The Use of Metabolic Inducers in Wheat to Increase the Nutritional and Functional Value of Grain
Abstract
1. Introduction
2. Results
2.1. Yield Parameters
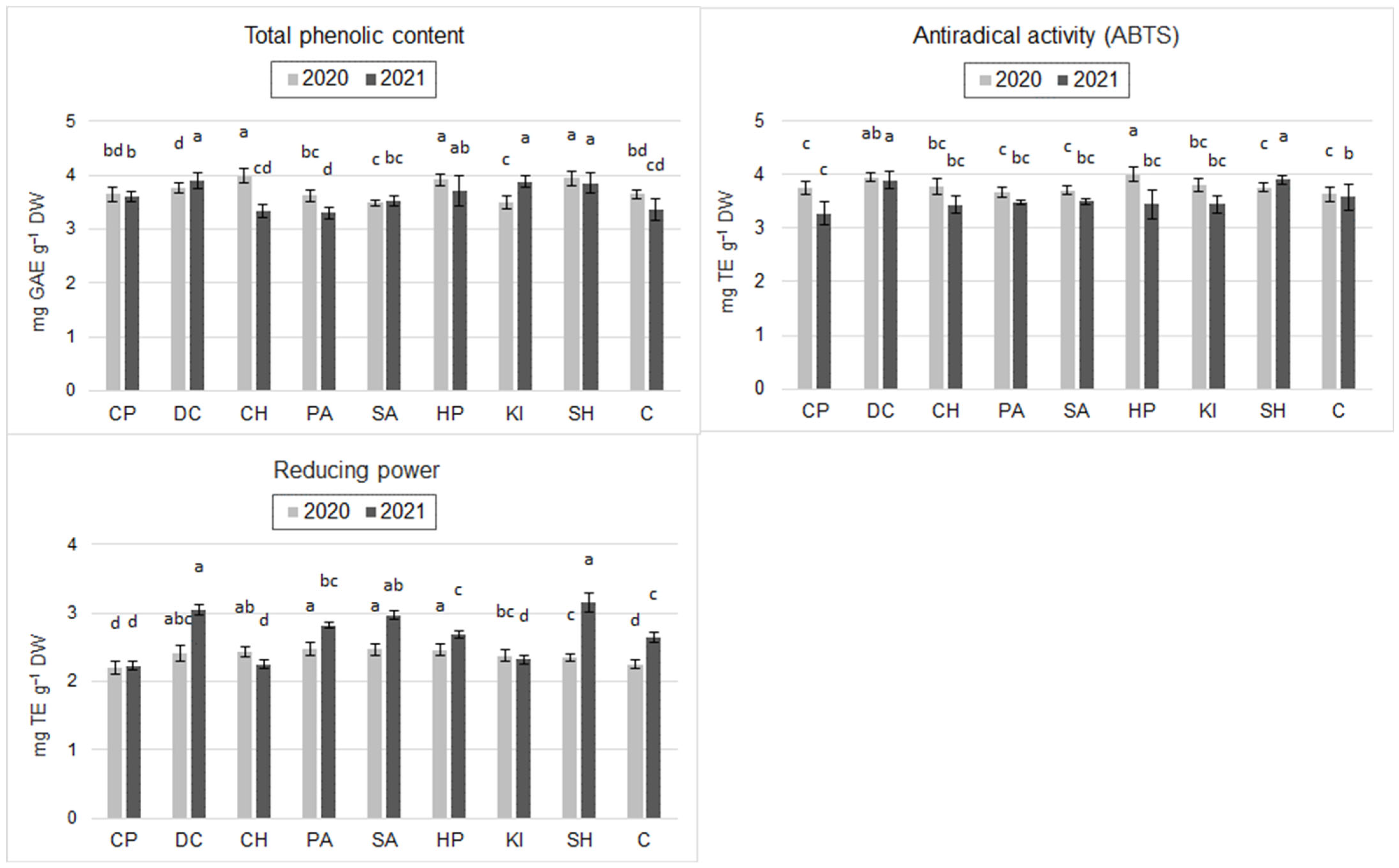
2.2. Antioxidant Value of Grains
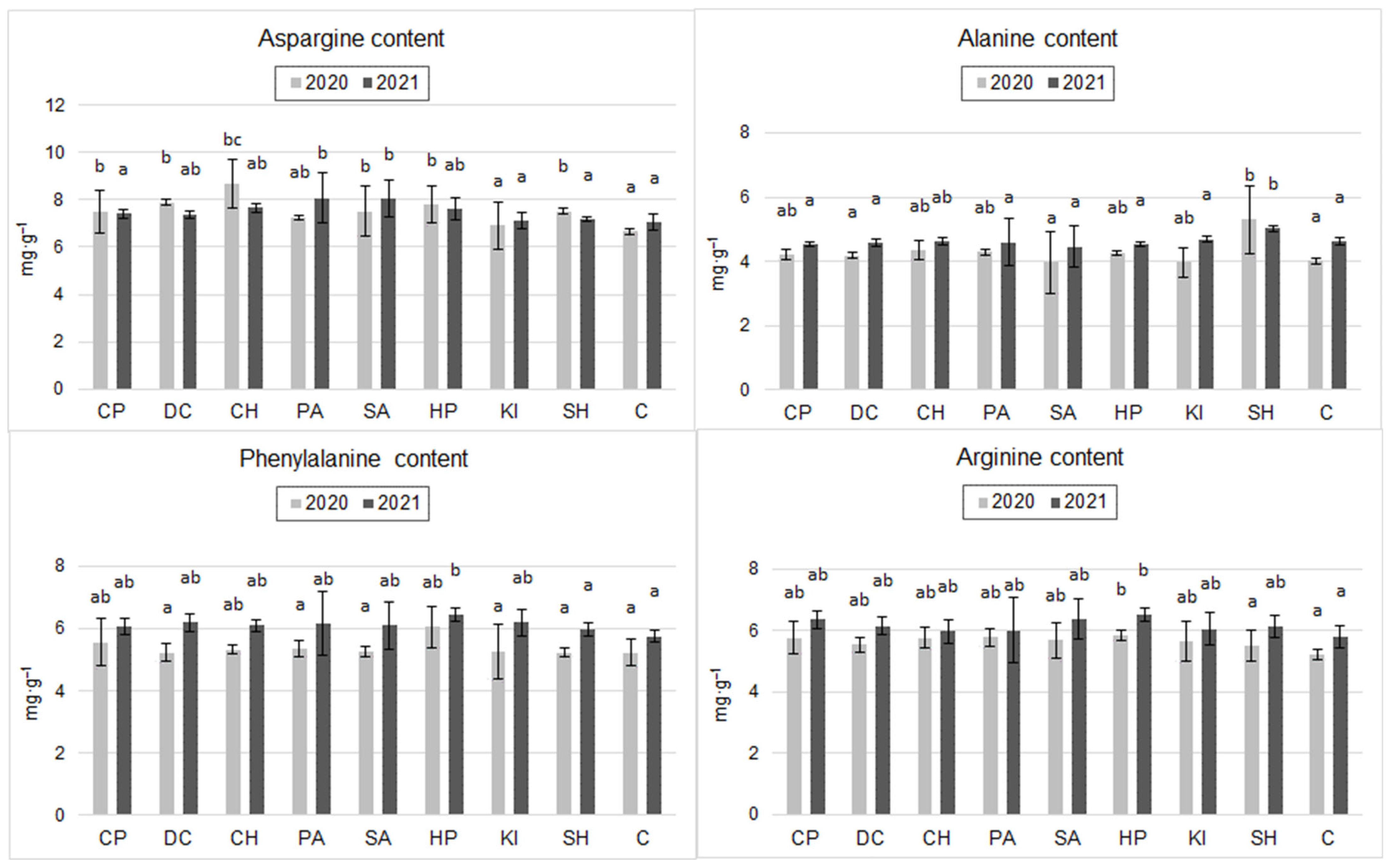
2.3. Amino Acid Content of the Grain
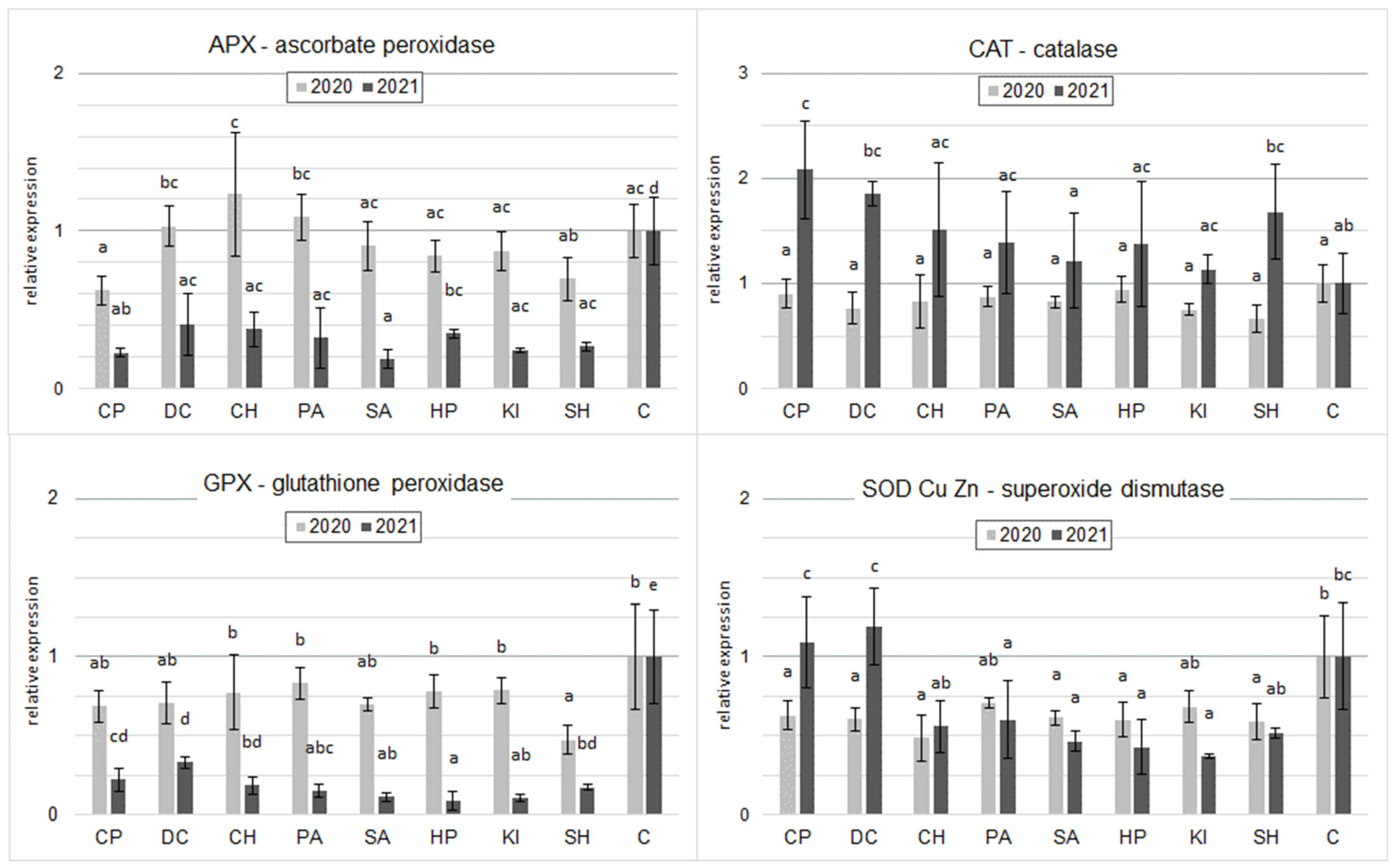
2.4. Gene Expression
3. Discussion
3.1. The Effect of Inducers on Yield and Nutritional Value
3.2. The Effect of Inducers on Functional Quality
3.3. The Effect of Inducers on the Gene Expression
4. Materials and Methods
4.1. Field Experiment
- (1)
- CP—micronized cricket flour extract (Acheta domesticus)—250 g dm·ha−1 (3.33 g dm·L−1; 300 L·ha−1–30 mL·m−2);
- (2)
- DC—chitin isolated from micronized cricket flour—250 g dm·ha−1 (3.33 g dm·L−1; 300 L·ha−1–30 mL·m−2);
- (3)
- CH—chitosan hydrochloride—250 g·ha−1 (1.33 g dm·L−1; 300 L·ha−1–30 mL·m−2);
- (4)
- PA—L-phenylalanine—10 g·ha−1 (0.033 g·L−1; 300 L·ha−1–30 mL·m−2);
- (5)
- SA—salicylic acid—41.4 g·ha−1 (1.0 mM; 0.138 g·L−1; 300 L·ha−1–30 mL·m−2);
- (6)
- HP—hydrogen peroxide—concentration 1.5% (294 mM; 300 L·ha−1–30 mL·1 m−2);
- (7)
- KI—potassium iodide—150 g·ha−1 (3 mM; 0.5%; 0.5 g·L−1 (0.498 g·L−1); 300 L·ha−1–30 mL·m−2);
- (8)
- SH—sodium hypochlorite—5% (45 L NaClO·ha−1; 300 L·ha−1–30 mL·1 m−2);
- (9)
- C—control (demineralized water without inducers).
4.2. Description of Inducers
4.3. Preparation of Cricket Powder
4.4. Yield Analysis
4.5. Analysis of Nutritional and Health-Promoting Quality of Wheat Grain
4.5.1. Sample Preparation for the Determination of Amino Acids
4.5.2. Extraction of Free and Bound Phenolic Compounds
4.5.3. Contents of Phenols (TPC)
4.5.4. Antiradical Activity (ABTS)
4.5.5. Reducing Power (RP)
4.6. Gene Expression of Antioxidant Enzymes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D. Comparison of phenolic acids profile and antioxidant potential of six varieties of spelt (Triticum spelta L.). J. Agric. Food Chem. 2012, 60, 4603–4612. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B. Influence of elicitation with H2O2 on phenolics content, antioxidant potential and nutritional quality of Lens culinaris sprouts. J. Sci. Food Agric. 2014, 94, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 49–60. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Kumar, V.; Singhal, R.K.; Bose, B.; Chauhan, J.; Alamri, S.; Sabagh, A.E. Seed priming with Mg(NO3)2 and ZnSO4 salts triggers the germination and growth attributes synergistically in wheat varieties. Agronomy 2021, 11, 2110. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Jwa, N.S.; Agrawal, G.K.; Tamogami, S.; Yonekura, M.; Han, O.; Iwahashi, H.; Rakwal, R. Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 2006, 44, 261–273. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Albert, M. Peptides as triggers of plant defence. J. Exp. Bot. 2013, 64, 5269–5279. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Production of chitosan oligosaccharides for inclusion in a plant biostimulant. Pure Appl. Chem. 2016, 88, 881–889. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Saharan, V. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 2019, 123, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Fischer, S.; Egan, D.; Doohan, F.M. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology 2006, 96, 386–394. [Google Scholar] [CrossRef]
- Khan, M.R.; Doohan, F.M. Bacterium-mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain. Biol. Control 2009, 48, 42–47. [Google Scholar] [CrossRef]
- Marquis, R.J.; Alexander, H.M. Evolution of resistance and virulence in plant-herbivore and plant-pathogen interactions. Trends Ecol. Evol. 1992, 7, 126–129. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Home Page. 2022. Available online: http://www.fao.org/home/en/ (accessed on 5 September 2025).
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic acid: Biosynthesis, metabolism and physiological role in plants. In Salicylic Acid A Plant Hormone; Springer: Dordrecht, The Netherlands, 2007; pp. 1–14. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; Ramadan, A.A.E.-M.; El-Bassiouny, H.M.S.; Bakry, B.A. Regulation of Antioxidant System in Wheat Cultivars by Using Chitosan or Salicylic Acid to Improve Growth and Yield Under Salinity Stress. Asian J. Plant Sci. 2020, 19, 114–126. [Google Scholar] [CrossRef]
- Kumar, P.; Dube, S.D.; Chauhan, V.S. Effect of salicylic acid on growth, development and some biochemical aspects of soybean (Glycine max L. Merrill). Indian J. Plant Physiol. 1999, 4, 327–330. [Google Scholar] [CrossRef]
- Olgun, M.; Kumlay, A.M.; Karadas, K.; Turan, M.; Tomar, O.; Çaglar, A. Effect of water stress and potassium iodide on yield and yield components in two wheat varieties. Acta Agric. Scand. B Soil Plant Sci. 2006, 56, 230–234. [Google Scholar] [CrossRef]
- Salazar-Mercado, S.A.; Torres-León, C.A.; Rojas-Suárez, J.P. Cytotoxic evaluation of sodium hypochlorite, using Pisum sativum L. as effective bioindicator. Ecotoxicol. Environ. Saf. 2019, 173, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Causil, V.L.A.; Coronado, G.J.L.; Verbel, M.L.F.; Vega, J.M.F.; Donado, E.K.A.; Pacheco, G.C. Cytotoxic effect of sodium hypochlorite (NaClO) in apical cells of onion roots (Allium cepa L.). Rev. Colom. Cienc. Hortí. 2017, 11, 97–104. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Elsevier: Amsterdam, The Netherlands, 2006; pp. 23–67. [Google Scholar]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Tech. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J. APriming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of potato (Solanum tuberosum L.) plants to spraying by hydrogen peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Afzal, I.; Farooq, M.; Wahid, A. Growth Improvement in Spring Maize through Exogenous Application of Ascorbic Acid, Salicylic Acid and Hydrogen Peroxide. Int. J. Agric. Biol. 2013, 15, 95–100. [Google Scholar]
- El-Rahman, A.; Lamyaa, A.; Omara, R.I.; Gad, M.A. Influence of Hydrogen Peroxide and Nanofertilizer on Rusts Development and Wheat Productivity. Egypt. J. Agron. 2021, 43, 295–306. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, D.; Wang, C.; Zhao, H.; Zhu, Y.; Guo, T. Responses of Amino Acid Composition to Nitrogen Application in High- and Low-Protein Wheat Cultivars at Two Planting Environments. Crop Sci. 2016, 56, 1277–1287. [Google Scholar] [CrossRef]
- Garcia Del Moral, L.F.; Rharrabti, Y.; Martos, V.; Royo, C. Environmentally induced changes in amino acid composition in the grain of durum wheat grown under different water and temperature regimes in a Mediterranean environment. J. Agric. Food Chem. 2007, 55, 8144–8151. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Simon-Sarkadi, L.; Sovány, C.; Kirsch, K.; Galiba, G.; Kocsy, G. Differential effects of cold acclimation and abscisic acid on free amino acid composition in wheat. Plant Sci. 2011, 180, 61–68. [Google Scholar] [CrossRef]
- Jaśkiewicz, B.; Szczepanek, M. Amino acids content in triticale grain depending on meteorological, agrotechnical and genetic factors. Res. Rural. Dev. Agric. Sci. (Crop Sci. Anim. Sci.) 2018, 2, 28–34. [Google Scholar] [CrossRef]
- Younis, U.; Qayyum, M.F.; Shah, M.H.R.; Danish, S.; Shahzad, A.N.; Malik, S.A.; Mahmood, S. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J. Plant Nutr. Soil Sci. 2015, 178, 209–217. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Delledonne, M.; Lamattina, L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013, 64, 3339–3349. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013, 11, 976–985. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Marine Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Liu, K. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.N.; Siddique, K.H.M.; Ahmad, P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt-and temperature-induced oxidative stress in Brassica juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Wang, M.; Wei, Y.; Xia, Z. Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 2016, 6, 1223. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Dudziak, K.; Zapalska, M.; Börner, A.; Szczerba, H.; Kowalczyk, K.; Nowak, M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Scient. Rep. 2019, 9, 274. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754–9765. [Google Scholar] [CrossRef]
- Ushimaru, T.; Kanematsu, S.; Shibasaka, M.; Tsuji, H. Effect of hypoxia on antioxidant enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 107, 181–187. [Google Scholar] [CrossRef]
- Jiang, W.Q.; Yang, L.; He, Y.Q.; Zhang, H.T.; Li, W.; Chen, H.G.; Ma, D.F.; Yin, J.L. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). Peer. J. 2019, 7, e8062. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Buivydaite, V.V. Soil survey and available soil data in Lithuania. Soil Resources of Europe: 2nd ed. Eur. Soil Bur. Rep. 2005, 9, 211–233. [Google Scholar]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.; Preston, G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 2012, 327, 1–8. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Induction of H2O2 and related enzymes in tomato roots infected with root knot nematode (M. javanica) by several chemical and microbial elicitors. Biocontrol Sci. Technol. 2009, 19, 301–313. [Google Scholar] [CrossRef]
- Urban, L.; Lauri, F.; Ben Hdech, D.; Aarrouf, J. Prospects for increasing the efficacy of plant resistance inducers stimulating salicylic acid. Agronomy 2022, 12, 3151. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Bahadur, A.; Singh, D.P.; Sarma, B.K.; Singh, U.P. Foliar application of l-phenylalanine and ferulic acids to pea plants: Induced phenylalanine ammonia lyase activity and resistance against Erysiphe pisi. Arch. Phytopathol. Plant Prot. 2012, 45, 398–403. [Google Scholar] [CrossRef]
- Singh, A.; Gairola, K.; Upadhyay, V.; Kumar, J. Chitosan: An elicitor and antimicrobial Bio-resource in plant protection. Agric. Rev. 2018, 39, 163–168. [Google Scholar] [CrossRef]
- Copes, W.E.; Ojiambo, P.S. Efficacy of hypochlorite as a disinfestant against fungal pathogens in agricultural and horticultural plant production: A systematic review and meta-analysis. Phytopathology 2021, 111, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Available online: https://search.worldcat.org/title/Official-methods-of-analysis-of-AOAC-international.-Vol.-1-Agricultural-chemicals-contaminants-drugs/oclc/59478847 (accessed on 5 September 2025).
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Babiker, E.E.; Hassan, A.B.; Eltayeb, M.M.; Osman, G.A.; El Hassan, N.M.; Hassan, K.A. Solubility and functional properties of boiled and fried Sudanese tree locust flour as a function of NaCl concentration. J. Food Technol. 2007, 5, 210–214. [Google Scholar]
- Kim, H.W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of house cricket (Acheta domesticus) flour addition on physicochemical and textural properties of meat emulsion under various formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef]
- ISO 16634-2:2016; Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content, Part 2: Cereals, Pulses and Milled Cereal Products. International Standardization Organization: Geneva, Switzerland, 2016.
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- ISO 21415-2:2015; Wheat and Wheat Flour—Gluten Content, Part 2: Determination of Wet Gluten and Gluten Index by Mechanical Means. International Standardization Organization: Geneva, Switzerland, 2015.
- ICC Standard No. 122/1; Determination of Starch Content by Calcium Chloride Dissolution. International Association for Cereal Science and Technology: Vienna, Austria, 1994.
- ISO 5529:2007; Wheat—Determination of the Sedimentation Index—Zeleny Test, International Standard, Third Edition 2007-09-01. International Standardization Organization: Geneva, Switzerland, 2007.
- Davis, M.G.; Thomas, A.J. An investigation of hydrolytic techniques for the amino acid analysis of foodstuffs. J. Sci. Food Agric. 1973, 24, 1525–1540. [Google Scholar] [CrossRef]
- Rigas, P.G. Review: Liquid chromatography—Post-column derivatization for amino acid analysis: Strategies, instrumentation, and applications. Instrum. Sci. Technol. 2012, 40, 161–193. [Google Scholar] [CrossRef]
- Peace, R.W.; Gilani, G.S. Chromatographic Determination of Amino Acids in Foods. J. AOAC Int. 2005, 88, 877–887. [Google Scholar] [CrossRef]
- Besman, M.; Matwiejczyk, M.; Cywoniuk, P.; Mieloch, A.A.; Porzucek, P.; Rybka, J.D.; Miedzianka, J.; Juchniewicz, S.; Zambrowicz, A. Evaluation of the Physicochemical Properties and Potential Immunomodulatory Effects of Bovine Thymic Peptide-Based Preparations. J. Pept. Res. Ther. 2025, 31, 108. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Wang, G.; Wang, G.; Zhang, X.; Wang, F.; Song, R. Isolation of high quality RNA from cereal seeds containing high levels of starch. Phytochem. Anal. 2012, 23, 159–163. [Google Scholar] [CrossRef]
| Trait | Year | Factor | HSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | DC | CH | PA | SA | HP | KI | SH | C | |||
| Grain yield (t·ha−1) | 2020 | 4.18 ab | 4.41 c | 4.19 b | 4.16 ab | 4.06 ab | 4.34 b | 3.75 a | 3.90 a | 4.28 bc | 0.24 |
| 2021 | 4.13 b | 4.25 b | 3.97 b | 4.10 b | 4.08 b | 4.21 b | 3.84 b | 3.36 a | 4.19 b | 0.36 | |
| Mean | 4.16 bc | 4.29 c | 3.98 b | 4.13 b | 4.07 ab | 4.28 b | 3.79 a | 3.63 a | 4.24 bc | 0.32 | |
| Harvest residue (t·ha−1) | 2020 | 6.62 a | 6.56 a | 6.55 a | 6.64 a | 6.60 a | 7.06 b | 6.59 a | 6.53 a | 6.73 ab | 0.21 |
| 2021 | 6.44 b | 6.26 a | 6.29 a | 6.17 a | 6.31 a | 6.54 bc | 6.17 a | 6.05 a | 6.69 bc | 0.29 | |
| Mean | 6.53 ab | 6.41 a | 6.42 a | 6.41 a | 6.46 a | 6.80 b | 6.38 a | 6.29 a | 6.71 b | 0.24 | |
| Harvest Index | 2020 | 0.39 a | 0.40 ab | 0.38 a | 0.39 a | 0.38 a | 0.37 a | 0.36 a | 0.37 a | 0.39 b | 0.03 |
| 2021 | 0.39 b | 0.41 b | 0.39 b | 0.40 b | 0.40 b | 0.38 a | 0.38 a | 0.35 a | 0.40 b | 0.03 | |
| Mean | 0.39 ab | 0.41 b | 0.38 ab | 0.39 b | 0.39 ab | 0.38 a | 0.37 a | 0.36 a | 0.39 b | 0.03 | |
| TGW (g) | 2020 | 30.26 a | 29.87 aa | 30.20 a | 30.24 a | 28.82 a | 29.9 ab | 29.68 a | 29.95 a | 29.48 a | n.s. |
| 2021 | 30.0 a | 29.9 a | 30.0 a | 30.3 a | 29.2 a | 30.1 a | 29.8 a | 29.7 a | 29.5 a | n.s. | |
| Mean | 30.1 a | 29.9 a | 30.1 a | 30.3 a | 29.0 a | 30.0 a | 29.7 a | 29.8 a | 29.5 a | n.s | |
| Trait | Year | Factor | HSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | DC | CH | PA | SA | HP | KI | SH | C | |||
| Protein content (g·kg−1) | 2020 | 143.0 ab | 139.0 a | 143.0 ab | 145.9 c | 146.0 c | 144.3 bc | 140.6 ab | 142.1 ab | 142.0 ab | 2.8 |
| 2021 | 142.1 a | 143.6 ab | 142.6 ab | 143.3 ab | 142.3 a | 143.6 ab | 140.5 a | 147.7 b | 141.07 a | 4.6 | |
| Mean | 142.6 a | 141.3 a | 142.8 a | 144.6 ab | 144.1 ab | 143.9 a | 140.5 a | 144.9 b | 141.5 a | 3.2 | |
| Wet gluten content (%) | 2020 | 34.1 a | 33.5 ab | 34.9 a | 35.3 b | 36.1 bc | 33.9 a | 33.3 a | 34.0 a | 32.8 a | 2.4 |
| 2021 | 35.4 a | 35.2 a | 35.2 a | 35.3 a | 35.6 a | 35.6 a | 35.3 a | 38.4 b | 34.3 a | 1.6 | |
| Mean | 34.7 ab | 34.3 ab | 35.0 ab | 35.3 ab | 35.7 ab | 34.8 ab | 34.3 ab | 36.2 b | 33.6 a | 2.0 | |
| Starch content (g·kg−1) | 2020 | 524.4 a | 526.1 a | 520.9 a | 524.3 a | 521.5 a | 524.3 a | 525.9 a | 519.0 a | 520.8 a | n.s. |
| 2021 | 528.3 b | 528.8 b | 529.4 b | 527.4 b | 531.8 b | 529.8 b | 530.1 b | 515.4 a | 531.0 b | 4.7 | |
| Mean | 526.4 a | 527.5 a | 525.2 a | 525.8 a | 526.7 a | 527.0 a | 528.0 a | 517.2 a | 525.9 a | n.s | |
| Zeleny sedimentation value | 2020 | 35.0 ab | 34.6 ab | 34.9 ab | 34.8 ab | 36.8 bc | 35.8 b | 30.2 a | 31.1 ab | 39.0 c | 5.0 |
| 2021 | 35.8 a | 37.7 ab | 36.8 a | 37.4 ab | 38.4 b | 37.7 ab | 36.2 a | 35.8 a | 37.8 b | 1.5 | |
| Mean | 35.4 ab | 36.2 b | 35.9 ab | 36.1 ab | 37.6 b | 36.8 ab | 33.2 a | 33.5 a | 38.4 b | 3.2 | |
| Macroelements | Content (mg·kg−1) | Abundance |
|---|---|---|
| N-NO3 | 17.04 | medium |
| N-NH4 | 1.05 | medium |
| P2O5 | 475 | very high |
| K2O | 368 | very high |
| S-SO4 | 215 | very high |
| Mg | 44 | low |
| Microelements | Content (mg·kg−1) | Abundance |
| Mn | 245.6 | medium |
| Cu | 2.9 | medium |
| Zn | 5.5 | medium |
| Fe | 1592 | medium |
| B | 1.08 | low |
| pH in 1 M KCl | 6.3 | slightly acidic |
| C org. % d.m. | 0.81 | low |
| Months | Years | |||||
|---|---|---|---|---|---|---|
| 2020 | 2021 | LTA * 1991–2020 | ||||
| Precipitation (mm)/Temperature (°C) | ||||||
| mm | °C | mm | °C | mm | °C | |
| March | 26 | 4.6 | 14.9 | 2.6 | 37.9 | 2.4 |
| April | 19 | 8.6 | 58.3 | 6.4 | 42.3 | 8.6 |
| May | 111.4 | 11.2 | 68 | 11.6 | 70.7 | 13.6 |
| June | 170.3 | 17.4 | 68.3 | 18.6 | 66.8 | 16.9 |
| July | 67.8 | 18.8 | 82.4 | 22 | 82.2 | 18.9 |
| August | 59.3 | 20.4 | 197.8 | 17.2 | 54.9 | 18.4 |
| Sum/Mean (September–August) | 453.8.7 | 13.5 | 489.7 | 13 | 354.8 | 13.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biszczak, W.; Jośko, I.; Świeca, M.; Kraska, K.; Haliniarz, M.; Różyło, K. The Use of Metabolic Inducers in Wheat to Increase the Nutritional and Functional Value of Grain. Molecules 2025, 30, 4699. https://doi.org/10.3390/molecules30244699
Biszczak W, Jośko I, Świeca M, Kraska K, Haliniarz M, Różyło K. The Use of Metabolic Inducers in Wheat to Increase the Nutritional and Functional Value of Grain. Molecules. 2025; 30(24):4699. https://doi.org/10.3390/molecules30244699
Chicago/Turabian StyleBiszczak, Wojciech, Izabela Jośko, Michał Świeca, Karol Kraska, Małgorzata Haliniarz, and Krzysztof Różyło. 2025. "The Use of Metabolic Inducers in Wheat to Increase the Nutritional and Functional Value of Grain" Molecules 30, no. 24: 4699. https://doi.org/10.3390/molecules30244699
APA StyleBiszczak, W., Jośko, I., Świeca, M., Kraska, K., Haliniarz, M., & Różyło, K. (2025). The Use of Metabolic Inducers in Wheat to Increase the Nutritional and Functional Value of Grain. Molecules, 30(24), 4699. https://doi.org/10.3390/molecules30244699









