Visible-Light-Mediated Aerobic α-Oxygenation of Tetrahydroisoquinolines and Isoindolines Without External Photocatalysts
Abstract
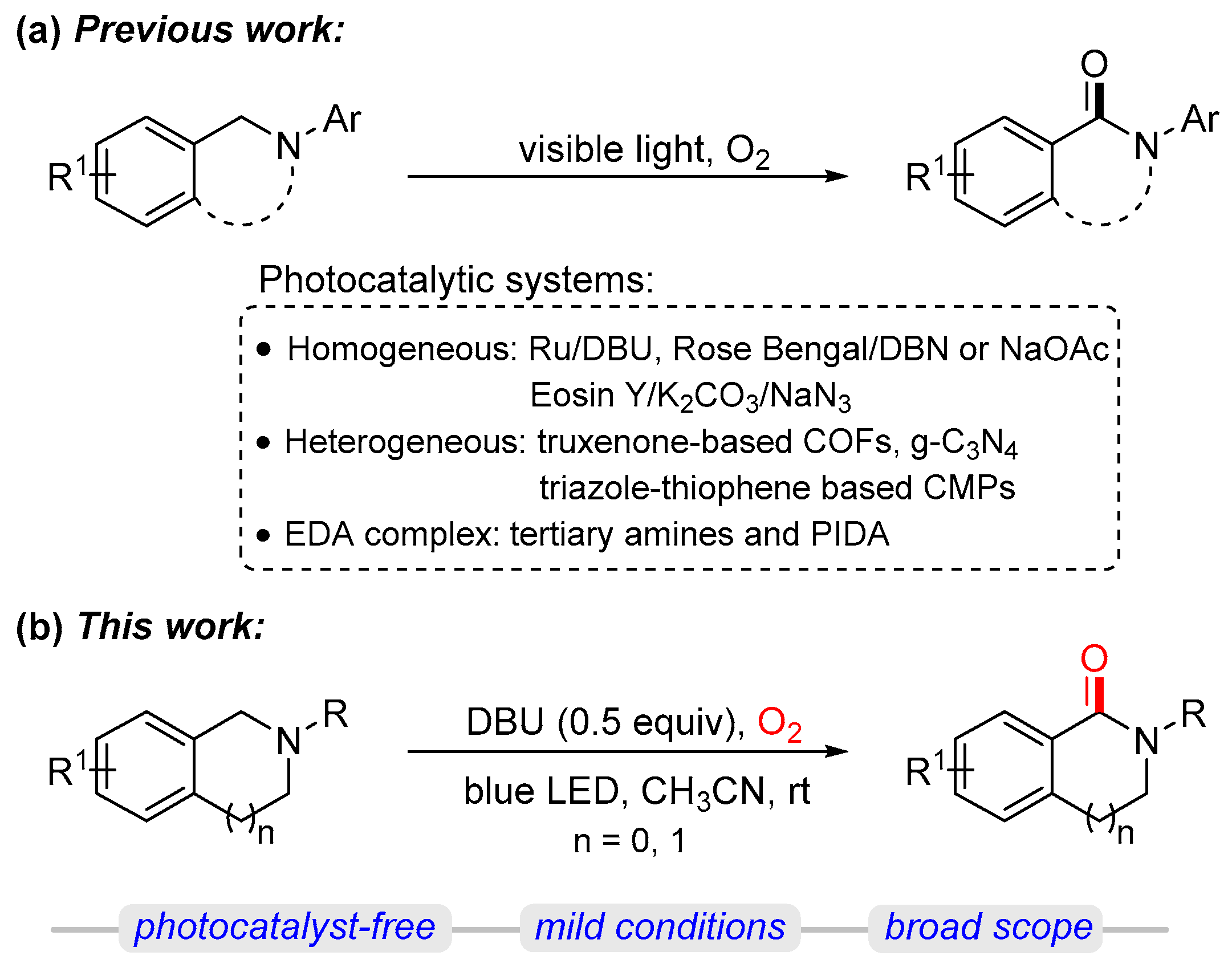
1. Introduction
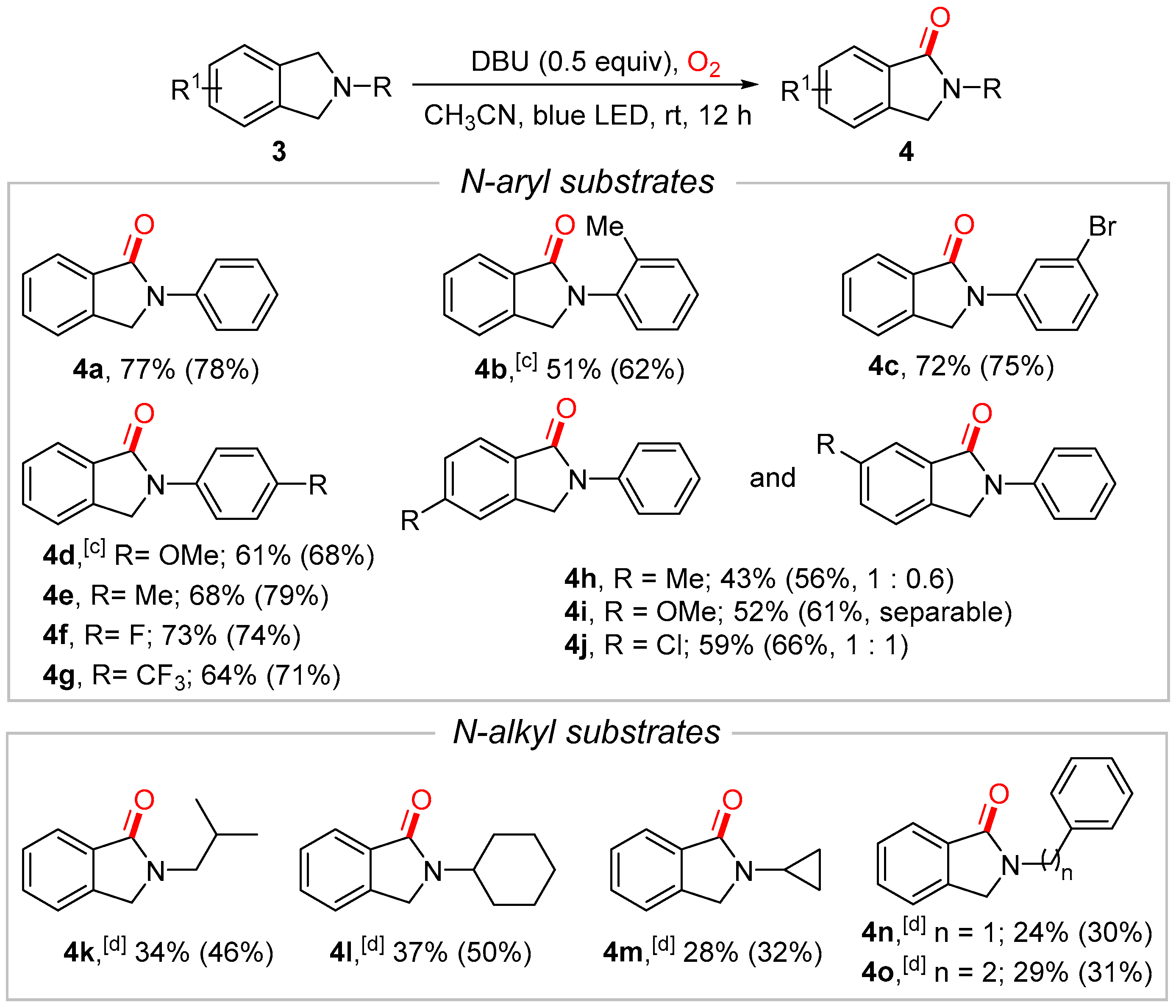
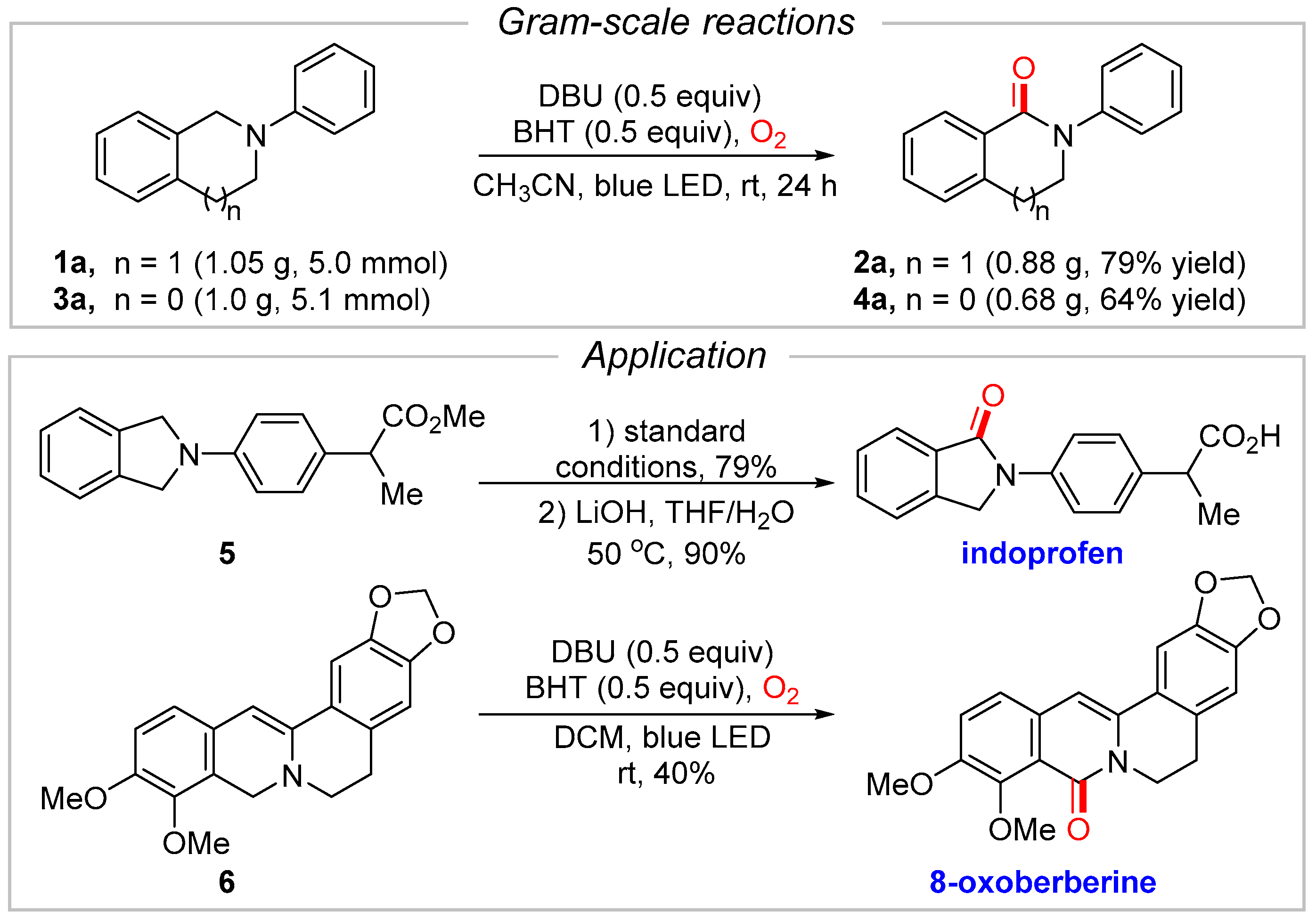
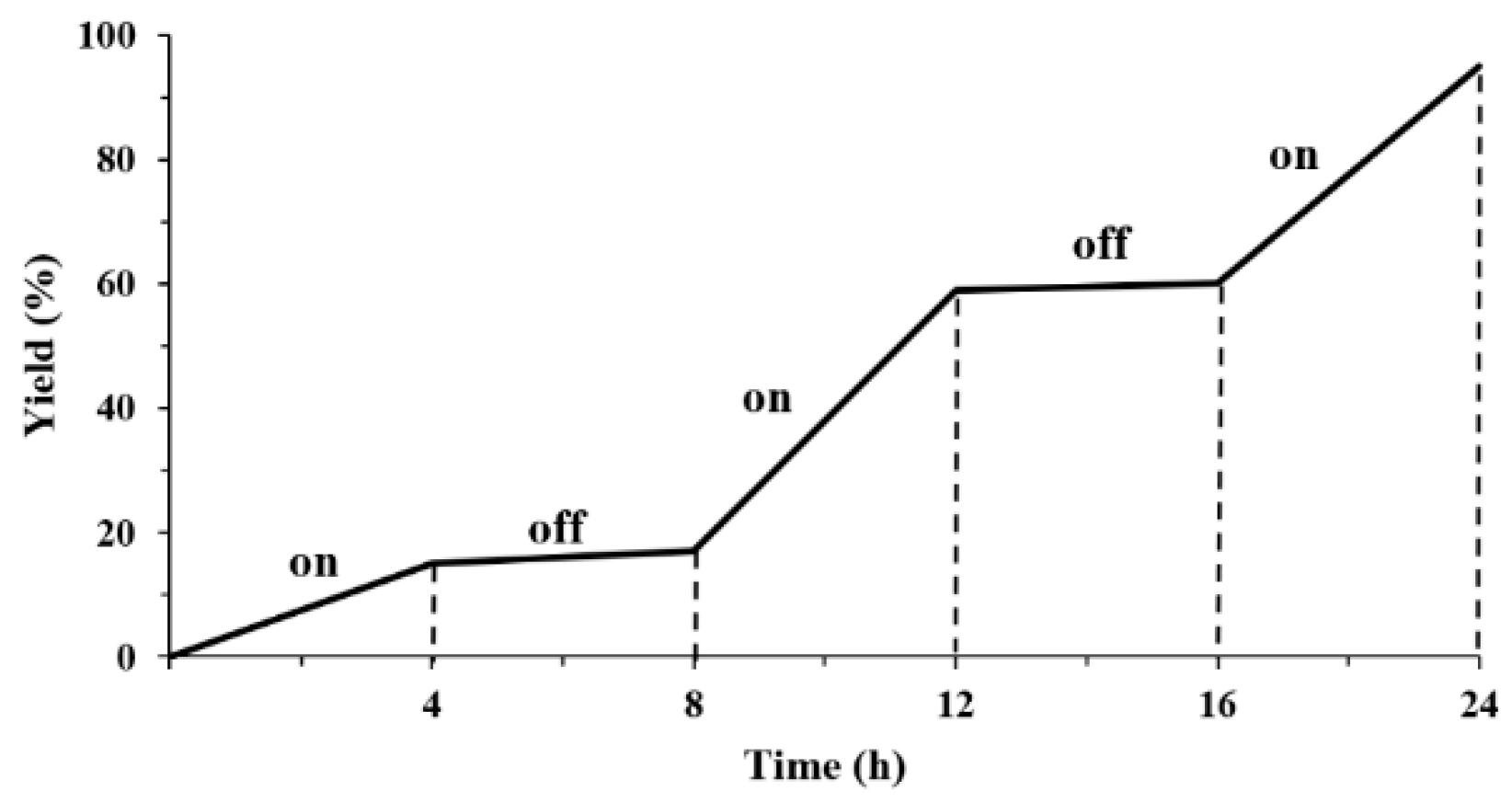
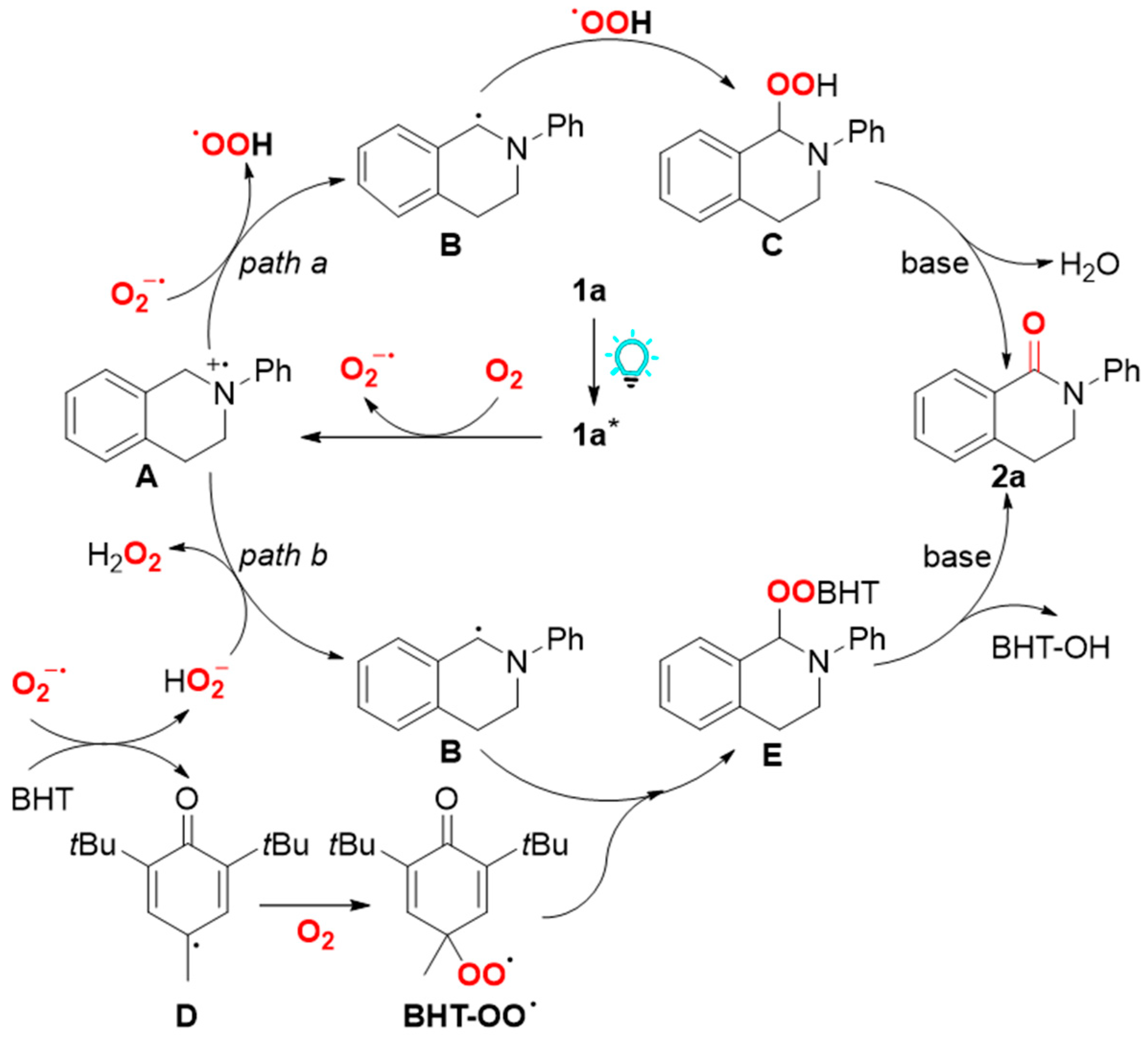
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Photocatalytic Late-Stage C–H Functionalization. Chem. Rev. 2023, 123, 4237–4352. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospectsin Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Fan, Q.; Zhu, L.; Li, X.; Ren, H.; Wu, G.; Zhu, H.; Sun, W. Catalyst-Free Visible Light-mediated Selective Oxidation of Sulfides into Sulfoxides under Clean Conditions. Green Chem. 2021, 23, 7945–7949. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef]
- Bjerg, E.E.; Marchán-García, J.; Buxaderas, E.; Moglie, Y.; Radivoy, G. Oxidative α-Functionalization of 1,2,3,4-Tetrahydroisoquinolines Catalyzed by a Magnetically Recoverable Copper Nanocatalyst. Application in the Aza-Henry Reaction and the Synthesis of 3,4-Dihydroisoquinolones. J. Org. Chem. 2022, 87, 13480–13493. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, F.; Wang, W.; Yao, M.; Lin, X.; Yang, F.; Qian, Y.; Chen, Z. Iron(III)-Catalyzed α-Cyanation and Carbonylation with 2-Pyridylacetonitrile: Divergent Synthesis of α-Amino Nitriles and Tetrahydroisoquinolinones. Org. Biomol. Chem. 2022, 20, 7031–7035. [Google Scholar] [CrossRef]
- Geng, S.; Xiong, B.; Zhang, Y.; Zhang, J.; He, Y.; Feng, Z. Thiyl Radical Promoted Iron-Catalyzed-Selective Oxidation of Benzylic sp3 C–H Bonds with Molecular Oxygen. Chem. Commun. 2019, 55, 12699–12702. [Google Scholar] [CrossRef]
- Thatikonda, T.; Deepake, S.K.; Das, U. α-Angelica Lactone in a New Role: Facile Access to N Aryl Tetrahydroisoquinolinones and Isoindolinones via Organocatalytic α-CH2 Oxygenation. Org. Lett. 2019, 21, 2532–2535. [Google Scholar] [CrossRef]
- Thapa, P.; Corral, E.; Sardar, S.; Pierce, B.S.; Foss, F.W., Jr. Isoindolinone Synthesis: Selective Dioxane-Mediated Aerobic Oxidation of Isoindolines. J. Org. Chem. 2019, 84, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Xue, D.; Xiao, M.; Liu, J.; Li, C.; Xiao, J. Reactions Catalysed by a Binuclear Copper Complex: Relay Aerobic Oxidation of N-Aryl Tetrahydroisoquinolines to Dihydroisoquinolones with a Vitamin B1 Analogue. Chem.-Eur. J. 2017, 23, 3062–3066. [Google Scholar] [CrossRef]
- Potter, T.J.; Ellman, J.A. Total Synthesis of (+)-Pancratistatin by the Rh(III)-Catalyzed Addition of a Densely Functionalized Benzamide to a Sugar-Derived Nitroalkene. Org. Lett. 2017, 19, 2985–2988. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, X.; Fu, J.; Zhang, L.; Cheng, M.; Yang, L.; Liu, Y. Collective Syntheses of 8-Oxoprotoberberines via Sequential In(OTf)3-Catalyzed Cyclization and Pd(OAc)2-Catalyzed Heck Coupling. J. Org. Chem. 2023, 88, 7179–7187. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Funk, R.L. Total Synthesis of (±)-Lennoxamine and (±)-Aphanorphine by Intramolecular Electrophilic Aromatic Substitution Reactions of 2-Amidoacroleins. Org. Lett. 2001, 24, 3923–3925. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, J.A.; Ancellin, N.; Bertrand, S.M.; Bingham, R.P.; Carter, P.S.; Chung, C.-W.; Churcher, I.; Dodic, N.; Fournier, C.; Francis, P.L.; et al. Structurally Diverse Mitochondrial Branched Chain Aminotransferase (BCATm) Leads with Varying Binding Modes Identified by Fragment Screening. J. Med. Chem. 2016, 59, 2452–2467. [Google Scholar] [CrossRef]
- Zeldis, J.B.; Knight, R.; Hussein, M.; Chopra, R.; Muller, G. A review of the History, Properties, and Use of the Immunomodulatory Compound Lenalidomide. Ann. N. Y. Acad. Sci. 2011, 1222, 76–82. [Google Scholar] [CrossRef]
- Lunn, M.R.; Root, D.E.; Martino, A.M.; Flaherty, S.P.; Kelley, B.P.; Coovert, D.D.; Burghes, A.H.; Man, N.T.; Morris, G.E.; Zhou, J.; et al. Indoprofen Upregulates the Survival Motor Neuron Protein through a Cyclooxygenase-Independent Mechanism. Chem. Biol. 2004, 11, 1489–1493. [Google Scholar] [CrossRef]
- Peng, K.; Dong, Z.-B. Recent Advances in Cross-Dehydrogenative Couplings (CDC) of C−H Bond in Aqueous Media. Adv. Synth. Catal. 2021, 363, 1185–1201. [Google Scholar] [CrossRef]
- Patil, M.R.; Dedhia, N.P.; Kapdi, A.R.; Kumar, A.V. Cobalt(II)/N-Hydroxyphthalimide-Catalyzed Cross-Dehydrogenative Coupling Reaction at Room Temperature under Aerobic Condition. J. Org. Chem. 2018, 83, 4477–4490. [Google Scholar] [CrossRef]
- Girard, S.A.; Knauber, T.; Li, C.-J. The Cross-Dehydrogenative Coupling of Csp3−H Bonds: A Versatile Strategy for C−C Bond Formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef]
- Kumar, I.; Thakur, A.; Manisha; Sharma, U. α-Oxygenation of N-Aryl/Alkyl Heterocyclic Compounds via Ruthenium Photocatalysis. React. Chem. Eng. 2021, 6, 2087–2091. [Google Scholar] [CrossRef]
- Guryev, A.A.; Hahn, F.; Marschall, M.; Tsogoeva, S.B. Visible-Light-Driven C-H Oxidation of Cyclic Tertiary Amines: Access to Synthetic Strychnos Alkaloids with Antiviral Activity. Chem.-Eur. J. 2019, 25, 4062–4066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riemer, D.; Schilling, W.; Kollmann, J.; Das, S. Visible-Light-Mediated Efficient Metal-Free Catalyst for α-Oxygenation of Tertiary Amines to Amides. ACS Catal. 2018, 8, 6659–6664. [Google Scholar] [CrossRef]
- Aganda, K.C.C.; Hong, B.; Lee, A. Aerobic α-Oxidation of N-Substituted Tetrahydroisoquinolines to Dihydroisoquinolones via Organo-photocatalysis. Adv. Synth. Catal. 2019, 361, 1124–1129. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Li, J.; Zhao, Z.; Zhou, K.; Li, Y.; He, P.; Wu, J.; Bao, Z.; Yang, Q.; et al. Blending Aryl Ketone in Covalent Organic Frameworks to Promote Photoinduced Electron Transfer. J. Am. Chem. Soc. 2023, 145, 9198–9206. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Zeng, Y.; Liu, J.; Nie, J.; Lu, C.; Ma, C.; Wang, F.; Yang, G. Click-Based Conjugated Microporous Polymers as Efficient Heterogeneous Photocatalysts for Organic Transformations. Catal. Sci. Technol. 2022, 12, 1202–1210. [Google Scholar] [CrossRef]
- Geng, P.; Tang, Y.; Pan, G.; Wang, W.; Hu, J.; Cai, Y. A g-C3N4-Based Heterogeneous Photocatalyst for Visible Light Mediated Aerobic Benzylic C–H Oxygenations. Green Chem. 2019, 21, 6116–6122. [Google Scholar] [CrossRef]
- Xie, X.; Guo, X.; Qiao, K.; Shi, L. Visible-Light Promoted Photocatalyst-free Aerobic α-Oxidation of Tertiary Amines to Amides. Org. Biomol. Chem. 2022, 20, 8031–8036. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Ma, Y.; Tan, S.; Li, F. Aerobic Benzylic C(sp3)−H Bond Oxygenations Catalyzed by NBS under Visible Light Irradiation. J. Org. Chem. 2024, 89, 534–540. [Google Scholar] [CrossRef]
- Stanforth, S.P. Synthesis of isocyanides through dehydration of formamides using XtalFluor-E. Tetrahedron 2000, 56, 461–464. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, R.; Yao, J.; Li, H. 1,5,7-Triazabicyclo [4.4.0] dec-5-ene Enhances Activity of Peroxide Intermediates in Phosphine-Free α-Hydroxylation of Ketones. Angew. Chemie. 2021, 133, 6705–6712. [Google Scholar] [CrossRef]
- Wang, X.-F.; Yu, S.-S.; Wang, C.; Xue, D.; Xiao, J. BODIPY catalyzed amide synthesis promoted by BHT and air under visible light. Org. Biomol. Chem. 2016, 14, 7028–7037. [Google Scholar] [CrossRef]
- Lambert, C.R.; Black, H.S.; Truscott, T.G. Reactivity of butylated hydroxytoluene. Free Radic. Biol. Med. 1996, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bansode, A.H.; Suryavanshi, G. Visible-Light-Induced Controlled Oxidation of N-Substituted 1,2,3,4-Tetrahydroisoquinolines for the Synthesis of 3,4-Dihydroisoquinolin-1(2H)-ones and Isoquinolin-1(2H)-ones. Adv. Synth. Catal. 2021, 363, 1390–1400. [Google Scholar] [CrossRef]
- Sahoo, B.; Hopkinson, M.N.; Glorius, F. External-Photocatalyst-Free Visible-Light-Mediated Synthesis of Indolizines. Angew. Chem. Int. Ed. 2015, 54, 15545–15549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, S.; Shena, Z. Copper-Catalyzed Cyanomethylation of Substituted Tetrahydroisoquinolines with Acetonitrile. Adv. Synth. Catal. 2016, 358, 2392–2397. [Google Scholar] [CrossRef]
- Shigeno, M.; Hayashi, K.; Nozawa-Kumada, K.; Kondo, Y. Organic Superbase t-Bu-P4 Catalyzes Amination of Methoxy(hetero)arenes. Org. Lett. 2019, 21, 5505–5508. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, Z.; Wang, Y. Oxidative α-Trichloromethylation of Tertiary Amines: An Entry to α-Amino Acid Esters. J. Org. Chem. 2019, 84, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Oss, G.; de Vos, S.D.; Luc, K.N.H.; Harper, J.B.; Nguyen, T.V. Tropylium-Promoted Oxidative Functionalization of Tetrahydroisoquinolines. J. Org. Chem. 2018, 83, 1000–1010. [Google Scholar] [CrossRef]
- Asamdi, M.; Chauhan, P.M.; Patel, J.J.; Chikhalia, K.H. Palladium catalyzed annulation of benzylamines and arynes via C–H activation to construct 5,6-dihydrophenanthridine derivatives. Tetrahedron 2019, 75, 3485–3494. [Google Scholar] [CrossRef]
- Wen, T.; Liang, B.; Liang, J.; Wang, D.; Shi, J.; Xu, S.; Zhu, W.; Chen, X.; Zhu, Z. Copper-Promoted N-Alkylation and Bromination of Arylamines/Indazoles Using Alkyl Bromides as Reagents for Difunctionalization. J. Org. Chem. 2022, 87, 12214–12224. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, X.; Ni, S.; Pan, Y.; Wang, Y. Nickel-Catalyzed Amination of Alkyl Electrophiles. Org. Lett. 2023, 25, 5822–5826. [Google Scholar] [CrossRef]
- Shen, Z.; Ni, Z.; Mo, S.; Wang, J.; Zhu, Y. Palladium-Catalyzed Intramolecular Decarboxylative Coupling of Arene Carboxylic Acids/Esters with Aryl Bromides. Chem. Eur. J. 2012, 18, 4859–4865. [Google Scholar] [CrossRef]



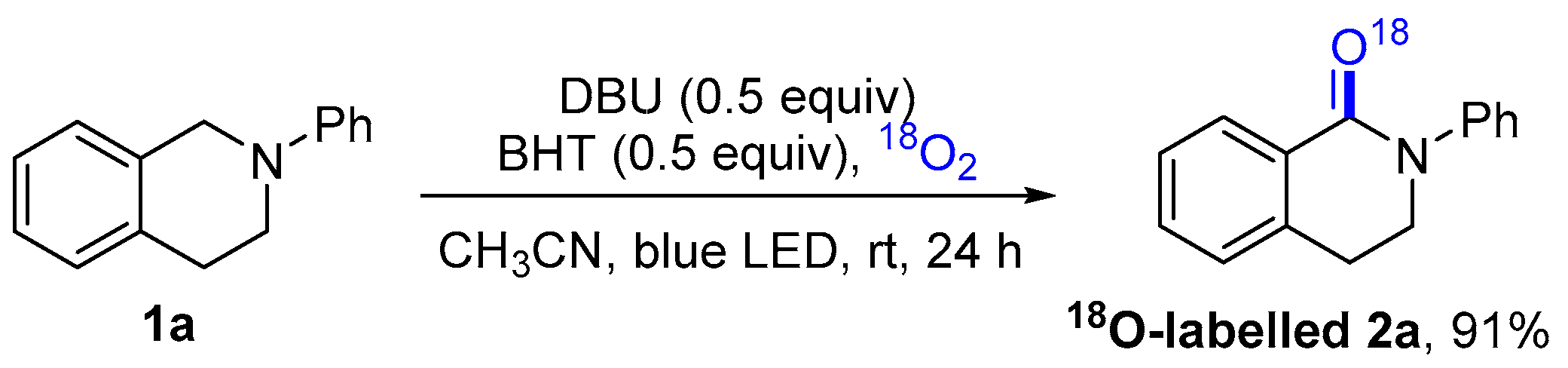

 | ||||
|---|---|---|---|---|
| Entry | Base (Equiv) | Solvent | Time (h) | Yield (%) [b] |
| 1 | - | DCM | 12 | 16 |
| 2 | DBU (0.2) | DCM | 12 | 50 |
| 3 | DBU (0.2) | THF | 12 | 71 |
| 4 | DBU (0.2) | EA | 24 | 61 |
| 5 | DBU (0.2) | CH3CN | 24 | 74 |
| 6 | DBU (0.2) | DMF | 24 | 65 |
| 7 | DBN (0.2) | CH3CN | 24 | 50 |
| 8 | TBD (0.2) | CH3CN | 24 | 46 |
| 9 | DBU (0.5) | CH3CN | 12 | 80 |
| 10 | DBU (1.0) | CH3CN | 12 | 78 |
| 11 [c] | DBU (0.5) | CH3CN | 48 | 85 |
| 12 [d] | DBU (0.5) | CH3CN | 12 | 0 |
| 13 [e] | DBU (0.5) | CH3CN | 12 | trace |
| 14 [f] | DBU (0.5) | CH3CN | 12 | 60 |
| 15 [g] | DBU (0.5) | CH3CN | 12 | trace |
| 16 [h] | DBU (0.5) | CH3CN | 24 | 95 |
| Entry | Quencher (1 Equiv) | Yield (%) [b] |
|---|---|---|
| 1 | TEMPO | 44 |
| 2 | BQ | 19 |
| 3 | CuCl2 | 10 |
| 4 | DABCO | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Li, Y.; Zhao, F.; Song, A.; Zhong, Z.; Tan, S.; Li, F. Visible-Light-Mediated Aerobic α-Oxygenation of Tetrahydroisoquinolines and Isoindolines Without External Photocatalysts. Molecules 2025, 30, 743. https://doi.org/10.3390/molecules30030743
Ye T, Li Y, Zhao F, Song A, Zhong Z, Tan S, Li F. Visible-Light-Mediated Aerobic α-Oxygenation of Tetrahydroisoquinolines and Isoindolines Without External Photocatalysts. Molecules. 2025; 30(3):743. https://doi.org/10.3390/molecules30030743
Chicago/Turabian StyleYe, Taiqiang, Yuzheng Li, Feng Zhao, Aorou Song, Zhaoxia Zhong, Shenpeng Tan, and Feng Li. 2025. "Visible-Light-Mediated Aerobic α-Oxygenation of Tetrahydroisoquinolines and Isoindolines Without External Photocatalysts" Molecules 30, no. 3: 743. https://doi.org/10.3390/molecules30030743
APA StyleYe, T., Li, Y., Zhao, F., Song, A., Zhong, Z., Tan, S., & Li, F. (2025). Visible-Light-Mediated Aerobic α-Oxygenation of Tetrahydroisoquinolines and Isoindolines Without External Photocatalysts. Molecules, 30(3), 743. https://doi.org/10.3390/molecules30030743






