1. Introduction
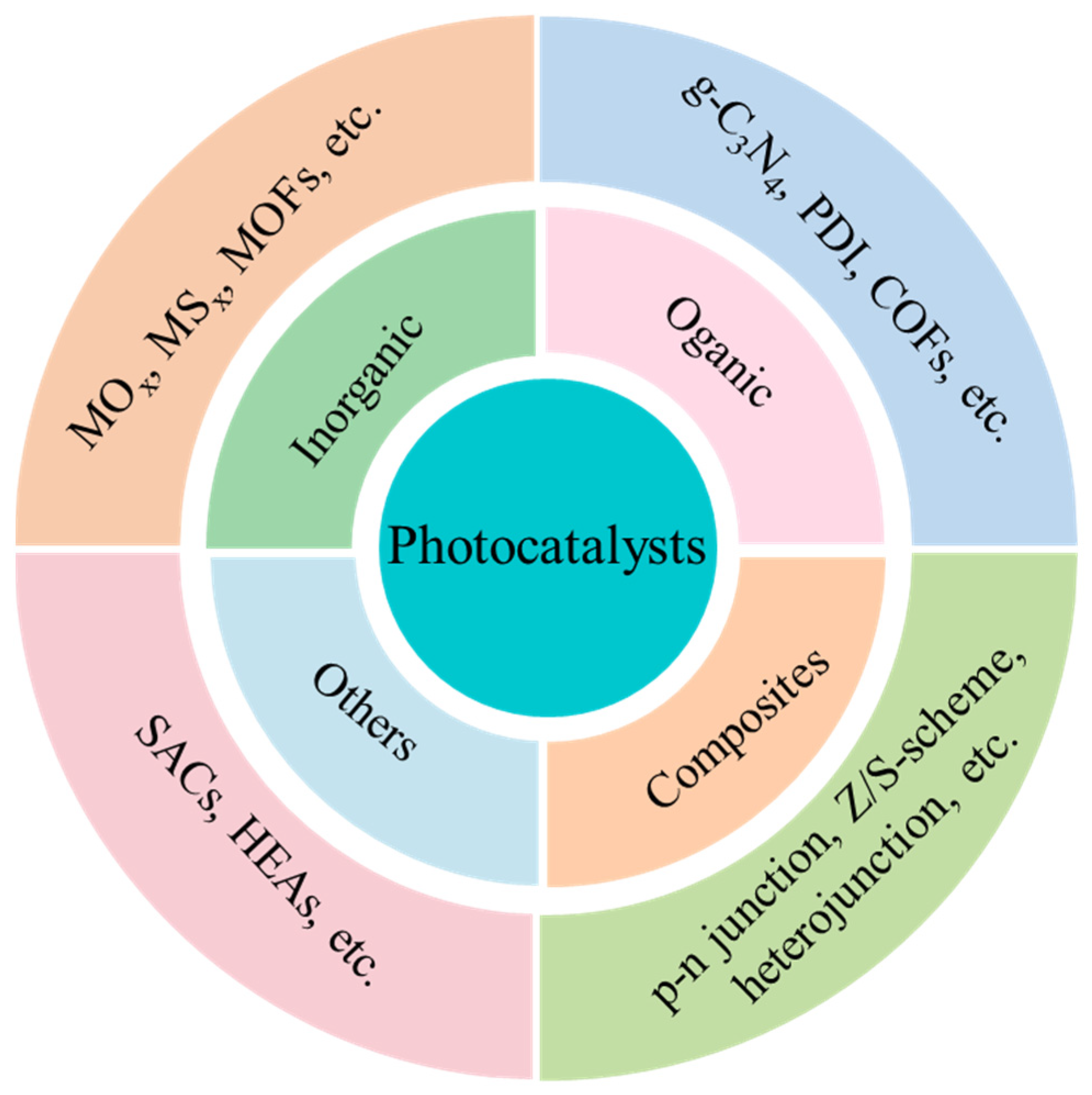
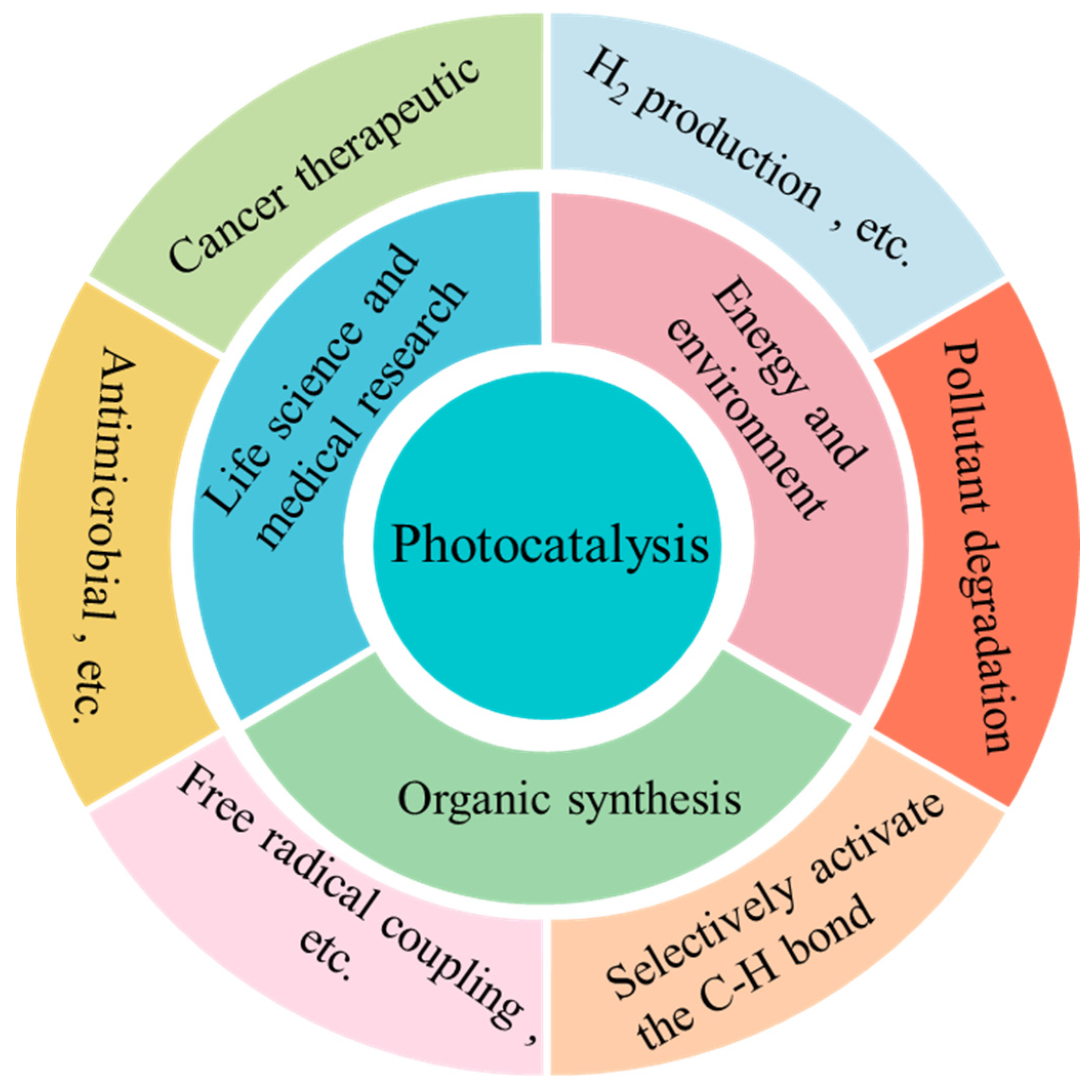
This Special Issue, titled “Photocatalytic Materials and Photocatalytic Reactions”, focuses on designing advanced photocatalysts, understanding their structure-dependent properties, and seeking to exploit them in the fields of energy conversion, pollutant degradation, artificial photosynthesis, organic synthesis, etc. As early as 1912, in the age of coal, Giacomo Ciamician [1] proposed the theory that photochemistry could be a potential and promising strategy to realize the harmonious development of human society and nature. However, the pioneering work on photosynthesis was a study on photo-electrochemically water splitting, published in 1972 by Fujishima and Honda [2]. Throughout the next few years, photocatalysis was mainly studied in regard to the degradation of toxic compounds. In recent decades, photocatalysis has attracted extensive and ongoing attention, because it exhibits great potential for applications in artificial photosynthesis, including H2 production and CO2 reduction, organic synthesis, pollutant degradation, N2 fixation, precious metal recovery, H2O2 photosynthesis, life science and medical research, space exploration, and other related fields (Figure 1) [3,4,5,6,7,8,9]. Meanwhile, photocatalysts have evolved from inorganic substances to new nanomaterials such as graphitic carbon nitride (g-C3N4), polymers, piezoelectric materials, ferroelectric materials, metal–organic frameworks (MOFs), covalent organic frameworks (COFs), single-atom catalysts (SACs), high-entropy alloys (HEAs), supramolecules, superlattices, topological insulators, localized surface plasmon resonance (LSPR), and diverse composite materials/heterojunctions, among other things (Figure 2) [6,7,8,9,10,11,12,13,14,15,16].
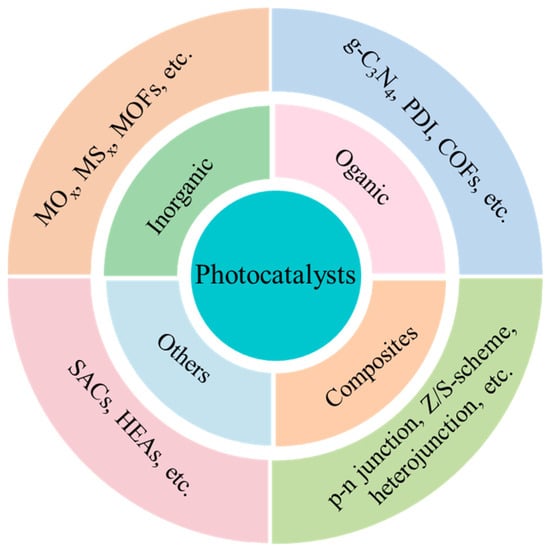
Figure 1.
The types of materials explored for use as potential photocatalysts.
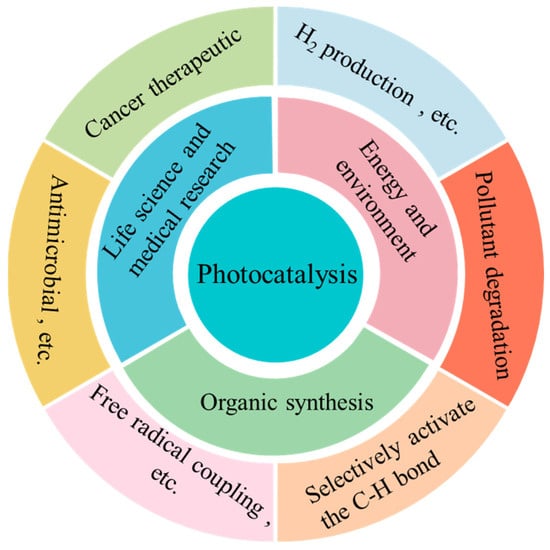
Figure 2.
Various photocatalytic reactions.
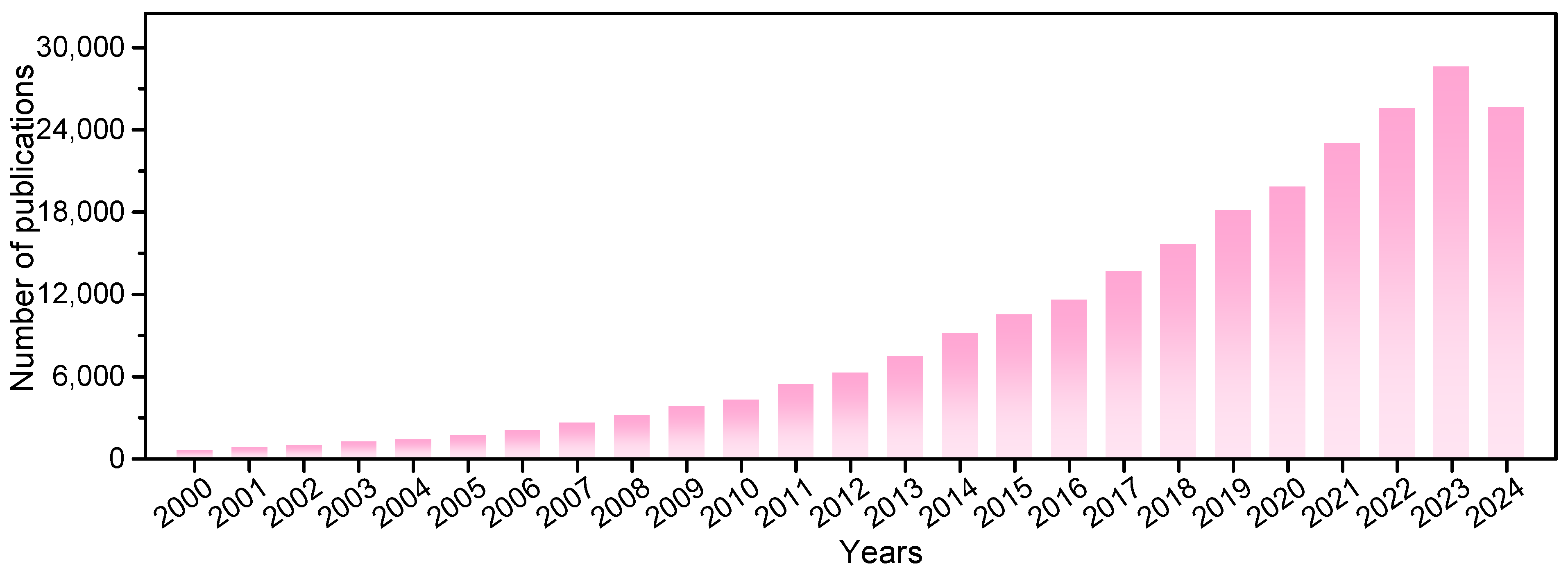
Our search results indicated a recent boom in photocatalysis research (Figure 3). About 243,000 studies on this topic have been published in the last 25 years. A total of 146 research areas and 212 countries/regions are involved. Photocatalysis is notable because it is driven by inexhaustible solar energy under mild reaction conditions. The design of advanced photocatalytic materials with good performance and the exploration of green photocatalytic reactions with carbon neutrality are significant for sustainability.
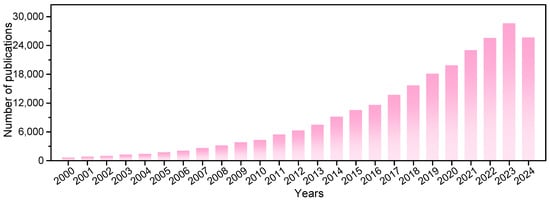
Figure 3.
The number of annual journal publications that covered “photocatalysts*” as a subject prior to 9 December 2024, as recorded in the Web of Science database.
This Special Issue contains 23 original research articles related to photocatalytic materials, including metal oxides, metal sulfides, metal nitrides, metallo-organic compounds, g-C3N4, clusters, LSPR, and heterojunction/composite materials. Their applications included H2 production, CO2 reduction, organic synthesis, environmental remediation, disinfection, toxicity, and dual-function photoredox reactions.
2. An Overview of the Published Articles
In the Special Issue’s first contribution, Li et al. synthesized a new magnetic nanocomposite, Ag2O/Fe3O4, to achieve the photocatalytic degradation of methyl orange (MO) under visible light irradiation. They observed that 99.5% of MO could be degraded by the Ag2O/Fe3O4 (10%) photocatalyst within 15 min. In addition, the designed Ag2O/Fe3O4 photocatalyst also exhibited broad applicability and stability. Their work demonstrated successful photocatalysis–Fenton coupling and overcame the challenges involved in a difficult catalyst recovery process and regarding low photocatalytic efficiency.
Next, in the study by Wang et al. (Contribution 2), a multicomponent composite MoP/a-TiO2/Co-ZnIn2S4 was prepared through multiple hydrothermal processes. The effects of Co dopants, i.e., amorphous TiO2 and MoP, increased visible light absorption, improving the separation of photoexcited charge carriers via heterojunction and hydrogen production sites, respectively. Thus, the high efficiency of photocatalytic H2 production was realized on MoP/a-TiO2/Co-ZnIn2S4. Compared to the pristine ZnIn2S4, the H2 production of MoP/a-TiO2/Co-ZnIn2S4 was enhanced by about three times.
Furthermore, in the research work by Meng et al. (Contribution 3), nanostructured polymeric carbon nitride (g-C3N4, PCN) was synthesized using a one-step thermal polymerization process with the assistance of hot water vapor. Vapor has a dual function used to prepare nanostructured PCN: besides being a green etching reagent, it can act as a gas bubble template. Moreover, reaction times, temperatures, mechanisms were also studied in the precursors of PCN. The H2 production of nanostructured PCN increased by about four times in contrast to bulk PCN. This study offers a new and versatile strategy for fabricating nanostructured g-C3N4 with high photocatalytic performance.
Wang et al. (Contribution 4) outlined a visible light-induced regioselective cascade and the sulfonylation–cyclization of a cascade of 1,5-dienes under mild conditions. An array of 3-sulfonylated pyrrolin-2-one derivatives was constructed through lower catalyst loading and achieved good to excellent yields at room temperature. Importantly, this protocol can be used in large-scale synthesis.
Meanwhile, in the research paper by Meng et al. (Contribution 5), a series of ZnmIn2S3+m photocatalysts (m = 1, 2, 3, 4 and 5) was prepared and applied in dual-function photoredox reactions: the selective oxidation of alcohols and the reduction of CO2 in one reaction system. In Zn5In2S8, Zn4In2S7, Zn3In2S6, Zn2In2S5, and ZnIn2S4, structures and properties was studied using experiments and theoretical calculation. The morphology, light absorption, and band structures were tuned by changing the Zn/In molar ratio. Moreover, the selectivity of gas products (H2 and CO) and liquid products (hydrobenzoin and benzaldehyde) could also be regulated.
In the research work by Chen et al. (Contribution 6), Ag/PW12/TiO2 composed of TiO2, polyoxometalates (POMs) [H3PW12O40] (PW12), and Ag nanoparticles was fabricated through successive electrospinning and photoreduction processes. Ag/PW12/TiO2 exhibited high degradation efficiencies for methyl orange (MO, 99.29%), enrofloxacin (ENR, 93.65%), and tetracycline (TC, 78.19%). Moreover, for TC degradation and TC concentration, the Ag/PW12/TiO2 dosage, the pH of the TC solution, and the intermediates and toxicities of the products were also investigated in detail.
Furthermore, in the study by Zhou et al. (Contribution 7), the Bi2S3-ZnO/CA film was fabricated by assembling Bi2S3, ZnO, and cellulose acetate (CA). In the Bi2S3-ZnO/CA film, the addition of Bi2S3 improved cavity density and uniformity. On the other hand, the addition of ZnO enabled the formation of heterojunctions with Bi2S3 to promote the separation and migration of photogenerated electron–hole pairs, thus improving the photocatalytic activity and stability of Bi2S3. This is evidenced by the fact that the RhB (rhodamine B) degradation efficiencies of ZnO/CA, 4Bi2S3/CA, and 4Bi2S3-ZnO/CA were about 26.51%, 50.26%, and 90.2%, respectively.
Shahid et al. (Contribution 8) prepared a 2D I-FeWO4/GO composite photocatalyst by combining halogen-doped FeWO4 (I-FeWO4) and graphene oxide (GO). In the designed I-FeWO4/GO composite, GO acted as a supporter, halogen facilitated H2O2 production, and the interface of the I-FeWO4/GO heterostructure promoted charge separation and migration. Thus, the I-FeWO4 displayed good photocatalytic performance for the degradation of methylene blue (MB). Under sunlight irradiation for 120 min, 97.0% of MB could be degraded by the I-FeWO4.
Furthermore, Ai et al. (Contribution 9) fabricated 2D heterojunction g-C3N4@CdS using a water bath method. Interestingly, tiny CdS nanorods were grown in situ in the gaps between the 2D g-C3N4 nanosheets. Due to the band-band transfer mechanism of the g-C3N4@CdS photocatalyst, the charge carriers could be separated efficiently, and thus excellent H2 production with the assistance of visible light and lactic acid was observed. The H2 production rate of G-CdS-3 could reach up to 1611.4 μmol·g−1·h−1, which was about 10 times that of CdS and 76 times that of g-C3N4.
In the research work by Wang et al. (Contribution 10), a 2D–3D hybrid junction In2S3/CdS/N-rGO was synthesized using a one-step pyrolysis method. The rational 2D–3D In2S3/CdS/N-rGO hybrid junctions not only provided more active sites but also formed multiple tight interfaces, which facilitated charge separation and migration. Thus, a high H2 evolution rate (10.9 mmol·g−1·h−1) could be obtained with the assistance of visible light and Na2S/Na2SO3 aqueous solution.
To remove high levels of toxic 4-nitrophenol (4-NP), Ma et al. (Contribution 11) designed the alkaline earth metal ion-doped photocatalyst Ca2+-doped AgInS2. The charge recombination and inactive production of superoxide radicals over AgInS2 could be improved by doping Ca2+. Thus, 63.2% of 4-NP was degraded under visible light for 120 min. In addition, capturing tests demonstrated that photoexcited holes and hydroxyl radicals were the main active species.
In the research work by Lu et al. (Contribution 12), cobalt phosphate (Co-Pi) was developed to modify ZnIn2S4 (ZnIn2S4/Co-Pi), aiming to suppress its charge recombination. Due to the transfer of photoexcited holes, ZnIn2S4/Co-Pi exhibited sustainable H2 production with assistance of visible light and triethanolamine (TEOA). Specifically, 3593 μmol·g−1·h−1 of H2 production was reached using ZnIn2S4/5%Co-Pi.
To remove hexavalent chromium, Guo et al. (Contribution 13) prepared a 2D g-C3N4/MoS2 nanocomposite using an ultrasonic method. Due to the Z-scheme transfer mechanism, photoexcited charges could not only be separated efficiently at the g-C3N4/MoS2 heterojunction but they also retained strong redox abilities. Thus, hexavalent chromium could be removed with high photocatalytic efficiency whenever it was exposed to UV light, visible light, or sunlight.
Furthermore, Garcia et al. (Contribution 14) fabricated a PdIn/TiO2 hybrid photoelectrocatalyst for wastewater treatment with simultaneously clean energy production. In the reaction system, on the one hand, paracetamol was degraded by oxidation at the photoanode; on the other hand, hydrogen was produced through reduction at the photocathode. Thus, a dual-function redox reaction system was established.
To use sunlight for CO2 reduction, Wang et al. (Contribution 15) designed TiO2/CuPc heterojunctions by combining TiO2 microspheres and copper phthalocyanines (CuPc). Benefiting from the heterojunction effect and the additional light-absorbing properties of TiO2 and CuPc, a good reduction rate in 32.4 μmol·g−1·h−1 of CO2 was achieved with TiO2/CuPc at about 3.7 times that of the pristine TiO2.
Unlike the experiments above, Gao et al. (Contribution 16) utilized the extended broken symmetry (EBS) method to investigate the low-lying spin states of the [Fe3S4] cluster, which is important for photosynthetic H2O splitting. The results indicated that the EBS results matched well with the experimental data. The weaknesses of the BS method could be compensated for through the developed EBS method.
Meanwhile, Tang et al. (Contribution 17) investigated the Sc2CX2/Sc2CY2 (X, Y = F, Cl, Br) Janus heterojunction for photocatalysis and photovoltaics using first-principles calculations. The calculated results indicate that these Janus heterojunctions possess type-II band structures and direct Z-scheme transfer mechanisms, which thus facilitates their application in photocatalysis and photovoltaics.
In their research paper, P. Ávila-Torres et al. (Contribution 18) prepared metal-yielded coordination compounds Cu-g-C3N4, Ni-g-C3N4, and Mn-g-C3N4. The structural properties of disinfected E. coli bacteria were investigated. The results indicate that the textural property is a key characteristic.
Liu et al. (Contribution 19) studied hydroxyl groups on the g-C3N4 (HCN) for O2 activation and pollutant degradation. The results show that hydroxyl groups can increase hydrophilicity and surface area, decrease interlayer distances, and promote the charge separation and transportation of the pristine g-C3N4, thus improving rhodamine B degradation.
Furthermore, A. Pavlatou et al. (Contribution 20) developed a new photocatalyst, namely magnesium oxide (MgO). MgO has a wide band gap and can produce hydroxyl radicals. It showed selectivity for rhodamine 6G and rhodamine B degradation under UV light. A total of 100% of rhodamine B could be degraded over MgO under UV light for 180 min.
In the research paper by Narkiewicz et al. (Contribution 21), triethylamine (TEA), diethylamine (DEA), and ethylenediamine (EDA) were used to modify TiO2. The effect of amines and temperature on the photocatalytic reduction of CO2 was investigated in detail. The results demonstrated that TEA-TiO2 treated in the microwave reactor exhibited the highest activity.
Furthermore, in the research by Wang et al. (Contribution 22), the 2D GaN/g-C3N4 heterojunction was investigated using first-principle calculations. The calculated results indicate that the GaN/g-C3N4 heterojunction possesses type-II band structures, broad light absorption capabilities, and direct Z-scheme transfer mechanisms, and thus has potential applications in the field of photocatalysis.
In the final research paper, Gerken et al. (Contribution 23) investigated the interplay between photocatalytic growth and the chemical dissolution of gold structures on TiO2/ITO patterns as a novel approach to mimic axonal dynamic connections. By optimizing gold growth and dissolution parameters, we demonstrated the potential for the precise control of the formation and removal of conductive pathways. This work bridges photocatalytic materials research and bio-inspired system development, offering new insights into the application of photodeposition and chemical etching in dynamic, adaptive systems.
3. Conclusions
In the face of increasingly severe environmental problems and resource challenges, green and sustainable development has become a global consensus and a guide for action. Photocatalysis is one of the most promising strategies for addressing the severe issues facing the environment and energy production and has attracted extensive and ongoing attention due to its inexhaustible, green, safe and economically viable characteristics. However, there are still many challenges involved in the practical application of photocatalysis, such as quantum efficiency, stability and reusability, selectivity, output-to-input ratio, and scaling-up. Fortunately, as a result of decades of hard work, photocatalysis has progressed to a new stage. Various and ingenious photocatalytic materials and photocatalytic reactions have been developed. Photocatalytic materials include but are not limited to nonmetallic LSPR materials, MOFs, COFs, SACs, SCCs, HEAs, heterojunctions, and composite materials. Photocatalytic reactions are involved in the fields of environmental, life science, medical research, space exploration, agriculture and food, energy, etc. However, designing advanced photocatalytic materials with good performance and exploring green photocatalytic reactions with carbon neutrality will require further progress.
Acknowledgments
The editors would like to express their great appreciation to all the authors, reviewers, and technical assistants who contributed to the Special Issue.
Conflicts of Interest
The author declares no conflicts of interest.
List of Contributions
- Shan, C.; Su, Z.; Liu, Z.; Xu, R.; Wen, J.; Hu, G.; Tang, T.; Fang, Z.; Jiang, L.; Li, M. One-Step Synthesis of Ag2O/Fe3O4 Magnetic Photocatalyst for Efficient Organic Pollutant Removal via Wide-Spectral-Response Photocatalysis–Fenton Coupling. Molecules 2023, 28, 4155.
- Wu, K.; Shang, Y.; Li, H.; Wu, P.; Li, S.; Ye, H.; Jian, F.; Zhu, J.; Yang, D.; Li, B.; et al. Synthesis and Hydrogen Production Performance of MoP/a-TiO2/Co-ZnIn2S4 Flower-like Composite Photocatalysts. Molecules 2023, 28, 4350.
- Long, B.; He, H.; Yu, Y.; Cai, W.; Gu, Q.; Yang, J.; Meng, S. Bifunctional hot water vapor template-mediated synthesis of nanostructured polymeric carbon nitride for efficient hydrogen evolution. Molecules 2023, 28, 4862.
- Ding, R.; Li, L.; Yu, Y.T.; Zhang, B.; Wang, P.L. Photoredox-Catalyzed Synthesis of 3-Sulfonylated Pyrrolin-2-ones via a Regioselective Tandem Sulfonylation Cyclization of 1, 5-Dienes. Molecules 2023, 28, 5473.
- Du, Z.; Gong, K.; Yu, Z.; Yang, Y.; Wang, P.; Zheng, X.; Wang, Z.; Zhang, S.; Chen, S.; Meng, S. Photoredox Coupling of CO2 Reduction with Benzyl Alcohol Oxidation over Ternary Metal Chalcogenides (ZnmIn2S3+m, m = 1–5) with Regulable Products Selectivity. Molecules 2023, 28, 6553.
- Shi, H.; Wang, H.; Zhang, E.; Qu, X.; Li, J.; Zhao, S.; Gao, S.; Chen, Z. Boosted photocatalytic performance for antibiotics removal with Ag/PW12/TiO2 composite: degradation pathways and toxicity assessment. Molecules 2023, 28, 6831.
- Dan, Y.; Xu, J.; Jian, J.; Meng, L.; Deng, P.; Yan, J.; Yuan, Z.; Zhang, Y.; Zhou, H. In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation. Molecules 2023, 28, 6882.
- Irfan, M.; Tahir, N.; Zahid, M.; Noreen, S.; Yaseen, M.; Shahbaz, M.; Mustafa, G.; Shakoor, R.A.; Shahid, I. The Fabrication of Halogen-Doped FeWO4 Heterostructure Anchored over Graphene Oxide Nanosheets for the Sunlight-Driven Photocatalytic Degradation of Methylene Blue Dye. Molecules 2023, 28, 7022.
- Ma, L.; Jiang, W.; Lin, C.; Xu, L.; Zhu, T.; Ai, X. CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production. Molecules 2023, 28, 7846.
- Zhang, M.; Wu, X.; Liu, X.; Li, H.; Wang, Y.; Wang, D. Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution. Molecules 2023, 28, 7878.
- Wang, X.; Liu, S.; Lin, S.; Qi, K.; Yan, Y.; Ma, Y. Visible Light Motivated the Photocatalytic Degradation of P-Nitrophenol by Ca2+-Doped AgInS2. Molecules 2024, 29, 361.
- Wu, Y.; Wang, Z.; Yan, Y.; Wei, Y.; Wang, J.; Shen, Y.; Yang, K.; Weng, B.; Lu, K. Rational Photodeposition of Cobalt Phosphate on Flower-like ZnIn2S4 for Efficient Photocatalytic Hydrogen Evolution. Molecules 2024, 29, 465.
- Tian, C.; Yu, H.; Zhai, R.; Zhang, J.; Gao, C.; Qi, K.; Zhang, Y.; Ma, Q.; Guo, M. Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium. Molecules 2024, 29, 637.
- Sacco, N.; Iguini, A.; Gamba, I.; Marchesini, F.A.; García, G. Pd: In-Doped TiO2 as a Bifunctional Catalyst for the Photoelectrochemical Oxidation of Paracetamol and Simultaneous Green Hydrogen Production. Molecules 2024, 29, 1073.
- Wang, J.; Fu, S.; Hou, P.; Liu, J.; Li, C.; Zhang, H.; Wang, G. Construction of TiO2/CuPc Heterojunctions for the Efficient Photocatalytic Reduction of CO2 with Water. Molecules 2024, 29, 1899.
- Chu, S.; Gao, Q. Unveiling the Low-Lying Spin States of [Fe3S4] Clusters via the Extended Broken-Symmetry Method. Molecules 2024, 29, 2152.
- He, X.; Wu, Y.; Luo, J.; Dai, X.; Song, J.; Tang, Y. First-Principles Study on Janus-Structured Sc2CX2/Sc2CY2 (X, Y= F, Cl, Br) Heterostructures for Solar Energy Conversion. Molecules 2024, 29, 2898.
- Lasso-Escobar, A.V.; Castrillon, E.D.C.; Acosta, J.; Navarro, S.; Correa-Penagos, E.; Rojas, J.; Ávila-Torres, Y.P. Modulation of Electronic Availability in g-C3N4 Using Nickel (II), Manganese (II), and Copper (II) to Enhance the Disinfection and Photocatalytic Properties. Molecules 2024, 29, 3775.
- Chen, J.; Yang, M.; Zhang, H.; Chen, Y.; Ji, Y.; Yu, R.; Liu, Z. Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4. Molecules 2024, 29, 3836.
- Gatou, M.A.; Bovali, N.; Lagopati, N.; Pavlatou, E.A. MgO Nanoparticles as a Promising Photocatalyst towards Rhodamine B and Rhodamine 6G Degradation. Molecules 2024, 29, 4299.
- Pełech, I.; Staciwa, P.; Sibera, D.; Sobczuk, K.S.; Majewska, W.; Kusiak-Nejman, E.; Morawski, A.T.; Wang, K.; Narkiewicz, U. The Influence of Heat Treatment on the Photoactivity of Amine-Modified Titanium Dioxide in the Reduction of Carbon Dioxide. Molecules 2024, 29, 4348.
- Dai, M.Y.; Zhao, X.C.; Lei, B.C.; Huang, Y.N.; Zhang, L.L.; Guo, H.; Wang, H.G. First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction. Molecules 2024, 29, 5355.
- Abshari, F.; Paulsen, M.; Veziroglu, S.; Vahl, A.; Gerken, M. Mimicking Axon Growth and Pruning by Photocatalytic Growth and Chemical Dissolution of Gold on TiO2 Patterns. Molecules 2024, 30, 99.
References
- Ciamician, G. The photochemistry of the future. Science 1912, 36, 385. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lei, J.; Zhou, N.; Sang, S.; Meng, S.; Low, J.; Li, Y. Unraveling the roles of atomically-dispersed Au in boosting photocatalytic CO2 reduction and aryl alcohol oxidation. Chin. J. Catal. 2024, 65, 163–173. [Google Scholar] [CrossRef]
- Che, Y.; Weng, B.; Li, K.; He, Z.; Chen, S.; Meng, S. Chemically bonded nonmetallic LSPR S-scheme hollow heterostructure for boosting photocatalytic performance. Appl. Catal. B Environ. Energy 2025, 361, 124656. [Google Scholar] [CrossRef]
- Candish, L.; Collins, K.D.; Cook, G.C.; Douglas, J.J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Photocatalysis in the life science industry. Chem. Rev. 2021, 122, 2907–2980. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, G.; Guan, X.; Lee, J.; Bahadur, R.; Ramadass, K.; Kumar, P.; Kibria, M.G.; Vidyasagar, D.; Yi, J.; et al. Multifunctional carbon nitride nanoarchitectures for catalysis. Chem. Soc. Rev. 2023, 52, 7602–7664. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Li, Z.; Yang, S.; Garcia, H. Metal–organic framework heterojunctions for photocatalysis. Chem. Soc. Rev. 2024, 53, 3002–3035. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mitchell, S.; Fang, Y.; Li, J.; Perez-Ramirez, J.; Lu, J. Advances in heterogeneous single-cluster catalysis. Nat. Rev. Chem. 2023, 7, 754–767. [Google Scholar] [CrossRef]
- Ham, R.; Nielsen, C.J.; Pullen, S.; Reek, J.N. Supramolecular coordination cages for artificial photosynthesis and synthetic photocatalysis. Chem. Rev. 2023, 123, 5225–5261. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Yu, J.; Liu, G.; Jaroniec, M. Non-noble plasmonic metal-based photocatalysts. Chem. Rev. 2022, 122, 10484–10537. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Meng, S.; Wang, Z.; Wang, P.; Wang, Z.; Chen, S.; Weng, B.; Zheng, Y.-M. Metal sulfide S-scheme homojunction for photocatalytic selective phenylcarbinol oxidation. Adv. Sci. 2024, 11, 2400099. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Kong, Y.; Wang, S.; Zuo, S.; Lin, W.; Fang, Y.; Hou, Y.; Zhang, G.; Zhang, H.; Wang, X. Hydroxyl-Bonded Ru on Metallic TiN Surface Catalyzing CO2 Reduction with H2O by Infrared Light. J. Am. Chem. Soc. 2023, 145, 27415–27423. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Effcient photocatalytic H2 evolution, CO2 reduction and N2 fxation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B Environ. 2021, 285, 119789. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Xiao, C.; Xie, Y. Dual-Plasmon Resonance Coupling Promoting Directional Photosynthesis of Nitrate from Air. Angew. Chem. Int. Ed. 2023, 62, e202311911. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, H.; Shen, J.; Wang, X.; Qu, C.; Lin, H.; Long, J.; Wang, Y.; Dai, W.; Fang, Y.; et al. Decoupling H2 and O2 Release in Particulate Photocatalytic Overall Water Splitting Using a Reversible O2 Binder. Angew. Chem. Int. Ed. 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).