. [Google Scholar] [CrossRef]
- Platella, C.; Criscuolo, A.; Riccardi, C.; Gaglione, R.; Arciello, A.; Musumeci, D.; DellaGreca, M.; Montesarchio, D. Exploring the Binding of Natural Compounds to Cancer-Related G-Quadruplex Structures: From 9,10-Dihydrophenanthrenes to Their Dimeric and Glucoside Derivatives. Int. J. Mol. Sci. 2023, 24, 7765. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Zarrelli, A. A New Dimeric 9,10-Dihydrophenanthrenoid from the rhizome of Juncus acutus. Tetrahedron Lett. 2002, 43, 2573–2575. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New Dimeric Phenanthrenoids from the Rhizomes of Juncus acutus. Structure Determination and Antialgal Activity. Tetrahedron Lett. 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- Behery, F.A.A.; Naeem, Z.E.M.; Maatooq, G.T.; Amer, M.M.A.; Ahmed, A.F. A Novel Antioxidant Phenanthrenoid Dimer from Juncus acutus L. Nat. Prod. Res. 2013, 27, 155–163. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Zarrelli, A. Dimeric Phenanthrenoids from the Juncus acutus. Nat. Prod. Res. 2005, 19, 69–74. [Google Scholar] [CrossRef]
- Ma, W.; Liu, F.; Ding, Y.Y.; Zhang, Y.; Li, N. Four New Phenanthrenoid Dimers from Juncus effusus L. with Cytotoxic and Anti-Inflammatory Activities. Fitoterapia 2015, 105, 83–88. [Google Scholar] [CrossRef]
- Bús, C.; Kúsz, N.; Jakab, G.; Senobar Tahaei, S.; Zupkó, I.; Endrész, V.; Bogdanov, A.; Burián, K.; Csupor-Löffler, B.; Hohmann, J.; et al. Phenanthrenes from Juncus compressus Jacq. with Promising Antiproliferative and Anti-HSV-2 Activities. Molecules 2018, 23, 2085. [Google Scholar] [CrossRef]
- Luo, Q.; Tang, Z.; Zhang, X.; Zhong, Y.; Yao, S.; Wang, L.; Lin, C.; Luo, X. Chemical Properties and Antioxidant Activity of a Water-Soluble Polysaccharide from Dendrobium Officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Zheng, C.J.; Gan, L.S.; Chen, G.Y.; Zhang, X.P.; Song, X.P.; Li, G.N.; Sun, C.G. Bioactive Phenanthrene and Bibenzyl Derivatives from the Stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Yang, D.; Liu, L.Y.; Cheng, Z.Q.; Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Zhou, J.; Ding, Z.T.; Hu, J.M. Five New Phenolic Compounds from Dendrobium aphyllum. Fitoterapia 2015, 100, 11–18. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Q.; Tan, X.; Jiang, H.; Li, X.; Chen, K.; Kinghorn, A.D. Three New Sesquiterpene Glycosides from Dendrobium nobile with Immunomodulatory Activity. J. Nat. Prod. 2001, 64, 1196–1200. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In Vitro Antioxidant Activities of a Water-Soluble Polysaccharide Derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, C.; Zhao, X.; Wang, Y.; Feng, D.; Zhang, M.; Xie, H. (±)-Homocrepidine A, a Pair of Anti-Inflammatory Enantiomeric Octahydroindolizine Alkaloid Dimers from Dendrobium crepidatum. J. Nat. Prod. 2016, 79, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wang, F.; Dong, H.; Guo, S.; Yang, J.; Xiao, P. Chemical Constituents of Dendrobium candidum. China J. Chin. Mater. Medica 2010, 35, 1715–1719. [Google Scholar]
- Li, R.; Yang, X.; He, P.; Gan, N. Studies on phenanthrene constituents from stems of Dendrobium candidum. J. Chin. Med. Mater. 2009, 32, 220–223. [Google Scholar]
- Cheng, L.; Guo, D.-L.; Zhang, M.-S.; Linghu, L.; Fu, S.-B.; Deng, Y.; He, Y.Q.; Xiao, S.J. Dihydrophenanthrofurans and Bisbibenzyl Derivatives from the Stems of Dendrobium nobile. Fitoterapia 2020, 143, 104586. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, W. New Alloaromadendrane, Cadinene and Cyclocopacamphane Type Sesquiterpene Derivatives and Bibenzyls from Dendrobium nobile. Planta Med. 2002, 68, 723–729. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical Constituents of Dendrobium venustum and Their Antimalarial and Anti-Herpetic Properties. Nat. Prod. Commun. 2014, 9, 825–827. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Lian, X. Isolation of Stilbenoids and Lignans from Dendrobium Hongdie. Trop. J. Pharm. Res. 2015, 14, 2055. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.G.; Li, J.T.; Yin, B.L.; Liu, Y. Bibenzyls, 9,10-Dihydrophenanthrenes, and Phenanthraquinone from Dendrobium longicornu. Chem. Nat. Compd. 2010, 46, 790–791. [Google Scholar] [CrossRef]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Five New Compounds from Dendrobium longicornu. Planta Med. 2008, 74, 535–539. [Google Scholar] [CrossRef]
- Chen, D.-N.; Wang, Y.Y.; Liu, W.J.; Chen, Y.J.; Wu, Y.P.; Wang, J.X.; He, F.; Jiang, L. Stilbenoids from Aerial Parts of Dendrobium plicatile. Nat. Prod. Res. 2020, 34, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Lv, X.; Chen, J.; Peng, M.; Cai, J. Recent Research Progress on Natural Stilbenes in Dendrobium Species. Molecules 2022, 27, 7233. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.L.; Pal (Née Ray), S. Rotundatin, a New 9,10-Didydrophenanthrene Derivative from Dendrobium rotundatum. Phytochemistry 1992, 31, 3225–3228. [Google Scholar] [CrossRef]
- Li, J.; Feng, W.; Dai, R.; Li, B. Recent Progress on the Identification of Phenanthrene Derivatives in Traditional Chinese Medicine and Their Biological Activities. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100078. [Google Scholar] [CrossRef]
- Mittraphab, A.; Muangnoi, C.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A New Bibenzyl-Phenanthrene Derivative from Dendrobium signatum and Its Cytotoxic Activity. Nat. Prod. Commun. 2016, 11, 657–659. [Google Scholar] [CrossRef]
- Zhan, R.; Wang, Z.C.; Yin, B.L.; Liu, Y.; Chen, Y.G. Novel 9, 10-Dihydrophenanthrene Derivatives from Eria bambusifolia with Cytotoxicity Aganist Human Cancer Cells In Vitro. Chin. J. Nat. Med. 2016, 14, 621–625. [Google Scholar] [CrossRef]
- Sun, A.; Liu, J.; Pang, S.; Lin, J.; Xu, R. Two Novel Phenanthraquinones with Anti-Cancer Activity Isolated from Bletilla striata. Bioorg. Med. Chem. Lett. 2016, 26, 2375–2379. [Google Scholar] [CrossRef]
- Kumar, N.P.; Nekkanti, S.; Sujana Kumari, S.; Sharma, P.; Shankaraiah, N. Design and Synthesis of 1,2,3-Triazolo-Phenanthrene Hybrids as Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2369–2376. [Google Scholar] [CrossRef]
- Li, J.Y.; Kuang, M.T.; Yang, L.; Kong, Q.H.; Hou, B.; Liu, Z.H.; Chi, X.Q.; Yuan, M.Y.; Hu, J.M.; Zhou, J. Stilbenes with Anti-Inflammatory and Cytotoxic Activity from the Rhizomes of Bletilla ochracea Schltr. Fitoterapia 2018, 127, 74–80. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Vizetto-Duarte, C.; Gangadhar, K.N.; Zengin, G.; Mollica, A.; Varela, J.; Barreira, L.; Custódio, L. In Vitro and In Silico Approaches to Unveil the Mechanisms Underlying the Cytotoxic Effect of Juncunol on Human Hepatocarcinoma Cells. Pharmacol. Rep. 2018, 70, 896–899. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.; Yang, J.; Zhang, D.D.; Wang, Y.L.; Li, S.H.; Pan, Y.N.; Zhang, H.M.; Sun, Y. Comparative Analysis of Aristolochic Acids in Aristolochia Medicinal Herbs and Evaluation of Their Toxicities. Toxins 2022, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, S.; Wen, D.; Che, B.; Liao, Y.; Liu, H.; Feng, X.; Hu, S. Rapid Determination of Aristolochic Acid I and II in Aristolochia Plants from Different Regions by β-Cyclodextrin-Modified Capillary Zone Electrophoresis. J. Chromatogr. A 2004, 1049, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.; Fernandes, A. Ancient Medicinal Use of Aristolochia: Birthwort’s Tradition and Toxicity. Pharm. Hist. 2011, 53, 3–21. [Google Scholar] [PubMed]
- Dey, A.; Hazra, A.K.; Mukherjee, A.; Nandy, S.; Pandey, D.K. Chemotaxonomy of the Ethnic Antidote Aristolochia indica for Aristolochic Acid Content: Implications of Anti-Phospholipase Activity and Genotoxicity Study. J. Ethnopharmacol. 2021, 266, 113416. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, P.C.; Wang, J.D. Chinese Herbs Containing Aristolochic Acid Associated with Renal Failure and Urothelial Carcinoma: A Review from Epidemiologic Observations to Causal Inference. BioMed Res. Int. 2014, 2014, 569325. [Google Scholar] [CrossRef]
- Dickman, K.G.; Sweet, D.H.; Bonala, R.; Ray, T.; Wu, A. Physiological and Molecular Characterization of Aristolochic Acid Transport by the Kidney. J. Pharmacol. Exp. Ther. 2011, 338, 588–597. [Google Scholar] [CrossRef]
- Han, J.; Xian, Z.; Zhang, Y.; Liu, J.; Liang, A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front. Pharmacol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Arlt, V.M. Aristolochic Acid as a Probable Human Cancer Hazard in Herbal Remedies: A Review. Mutagenesis 2002, 17, 265–277. [Google Scholar] [CrossRef]
- Luciano, R.L.; Perazella, M.A. Aristolochic Acid Nephropathy: Epidemiology, Clinical Presentation, and Treatment. Drug Saf. 2015, 38, 55–64. [Google Scholar] [CrossRef]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic Acids and Their Derivatives Are Widely Implicated in Liver Cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, Q.; Ma, G.; Zhu, L.; Wang, Y.; Chen, Z.; Wang, Y.; Zhao, L. New Dual-Spectroscopic Strategy for the Direct Detection of Aristolochic Acids in Blood and Tissue. Anal. Chem. 2019, 91, 8154–8161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Zhou, C.; Jia, Y.; Liu, S.; Xiong, Z.; Guo, X.; Fei, X.; Jiang, X.; Yu, W. Aristolochic Acid Induces Mitochondrial Apoptosis through Oxidative Stress in Rats, Leading to Liver Damage. Toxicol. Mech. Methods 2021, 31, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Jin, L.; Luo, K.; Jin, J.; Lim, S.W.; Shin, Y.J.; Ko, E.J.; Chung, B.H.; Yang, C.W. Assessment of Nephrotoxicity of Herbal Medicine Containing Aristolochic Acid in Mice. Korean J. Intern. Med. 2020, 35, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The Ameliorations of Ganoderma applanatum Residue Polysaccharides against CCl4 Induced Liver Injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; De Plaen, J.F.; Ferluga, D.; Van Ypersele De Strihou, C. Chinese Herbs Nephropathy: A Clue to Balkan Endemic Nephropathy? Kidney Int. 1994, 45, 1680–1688. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.-L.; Nortier, J.L. Aristolochic Acid Nephropathy: A Worldwide Problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic Aspects of a Newly Described Nephropathy Related to the Prolonged Use of Chinese Herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen-Fastre, R.; But, P.; Vanherweghem, J.-L. Identification of Aristolochic Acid in Chinese Herbs. Lancet 1994, 343, 174. [Google Scholar] [CrossRef]
- Anandagoda, N.; Lord, G.M. Preventing Aristolochic Acid Nephropathy. Clin. J. Am. Soc. Nephrol. 2015, 10, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, J.P. Aristolochic Acid and ‘Chinese Herbs Nephropathy’. Drug. Safety 2003, 26, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Pozdzik, A.A.; Salmon, I.J.; Debelle, F.D.; Decaestecker, C.; Van Den Branden, C.; Verbeelen, D.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic Acid Induces Proximal Tubule Apoptosis and Epithelial to Mesenchymal Transformation. Kidney Int. 2008, 73, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Jadot, I.; Declèves, A.E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Carlson, B.A.; Tobe, R.; Yefremova, E.; Tsuji, P.A.; Hoffmann, V.J.; Schweizer, U.; Gladyshev, V.N.; Hatfield, D.L.; Conrad, M. Glutathione Peroxidase 4 and Vitamin E Cooperatively Prevent Hepatocellular Degeneration. Redox Biol. 2016, 9, 22–31. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of Ferroptosis Regulator Glutathione Peroxidase 4 in Forebrain Neurons Promotes Cognitive Impairment and Neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Huang, X.; Liu, R.; Zhan, C.; Wu, H.; Fan, J.; Li, Z.; Yang, X. Aristolochic Acid Induces Acute Kidney Injury through Ferroptosis. Front. Pharmacol. 2024, 15, 1330376. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of P53 Promotes Renal Injury in Acute Aristolochic Acid Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 31–41. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-Y.; Wu, T.S.; Chen, T.W.; Liu, B.H. Aristolochic Acid I Induced Oxidative DNA Damage Associated with Glutathione Depletion and ERK1/2 Activation in Human Cells. Toxicol. Vitro 2011, 25, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.; Jadot, I.; Colombaro, V.; Martin, B.; Voisin, V.; Nortier, J.; Caron, N. Protective Effect of Nitric Oxide in Aristolochic Acid-induced Toxic Acute Kidney Injury: An Old Friend with New Assets. Exp. Physiol. 2016, 101, 193–206. [Google Scholar] [CrossRef]
- Kim, J.Y.; Leem, J.; Jeon, E.J. Protective Effects of Melatonin Against Aristolochic Acid-Induced Nephropathy in Mice. Biomolecules 2019, 10, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, X.; Fang, L.; He, W.; Dai, C.; Yang, J. Aristolochic Acid Causes Albuminuria by Promoting Mitochondrial DNA Damage and Dysfunction in Podocyte. PLoS ONE 2013, 8, e83408. [Google Scholar] [CrossRef]
- Xie, X.C.; Zhao, N.; Xu, Q.H.; Yang, X.; Xia, W.K.; Chen, Q.; Wang, M.; Fei, X. Relaxin Attenuates Aristolochic Acid Induced Human Tubular Epithelial Cell Apoptosis In Vitro by Activation of the PI3K/Akt Signaling Pathway. Apoptosis 2017, 22, 769–776. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, Y.; Shi, B.; Jin, Y.; Wang, Y.; Jiang, X.; Song, M.; Yu, W. Grape Seed Extract Proanthocyanidin Antagonizes Aristolochic Acid I-Induced Liver Injury in Rats by Activating PI3K-AKT Pathway. Toxicol. Mech. Methods 2023, 33, 131–140. [Google Scholar] [CrossRef]
- Yang, X.; Thorngren, D.; Chen, Q.; Wang, M.; Xie, X. Protective Role of Relaxin in a Mouse Model of Aristolochic Acid Nephropathy. Biomed. Pharmacother. 2019, 115, 108917. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Jin, J.; Guan, C.; Li, M.; Xi, C.; Ouyang, Z.; Chen, M.; Qiu, Y.; Huang, M.; et al. Endoplasmic Reticulum Stress Mediates Aristolochic Acid I-Induced Apoptosis in Human Renal Proximal Tubular Epithelial Cells. Toxicol. In Vitro 2012, 26, 663–671. [Google Scholar] [CrossRef]
- Hsin, Y.H.; Cheng, C.H.; Tzen, J.T.C.; Wu, M.J.; Shu, K.H.; Chen, H.C. Effect of Aristolochic Acid on Intracellular Calcium Concentration and Its Links with Apoptosis in Renal Tubular Cells. Apoptosis 2006, 11, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Debelle, F.A.A.A.A.D.; Nortier, J.D.L.; De Prez, E.G.; Garbar, C.H.; Vienne, A.R.; Salmon, I.J.; Deschodt-Lanckman, M.M.; Vanherweghem, J.-L. Aristolochic Acids Induce Chronic Renal Failure with Interstitial Fibrosis in Salt-Depleted Rats. J. Am. Soc. Nephrol. 2002, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, M.; Foresto-Neto, O.; Steiger, S.; Kraft, F.; Koehler, P.; Von Rauchhaupt, E.; Potempa, J.; Adamowicz, K.; Koziel, J.; Lech, M. Aristolochic Acid I Determine the Phenotype and Activation of Macrophages in Acute and Chronic Kidney Disease. Sci. Rep. 2018, 8, 12169. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J.; Chen, Y.H. Developmental Nephrotoxicity of Aristolochic Acid in a Zebrafish Model. Toxicol. Appl. Pharmacol. 2012, 261, 59–65. [Google Scholar] [CrossRef]
- Jadot, I.; Colombaro, V.; Martin, B.; Habsch, I.; Botton, O.; Nortier, J.; Declèves, A.-E.; Caron, N. Restored Nitric Oxide Bioavailability Reduces the Severity of Acute-to-Chronic Transition in a Mouse Model of Aristolochic Acid Nephropathy. PLoS ONE 2017, 12, e0183604. [Google Scholar] [CrossRef]
- Wang, S.; Fan, J.; Mei, X.; Luan, J.; Li, Y.; Zhang, X.; Chen, W.; Wang, Y.; Meng, G.; Ju, D. Interleukin-22 Attenuated Renal Tubular Injury in Aristolochic Acid Nephropathy via Suppressing Activation of NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2277. [Google Scholar] [CrossRef]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front. Immunol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Wang, L.; Liu, N.; Xue, X.; Zhou, S. The Effect of Overexpression of the Enhancer of Zeste Homolog 1 (EZH1) Gene on Aristolochic Acid-Induced Injury in HK-2 Human Kidney Proximal Tubule Cells In Vitro. Med. Sci. Monit. 2019, 25, 801–810. [Google Scholar] [CrossRef]
- Lemy, A.; Wissing, K.M.; Rorive, S.; Zlotta, A.; Roumeguere, T.; Muniz Martinez, M.-C.; Decaestecker, C.; Salmon, I.; Abramowicz, D.; Vanherweghem, J.-L.; et al. Late Onset of Bladder Urothelial Carcinoma After Kidney Transplantation for End-Stage Aristolochic Acid Nephropathy: A Case Series With 15-Year Follow-Up. Am. J. Kidney Dis. 2008, 51, 471–477. [Google Scholar] [CrossRef]
- Kwok, H.-C.; Chan, W. Aristolochic Acid Exposure via Dermal Contact or Inhalation of Herbal Powders: Evidence of Occupational Exposure in Herbalists with Urothelial Cancer. Chem. Res. Toxicol. 2024, 37, 873–877. [Google Scholar] [CrossRef]
- Chen, C.J.; Chiu, W.C.; Tseng, Y.H.; Lin, C.M.; Yang, H.Y.; Yang, Y.H.; Chen, P.C. Aristolochic Acid and the Risk of Cancers in Patients with Type 2 Diabetes: Nationwide Population-Based Cohort Study. Phytomedicine 2022, 99, 154023. [Google Scholar] [CrossRef]
- Au, C.K.; Chan, C.K.; Tung, K.K.; Zhang, J.; Chan, W. Quantitation of DNA Adducts of Aristolochic Acids in Repair-Deficient Cells: A Mechanistic Study of the DNA Repair Mechanism. Chem. Res. Toxicol. 2020, 33, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Bárta, F.; Dedíková, A.; Bebová, M.; Dušková, Š.; Mráz, J.; Schmeiser, H.H.; Arlt, V.M.; Hodek, P.; Stiborová, M. Co-Exposure to Aristolochic Acids I and II Increases DNA Adduct Formation Responsible for Aristolochic Acid I-Mediated Carcinogenicity in Rats. Int. J. Mol. Sci. 2021, 22, 10479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; He, H.; Zhao, W.; Wong, Y.; Li, W.; Feng, S.; Liu, B.; Wang, J.; Luo, P. Hepatotoxic effects of aristolochic acid: Mechanisms and implications. Acta Mater. Medica 2024, 3, 349–362. [Google Scholar] [CrossRef]
- Sidorenko, V.S. Biotransformation and Toxicities of Aristolochic Acids. Adv. Exp. Med. Biol. 2020, 1241, 139–166. [Google Scholar]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC Technology: From Drug Development to Probe Technology for Target Deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef] [PubMed]
 indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects.
indicate the inhibitory effects.
 indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects.
indicate the inhibitory effects.







 indicate the promotional effects.
indicate the promotional effects.
 indicate the promotional effects.
indicate the promotional effects.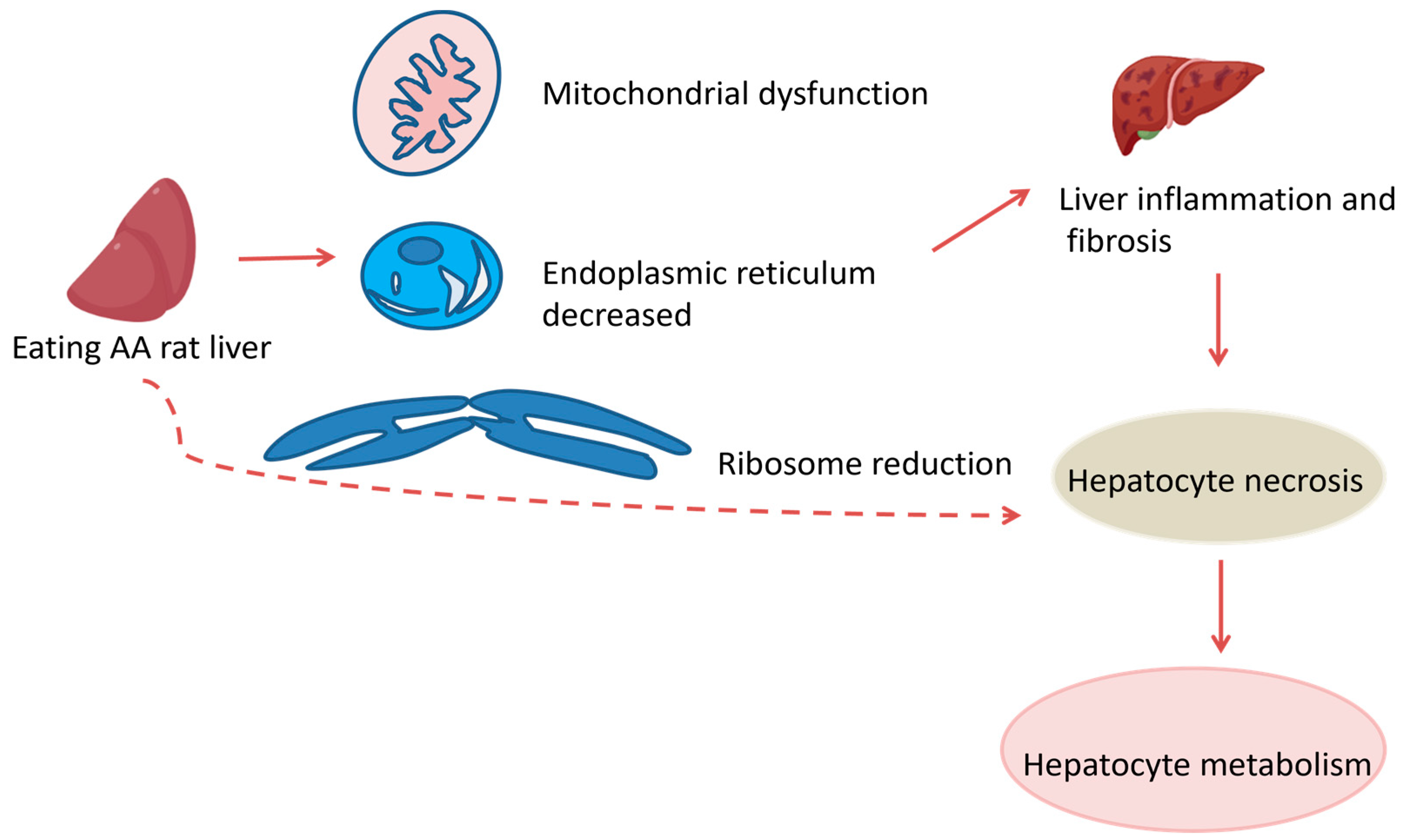
 indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects,
indicate the inhibitory effects,  indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.
 indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects,
indicate the inhibitory effects,  indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.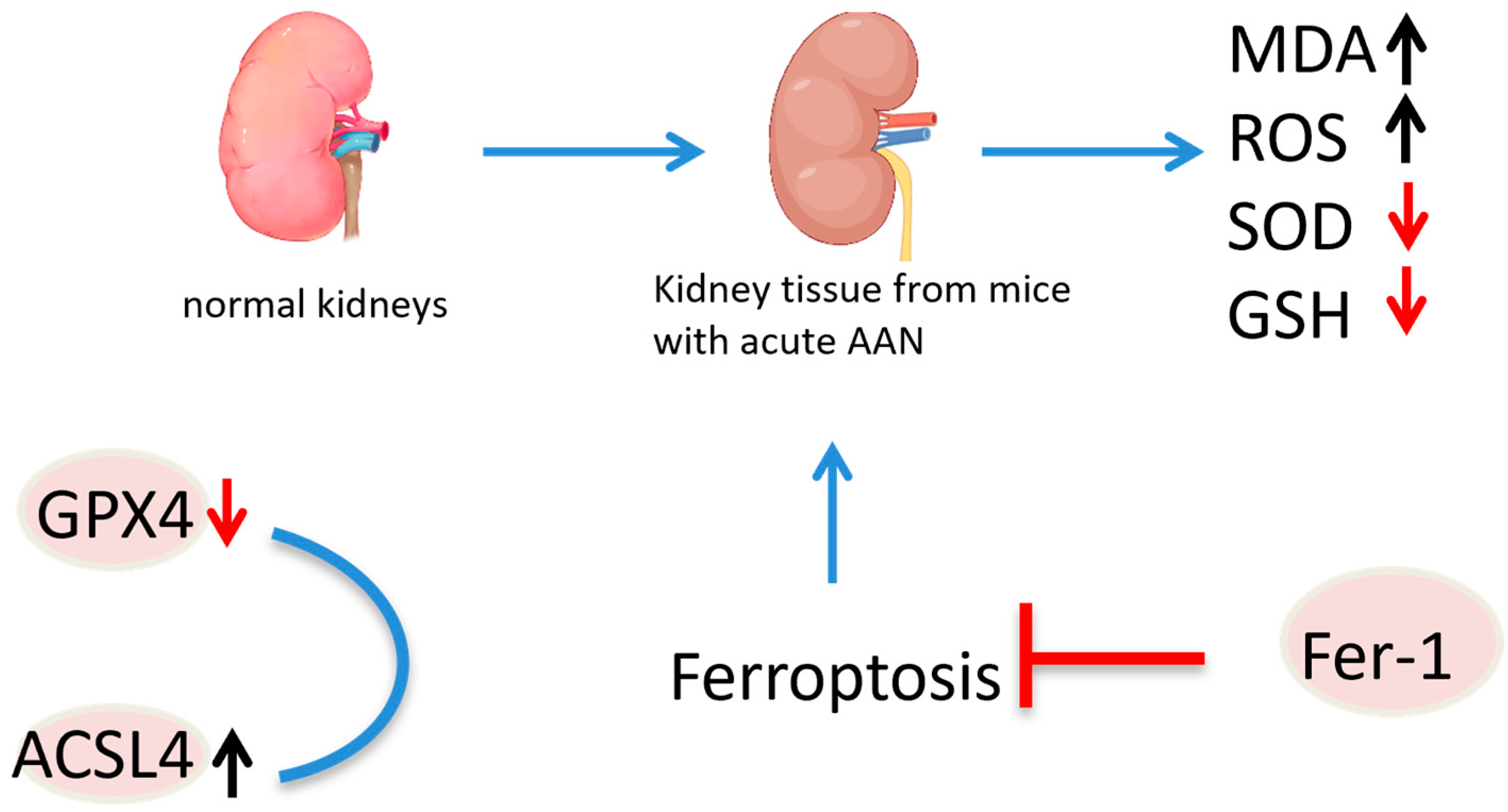
 indicate the promotional effects and
indicate the promotional effects and  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.
 indicate the promotional effects and
indicate the promotional effects and  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.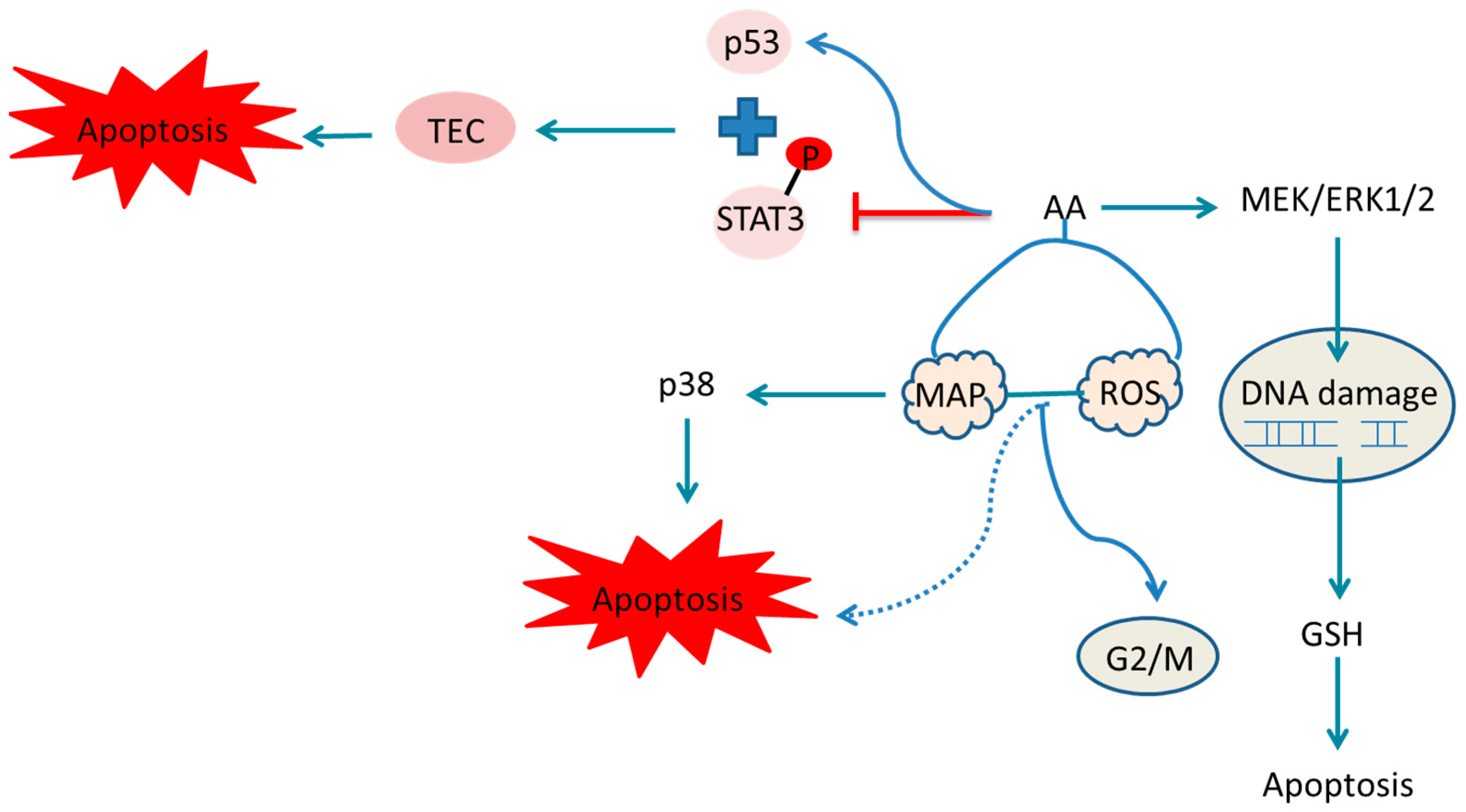
| Compounds | Anticancer Activity | Source | References |
|---|---|---|---|
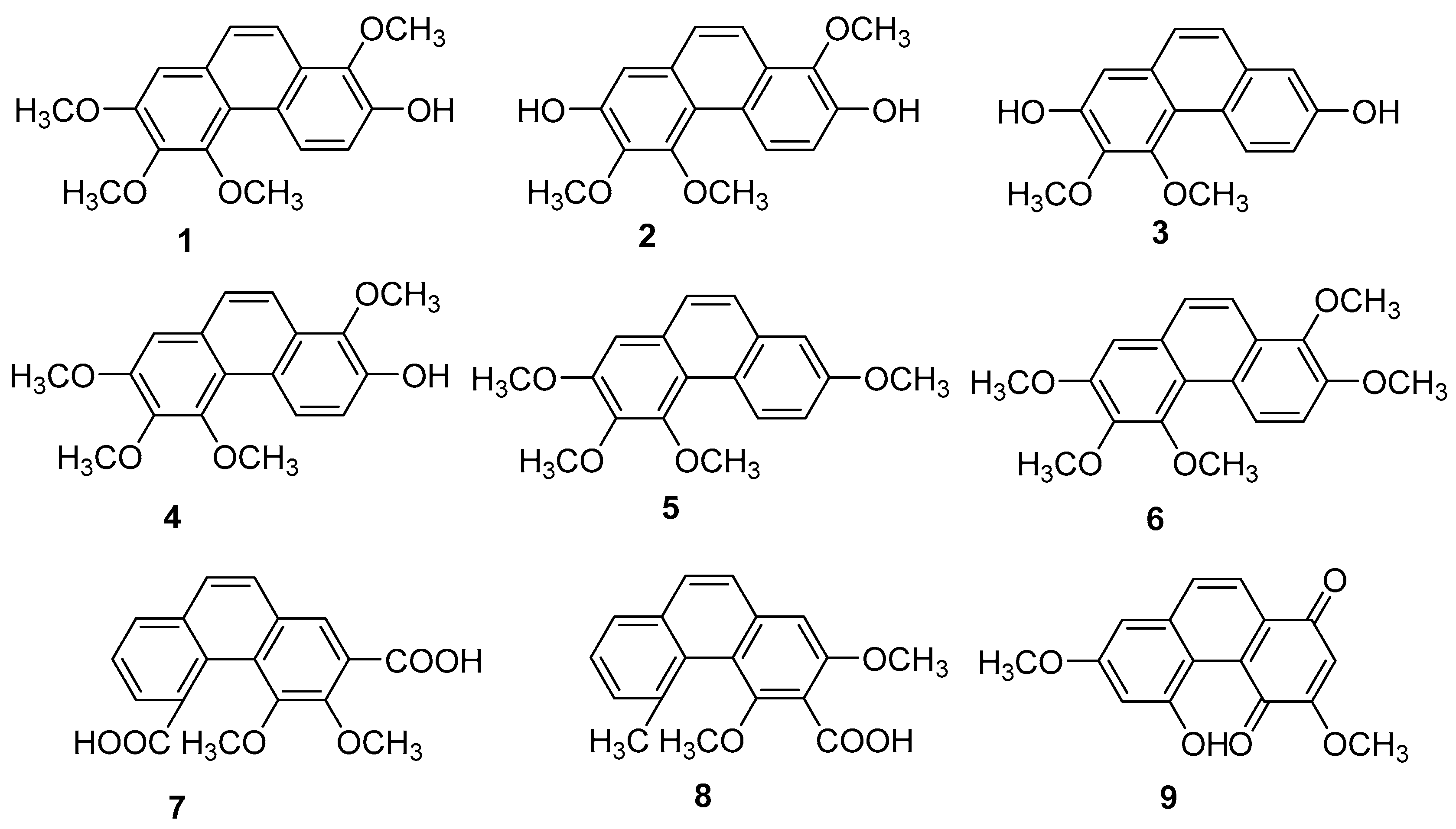
| Chrysotoxene (1) | IC50 (HepG2) = 19.64 µM. | Dendrobium genus | [12,13,14,15,16] |
| Confusarin (2) | IC50 (HI-60) = 18.95 ± 0.70 µM; IC50 (THP-1) = 11.51 ± 0.12 µM. | Dendrobium genus | [12,13,14,15,16] |
| Nudol (3) | IC50 (MG63) = 12.97 ± 0.28 µM; IC50 (U2OS) = 11.29 ± 0.21 µM. | Dendrobium genus | [12,13,14,15,16] |
| 7-dihydroxy-3,4,8-trimethoxyphenan threne (12) | IC50 (HeLa) = 0.97 µM. | Tamus communis | [20] |
| Moscatin (17) | IC50 (MCF-7) = 23.75 ± 0.28 µM; IC50 (A549) = 16.29 ±0.25 µM; IC50 (SW480) = 18.97 ± 1.04 µM. | Dendrobium nobile | [21,22] |
| 1,5,6-trimethoxy-2,7-dihydroxy- phenanthrene (19) | IC50 (HeLa) = 0.42 µM; IC50 (HepG2) = 0.2 µM. | Dendrobium officinale | [23] |
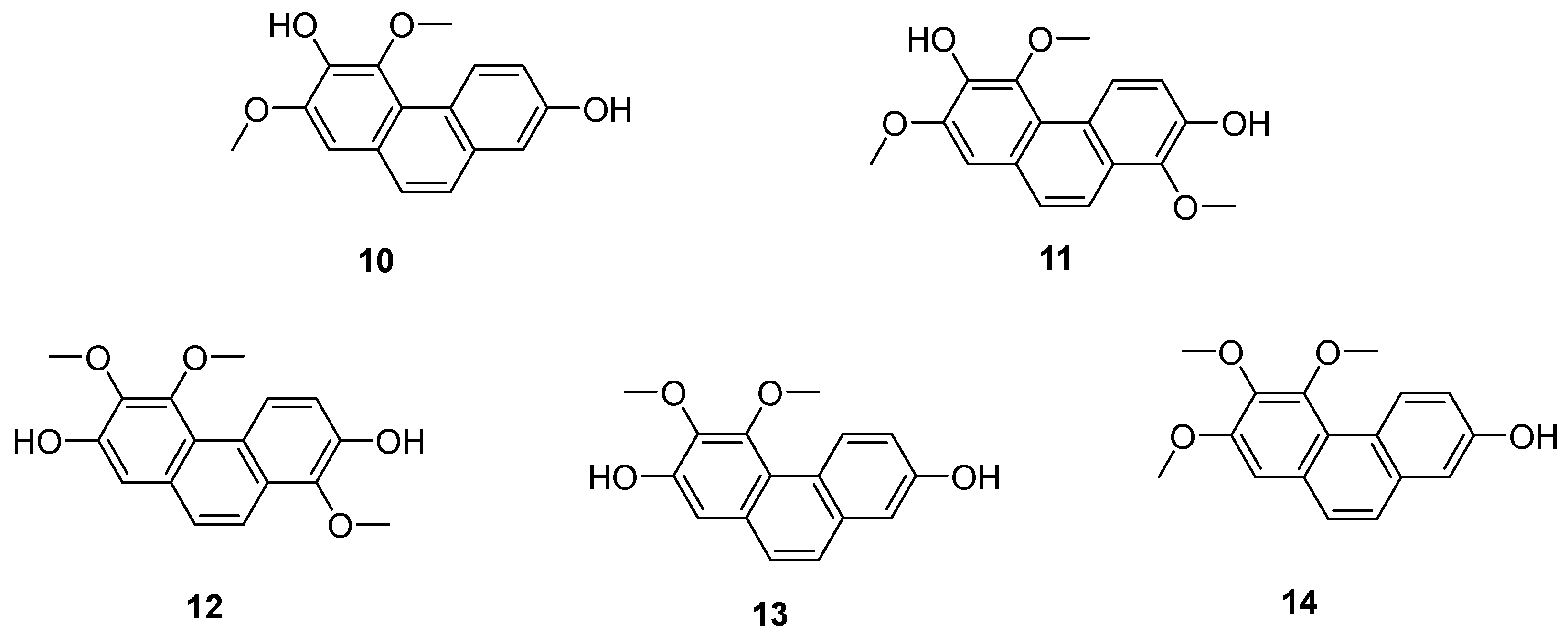
| Hydrojuncinol (22) | IC50 (THP-1) = 3 µM. | L. sylvatica | [25,26,27,28] |
| Hydrojuncuenin (23) | IC50 (THP-1) = 5 µM. | L. sylvatica | [25,26,27,28] |
| Dehydrogenated rush alcohol (24) | IC50 (SGC-7901) = 35.89 µM; IC50 (AGS) = 32.92 µM. | Traditional Chinese herbal medicine rush | [29] |
| 5-(1-methoxyethyl)-1-methyl-phenanthrene-2,7-diol (25) | IC50 (MCF-7) = 10.87 ± 0.82 µM; IC50 (HepG2) = 37.03 ± 2.44 µM; IC50 (HeLa) = 52.82 ± 5.58 µM; IC50 (SHSY-5Y) = 31.98 ± 2.64 µM; IC50 (SMMC-7721) = 42.94 ± 2.95 µM. | J. effuses | [30] |
| Dehydroeffusal (26) | IC50 (MCF-7) = 45.83 ± 5.54 µM; IC50 (HepG2) = 12.43 ± 2.56 µM; IC50 (HeLa) = 13.07 ± 2.56 µM; IC50 (SHSY-5Y) = 30.05 ± 1.64 µM; IC50 (SMMC-7721) = 25.35 ± 2.08 µM. | J. effuses | [30] |
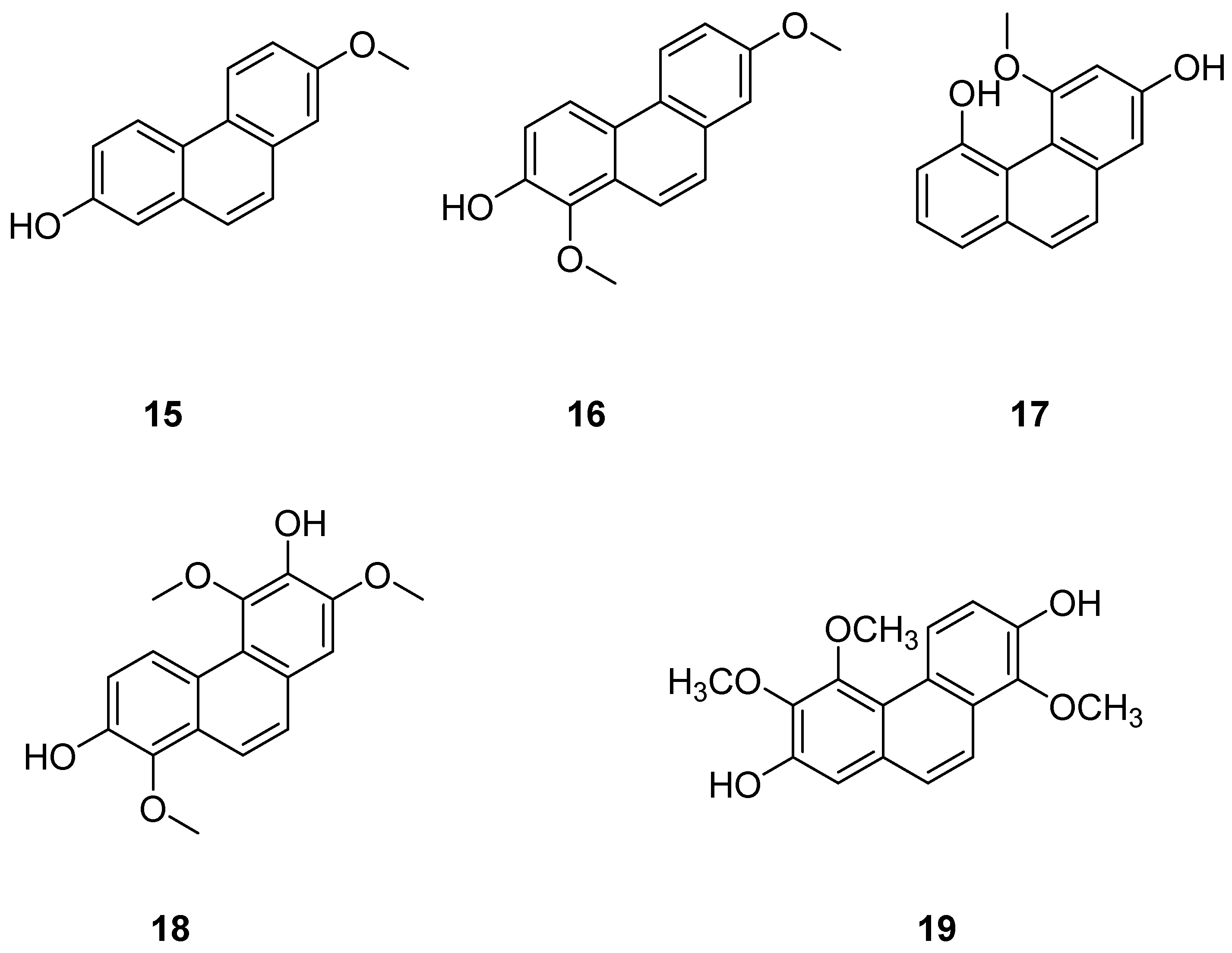
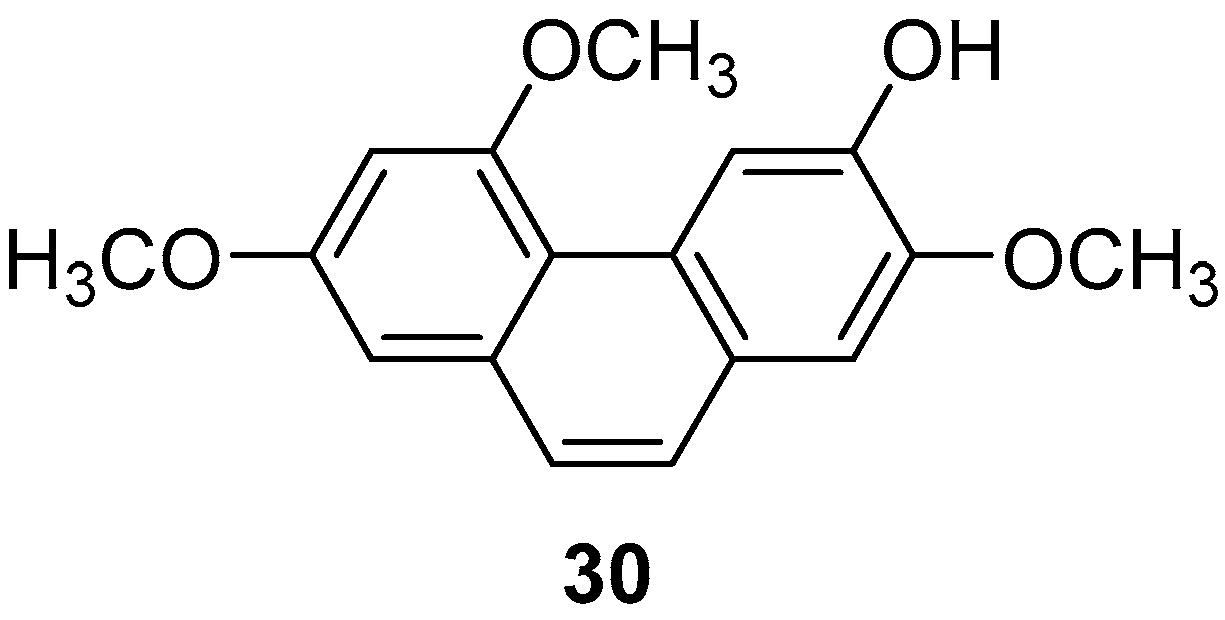
| Extract I (30) | IC50 (prostaglandin D2 and leukotriene C4) = 1.78 µM. | Batatasins | [33,37,38] |
| 7-methoxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthren-2-ol (32) | IC50 (A2780) = 22.3 ± 2.7 µM; IC50 (A2780 Cis) = 16.9 ± 4.7 µM; IC50 (KCR) = 24.2 ± 2.1 µM; IC50 (MCF-7) = 12.9 ± 0.7 µM; IC50 (HeLa) = 24.7 ± 0.3 µM; IC50 (HTB-26) = 22.8 ± 0.2 µM; IC50 (T47D) = 14.2 ± 1.1 µM; IC50 (MRC-5) = 18.9 ± 4.0 µM. | Juncus acutus | [24,41] |
| 1,6-dimethyl-5-vinyl-9,10-dihydrophenanthrene-2,7-diol (34) | IC50 (A2780) = 23.8 ± 1.3 µM; IC50 (A2780 Cis) = 37.1 ± 2.8 µM; IC50 (KCR) = 35.8 ± 1.7 µM; IC50 (MCF-7) = 37.1 ± 1.1 µM; IC50 (HeLa) = 0.5 ± 0.0 µM; IC50 (HTB-26) = 41.7 ± 3.5 µM; IC50 (T47D) = 25.0 ± 0.4 µM; IC50 (MRC-5) = 40.9 µM. | Juncus acutus | [24,41] |
| Juncuenins E (40) | IC50 (MCF-7) = 21.3 µM; IC50 (HeLa) = 60.5 µM. | Juncus effuses L. | [42] |
| Juncuenins F (41) | IC50 (MCF-7) > 100 µM; IC50 (HeLa) > 100 µM. | Juncus effuses L. | [42] |
| Juncuenins G (42) | IC50 (MCF-7) > 100 µM; IC50 (HeLa) > 100 µM. | Juncus effuses L. | [42] |
| 4,7-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (43) | IC50 (MCF-7) = 9.17 µM; IC50 (HeLa) = 19.6 µM. | Juncus effuses L. | [42] |
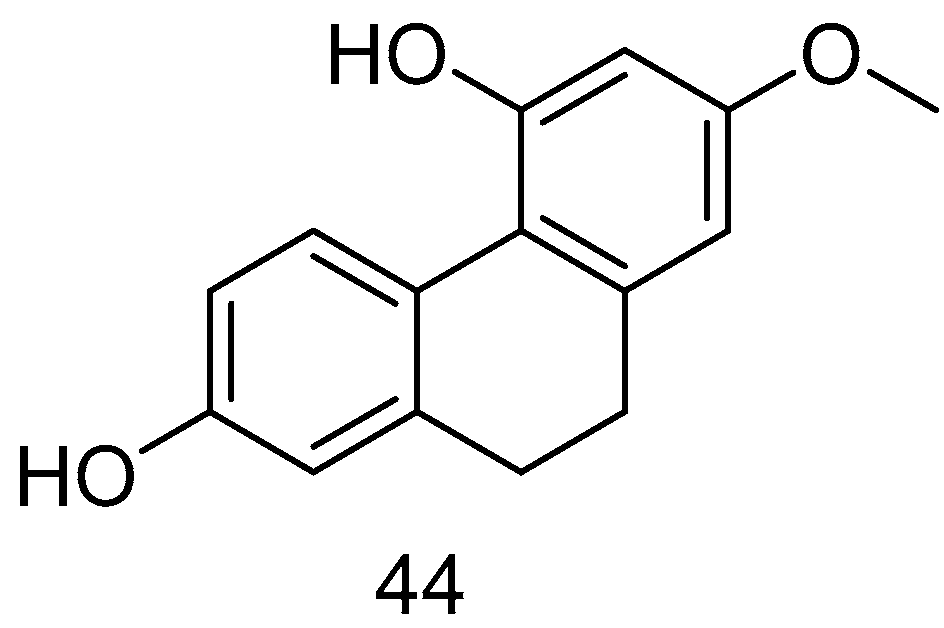
| Lusianthridin (44) | IC50 (A549) = 7.7 µM; IC50 (SK-OV-3) = 37.1 ± 2.8 µM; IC50 (KCR) = 35.8 ± 1.7 µM | Dendrobium nobile Lindl. | [44] |
| Orchinol (45) | IC50 (HI-60) = 11.96 µM; IC50 (THP-1) = 8.92 µM. | Dendrobium officinale Kimura & Migo | [13] |
| Spiranthesphenanthrine A (46) | IC50 (B16-F10) = 19.0 ± 7.3 µM. | Orchid plant Panlongshen | [45] |
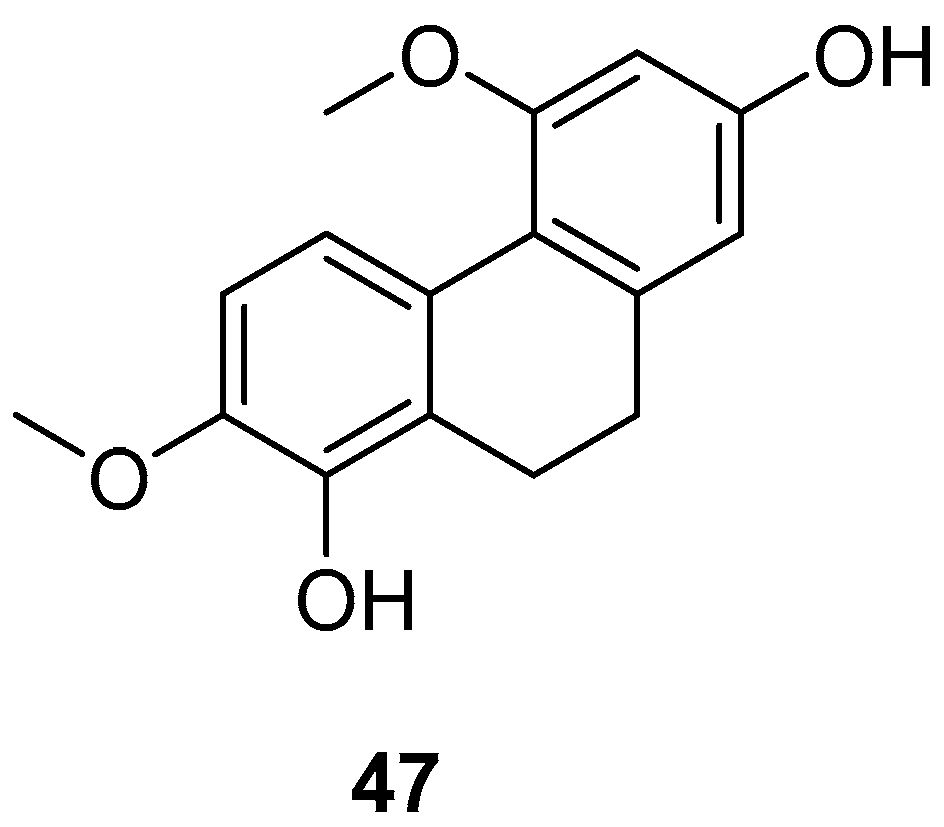
| 9,10-dihydro-4,7-dimethoxyphenanthrene-2,8-diol (47) | IC50 (RAW) = 25.0 to 87.2 µM. | Bai Ji | [46] |
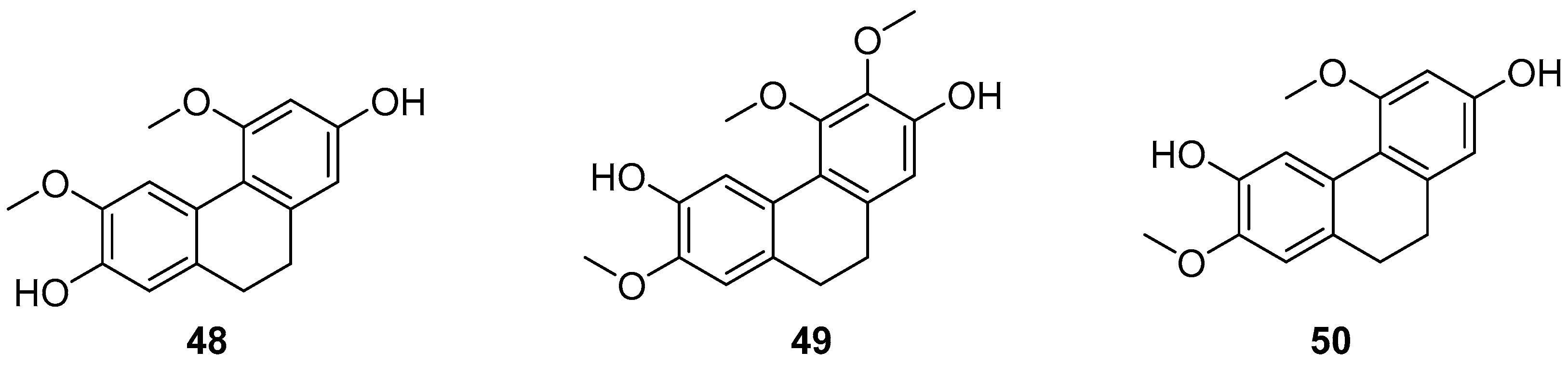
| 2,7-dihydroxy-4,6-dimethoxyphenylene (48) | IC50 (786-0) = 56.98 ± 9.29 µM; IC50 (MCF-7) = 46.99 ± 5.55 µM; IC50 (Hep2) > 100 µM; IC50 (UACC-62) = 2.59 ± 0.11 µM; IC50 (NCI/ADR-RES) = 58.83 ± 2.33 µM. | Combretum laxum | [47] |
| 2,6-dihydroxy-3,4,7-trimethoxy-9,10-dihydrophenanthrene (49) | IC50 (786-0) > 100 µM; IC50 (MCF-7) = 42.01 ± 9.33 µM; IC50 (Hep2) > 100 µM; IC50 (UACC-62) > 100 µM; IC50 (NCI/ADR-RES) > 100 µM. | Combretum laxum | [47] |
| 2,6-dihydroxy-4,7-dimethoxy-9,10- dihydrophenanthrene (50) | IC50 (786-0) > 100 µM; IC50 (MCF-7) > 100 µM; IC50 (Hep2) = 47.58 ± 0.11µM; IC50 (UACC-62) = >100 µM; IC50 (NCI/ADR-RES) > 100 µM. | Combretum laxum | [47] |
| Phytol (51) | IC50 (P388D1) = 75.0 µM. | Pholidota cantonensis Rolfe | [48] |
| Phocantone (52) | IC50 (P388D1) = 27.5 µM. | Pholidota cantonensis Rolfe | [48] |
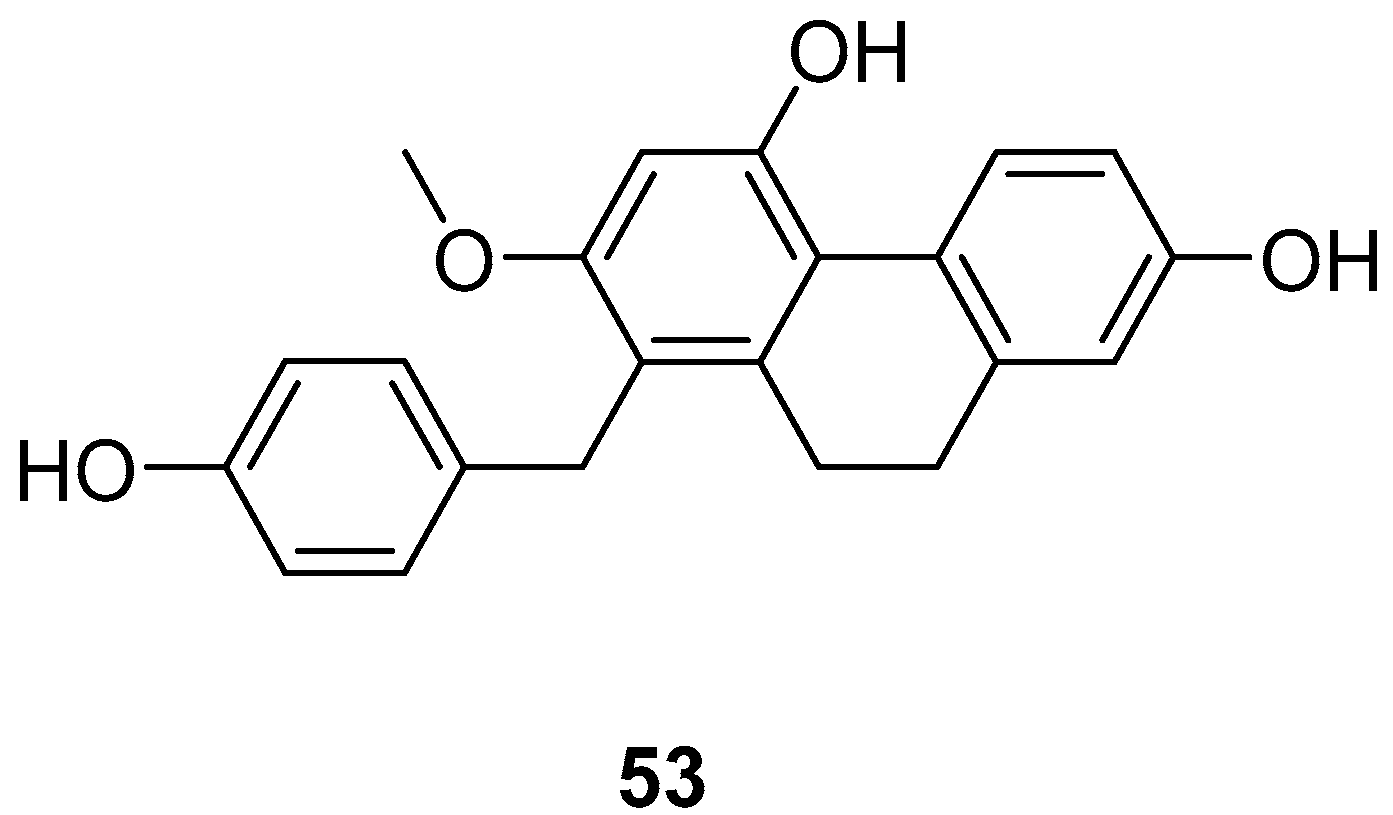
| Shancidin (53) | IC50 (SMMC-7721) = 12.57 µM; IC50 (A549) = 18.21 µM; IC50 (MGC80-3) = 11.6 µM | Cymbidium hybridum | [49,50] |
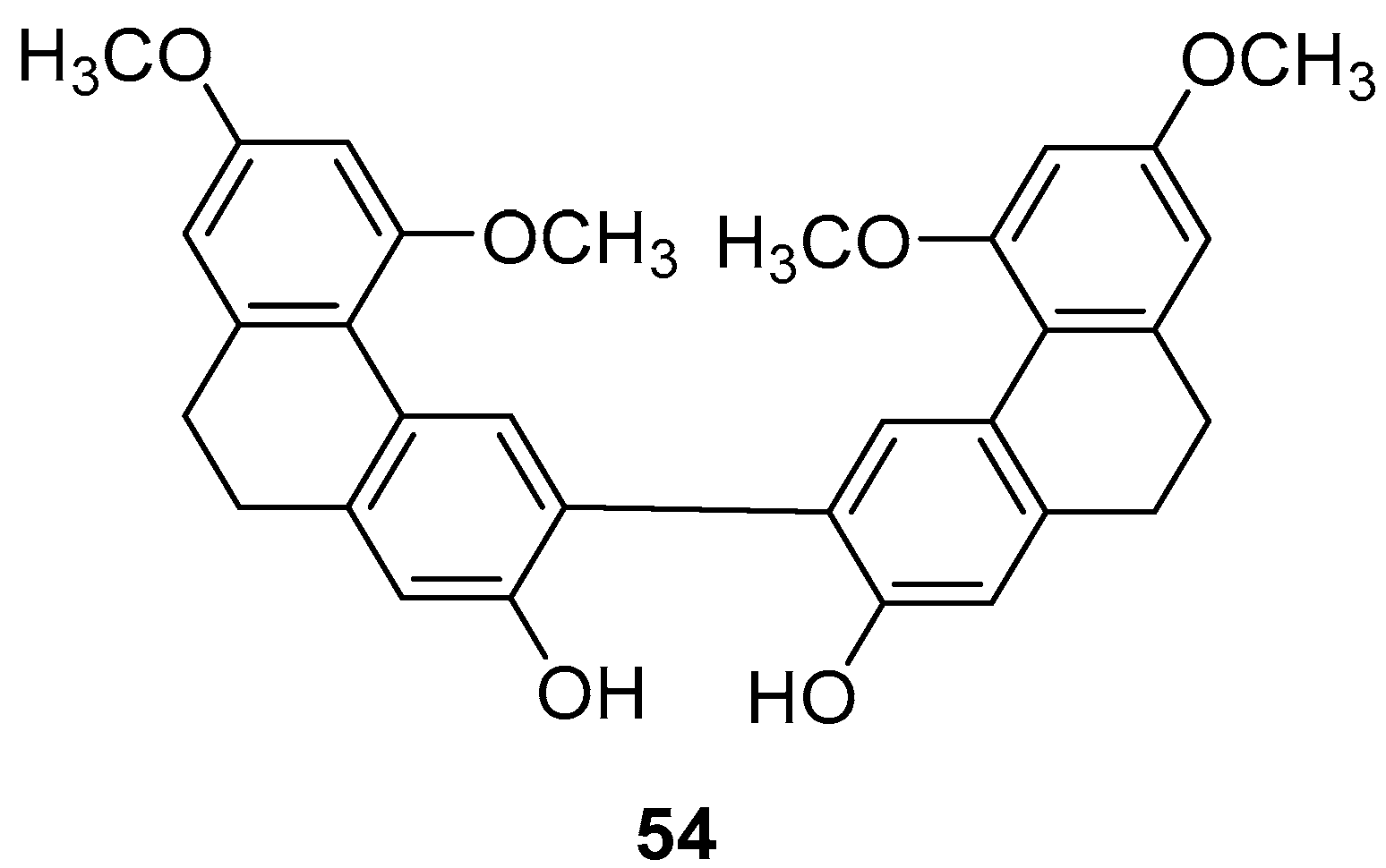
| 9,9′,10,10′-tetrahydro-3,3′-biphenanthrene (54) | IC50 (SGC-7901) = 63.8 ± 3.6 µM; IC50 (HepG2) = 78.4 ± 29.0 µM; IC50 (KCR) = 58.2 ± 2.6 µM | Orchid plant Panlongshen | [45] |
| 56 | IC50 (HeLa) = 25 µM; IC50 (MCF-7) = 31 µM; IC50 (A431) = 42 µM | / | [51] |
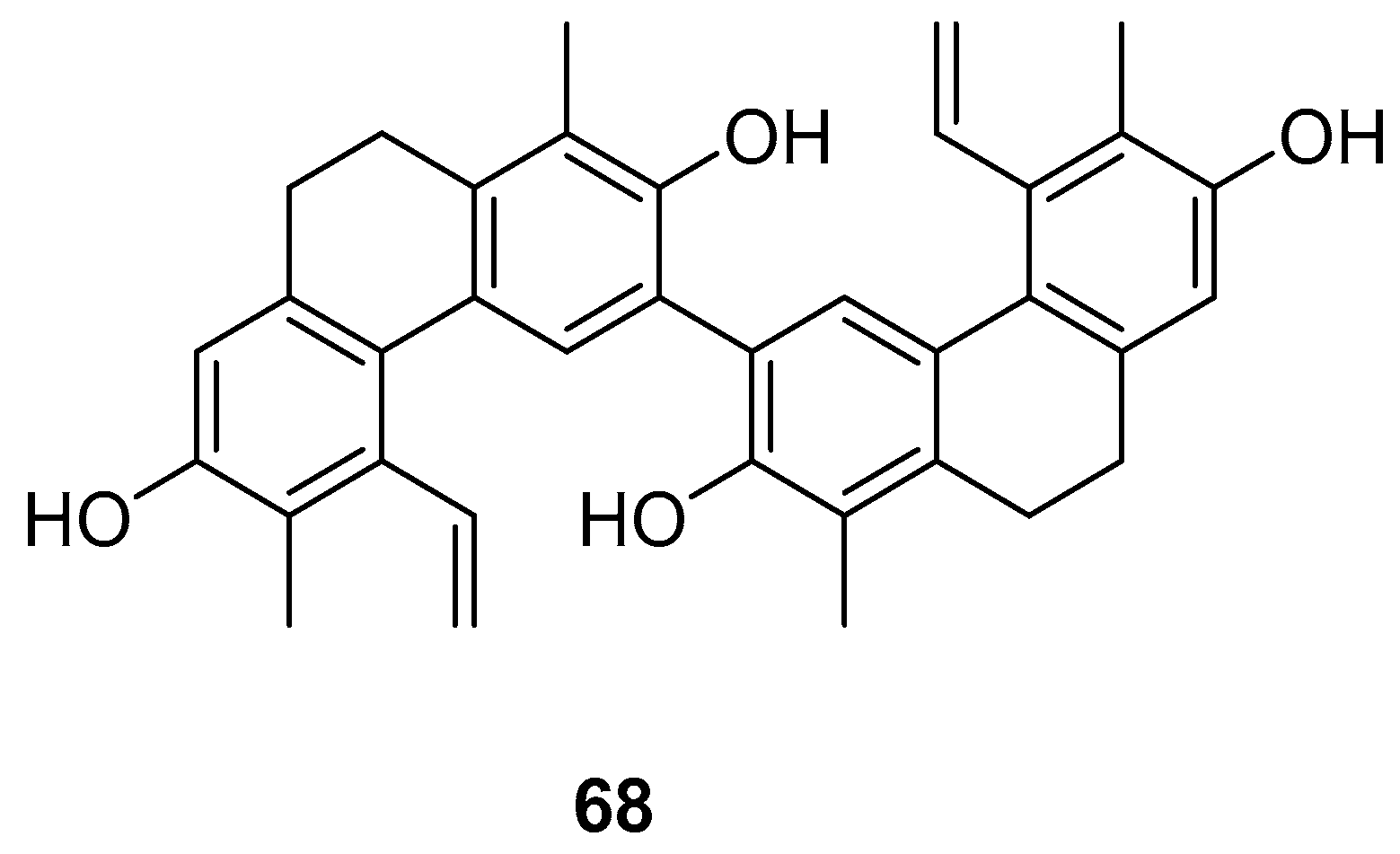
| Compressin B (68) | IC50 (HeLa) = 1.86 µM. | J. compressus | [57] |
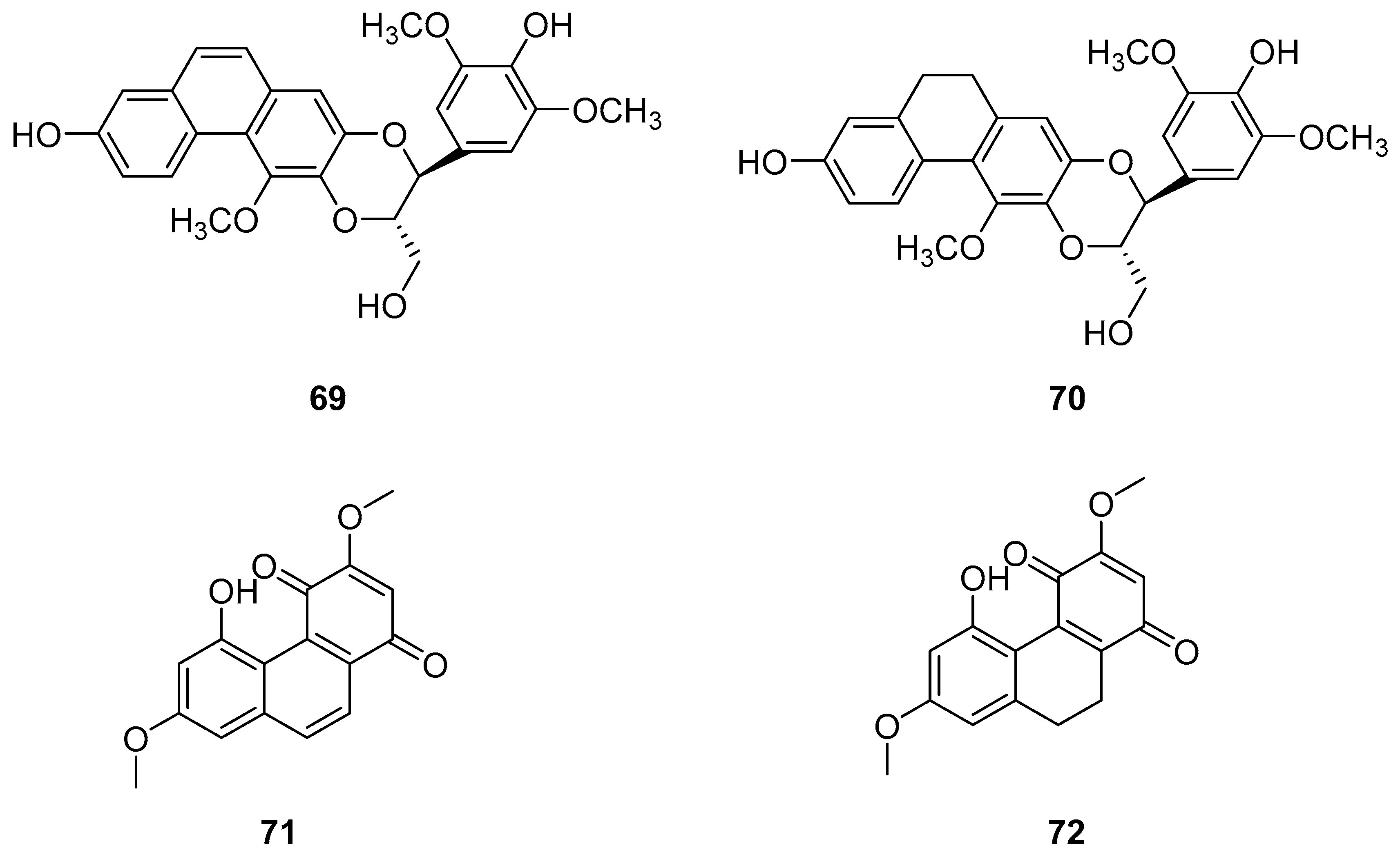
| Dendrocandin P1 (69) | IC50 (HI-60) = 35.32 ± 1.76 µM; IC50 (THP-1) = 20.78 ± 1.80 µM. | Dendrobium officinale stem | [13,58,59,60,61,62,63] |
| Dendrocandin P2 (70) | IC50 (HI-60) > 50 µM; IC50 (THP-1) = 45.32 ± 2.39 µM. | Dendrobium officinale stem | [13,58,59,60,61,62,63] |
| Denbinobin (71) | IC50 (MCF-7) = 13.13 ± 0.47 µM; IC50 (HL-60) = 3.08 ± 0.12 µM; IC50 (A549) = 19.68 ± 1.12 µM; IC50 (SW480) = 16.81 ± 0.13 µM. | D. candidum; D. nobile; D. venustum | [64,65,66,67,68,73] |
| Ephemeranthoquinone (72) | IC50 (MCF-7) = 3.63 ± 0.03 µM; IC50 (HL-60) = 2.33 ± 0.12 µM; IC50 (A549) = 14.97 ± 0.64 µM; IC50 (SW480) = 6.66 ± 0.71 µM. | D. hancockii, D. hongdie; D. longicornu; D. plicatile | [69,70,71,72,73] |
| 8-methoxy-12-(4-methoxybenzyl)-13,14-dihydro-12H-naphtho [2,1-a]xanthene- 2,5,9,10-tetraol (74) | IC50 (MDA-231) = 25.2 µM; IC50 (HepG2) = 51.3 µM; IC50 (HT-29) = 30.4 µM. | Dendrobium officinale | [76] |
| Erathrin A (75) | IC50 (HeLa) = 14.5 µM. | Wild peony | [77] |
| 3′,7′,7-trihydroxy-2,2′,4′-trimethoxy-[1,8′-biphenanthrene]-3,4-dione (76) | IC50 (MCF-7) = 12.6 µM; IC50 (HT-29) = 22.7 µM; IC50 (HUVEC) = 33.5 µM; IC50 (A549) = 22.6 µM. | Bai ji | [78] |
| 77 | IC50 (DU145) = 1.5 ± 0.09 µM; IC50 (HeLa) = 2.9 ± 0.19 µM. | / | [79] |
| Bleochranol A (78) | IC50 (HL-60) = 0.24 ± 0.03 µM; IC50 (SMMC-7721) = 12.22 ± 0.26 µM; IC50 (A549) = 3.51 ± 0.09 µM; IC50 (MCF-7) = 3.33 ± 0.09 µM; IC50 (SW480) = 12.97 ± 0.34 µM. | Bletilla striata | [80] |
| Bleochranol B (79) | IC50 (HL-60) => 40 µM; IC50 (SMMC-7721) > 40 µM; IC50 (A549) = 34.87 ± 0.40 µM; IC50 (MCF-7) = 29.07 ± 1.34 µM; IC50 (SW480) > 40 µM. | Bletilla striata | [80] |
| Bleochranol C (80) | IC50 (HL-60) = 15.05 ± 0.33 µM; IC50 (SMMC-7721) = 19.85 ± 0.42 µM; IC50 (A549) = 19.16 ± 0.41 µM; IC50 (MCF-7) = 18.84 ± 0.41 µM; IC50 (SW480) = 18.61 ± 0.68 µM. | Bletilla striata | [80] |
| Bleochranol D (81) | IC50 (HL-60) = 10.65 ± 0.09 µM; IC50 (SMMC-7721) = 17.95 ± 0.44 µM; IC50 (A549) = 18.32 ± 0.44 µM; IC50 (MCF-7) = 17.62 ± 0.81 µM; IC50 (SW480) = 18.60 ± 0.99 µM. | Bletilla striata | [80] |
| Juncunol (82) | IC50 (HepG2) = 18 µM. | Juncus effuses L. | [81] |
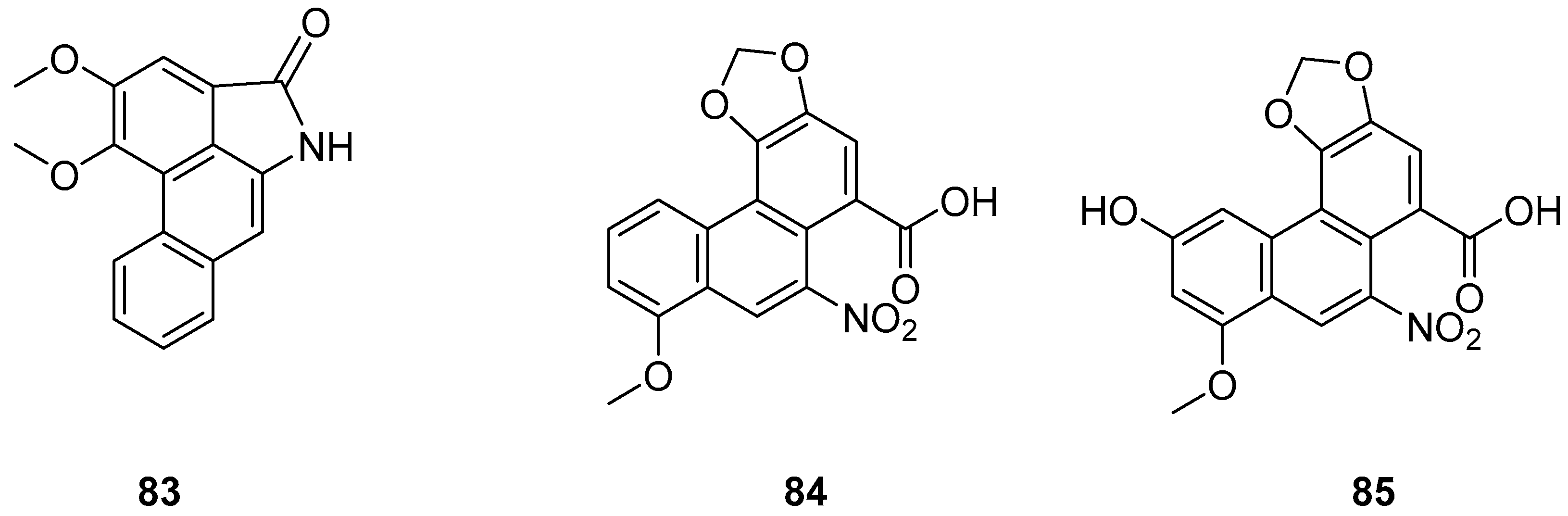
| AL-BII (83) | IC50 (HepG2) = 0.2 µM. | Aristolochia; Asarum; Akebia; Clematis, Stephania; Menispermum; Dauricum; Asteraceae | [82,84] |
| AAI (84) | IC50 (HepG2) = 9.7 µM. | Clematis, Stephania; Menispermum; Dauricum; Asteraceae | [82,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Du, Z.; Sheng, C.; Zhang, G.; Yan, S.; Zhang, Z.; Qin, S. The Double-Edge Sword of Natural Phenanthrenes in the Landscape of Tumorigenesis. Molecules 2025, 30, 1204. https://doi.org/10.3390/molecules30061204
Liu Y, Du Z, Sheng C, Zhang G, Yan S, Zhang Z, Qin S. The Double-Edge Sword of Natural Phenanthrenes in the Landscape of Tumorigenesis. Molecules. 2025; 30(6):1204. https://doi.org/10.3390/molecules30061204
Chicago/Turabian StyleLiu, Yan, Ziwei Du, Chen Sheng, Guangshuai Zhang, Si Yan, Zhijun Zhang, and Shuanglin Qin. 2025. "The Double-Edge Sword of Natural Phenanthrenes in the Landscape of Tumorigenesis" Molecules 30, no. 6: 1204. https://doi.org/10.3390/molecules30061204
APA StyleLiu, Y., Du, Z., Sheng, C., Zhang, G., Yan, S., Zhang, Z., & Qin, S. (2025). The Double-Edge Sword of Natural Phenanthrenes in the Landscape of Tumorigenesis. Molecules, 30(6), 1204. https://doi.org/10.3390/molecules30061204







