Quercetin and Its Metabolites: Mechanistic Insights as the Basis of Their Therapeutic Potential in NAFLD and HCC
Abstract
1. Introduction
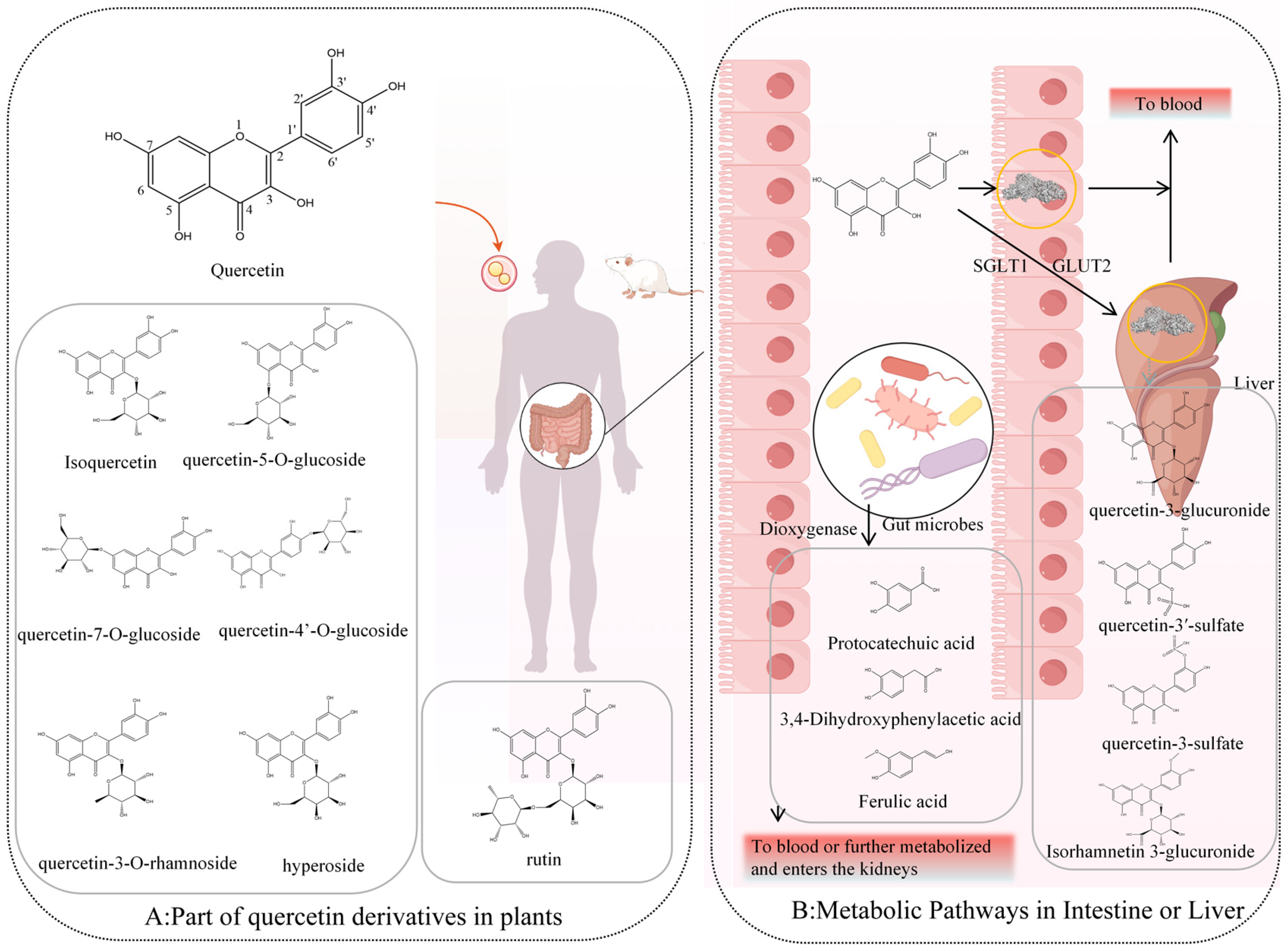
2. Sources of Quercetin and Its In Vivo Metabolic Pathways and Products
3. Mechanisms Insights
3.1. Antioxidant and Redox Modulation
3.1.1. Quercetin
3.1.2. Quercetin-3-O-Glucuronide
3.1.3. Quercetin-3′-Sulfate and Quercetin-3-Sulfate
3.1.4. Isorhamnetin-3-Glucuronide and Isorhamnetin
3.1.5. 3,4-Dihydroxyphenylacetic Acid
3.1.6. Protocatechuic Acid
3.1.7. Trans-4-Hydroxy-3-Methoxycinnamic Acid (Ferulic Acid)
3.2. Modulation of the Gut Microbiome
3.3. Other Mechanisms
4. Cell-Specific Effects and Targeting
5. Discussion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Fernandes, I.; Gonzalez-Manzano, S.; de Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797–803. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Tang, Y.; Yao, P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhou, N.; Wang, X.; Liu, Q.; Bai, Y.; Bai, Y.; Liu, Z.; Yang, H.; Zou, J. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed. Rep. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Sotiropoulou, M.; Katsaros, I.; Vailas, M.; Lidoriki, I.; Papatheodoridis, G.V.; Kostomitsopoulos, N.G.; Valsami, G.; Tsaroucha, A.; Schizas, D. Nonalcoholic fatty liver disease: The role of quercetin and its therapeutic implications. Saudi J. Gastroenterol. 2021, 27, 319. [Google Scholar] [CrossRef]
- Hernández-Ortega, L.D.; Alcántar-Díaz, B.E.; Ruiz-Corro, L.A.; Sandoval-Rodriguez, A.; Bueno-Topete, M.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J. Gastroenterol. Hepatol. 2012, 27, 1865–1872. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Xu, J.; Mi, M.; Wang, J.; Qin, Y. Quercetin intervention reduced hepatic fat deposition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled crossover clinical trial. Am. J. Clin. Nutr. 2024, 120, 507–517. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The therapeutic effects and mechanisms of quercetin on metabolic diseases: Pharmacological data and clinical evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A. Evolving global etiology of hepatocellular carcinoma (HCC): Insights and trends for 2024. J. Clin. Exp. Hepatol. 2025, 15, 102406. [Google Scholar] [CrossRef]
- Sethi, G.; Rath, P.; Chauhan, A.; Ranjan, A.; Choudhary, R.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Rani, I.; Tuli, H.S. Apoptotic mechanisms of quercetin in liver cancer: Recent trends and advancements. Pharmaceutics 2023, 15, 712. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Qian, Y.; Yu, L.; Ma, J.; Gu, B.; Tang, W.; Li, Y.; Li, H.; Wu, W. Quercetin induces apoptosis through downregulating P4HA2 and inhibiting the PI3K/Akt/mTOR axis in hepatocellular carcinoma cells: An In Vitro study. Cancer Rep. 2025, 8, e70220. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, W.; Yuan, X.; Liu, S.; He, Z. Mechanistic study of quercetin in the treatment of hepatocellular carcinoma with diabetes via MEK/ERK pathway. Int. Immunopharmacol. 2024, 142, 113194. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Ma, Y.-X.; Li, W.-T.; Li, L.; Zhu, H.-Z.; Wu, M.-H.; Zhou, J.-R. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J. Nutr. Biochem. 2019, 69, 108–119. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martiín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef]
- Frenț, O.-D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin—Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef]
- Vu, H.T.; Song, F.V.; Tian, K.V.; Su, H.; Chass, G.A. Systematic characterisation of the structure and radical scavenging potency of Pu’Er tea () polyphenol theaflavin. Org. Biomol. Chem. 2019, 17, 9942–9950. [Google Scholar] [CrossRef]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Mol. Cell. Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef]
- Li, Z.; Moalin, M.; Zhang, M.; Vervoort, L.; Hursel, E.; Mommers, A.; Haenen, G.R. The flow of the redox energy in quercetin during its antioxidant activity in water. Int. J. Mol. Sci. 2020, 21, 6015. [Google Scholar] [CrossRef]
- Liu, L.; Barber, E.; Kellow, N.J.; Williamson, G. Improving quercetin bioavailability: A systematic review and meta-analysis of human intervention studies. Food Chem. 2025, 477, 143630. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from Shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zang, E.-H.; Wang, C.-C.; Liu, Y.-C.; Niu, H.; Gao, Y.; Li, M.-H. Dianthi herba: A comprehensive review of its botany, traditional use, phytochemistry, and pharmacology. Chin. Med. 2022, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Waizumi, R.; Hirayama, C.; Tomita, S.; Iizuka, T.; Kuwazaki, S.; Jouraku, A.; Tsubota, T.; Yokoi, K.; Yamamoto, K.; Sezutsu, H. A major endogenous glycoside hydrolase mediating quercetin uptake in Bombyx mori. PLoS Genet. 2024, 20, e1011118. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef]
- Pico, J.; Martínez, M.M. Unraveling the inhibition of intestinal glucose transport by dietary phenolics: A review. Curr. Pharm. Des. 2019, 25, 3418–3433. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes 2025, 11, 12. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 1, 1–8. [Google Scholar]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The relationship of redox with hallmarks of cancer: The importance of homeostasis and context. Front. Oncol. 2022, 12, 862743. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Coriat, R.; Nicco, C.; Chéreau, C.; Mir, O.; Alexandre, J.; Ropert, S.; Weill, B.; Chaussade, S.; Goldwasser, F.; Batteux, F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 2284–2293. [Google Scholar] [CrossRef]
- Liu, X.; Song, L. Quercetin protects human liver cells from o, p’-DDT-induced toxicity by suppressing Nrf2 and NADPH oxidase-regulated ROS production. Food Chem. Toxicol. 2022, 161, 112849. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Deng, Y.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother. Res. 2021, 35, 4727–4747. [Google Scholar] [CrossRef]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M. Quercetin suppresses proliferation of liver cancer cell lines in vitro. Anticancer. Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M. A comprehensive analysis and anti-cancer activities of quercetin in ROS-mediated cancer and cancer stem cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.; Moalin, M.; Bast, A.; van der Vijgh, W.J.; Haenen, G.R. An essential difference between the flavonoids monoHER and quercetin in their interplay with the endogenous antioxidant network. PLoS ONE 2010, 5, e13880. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Mantas, A.; Chass, G.A.; Ferretti, F.H.; Estrada, M.; Zamarbide, G.; Csizmadia, I.G. Ab initio and DFT conformational analysis of selected flavones: 5, 7-dihydroxyflavone (chrysin) and 7, 8-dihydroxyflavone. Can. J. Chem. 2002, 80, 845–855. [Google Scholar] [CrossRef]
- Luo, X.; Weng, X.; Bao, X.; Bai, X.; Lv, Y.; Zhang, S.; Chen, Y.; Zhao, C.; Zeng, M.; Huang, J. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022, 57, 102511. [Google Scholar] [CrossRef]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef]
- Spiess, P.C.; Kasahara, D.; Habibovic, A.; Hristova, M.; Randall, M.J.; Poynter, M.E.; van der Vliet, A. Acrolein exposure suppresses antigen-induced pulmonary inflammation. Respir. Res. 2013, 14, 107. [Google Scholar] [CrossRef]
- Lemmens, K.J.; Herst, P.M.; Housmans, B.A.; Moalin, M.; van der Vijgh, W.J.; Bast, A.; Haenen, G.R. The contribution of the major metabolite 4′-O-methylmonoHER to the antioxidant activity of the flavonoid monoHER. Chem.-Biol. Interact. 2015, 239, 146–152. [Google Scholar] [CrossRef]
- Speisky, H.; Arias-Santé, M.F.; Fuentes, J. Oxidation of quercetin and kaempferol markedly amplifies their antioxidant, cytoprotective, and anti-inflammatory properties. Antioxidants 2023, 12, 155. [Google Scholar] [CrossRef]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H.n. Quercetin oxidation paradoxically enhances its antioxidant and cytoprotective properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef]
- Acosta-Quiroga, K.; Rocha-Valderrama, E.; Zúñiga-Bustos, M.; Mera-Adasme, R.; Cabrera-Barjas, G.; Olea-Azar, C.; Moncada-Basualto, M. Gross Antioxidant Capacity and Anti-Inflammatory Potential of Flavonol Oxidation Products: A Combined Experimental and Theoretical Study. Antioxidants 2025, 14, 479. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiao, N.; Li, X.W. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Scientific Reports 2016, 6, 35460. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Beak, S.-Y.; Choi, I.; Sung, J.-S. Quercetin and its metabolites protect hepatocytes against ethanol-induced oxidative stress by activation of Nrf2 and AP-1. Food Sci. Biotechnol. 2018, 27, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, C.; Yang, T.; Zeng, M.; Wang, Z.; Chen, Q.; Chen, J.; He, Z. Miquelianin, a main functional flavonoid of lotus leaf, induces thermogenic signature via p38-PINK1-PARKIN-mediated mitophagy and mimicking NRF2 signaling during brown adipocyte differentiation. Food Front. 2023, 4, 1831–1844. [Google Scholar] [CrossRef]
- Choi, D.W.; Jung, S.Y.; Kim, G.-D.; Lee, S.-Y.; Shin, H.S. Miquelianin inhibits allergic responses in mice by suppressing CD4+ T cell proliferation. Antioxidants 2021, 10, 1120. [Google Scholar] [CrossRef]
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008, 283, 9424–9434. [Google Scholar] [CrossRef]
- Soriano, C.M. Efectos Vasculares de la Quercetina y la Catequina: Interacciones y Papel de los Procesos de Conjugación y Desconjugación Metabólica. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2012. [Google Scholar]
- Santa, K.; Watanabe, K.; Kumazawa, Y.; Nagaoka, I. Phytochemicals and vitamin D for a healthy life and prevention of diseases. Int. J. Mol. Sci. 2023, 24, 12167. [Google Scholar] [CrossRef]
- Criel, H. Intracellular Accumulation of Quercetin and Its Impact on Cellular Stress: An in Vitro Study. Master’s Thesis, Ghent University, Chent, Belgium, 2019. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yang, K.; Li, C.; Zhang, M. Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization. Genes 2025, 16, 449. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, F.; Huiyao, Z.; Song, Z.; Li, D. Quercetin ameliorates hyperuricemic nephropathy by repressing uric acid synthesis and reabsorption in mice and cells. eFood 2024, 5, e139. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. A critical examination of human data for the biological activity of quercetin and its phase-2 conjugates. Crit. Rev. Food Sci. Nutr. 2025, 65, 1669–1705. [Google Scholar] [CrossRef]
- Lodi, F.; Jimenez, R.; Moreno, L.; Kroon, P.A.; Needs, P.W.; Hughes, D.A.; Santos-Buelga, C.; Gonzalez-Paramas, A.; Cogolludo, A.; Lopez-Sepulveda, R. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis 2009, 204, 34–39. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Pánovics, A.; Fliszár-Nyúl, E.; Schilli, G.; Hetényi, C.; Mladěnka, P.; Needs, P.W.; Kroon, P.A.; Pethő, G.; Poór, M. Inhibitory effects of quercetin and its human and microbial metabolites on xanthine oxidase enzyme. Int. J. Mol. Sci. 2019, 20, 2681. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, K.; Yamaki, K.; Tsushida, T. Effect of flavonoids on the differentiation of 3T3-L1 adipocytes. Food Sci. Technol. Res. 2001, 7, 154–160. [Google Scholar] [CrossRef]
- Eseberri, I.; Miranda, J.; Lasa, A.; Mosqueda-Solís, A.; González-Manzano, S.; Santos-Buelga, C.; Portillo, M.P. Effects of quercetin metabolites on triglyceride metabolism of 3T3-L1 preadipocytes and mature adipocytes. Int. J. Mol. Sci. 2019, 20, 264. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl-and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.-W.; Ohkohchi, N.; Isoda, H. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci. Rep. 2019, 9, 16210. [Google Scholar] [CrossRef]
- Choi, Y.H. Isorhamnetin induces ROS-dependent cycle arrest at G2/M phase and apoptosis in human hepatocarcinoma Hep3B cells. Gen. Physiol. Biophys. 2019, 38, 473–484. [Google Scholar] [CrossRef]
- Dong, G.-Z.; Lee, J.-H.; Ki, S.H.; Yang, J.H.; Cho, I.J.; Kang, S.H.; Zhao, R.J.; Kim, S.C.; Kim, Y.W. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2014, 740, 634–640. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Zhang, C.; Lan, S.; Liu, S.; Yao, X.; Guo, W.; Chen, H. Isorhamnetin Inhibits The Proliferation And Induces Apoptosis Of Hepatocellular Carcinoma by Targeting the GSK3-Β/PI3K/AKT Pathway. Clin. Oncol. 2024, 9, 2052. [Google Scholar]
- El-Rayyes, R.; Abbas, M.M.; Obeidat, R.; Abbas, M.A. Isorhamnetin decreased the expression of HMG-CoA reductase and increased LDL receptors in HepG2 cells. J. Appl. Pharm. Sci. 2023, 13, 155–161. [Google Scholar]
- Che, L.; Chi, W.; Qiao, Y.; Zhang, J.; Song, X.; Liu, Y.; Li, L.; Jia, J.; Pilo, M.G.; Wang, J. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut 2020, 69, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, N.; Song, D.; Steer, C.J.; Zheng, G.; Song, G. A positive feedback between cholesterol synthesis and the pentose phosphate pathway rather than glycolysis promotes hepatocellular carcinoma. Oncogene 2023, 42, 2892–2904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-L.; Medine, C.N.; Zhu, L.; Hay, D.C. Stem cell differentiation and human liver disease. World J. Gastroenterol. WJG 2012, 18, 2018. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Global gene expression profiling reveals isorhamnetin induces hepatic-lineage specific differentiation in human amniotic epithelial cells. Front. Cell Dev. Biol. 2020, 8, 578036. [Google Scholar] [CrossRef]
- Yamashita, T.; Honda, M.; Nio, K.; Nakamoto, Y.; Yamashita, T.; Takamura, H.; Tani, T.; Zen, Y.; Kaneko, S. Oncostatin m renders epithelial cell adhesion molecule–positive liver cancer stem cells sensitive to 5-fluorouracil by inducing hepatocytic differentiation. Cancer Res. 2010, 70, 4687–4697. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Gotteland, M.; Castillo, R.L.; Chen, C. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp. Cell Res. 2015, 334, 270–282. [Google Scholar] [CrossRef]
- Nunes, C.; Almeida, L.; Laranjinha, J. 3,4-Dihydroxyphenylacetic acid (DOPAC) modulates the toxicity induced by nitric oxide in PC-12 cells via mitochondrial dysfunctioning. Neurotoxicology 2008, 29, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.; Ferreira, J.; Carrasco-Pozo, C. The microbiota-derived metabolite of quercetin, 3,4-dihydroxyphenylacetic acid prevents malignant transformation and mitochondrial dysfunction induced by hemin in colon cancer and normal colon epithelia cell lines. Molecules 2020, 25, 4138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kurita, A.; Nakashima, S.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotechnol. Biochem. 2017, 81, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm 2015, 72, 643–650. [Google Scholar]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant effects of protocatechuic acid and protocatechuic aldehyde: Old wine in a new bottle. Evid.-Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Habib, S.A.; Suddek, G.M.; Rahim, M.A.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Luangsuphabool, T.; Wongpoomchai, R. Protocatechuic acid as a potent anticarcinogenic compound in purple rice bran against diethylnitrosamine-initiated rat hepatocarcinogenesis. Sci. Rep. 2022, 12, 10548. [Google Scholar] [CrossRef]
- Yip, E.; Chan, A.; Pang, H.; Tam, Y.; Wong, Y. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A.; Karin, M. A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Soga, M.; Matsuzawa, A.; Ichijo, H. Oxidative stress-induced diseases via the ASK1 signaling pathway. Int. J. Cell Biol. 2012, 2012, 439587. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Li, W.; Jia, R.; Meng, D.; Zhang, H.; Xia, L. Molecular mechanism of ferulic acid and its derivatives in tumor progression. Pharmacol. Rep. 2023, 75, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lai, X.; Yuan, D.; Liu, Y.; Wang, J.; Liang, Y. Effects of ferulic acid, a major component of rice bran, on proliferation, apoptosis, and autophagy of HepG2 cells. Food Res. Int. 2022, 161, 111816. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Yi, T.; Luo, H.; Jiang, L. Preclinical findings: The pharmacological targets and molecular mechanisms of ferulic acid treatment for COVID-19 and osteosarcoma via targeting autophagy. Front. Endocrinol. 2022, 13, 971687. [Google Scholar] [CrossRef]
- Abdulal, Z.A.; Altahhan, M.Y.; Qindil, A.F.; Al-Juhani, A.M.; Alatawi, M.A.; Hassan, H.M.; Al-Gayyar, M.M. Ferulic acid inhibits tumor proliferation and attenuates inflammation of hepatic tissues in experimentally induced HCC in rats. J. Investig. Med. 2024, 72, 900–910. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, L.; Liu, Q.; Wang, W.; Liu, Y. Molecular mechanism and research progress on pharmacology of ferulic acid in liver diseases. Front. Pharmacol. 2023, 14, 1207999. [Google Scholar] [CrossRef]
- Muthusamy, G.; Balupillai, A.; Ramasamy, K.; Shanmugam, M.; Gunaseelan, S.; Mary, B.; Prasad, N.R. Ferulic acid reverses ABCB1-mediated paclitaxel resistance in MDR cell lines. Eur. J. Pharmacol. 2016, 786, 194–203. [Google Scholar] [CrossRef]
- Xiao, K.; Li, K.; Xiao, K.; Yang, J.; Zhou, L. Gut Microbiota and Hepatocellular Carcinoma: Metabolic Products and Immunotherapy Modulation. Cancer Med. 2025, 14, e70914. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, L.; Ji, J.; Feng, J.; Dai, W.; Li, J.; Wu, J.; Guo, C. Gut microbiota, peroxisome proliferator-activated receptors, and hepatocellular carcinoma. J. Hepatocell. Carcinoma 2020, 2020, 271–288. [Google Scholar] [CrossRef]
- Li, X.; He, M.; Yi, X.; Lu, X.; Zhu, M.; Xue, M.; Tang, Y.; Zhu, Y. Short-chain fatty acids in nonalcoholic fatty liver disease: New prospects for short-chain fatty acids as therapeutic targets. Heliyon 2024, 10, e26991. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, X. The role of gut–liver axis in gut microbiome dysbiosis associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Liu, N.; Wu, H.; Cong, K.; Duan, L.; Chen, T.; Zhang, J. Health benefits of medicinal plant natural products via microbiota-mediated different gut axes. Pharmacol. Res. 2025, 215, 107730. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Liu, J.-R. Effect of Lactobacillus rhamnosus GG on energy metabolism, leptin resistance, and gut microbiota in mice with diet-induced obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Deng, Y.; Li, X.; Zhu, L.; Wang, X.; Li, L.; Li, X. Regulatory Roles of Quercetin in Alleviating Fructose--Induced Hepatic Steatosis: Targeting Gut Microbiota and Inflammatory Metabolites. Food Sci. Nutr. 2025, 13, e4612. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Wu, R.; Xiong, J.; Zhou, T.; Zhang, Z.; Huang, Z.; Tian, S.; Wang, Y. Quercetin/anti-PD-1 antibody combination therapy regulates the gut microbiota, impacts macrophage immunity and reshapes the hepatocellular carcinoma tumor microenvironment. Front. Biosci.-Landmark 2023, 28, 327. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; He, L.; Yang, C.; He, J.; Hua, Y.; Guo, J.; Liu, S. Ferulic acid alleviates hepatic lipid accumulation and inflammation by improving proximal and distal intestinal barriers in NAFLD mice. Tohoku J. Exp. Med. 2023, 260, 149–163. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Hao, W.; Zhu, H.; Liu, J.; Ma, K.Y.; He, W.-S.; Chen, Z.-Y. Cholesterol-lowering activity of protocatechuic acid is mediated by increasing the excretion of bile acids and modulating gut microbiota and producing short-chain fatty acids. Food Funct. 2021, 12, 11557–11567. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Xiong, S.; Cheng, Y.; Deng, J.; Zhang, J.; Yu, F.; Hao, L.; Li, S.; Hu, X. Causal impact of gut microbiota on five liver diseases: Insights from mendelian randomization and single-cell RNA sequencing. Front. Genet. 2024, 15, 1362139. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Wu, Y.; Wang, T.; Zhang, J.; You, L.; Li, H.; Zheng, C.; Gao, Y.; Sun, X. Bidirectional Mendelian randomization links gut microbiota to primary biliary cholangitis. Sci. Rep. 2024, 14, 28301. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hou, X.; Fan, F.; Wu, H. Quercetin stimulates mitochondrial apoptosis dependent on activation of endoplasmic reticulum stress in hepatic stellate cells. Pharm. Biol. 2016, 54, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sun, L.; Wei, J.; Shen, Y.; Wang, J.; Zhang, P.; Yang, X.; Ding, Y.; Yin, W.; Lu, X. Quercetin alleviates endothelial dysfunction in preeclampsia by inhibiting ferroptosis and inflammation through EGFR binding. Commun. Biol. 2025, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhang, W.; Liu, H.; Jiang, Y.; Li, F.; Wu, M.; Waterhouse, G.I.; Sun-Waterhouse, D.; Li, D. Quercetin induces apoptosis in HepG2 cells via directly interacting with YY1 to disrupt YY1-p53 interaction. Metabolites 2023, 13, 229. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; Ma, T.; Jiang, L.; Tang, L.; Shi, T.; Zhang, S.; Zhang, L.; Zhu, P.; Li, J. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine 2018, 43, 37–45. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Lesser, S.; Wolffram, S. Oral bioavailability of the flavonol quercetin a review. Curr. Top. Nutraceutical Res. 2006, 4, 239. [Google Scholar]
- Ding, K.; Jia, H.; Jiang, W.; Qin, Y.; Wang, Y.; Lei, M. A double-edged sword: Focusing on potential drug-to-drug interactions of quercetin. Rev. Bras. Farm. 2023, 33, 502–513. [Google Scholar] [CrossRef]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behaviour of their mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Goyal, A.; Rai, A.; Sharma, A.; Ugoeze, K.C.; Singh, I. Quercetin nanoformulations: Recent advancements and therapeutic applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 033002. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Filipa Brito, A.; Ribeiro, M.; Margarida Abrantes, A.; Salome Pires, A.; Jorge Teixo, R.; Guilherme Tralhao, J.; Filomena Botelho, M. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Y.; Jiang, T.; Wang, Y.; Li, C.; He, Z. Quercetin and Its Metabolites: Mechanistic Insights as the Basis of Their Therapeutic Potential in NAFLD and HCC. Molecules 2025, 30, 4441. https://doi.org/10.3390/molecules30224441
Li Z, Li Y, Jiang T, Wang Y, Li C, He Z. Quercetin and Its Metabolites: Mechanistic Insights as the Basis of Their Therapeutic Potential in NAFLD and HCC. Molecules. 2025; 30(22):4441. https://doi.org/10.3390/molecules30224441
Chicago/Turabian StyleLi, Zhengwen, Yongzuo Li, Tianqing Jiang, Yue Wang, Chujie Li, and Zhengyou He. 2025. "Quercetin and Its Metabolites: Mechanistic Insights as the Basis of Their Therapeutic Potential in NAFLD and HCC" Molecules 30, no. 22: 4441. https://doi.org/10.3390/molecules30224441
APA StyleLi, Z., Li, Y., Jiang, T., Wang, Y., Li, C., & He, Z. (2025). Quercetin and Its Metabolites: Mechanistic Insights as the Basis of Their Therapeutic Potential in NAFLD and HCC. Molecules, 30(22), 4441. https://doi.org/10.3390/molecules30224441





