Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Natural Deep Eutectic Solvents
2.2. Thermal Behavior of Prepared Natural Deep Eutectic Solvents
Effect of Water Addition on the Thermal Stability of Natural Deep Eutectic Solvents N1 to N4
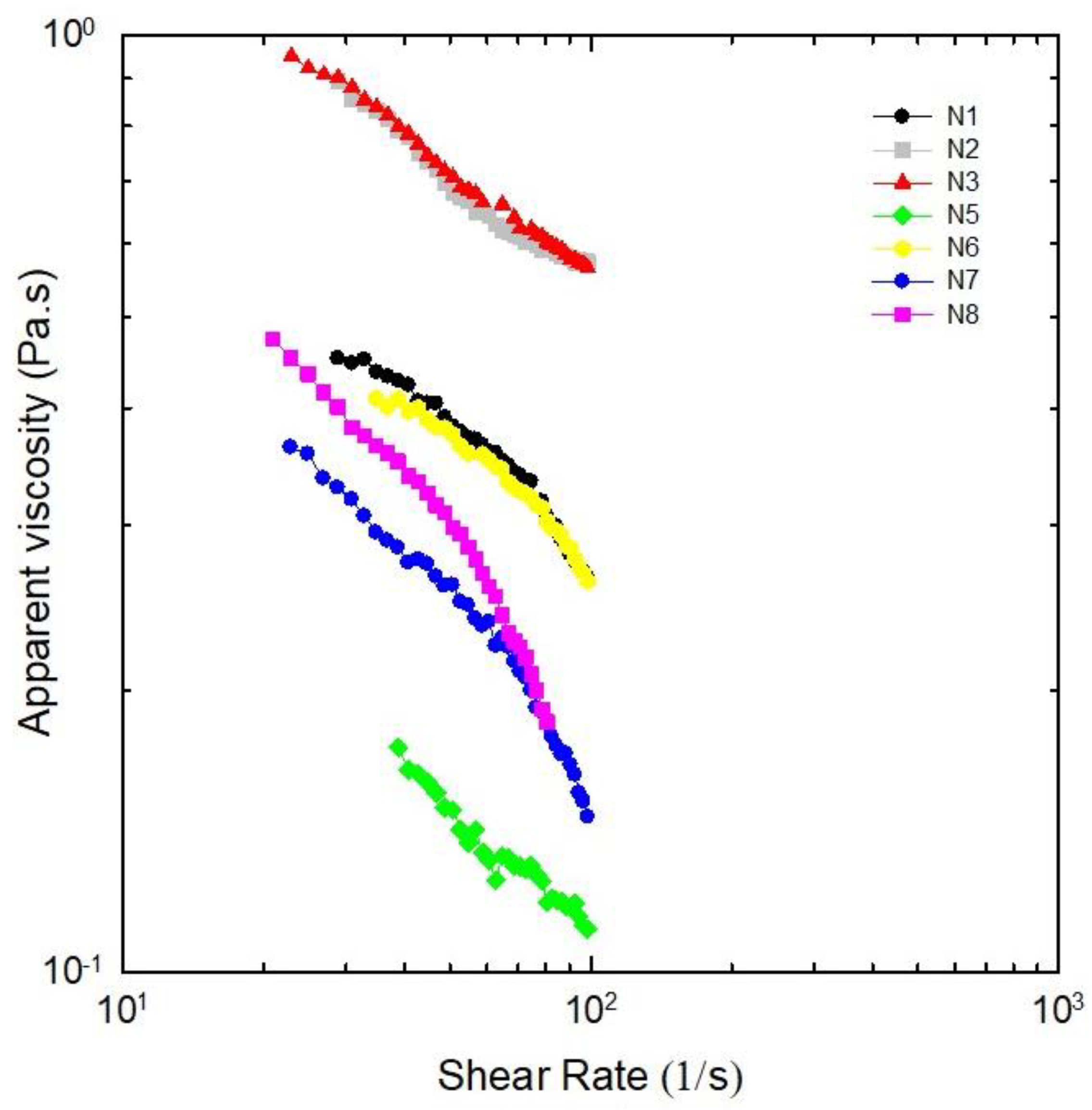
2.3. Rheological Measurements
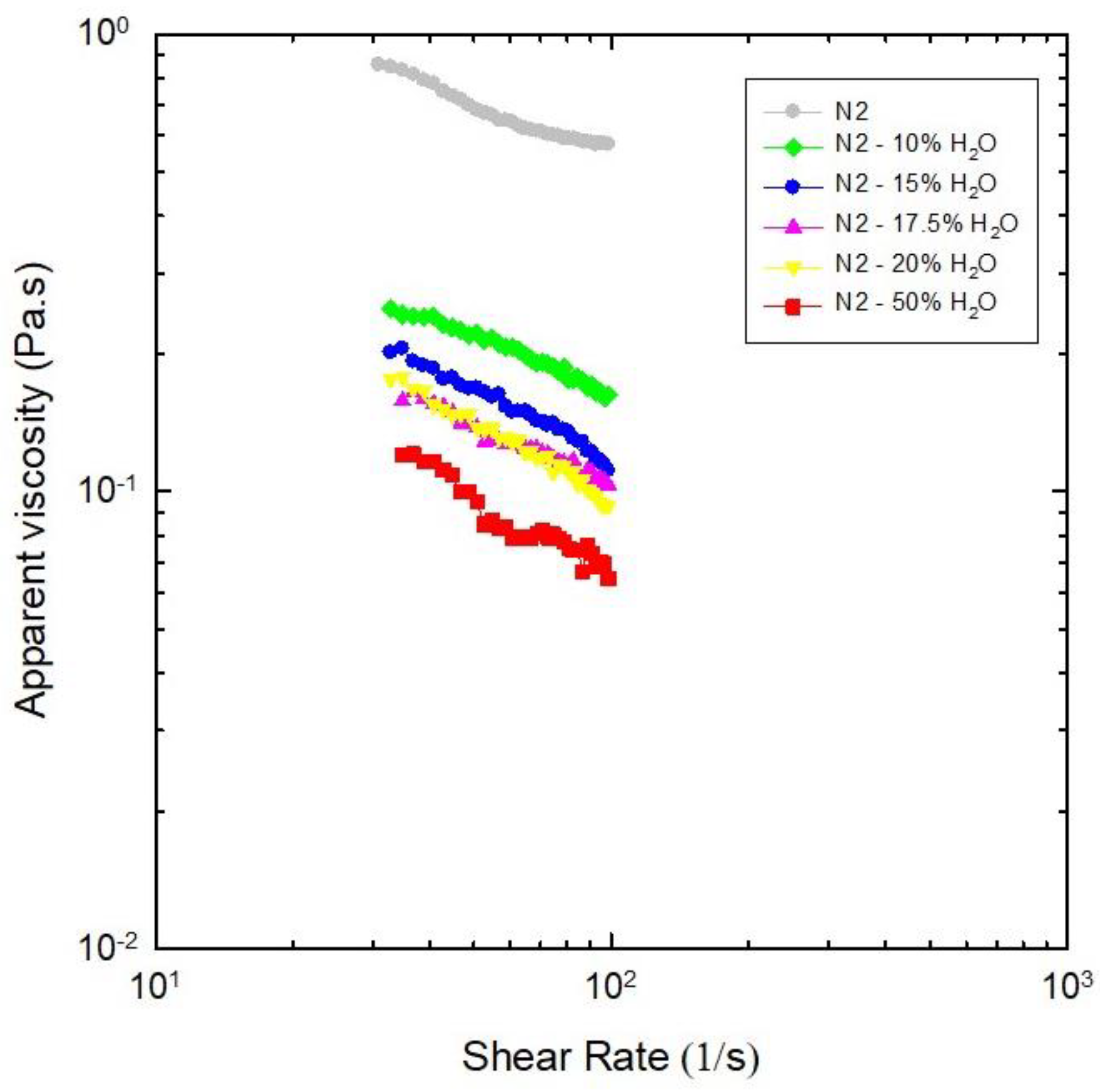
Effect of Water as Diluent on Viscosities of NADES N2
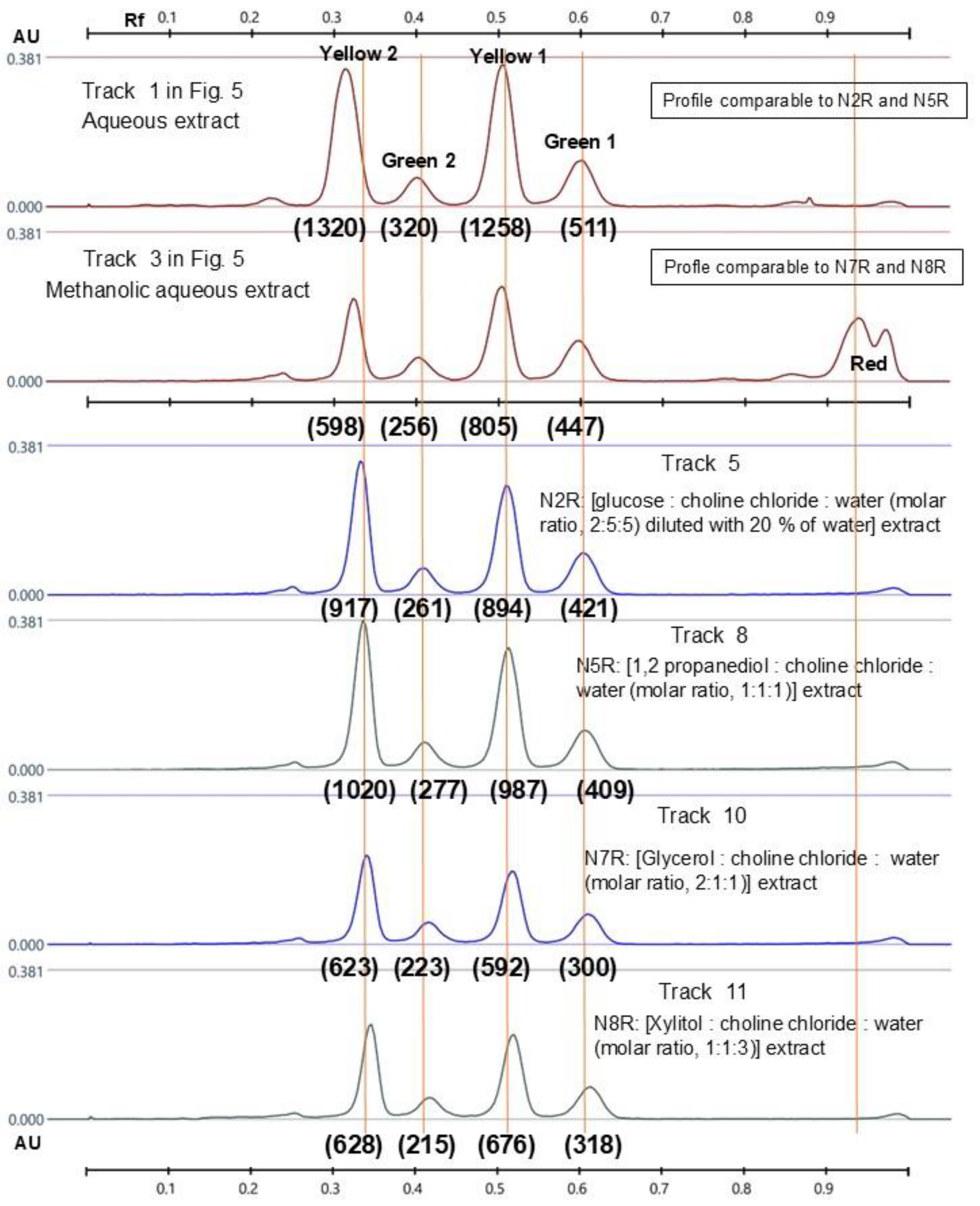
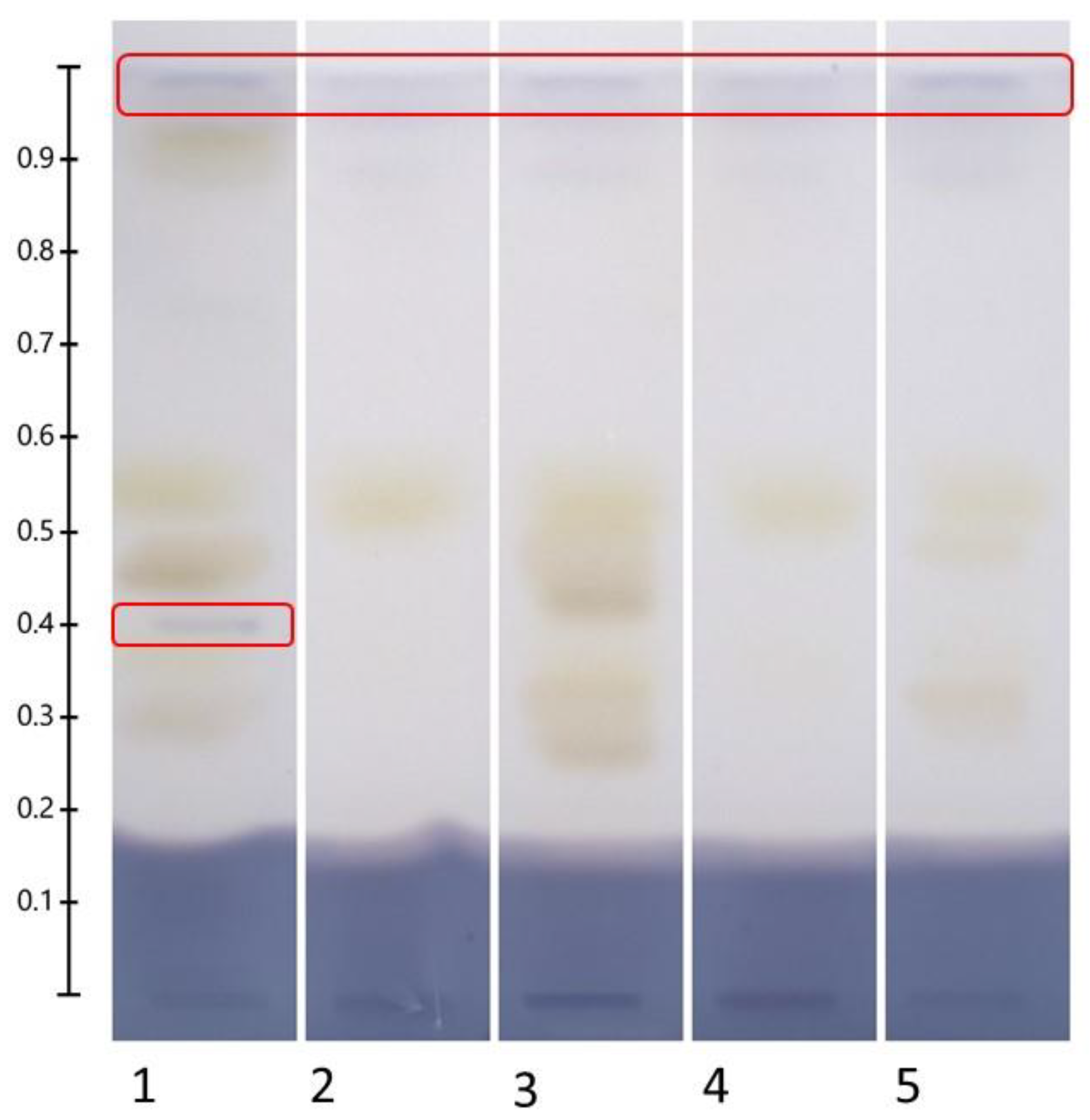
2.4. HPTLC Fingerprints of Flavonoids in Recovered NADES Extracts
2.5. Free Radical Scavenging Capacity of Recovered NADES Extracts
2.6. α-Amylase Inhibitory Activity of Recovered NADES Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. NADES Prepararion
3.4. Characterization of NADESs
3.4.1. Thermal Stability
3.4.2. Rheological Measurements
3.5. Preparation of Extracts and Sample Solutions for HPTLC Analysis
3.6. Characterization of Recovered NADES-Extracts
High-Performance Thin-Layer Chromatography
- Flavonoids: In total, 2 mL of aminoethyl diphenylborinate solution was applied on the warm plate (green nozzle, level 3) followed by 2 mL of polyethyleneglycol 400 solution (blue nozzle, level 2).
- Free radical scavenging activity (DPPH● assay): In total, 2 mL of DPPH solution was applied on the warm plate (green nozzle, level 3). Images were recorded 90 s and 120 min after derivatization.
- α-Amylase inhibitory activity assay: In total, 3 mL of α-amylase solution was applied (yellow nozzle, level 4) on the cooled plate that was then incubated at 37 °C for 30 min in a humid chamber [19]. Then, 2 mL of starch solution was applied (yellow nozzle, level 6), and the plate was incubated at 37 °C in a humid chamber for 10 min and treated with iodine vapors for 2 min (1 g of solid iodine in a 20 × 20 cm development chamber).
- Upon derivatization, the plates were documented as digital images under short-wave UV light (254 nm), long-wave UV light (365 nm), and white light using the TLC Visualizer 2. The Camag® systems were driven using the visionCATS software, version 2.5.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, V.I.B.; Mano, F.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Synthesis and physical and thermodynamic properties of lactic acid and malic acid-based natural deep eutectic solvents. J. Chem. Eng. Data 2018, 63, 2548–2556. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Savi, L.K.; Dias, M.C.G.C.; Carpine, D.; Waszczynskyj, N.; Ribani, R.H.; Haminiuk, C.W.I. Natural deep eutectic solvents (NADES) based on citric acid and sucrose as a potential green technology: A comprehensive study of water inclusion and its effect on thermal, physical and rheological properties. Int. J. Food Sci. Technol. 2018, 54, 898–907. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, J.; Qu, J.; Wang, B.; Xu, X.; Zhao, C. Deep Eutectic Solvents and Wall-Breaking Technique: A New Frontier in the Extraction of Oleuropein and Flavonoids from Olive Leaves with Superior Antioxidant and Antitumor Potential. Molecules 2025, 30, 1150. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insight into the nature of eutectic and deep eutectic mixtures. J. Solution Chem. 2014, 48, 962–982. [Google Scholar] [CrossRef]
- Lui, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollman, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Meng, L.; Ding, P.; Tan, Y.; Zhang, Y.; Zhao, J. Study on the Ultrasonic-Assisted Extraction Process of Anthocyanin from Purple Cabbage with Deep Eutectic Solvent. Molecules 2025, 30, 1281. [Google Scholar] [CrossRef] [PubMed]
- Terrigno, V.; Della Posta, S.; Pietrangeli, G.; Tonto, T.C.; Locato, V.; De Gara, L.; Fanali, C. Extraction and Analysis of Phenolic Compounds from Rocket: Development of a Green and Innovative DES-Based Extraction Method. Molecules 2025, 30, 1177. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Raynie, D.E. Thermal behavior, solvatochromic parameters, and metal halide solvation of the novel water-based deep eutectic solvents. J. Mol. Liq. 2020, 324, 114779. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Kowaluk, A.; Strzelec, M.; Sawicki, T.; Tańska, M. Evaluation of Bioactive Compounds and Chemical Elements in Herbs: Effectiveness of Choline Chloride-Based Deep Eutectic Solvents in Ultrasound-Assisted Extraction. Molecules 2025, 30, 368. [Google Scholar] [CrossRef]
- Khan, J.; Asaf, S.; Lubna; Abdelbacki, A.M.M.; Jan, R.; Kim, K.M. Green Extraction of Antioxidant Rich Flavonoids from Fagonia cretica Using Deep Eutectic Solvents. Molecules 2025, 30, 813. [Google Scholar] [CrossRef]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Swieca, M.; Sulkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef]
- Hemalatha, P.; Bomzan, D.P.; Sathyendra Rao, B.V.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on alpha-amylase and alpha-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef]
- Kumpun, S.; Maria, A.; Crouzet, S.; Evrard-Todeschi, N.; Girault, J.-P.; Lafont, R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011, 125, 1226–1234. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Taco, V.; Palmieri, C.; Duez, P.; Nachtergael, A. Optimized HPTLC bioautography screening of Ecuadorian Chenopodium quinoa Willd leaf extracts for inhibition of α-amylase. J. Planar. Chromatogr. Mod. TLC 2021, 34, 561–567. [Google Scholar] [CrossRef]
- Taco, V.; Palmieri, C.; Borja, D.; Villacrés, E.; Duez, P.; Nachtergael, A. Qualitative Analysis by High-Performance Thin-Layer Chromatography–Bioautography of Ecuado-rian Chenopodium quinoa Willd. Leaves: Influence of Variety, Phenological Stage, and Place of Cultivation on Free Radical Scavenging and α-Amylase Activity. Nutraceuticals 2025, 5, 1. [Google Scholar] [CrossRef]
- Liu, X.; Ahlgren, S.; Korthout, H.; Salome-Abarca, L.F.; Bayona, L.M.; Verpoorte, R.; Choi, Y.H. Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography-based method. J. Chromatogr. A 2018, 1532, 198–207. [Google Scholar] [CrossRef]
- Cunha, S.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trend Anal. Chem. 2018, 105, 225–238. [Google Scholar] [CrossRef]
- Caprin, B.; Charton, V.; Vogelgesang, B. The use of NADES to support innovation in the cosmetic industry. Adv. Bot. Res. 2021, 97, 309–332. [Google Scholar]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Elhamarnah, Y.A.; Nasser, M.; Qiblawey, H.; Benamor, A.; Atilhan, M.; Aparicio, S. A comprehensive review on the rheological behavior of imidazolium based ionic liquids and natural deep eutectic solvents. J. Mol. Liq. 2019, 277, 932–958. [Google Scholar] [CrossRef]
- Dehghan, S.A.; Sadeghi, M.A.; Ghorbani, M.; Kashani, N.M.; Maghsoudlou, Y. Optimization of Ultrasound-assisted Extraction of Quince Seed Gum through Response Surface Methodology. J. Agric. Sci. Technol. 2017, 19, 323–333. [Google Scholar]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Fraige, K.; Arrua, R.D.; Sutton, A.T.; Funari, C.S.; Cavalheiro, A.J.; Hilder, E.F.; Bolzani, V.D.S. Using natural deep eutectic solvents for the extraction of metabolites in Byrsonima intermedia leaves. J. Sep. Sci. 2019, 42, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718. [Google Scholar] [CrossRef]
- Taco, V.; Savarino, P.; Benali, P.; Villacrés, E.; Raquez, J.M.; Gerbaux, P.; Duez, P.; Nachtergael, A. Deep eutectic solvents for the extraction and stabilization of Ecuadorian quinoa (Chenopodium quinoa Willd.) saponins. J. Clean. Prod. 2022, 363, 132609. [Google Scholar] [CrossRef]
- Taco, V.; Semay, I.; Villacrés, E.; Santamaría, J.; Flores, R.; Gerbaux, P.; Duez, P.; Nachtergael, A. Deep Eutectic Solvents for the Extraction and Stabilization of Radical Scavengers From Ecuadorian Quinoa (Chenopodium quinoa Willd.) Leaves. Sep. Sci. Plus. 2025, 8, e70034. [Google Scholar] [CrossRef]
- Brahmi, F.; Guedouze, N.; Hauchard, D.; Okusa, P.; Kamagaju, L.; Madani, K.; Duez, P. Phenolic profile and biological activities of Micromeria graeca (L.) Benth. ex Rchb. Int. J. Food Prop. 2017, 20, 2070–2083. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W.; Adam, A.; Mizaton, H.H.; Zakaria, H. High-performance thin-layer chromatographic methods in the evaluation of the antioxidant and anti-hyperglycemic activity of Myrmecodia platytyrea as a promising opportunity in diabetes treatment. J. Chromatogr. A 2017, 1530, 192–196. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and validation of an HPTLC–DPPH assay and its application to the analysis of honey. J. Planar. Chromatogr. Mod. TLC 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Lui, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Guendouze-Bouchefa, N.; Madani, K.; Chibane, M.; Boulekbache-Makhlouf, L.; Hauchard, D.; Kiendrebeogo, M.; Stévigny, C.; Ndjolo Okusa, P.; Duez, P. Phenolic compounds, antioxidant and antibacterial activities of three Ericaceae from Algeria. Ind. Crops Prod. 2015, 70, 459–466. [Google Scholar] [CrossRef]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J. Antidiabetic potential of flavonoids from Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.; Moreira, M.T.; Lema, J.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Europe, C. High-Performance Thin-Layer Chromatography of Herbal Drugs and Herbal Drug Preparations (2.8.25). In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2019. [Google Scholar]





| NADES | Components for Preparation | Molar Ratio | T (°C) | tp (a) (min) | ts (b) (Months) | Proportion of Water to Dilute NADES Prior to Extraction Procedure (d) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| N1 | Choline chloride | Malic acid | Water | 1:1:2 | 50 | 440 | >33 (c) | 17.5% | |
| N2 | Choline chloride | Glucose | Water | 5:2:5 | 60 | 720 | >33 (c) | 20% | |
| N3 | Proline | Malic acid | Water | 1:1:3 | 50 | 360 | >33 (c) | 10% | |
| N4 | Fructose | Glucose | Saccharose | Water | 1:1:1:11 | 50 | 600 | <2 | 10% |
| N5 | Choline chloride | 1,2 Propanediol | Water | 1:1:1 | 50 | 30 | <6 | n.n. (e) | |
| N6 | Glucose | Lactic acid | Water | 1:5:3 | 60 | 60 | >20 (c) | n.n. (e) | |
| N7 | Choline chloride | Glycerol | Water | 1:2:1 | 50 | 20 | >20 (c) | n.n. (e) | |
| N8 | Choline chloride | Xylitol | Water | 2:1:3 | 50 | 60 | <6 | n.n. (e) | |
| NADES | k (Pa·sn) | n | R |
|---|---|---|---|
| N1 | 2.2 | 0.55 | 0.9844 |
| N2 | 3.0 | 0.63 | 0.9941 |
| N3 | 3.1 | 0.63 | 0.9990 |
| N4 (a) | ---- | ---- | ---- |
| N5 | 0.8 | 0.57 | 0.9878 |
| N6 | 2.0 | 0.57 | 0.9880 |
| N7 | 2.5 | 0.41 | 0.9611 |
| N8 | 3.9 | 0.33 | 0.9313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taco, V.; Almachi, D.; Bonilla, P.; Gijón-Arreortúa, I.; Benali, S.; Raquez, J.-M.; Duez, P.; Nachtergael, A. Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography. Molecules 2025, 30, 2620. https://doi.org/10.3390/molecules30122620
Taco V, Almachi D, Bonilla P, Gijón-Arreortúa I, Benali S, Raquez J-M, Duez P, Nachtergael A. Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography. Molecules. 2025; 30(12):2620. https://doi.org/10.3390/molecules30122620
Chicago/Turabian StyleTaco, Verónica, Dennys Almachi, Pablo Bonilla, Ixchel Gijón-Arreortúa, Samira Benali, Jean-Marie Raquez, Pierre Duez, and Amandine Nachtergael. 2025. "Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography" Molecules 30, no. 12: 2620. https://doi.org/10.3390/molecules30122620
APA StyleTaco, V., Almachi, D., Bonilla, P., Gijón-Arreortúa, I., Benali, S., Raquez, J.-M., Duez, P., & Nachtergael, A. (2025). Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography. Molecules, 30(12), 2620. https://doi.org/10.3390/molecules30122620








