Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrogel Synthesis
2.2. FT-IR Characterization Analysis of the Hydrogels
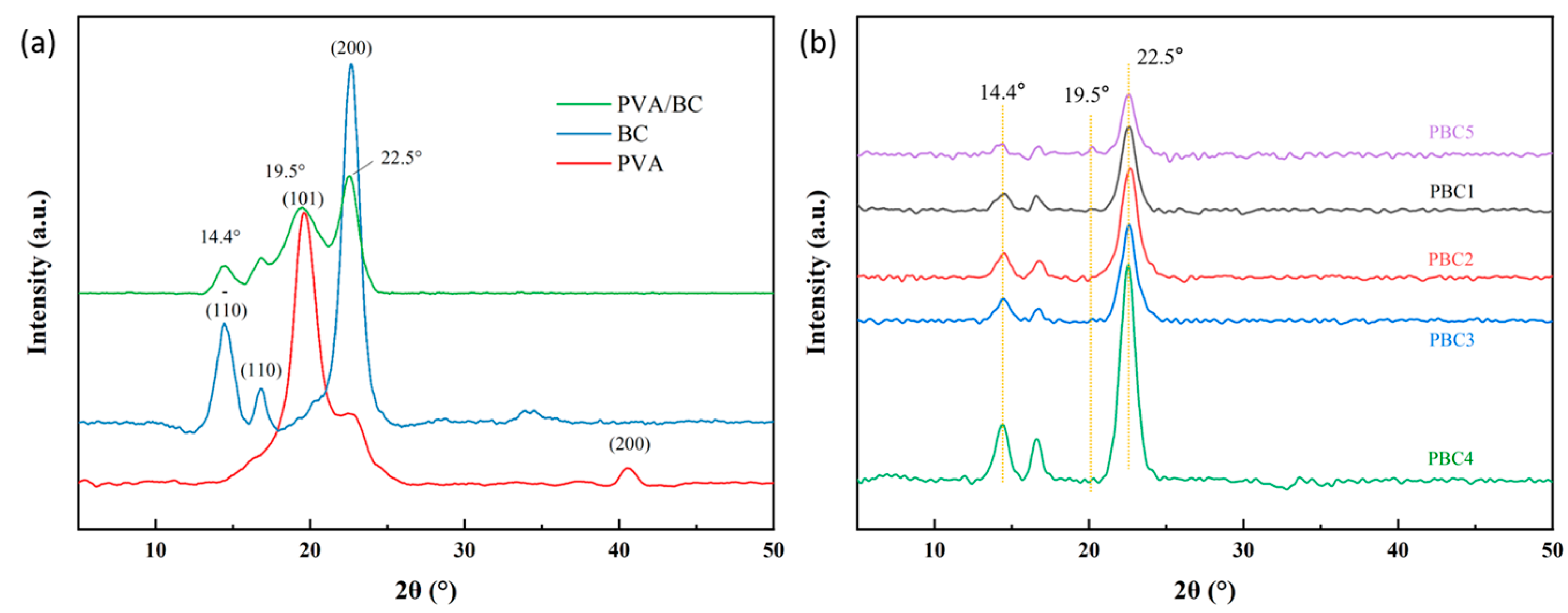
2.3. X-Ray Diffraction Analysis of the Hydrogels
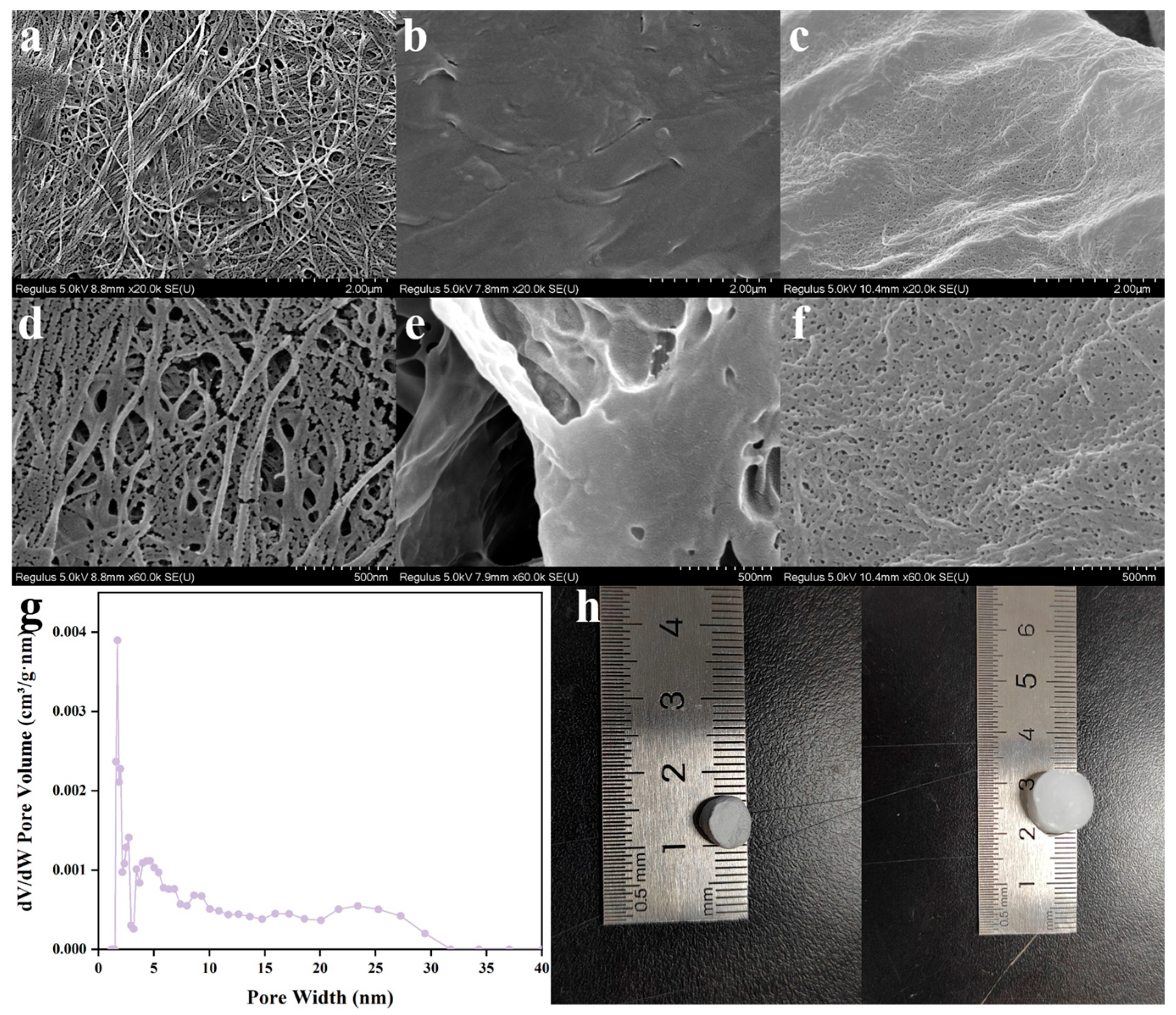
2.4. Morphology Characterization
2.5. Gel Fraction Analysis
2.6. Water Vapor Transmission Rate (WVTR)
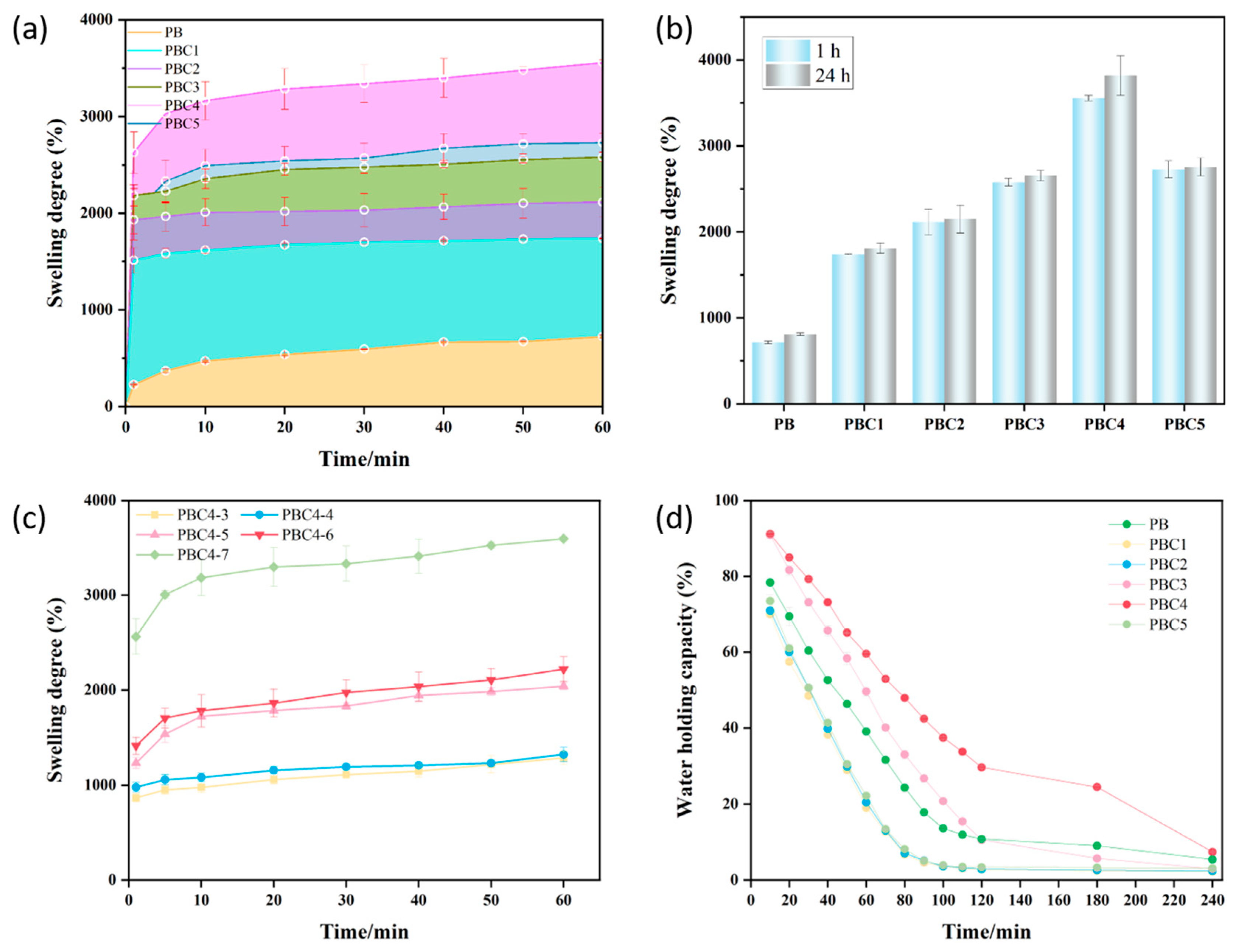
2.7. Swelling Capacity of Hydrogel
2.8. Water Holding Capacity
2.9. Cytotoxicity Assessment by CCK-8
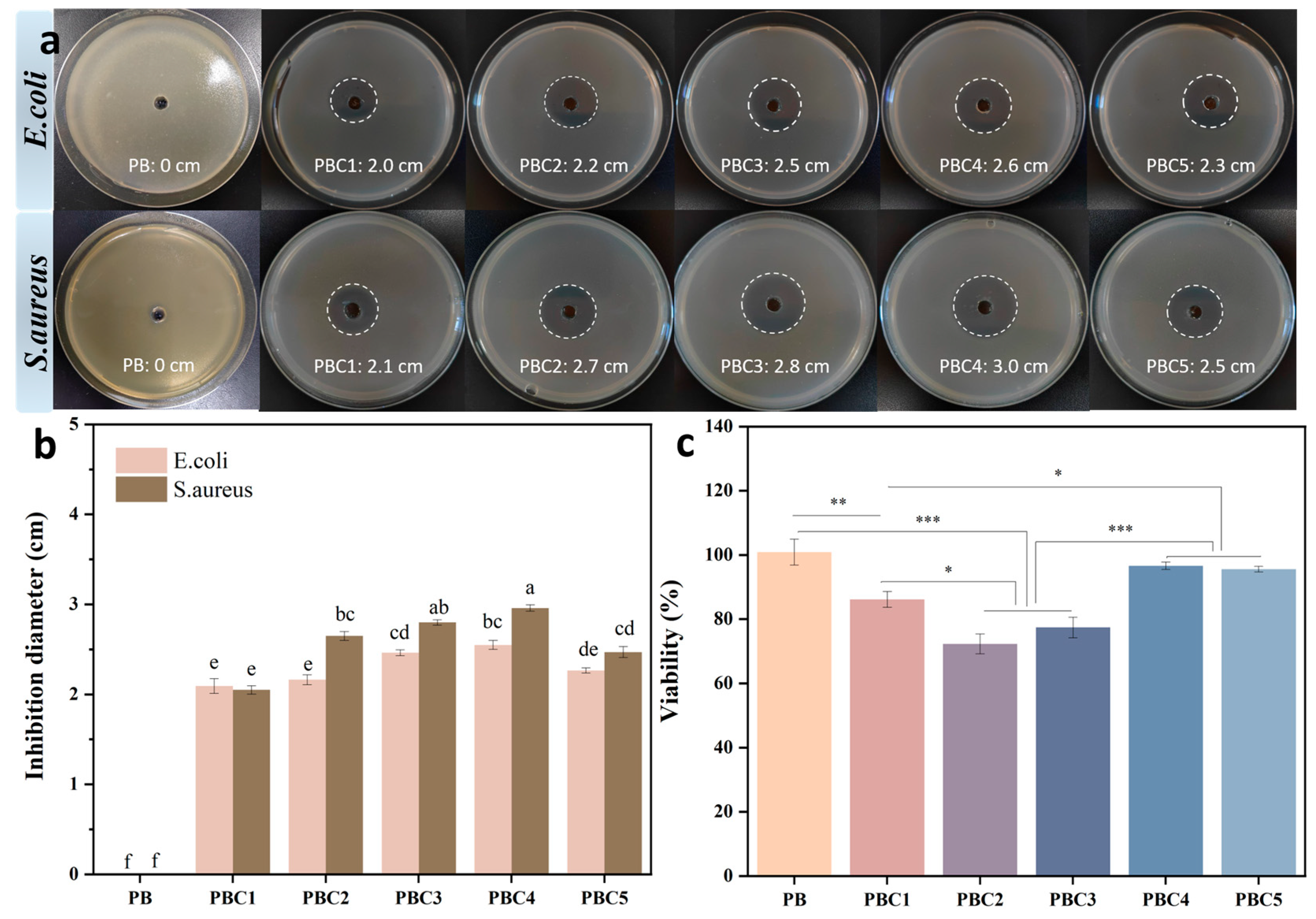
2.10. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hydrogels
3.3. Characterization of Hydrogels
3.4. Gel Fraction
3.5. Swelling Capacity
3.6. Water Holding Capacity Test
3.7. Water Vapor Transmission Rate
3.8. Cytocompatibility Analysis
3.8.1. Cell Culture
3.8.2. Cell Toxicity Assessment
3.9. In Vitro Antibacterial Performance
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, T.; Yu, J.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, e2201254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, X.; Zhang, R.; Zhang, K.; Li, Y.; Xu, F.-J. Self-Assembled Herbal Medicine Encapsulated by an Oxidation-Sensitive Supramolecular Hydrogel for Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 56898–56907. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Tian, P.; Hao, L.; Hu, C.; Liu, B.; Meng, F.; Yi, X.; Pan, X.; Hu, X.; Wang, H.; et al. Antioxidative bioactive glass reinforced injectable hydrogel with reactive oxygen species scavenging capacity for diabetic wounds treatment. Chem. Eng. J. 2024, 481, 148768. [Google Scholar] [CrossRef]
- Ning, T.; Zhang, R.; Zheng, Y.; Jing, W.; Muhammad, K.; Xue, J.; Cheng, Z.; Rawan, O.; Walaa, S.; Weiwei, W.; et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022, 34, 2106842. [Google Scholar]
- Hu, W.-W.; Lin, Y.-T. Alginate/polycaprolactone composite fibers as multifunctional wound dressings. Carbohydr. Polym. 2022, 289, 119440. [Google Scholar] [CrossRef]
- Panwar, V.; Sharma, A.; Murugesan, P.; Salaria, N.; Ghosh, D. Free-flowing, self-crosslinking, carboxymethyl starch and carboxymethyl cellulose microgels, as smart hydrogel dressings for wound repair. Int. J. Biol. Macromol. 2023, 246, 125735. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Liao, J.; Wang, F.; Jiang, J.; Cao, C.; Li, S. Novel bilayer wound dressing composed of SIS membrane with SIS cryogel enhanced wound healing process. Mater. Sci. Eng. C 2018, 85, 162–169. [Google Scholar] [CrossRef]
- Umar, M.; Ullah, A.; Nawaz, H.; Areeb, T.; Hashmi, M.; Kharaghani, D.; Kim, K.O.; Kim, I.S. Wet-spun bi-component alginate based hydrogel fibers: Development and in-vitro evaluation as a potential moist wound care dressing. Int. J. Biol. Macromol. 2021, 168, 601–610. [Google Scholar] [CrossRef]
- Han, X.; Su, Y.; Che, G.; Wei, Q.; Zheng, H.; Zhou, J.; Li, Y. Supramolecular Hydrogel Dressing: Effect of Lignin on the Self-Healing, Antibacterial, Antioxidant, and Biological Activity Improvement. ACS Appl. Mater. Interfaces 2022, 14, 50199–50214. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.-L.; Cheng, B.; Mo, X.-M.; Chen, C.; Pan, J.-F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels To Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Long, M.; Zheng, N.; Deng, Y.; Wang, Q.; Osire, T.; Xia, X. Microstructural, physicochemical properties, and interaction mechanism of hydrogel nanoparticles modified by high catalytic activity transglutaminase crosslinking. Food Hydrocoll. 2024, 147, 109384. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; Li, X.; Li, T.; Zheng, J.; Hu, M.; Yu, Z.; Luo, W.; Zhang, W.; Zheng, F.; et al. A self-assembled hydrogel dressing as multi-target therapeutics to promote wound healing. Chem. Eng. J. 2023, 477, 147145. [Google Scholar] [CrossRef]
- Raafat, A.I.; El-Sawy, N.M.; Badawy, N.A.; Mousa, E.A.; Mohamed, A.M. Radiation fabrication of Xanthan-based wound dressing hydrogels embedded ZnO nanoparticles: In vitro evaluation. Int. J. Biol. Macromol. 2018, 118, 1892–1902. [Google Scholar] [CrossRef]
- Lu, J.; Fan, X.; Hu, J.; Li, J.; Rong, J.; Wang, W.; Chen, Y.; Liu, W.; Chen, J.; Chen, Y. Construction and function of robust and moist bilayer chitosan-based hydrogel wound dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Zhou, N.; Tan, J.; Ashley, J.; Wang, W.; Wu, F.; Shen, J.; Zhang, M. Study on a novel poly (vinyl alcohol)/graphene oxide-citicoline sodium-lanthanum wound dressing: Biocompatibility, bioactivity, antimicrobial activity, and wound healing effect. Chem. Eng. J. 2020, 395, 125059. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Wei, X.; Li, H.; Liu, X.; Cheng, F. A polyphenol and ε-polylysine functionalized bacterial cellulose/PVA multifunctional hydrogel for wound healing. Int. J. Biol. Macromol. 2023, 247, 125663. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, D.; Lu, K.; Zhang, H.; Liu, C.; Lin, Y.; Cheng, J.; Zou, Y.; Xu, H.; Chen, H.; et al. Bacterial cellulose-based dressings with photothermal bactericidal activity and pro-angiogenic ability for infected wound healing. J. Mater. Sci. Technol. 2023, 160, 76–85. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.Y.; Yang, Q.; Lu, D.N. Bacterial cellulose–poly(vinyl alcohol) nanocomposite hydrogels prepared by chemical crosslinking. J. Appl. Polym. Sci. 2012, 126, E245–E251. [Google Scholar] [CrossRef]
- Tamahkar, E. Bacterial cellulose/poly vinyl alcohol based wound dressings with sustained antibiotic delivery. Chem. Pap. 2021, 75, 3979–3987. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. Bacterial cellulose-based membrane-like biodegradable composites using cross-linked and noncross-linked polyvinyl alcohol. J. Mater. Sci. 2012, 47, 6066–6075. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Q.; Lu, D.-N. Effect of chemical crosslinking degree on mechanical properties of bacterial cellulose/poly(vinyl alcohol) composite membranes. Monatshefte Chem.—Chem. Mon. 2014, 145, 91–95. [Google Scholar] [CrossRef]
- Wang, C.; Cao, H.; Jia, L.; Liu, W.; Liu, P. Characterization of antibacterial aerogel based on ɛ-poly-l-lysine/nanocellulose by using citric acid as crosslinker. Carbohydr. Polym. 2022, 291, 119568. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Wang, X.; Yan, B.; Peng, Y.; Ran, R. Fe3+-citric acid/sodium alginate hydrogel: A photo-responsive platform for rapid water purification. Carbohydr. Polym. 2021, 269, 118269. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- de Lima, G.G.; Ferreira, B.D.; Matos, M.; Pereira, B.L.; Nugent, M.J.D.; Hansel, F.A.; Magalhães, W.L.E. Effect of cellulose size-concentration on the structure of polyvinyl alcohol hydrogels. Carbohydr. Polym. 2020, 245, 116612. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Z.; Jia, Q.; Wang, Q.; Zhang, Q.; Li, X.; Yu, J.; Ding, B. Flexible Bioactive Glass Nanofiber-Based Self-Expanding Cryogels with Superelasticity and Bioadhesion Enabling Hemostasis and Wound Healing. ACS Nano 2023, 17, 11507–11520. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Zhou, Q.; Ran, F. Integrating supercapacitor with sodium hyaluronate based hydrogel as a novel All-In-One wound Dressing: Self-Powered electronic stimulation. Chem. Eng. J. 2023, 452, 139491. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–Thaw-Induced Physically Cross-linked Superabsorbent Polyvinyl Alcohol/Soy Protein Isolate Hydrogels for Skin Wound Dressing: In Vitro and In Vivo Characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Ou, K.; Hou, Y.; Yuan, P.; Yu, W.; Li, X.; Wang, B.; He, J.; Cui, S.; Chen, X. Unidirectional water-transport antibacterial trilayered nanofiber-based wound dressings induced by hydrophilic-hydrophobic gradient and self-pumping effects. Mater. Des. 2021, 201, 109461. [Google Scholar] [CrossRef]
- Eghbalifam, N.; Shojaosadati, S.A.; Hashemi-Najafabadi, S.; Khorasani, A.C. Synthesis and characterization of antimicrobial wound dressing material based on silver nanoparticles loaded gum Arabic nanofibers. Int. J. Biol. Macromol. 2020, 155, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Shen, Y.; Liu, F.; Zhou, Y.; Wu, H.; Liu, Q.; Deng, B. Preparation of Centella asiatica loaded gelatin/chitosan/nonwoven fabric composite hydrogel wound dressing with antibacterial property. Int. J. Biol. Macromol. 2021, 192, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Deng, L.; Huang, Y.; Chen, S.; Han, Z.; Han, Z.; Jin, M.; Qu, X.; Wang, B.; Wang, H.; Gu, S. Bacterial cellulose-based hydrogel with antibacterial activity and vascularization for wound healing. Carbohydr. Polym. 2023, 308, 120647. [Google Scholar] [CrossRef]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef]
- Harada, N.; Mitsukami, Y.; Uyama, H. Preparation and characterization of water-swellable hydrogel-forming porous cellulose beads. Polymer 2021, 215, 123381. [Google Scholar] [CrossRef]
- Ciecholewska-Jusko, D.; Zywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdal, P.; Kowalska, U.; Toporkiewicz, M.; Fijalkowski, K. Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr. Polym. 2021, 253, 117247. [Google Scholar] [CrossRef]
- Chang, A.; Ye, Z.; Ye, Z.; Deng, J.; Lin, J.; Wu, C.; Zhu, H. Citric acid crosslinked sphingan WL gum hydrogel films supported ciprofloxacin for potential wound dressing application. Carbohydr. Polym. 2022, 291, 119520. [Google Scholar] [CrossRef] [PubMed]
- Eswaramma, S.; Reddy, N.S.; Rao, K.S.V.K. Phosphate crosslinked pectin based dual responsive hydrogel networks and nanocomposites: Development, swelling dynamics and drug release characteristics. Int. J. Biol. Macromol. 2017, 103, 1162–1172. [Google Scholar] [CrossRef] [PubMed]




| Sample | R2 | Ks | W∞calc | W∞obs |
|---|---|---|---|---|
| PBC1 | 0.9999 | 0.0573 | 17.44 | 18.42 |
| PBC2 | 0.9998 | 0.0443 | 22.57 | 22.67 |
| PBC3 | 0.9999 | 0.0386 | 25.90 | 27.24 |
| PBC4 | 0.9990 | 0.0283 | 35.31 | 40.82 |
| PBC5 | 0.9997 | 0.0347 | 28.80 | 28.39 |
| Material | Swelling Rate at 24 h (%) | Water Vapor Transmission Rate (g m−2 day−1) | Reference |
|---|---|---|---|
| PVA/SPI | 838.8 | 2430.8 | [31] |
| PU/PAN-SPA | 950 | 2200 | [32] |
| GA/AgNPs/PVA/PCL | 540.53 | 2193.27 | [33] |
| CA/Gel/CS | 450 | 3419 | [34] |
| PVA/CS/nZnO | 9.3 | 2200 | [35] |
| PVA/BC/CA | 3485.3 | 2332.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, M.; Wu, C.; Chen, Y. Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing. Molecules 2025, 30, 737. https://doi.org/10.3390/molecules30030737
Mo M, Wu C, Chen Y. Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing. Molecules. 2025; 30(3):737. https://doi.org/10.3390/molecules30030737
Chicago/Turabian StyleMo, Meiqing, Chaojun Wu, and Yehong Chen. 2025. "Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing" Molecules 30, no. 3: 737. https://doi.org/10.3390/molecules30030737
APA StyleMo, M., Wu, C., & Chen, Y. (2025). Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing. Molecules, 30(3), 737. https://doi.org/10.3390/molecules30030737





