Synthesis, Antibacterial Properties and Molecular Docking Studies of Nitrogen Substituted 9-(((4X-But-2-ynyloxy)methyl)-1,2,3-triazolyl)–Cinchona Alkaloid Conjugates
Abstract
1. Introduction
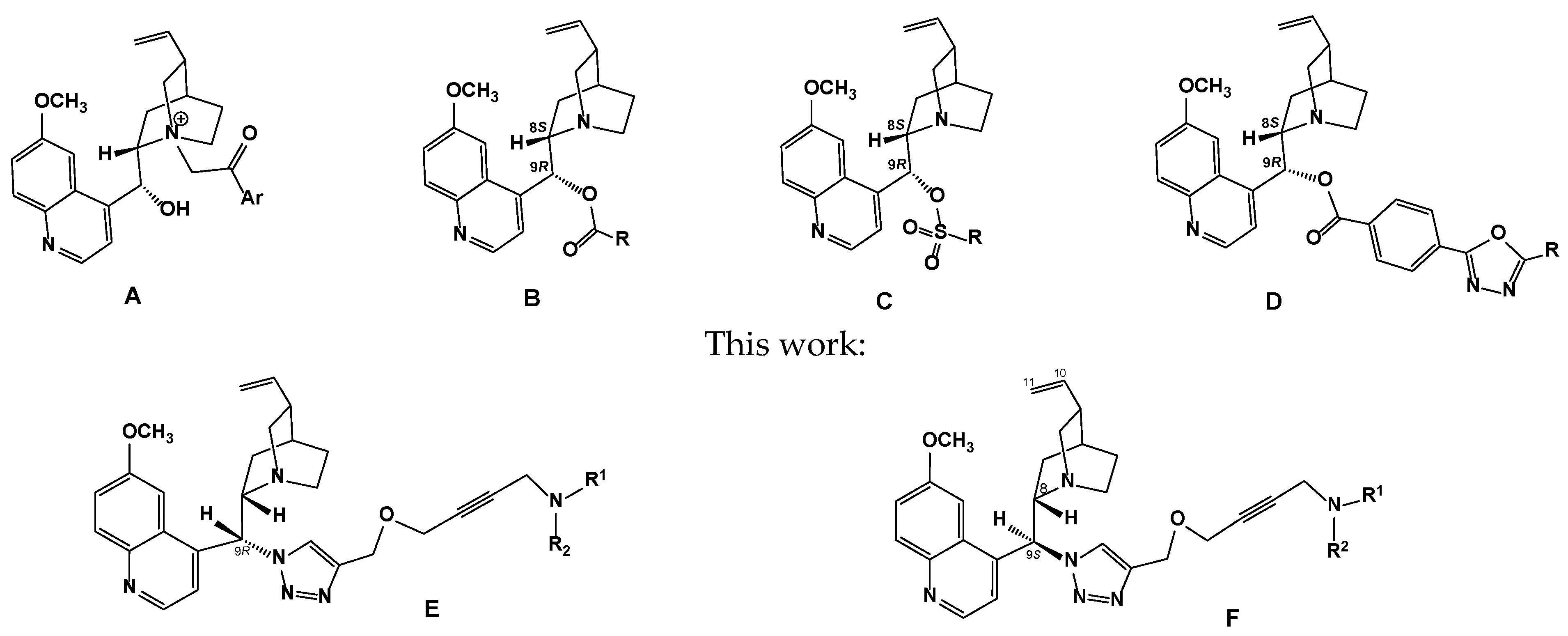
2. Results and Discussion
2.1. Biological Study
Antibacterial Activity
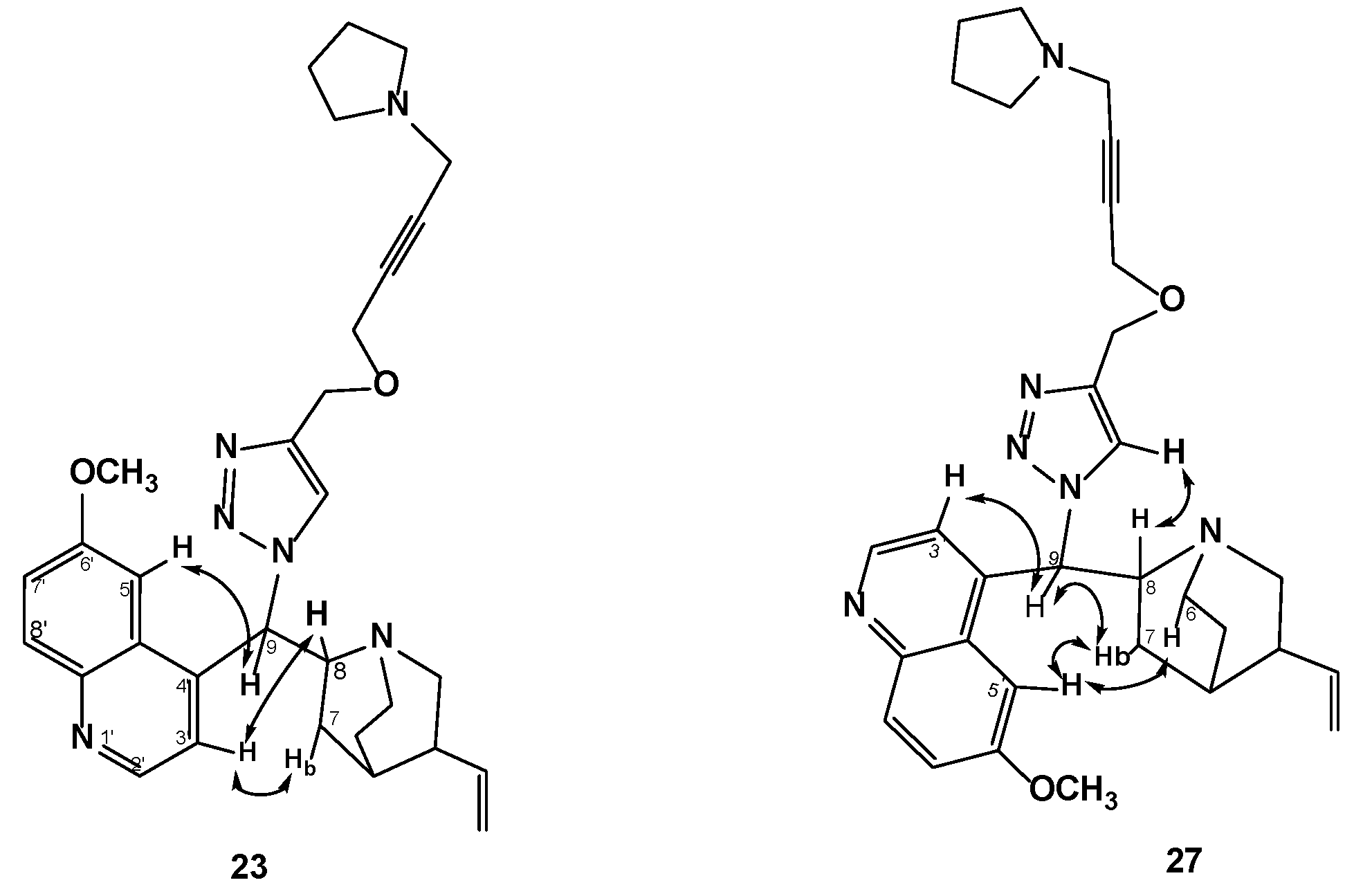
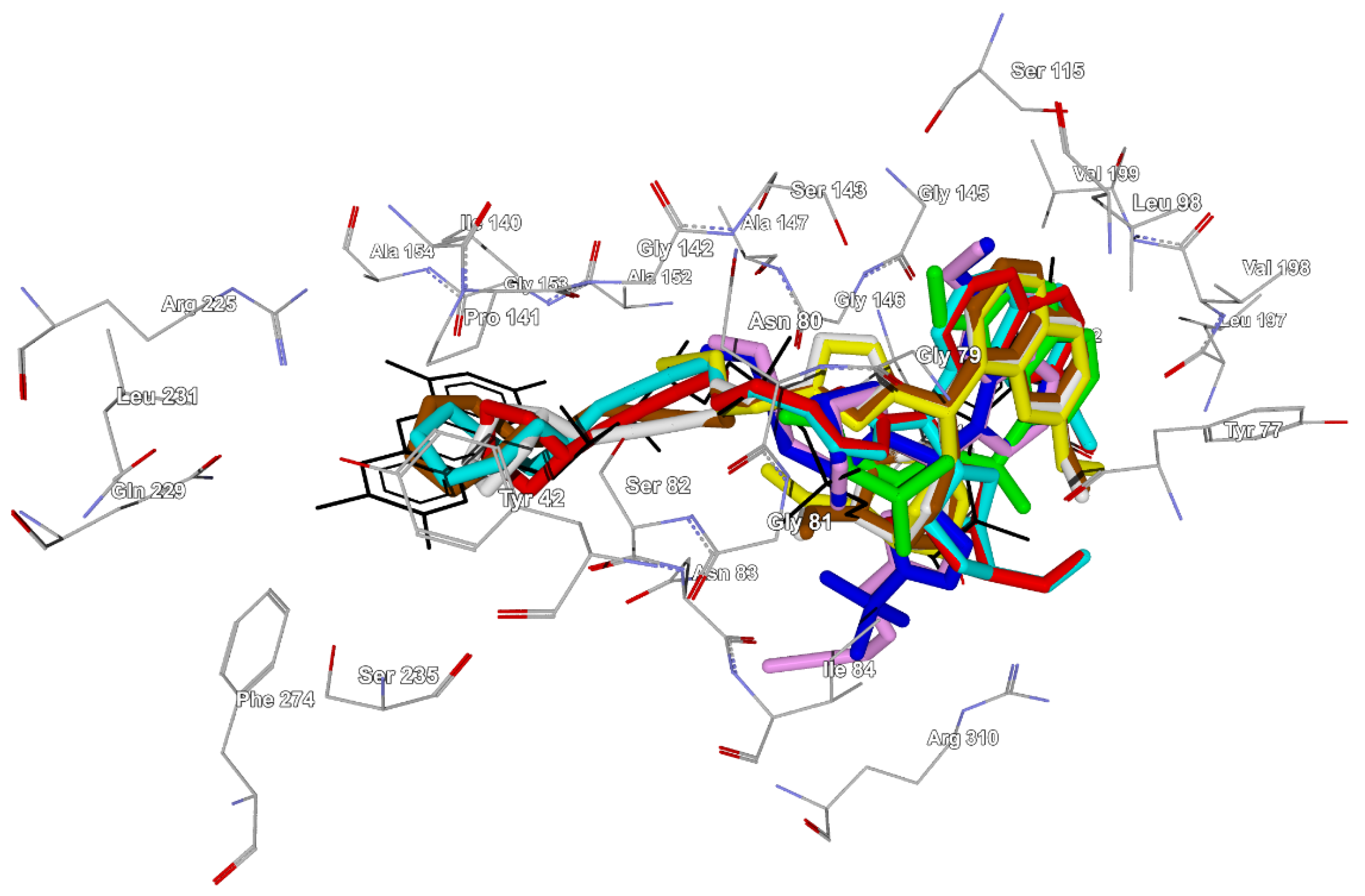
2.2. Docking Studies of Compounds 1, 4d, 11, 12, 23, 26, 27, and 30 in the FAD Site of MurB
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
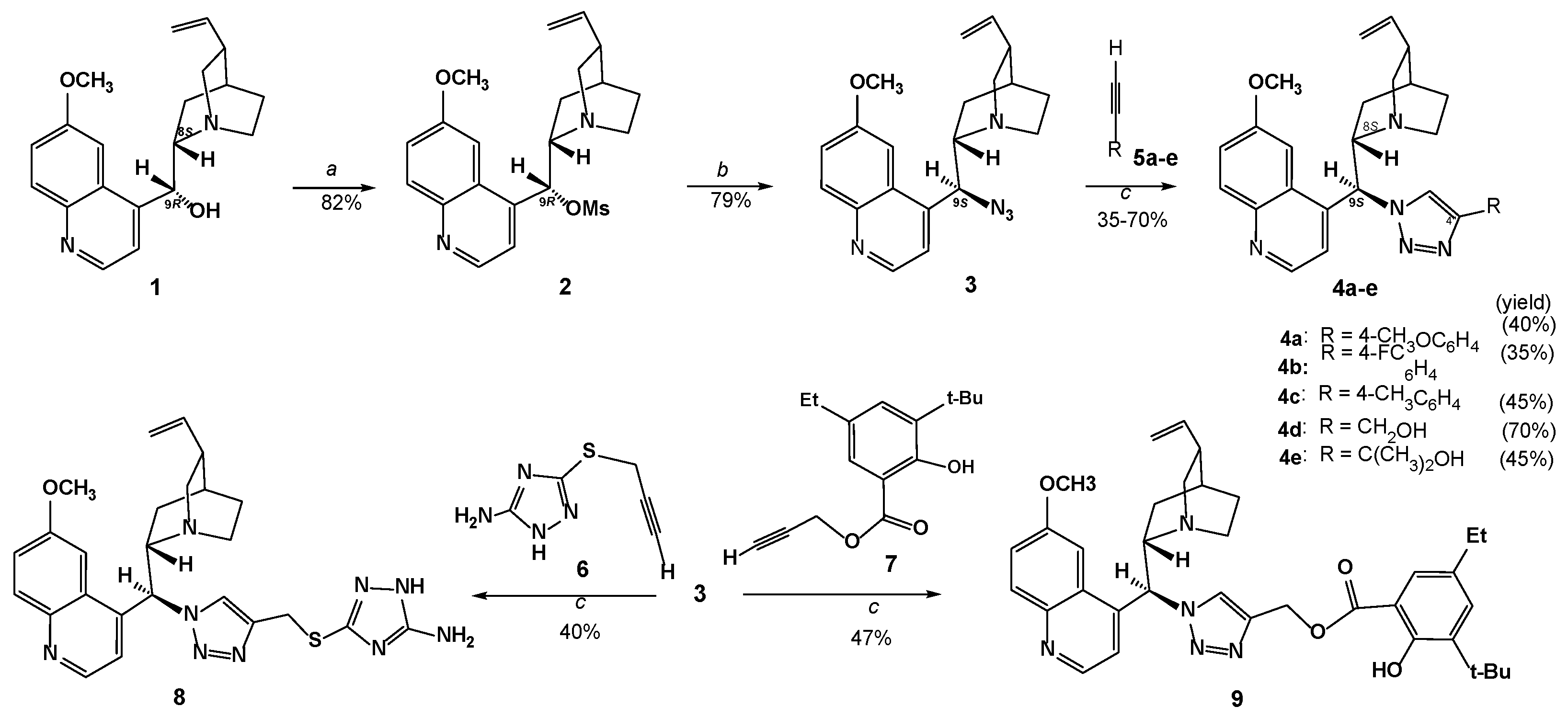
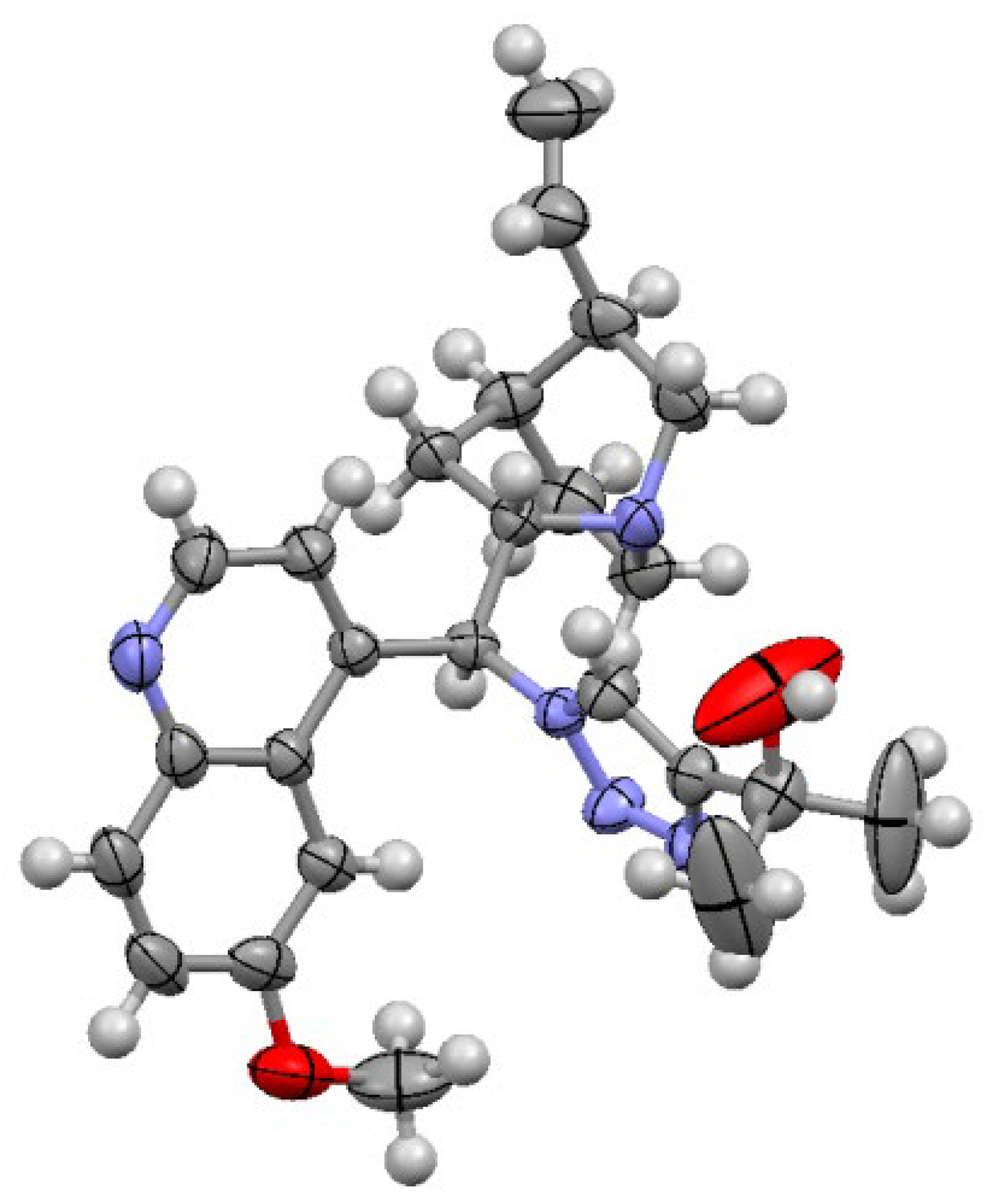
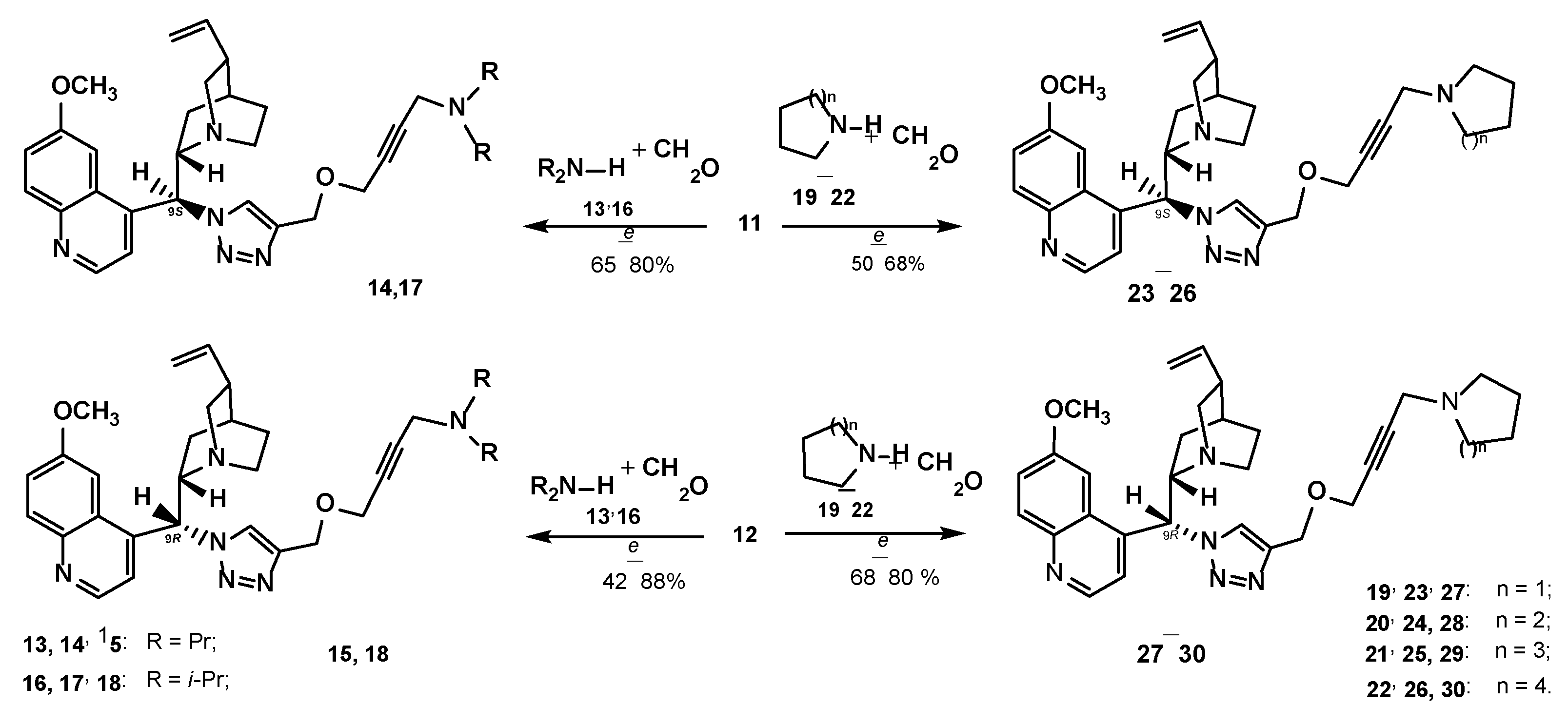
3.1.2. Experimental Procedure
Preparation of Compounds 4a–e,8,9
Preparation of Compounds 11, 12
General Procedure for the A3 Coupling Reaction
3.2. The Study of Antibacterial Activity
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufman, T.S.; Rúveda, E.A. The quest for quinine:Those who won the battles and those who won the war. Angew. Chem. Int. Ed. 2005, 44, 854–885. [Google Scholar] [CrossRef]
- Achan, J.; Tibenderana, J.K.; Kyabayinze, D.; Wabwire, M.F.; Kamya, M.R.; Dorsey, G.; D’Alessandro, U.; Rosenthal, P.J.; Talisuna, A.O. Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: Randomised trial. BMJ 2009, 339, b2763. [Google Scholar] [CrossRef]
- Gachelin, G.; Garner, P.; Ferroni, E.; Tröhler, U.; Chalmers, I. Evaluating Cinchona bark and quinine for treating and preventing malaria. J. R. Soc. Med. 2017, 110, 31–40. [Google Scholar] [CrossRef]
- Bozic, B.; Uzelac, T.V.; Kezic, A.; Bajcetic, M. The role of quinidine in the pharmacological therapy of ventricular arrhythmia ‘Quinidine’ Mini Rev. Med. Chem. 2018, 18, 468–475. [Google Scholar] [CrossRef]
- Große, M.; Ruetalo, N.; Layer, M.; Hu, D.; Businger, R.; Rheber, S.; Setz, C.; Rauch, P.; Auth, J.; Fröba, M.; et al. Quinine inhibits infection of human cell lines with SARS-CoV-2. Viruses 2021, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Latarissa, I.R.; Barliana, M.I.; Meiliana, A.; Lestari, K. Potential of quinine sulfate for COVID-19 treatment and its safety profile: Review. Clin. Pharmacol. 2021, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.P.; Yang, J.M.; Sun, D.; Tian, Y.E.; Liu, S.M.; Lin, X.M.; Jiang, J.; Chen, G.Q. Combinatorial synthesis of novel 9R-acyloxyquinine derivatives as insecticidal agents. Comb. Chem. High Throughput Screen. 2020, 23, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jew, S.-S.; Park, H.-H. Cinchona-based phase-transfer catalysts for asymmetric synthesis. Chem. Commun. 2009, 14, 7090–7103. [Google Scholar] [CrossRef]
- Yeboah, E.M.O.; Yeboah, S.O.; Singh, G.S. Recent applications of Cinchona alkaloids and their derivatives as catalysts in metal-free asymmetric synthesis. Tetrahedron 2011, 67, 1725–1762. [Google Scholar] [CrossRef]
- Duan, J.; Li, P. Asymmetric organocatalysis mediated by primary amines derived from cinchona alkaloids: Recent advances. Catal. Sci. Technol. 2014, 4, 311–320. [Google Scholar] [CrossRef]
- Li, M.; Wei, H.W.; Zhang, S.-Y. The use of Cinchona alkaloid derivatives as chiral ligands and organocatalysts in asymmetric catalysis. Mini-Rev. Org. Chem. 2022, 19, 146–165. [Google Scholar] [CrossRef]
- Majdecki, M.; Grodek, P.; Jurczak, J. Stereoselective α-chlorination of β-keto esters in the presence of hybrid amide-based cinchona alkaloids as catalysts. J. Org. Chem. 2021, 86, 995–1001. [Google Scholar] [CrossRef]
- Bajtai, A.; Ilisz, I.; Howan, D.H.O.; Tóth, G.K.; Scriba, G.K.E.; Lindner, W.; Pėter, A. Enantioselective resolution of biologically active dipeptide analogs by high-performance liquid chromatography applying Cinchona alkaloid-based ion-exchanger chiral stationary phases. J. Chromatogr. A 2020, 1611, 460574. [Google Scholar] [CrossRef]
- Laraia, L.; Garivet, G.; Foley, D.J.; Kaiser, N.; Müller, S.; Zinken, S.; Pinkert, T.; Wilke, J.; Corkery, D.; Pahl, A.; et al. Image-Based Morphological Profiling Identifies a Lysosomotropic, Iron-Sequestering Autophagy Inhibitor Angew. Chem. Int. Ed. 2020, 59, 5721–5729. [Google Scholar] [CrossRef]
- Dembo, A.; Ferenczi, E.; Jernei, T.; Bor, A.; Schelz, Z.; Zupkó, I.; Varga, S.; Csámpai, A. CuAAC-Based synthesis, copper-catalyzed aldehyde-forming hydrolytic fission and antiproliferative evaluation of novel ferrocenoylamino-substituted triazole-tethered quinine–chalcone hybrids. Molecules 2024, 29, 375. [Google Scholar] [CrossRef]
- Lawer, A.; Player, F.P.; Avery, V.M.; Foley, D.J. Synthesis of quinine-inspired antimalarials by Ni-catalysed cross electrophile coupling. Adv. Synth. Catal. 2024, 366, 2090–2100. [Google Scholar] [CrossRef]
- Bro, F.S.; Laraia, L. Unifying principles for the design and evaluation of natural product-inspired compound collections. Chem. Sci. 2025, 16, 2961–2979. [Google Scholar] [CrossRef] [PubMed]
- Player, F.P.; Foley, D.J. Site-Selective Synthetic Modifications of the Cinchona Alkaloids. Asian J. Org. Chem. 2024, 13, e202400397. [Google Scholar] [CrossRef]
- Wolf, R.; Baroni, A.; Greco, R.; Donnarumma, G.; Ruocco, E.; Tufano, M.A. Quinine sulfate and bacterial invasion. Ann. Clin. Microbiol. Antimicrob. 2002, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kharal, S.A.; Hussain, Q.; Ali, S.; Fakhruddin. Quinine is bactericidal. J. Pak. Med. Assoc. 2009, 59, 208–211. [Google Scholar] [PubMed]
- Wolf, R.; Tufano, M.A.; Rucco, V.; Grimaldi, E.; Ruocco, E.; Donnarumma, G.; Baroni, A. Quinine sulfate inhibits invasion of some bacterial skin pathogens. Int. J. Dermatol. 2006, 45, 661–663. [Google Scholar] [CrossRef]
- Rattanachak, N.; Weawsiangsang, S.; Jongjitvimol, T.; Baldock, R.A.; Jongjitwimol, J. Hydroquinine possesses antibacterial activity, and at half the MIC, induces the overexpression of RND-type efflux pumps using multiplex digital PCR in Pseudomonas aeruginosa. Trop. Med. Infect. Dis. 2022, 7, 156. [Google Scholar] [CrossRef]
- Lv, J.; Qian, Y.; Liu, T.; Wang, Y. Synthesis and evaluation of amphiphilic cationic quinine-derived for antibacterial activity against methicillin-resistance Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2007, 17, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.D.; Triana, D.; Ernawati, T. Antimicrobial activity of quinine derivatives against human pathogenic bacteria. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012006. [Google Scholar] [CrossRef]
- Wiles, J.A.; Phadke, A.S.; Bradbury, B.J.; Pucci, M.J.; Thanassi, J.A.; Deshpande, M. Selenophene-Containing Inhibitors of Type IIA Bacterial Topoisomerases. J. Med. Chem. 2011, 54, 3418–3425. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Y.; Sheng, L.; Larson, P.; Liu, X.; Zou, X.; Wang, S.; Guo, K.; Ma, C.; Zhang, G.; et al. A cinchona alkaloid antibiotic that appears to target ATP synthase in Streptococcus pneumoniae. J. Med. Chem. 2019, 62, 2305–2332. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Tian, Y.; Huang, X.; Zhou, L.; Liu, S.; Chen, G.; Che, Z. Synthesis, anti-oomycete and anti-fungal activities of novel cinchona alkaloid derivatives containing sulfonate moiety. Chem. Biodiver. 2023, 20, e202300607. [Google Scholar] [CrossRef]
- Tian, Y.; Yin, W.; Wang, R.; Sun, H.; Xu, S.; Huang, X.; Chen, G.; Che, Z. Non-food renewable and bioactive products for pesticides: Synthesis, anti-oomycete and anti-fungal activities of 9R-acyloxyquinine derivatives bearing an 1,3,4-oxadiazole moiety. Ind. Crops Prod. 2024, 222, 119751. [Google Scholar] [CrossRef]
- Roper, S.; Franz, M.H.; Wartchow, R.; Hoffmann, H.M.R. The first and second Cinchona rearrangement. Two fundamental transformations of alkaloid chemistry. Org. Lett. 2003, 5, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K.; Migas, M.; Plutecka, A.; Rychlewska, U.; Gawroński, J. The library of Cinchona alkaloids—1,2,3-triazole derivatives: Structure and facile access by “Click Chemistry”. Heterocycles 2005, 65, 1931–1938. [Google Scholar] [CrossRef]
- Skiera, I.; Antoszczak, M.; Trynda, J.; Wietrzyk, J.; Boratyński, P.; Kacprzak, K.; Huczyński, A. Antiproliferative activity of polyether antibiotic—Cinchona alkaloid conjugates obtained via click chemistry. Chem. Biol. Drug Des. 2015, 86, 911–917. [Google Scholar] [CrossRef]
- Gałęzowska, J.; Boratynński, P.; Kowalczyk, R.; Lipke, K.; Czapor-Irzabek, H. Copper(II) complexes of chiral 1,2,3-triazole biheterocyclic ‘click’ ligands equipped in Cinchona alkaloid moiety. Polyhedron 2017, 121, 1–8. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Gałęzowska, J.; Turkowiak, K.; Anisiewicz, A.; Kowalczyk, R.; Wietrzyk, J. Triazole biheterocycles from cinchona alkaloids: Coordination and antiproliferative properties. ChemistrySelect 2018, 3, 9368–9373. [Google Scholar] [CrossRef]
- Sahu, A.; Agrawal, R.K.; Pandey, R.K. Synthesis and systemic toxicity assessment of quinine-triazole scaffold with antiprotozoal potency. Bioorg. Chem. 2019, 88, 102939. [Google Scholar] [CrossRef]
- Mishra, N.; Agrahari, A.K.; Bose, P.; Singh, S.K.; Singh, A.S.; Tiwari, V.K. Click Inspired Synthesis of Novel Cinchonidine Glycoconjugates as Promising Plasmepsin Inhibitors. Sci. Rep. 2020, 10, 35861. [Google Scholar] [CrossRef]
- Barrulas, P.; Carreiro, E.P.; Veiros, L.F.; Amorim, A.C.; Gut, G.; Rosenthal, P.J.; López, Ó.; Puerta, A.; Padrón, J.M.; Fernández-Bolaños, J.G.; et al. Novel 1,2,3-triazole epicinchonas: Transitioning from organocatalysis to biological activities. Synth. Commmun. 2021, 51, 2954–2974. [Google Scholar] [CrossRef]
- Sahu, A.; Agrawal, R.K. Synthesis of quinine-triazole derivatives (QNTDS) with antifungal potency. Anti-Infect. Agents 2022, 20, e240122200465. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef]
- Carneiro, A.; Uriarte, E.; Borges, F.; Matos, M. Propargylamine: An important moiety in drug discovery. Future Med. Chem. 2023, 15, 211–224. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef]
- Martinez-Amezaga, M.; Giordano, R.A.; Gori, D.N.P.; Squizatto, C.P.; Giolito, M.V.; Scharovsky, O.G.; Rozados, V.R.; Rico, M.J.; Mata, E.G.; Delpiccolo, C.M.L. Synthesis of propargylamines via the A3 multicomponent reaction and their biological evaluation as potential anticancer agents. Org. Biomol. Chem. 2020, 18, 2475–2486. [Google Scholar] [CrossRef]
- Zielinska-Blajet, M.; Kucharska, M.; Skarzowski, J. Simple enantiospecific synthesis of sulfides of Cinchona alkaloids. Synthesis 2006, 7, 1176–1182. [Google Scholar] [CrossRef]
- Kacprzak, K.; Gierczyk, B. Clickable 9-azido-(9-deoxy)-Cinchona alkaloids: Synthesis and conformation. Tetrahedron Asymmetry 2010, 21, 2740–2745. [Google Scholar] [CrossRef]
- Dijkstra, G.D.H.; Kellogg, R.M.; Wynberg, H. Conformational study of cinchona alkaloids. A combined NMR and molecular orbital approach. J. Org. Chem. 1990, 55, 6121–6131. [Google Scholar] [CrossRef]
- Bürgi, T.; Baiker, A. Conformational behavior of cinchonidine in different solvents: A Combined NMR and ab initio investigation. J. Am. Chem. Soc. 1998, 120, 12920–12926. [Google Scholar] [CrossRef]
- Busygin, I.; Nieminen, V.; Taskinen, A.; Sinkkonen, J.; Toukoniitty, E.; Sillanpää, R.; Murzin, D.Y.; Leino, R. A Combined NMR, DFT, and X-ray Investigation of Some Cinchona Alkaloid O-Ethers. J. Org. Chem. 2008, 73, 6559–6565. [Google Scholar] [CrossRef] [PubMed]
- Yanuka, T.; Geryes, A.; Heller, M. Stereospecific epimerisation, oxidation and toxine rearrangement in cinchona alkaloids catalyzed by acetic acid. Tetrahedron 1987, 43, 911–922. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Skarżewski, J. New functional derivatives of 9-phenylquinine functional derivatives of 9-phenylquinine. Synthesis 2009, 18, 3113–3119. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Turowska-Tyrk, I.; Skarżewski, J. Synthetic approaches to 9-arylated Cinchona alkaloids: Stereoselective addition of Grignard reagents to cinchonanones and hydroxylation of 9-phenylcinchonanes. Tetrahedron Asymmetry 2012, 23, 876–883. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Yang, Z.; Xu, X.; Zhao, H.; Huang, Y.; Wang, Y. Synthesis and computation of diastereomeric phenanthroline–quinine ligands and their application in asymmetric Henry reaction. Tetrahedron 2013, 69, 10644–10652. [Google Scholar] [CrossRef]
- Ramić, A.; Skočibušić, M.; Odžak, R.; Cipak Gašparović, A.; Milković, L.; Mikelić, A.; Sović, K.; Primožić, I.; Hrenar, T. Antimicrobial activity of quasi-enantiomeric cinchona alkaloid derivatives and prediction model developed by machine learning. Antibiotics 2021, 10, 659. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef]
- Huang, M.; Brena, D.; Wu, J.Y.; Shelton, M.; Bond, V.C. SMR peptide antagonizes Staphylococcus aureus biofilm formation. Microbiol. Spectr. 2024, 12, e0258323. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. Medchemcomm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Skogman, M.E.; Kujala, J.; Busygin, I.; Leinob, R.; Vuorela, P.M.; Fallarero, A. Evaluation of antibacterial and anti-biofilm activities of cinchona alkaloid derivatives against Staphylococcus aureus. Nat. Prod. Commun. 2012, 7, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. The effects of culture concentration and age, time, and temperature on bacterial attachment to polystyrene. Can. J. Microbiol. 1977, 23, 1–6. [Google Scholar] [CrossRef]
- El Zoeiby, A.; Sanschagrin, F.; Levesque, R.C. Structure and function of the Mur enzymes: Development of novel inhibitors. Mol. Microbiol. 2003, 47, 1–12. [Google Scholar] [CrossRef]
- Benson, T.E.; Walsh, C.T.; Hogle, J.M. The structure of the substrate-free form of MurB, an essential enzyme for the synthesis of bacterial cell walls. Structure 1993, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.J.; Bronson, J.J.; D’Andrea, S.V.; Deshpande, M.S.; Falk, P.J.; Grant-Young, K.A.; Harte, W.E.; Ho, H.T.; Misco, P.F.; Robertson, J.G.; et al. 4-Thiazolidinones: Novel inhibitors of the bacterial enzyme MurB. Bioorg. Med. Chem. Lett. 2000, 10, 715–717. [Google Scholar] [CrossRef]
- Yang, Y.; Severin, A.; Chopra, R.; Krishnamurthy, G.; Singh, G.; Hu, W.; Keeney, D.; Svenson, K.; Petersen, P.J.; Labthavikul, P.; et al. 3,5-Dioxopyrazolidines, novel inhibitors of UDP-N-acetylenolpyruvylglucosamine reductase (MurB) with activity against gram-positive bacteria. Antimicrob. Agents Chemother. 2006, 50, 556–564. [Google Scholar] [CrossRef]
- Hervin, V.; Roy, V.; Agrofoglio, L.A. Antibiotics and antibiotic resistance-Mur ligases as an antibacterial target. Molecules 2023, 28, 8076. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kumar, V.; Naik, B.; Masood Khan, J.; Singh, P.; Saris, P.E.J.; Gupta, S. Screening and molecular dynamics simulation of compounds inhibiting MurB enzyme of drug-resistant Mycobacterium tuberculosis: An in-silico approach. Saudi J. Biol. Sci. 2023, 30, 103730. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef]
- Lipeeva, A.V.; Zakharov, D.O.; Burova, L.G.; Frolova, T.S.; Baev, D.S.; Shirokikh, I.V.; Evstropov, A.N.; Sinitsyna, O.I.; Tolsikova, T.G.; Shults, E.E. Design, synthesis and antibacterial activity of coumarin-1,2,3-triazole hybrids obtained from natural furocoumarin peucedanin. Molecules 2019, 24, 2126. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Mironov, A.N. (Ed.) Guidelines for Conducting Preclinical Studies of Drugs; GRIF-K: Moscow, Russia, 2012; Part 1; p. 206. [Google Scholar]
- Coenye, T.; Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.E.; Harris, M.S.; Choi, G.H.; Cialdella, J.I.; Herberg, J.T.; Martin, J.P., Jr.; Baldwin, E.T. A structural variation for MurB: X-ray crystal structure of Staphylococcus aureus UDP-N-acetylenolpyruvylglucosamine reductase (MurB). Biochemistry 2001, 40, 2340–2350. [Google Scholar] [CrossRef]

| Compound | Diameters of the Inhibition Zone (mm) [a] | Minimum Bactericidal Concentration, MBC (μg/mL) | ||||
|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 6538-P | Bacillus subtilis ATCC 6633 | Escherichia coli ATCC 25922 | Staphylococcus aureus ATCC 6538-P | Bacillus subtilis ATCC 6633 | Escherichia coli ATCC 25922 | |
| 4a | 12 ± 0.2 | 15 ± 0.1 * | 15 ± 0.1 * | 25 | 25 | |
| 4b | 14 ± 0.1 * | 13 ± 0.2 | 16 ± 0.2 | 25 | 12.5 | |
| 4c | - | - | 14 ± 0.2 | 50 | ||
| 4d | 13 ± 0.2 | - | 14 ± 0.1 | 50 | ||
| 8 | 15 ± 0.2 | - | 15 ± 0.1 * | 25 | 25 | |
| Gentamicin | 24 ± 0.1 | 21 ± 0.2 | 26 ± 0.1 | 11.8 | 22.5 | 11.3 |
| Compound | MIC, μg/mL [a,b] | Compound | MIC, μg/mL [a] | ||
|---|---|---|---|---|---|
| S. aureus 209 ATCC 6538-P [b] | B. cereus ATCC 10702 [c] | S. aureus 209 ATCC 6538-P [b] | B. cereus ATCC 10702 [c] | ||
| 11 | >1000 | 596.5 ± 3.2 | 24 | 385 ± 14.3 | 366.7 ± 33.3 |
| 12 | >1000 | 456.7 ± 29.6 | 28 | 250 ± 26.7 | 291.7 ± 8.3 |
| 14 | >1000 | 481.7 ± 18.3 | 25 | 350 ± 34.3 | 296.5 ± 23.2 |
| 15 | >1000 | 750 ± 76.4 | 29 | 200 ± 16.4 | 283.2 ± 28.8 |
| 17 | 646.7 ± 33.3 | 533.3 ± 33.3 | 26 | 181 ± 12.8 | 218.8 ± 18.75 |
| 18 | 683.3 ± 92.8 | 633.3 ± 66.7 | 30 | 179.2 ± 18.4 | 107.5 ± 10.9 |
| 23 | 416.7 ± 22.6 | 498.3 ± 30.3 | Quinine 1 | >1000 | 956.7 ± 109.6 |
| 27 | 303.3 ± 43.3 | 545.8 ± 26.7 | Ceftriaxonum | 2.7 ± 0.7 | 6.03 ± 0.52 |
| Doses (M ± m), µg/mL | 250 | 125 | 62.5 | 31.25 | Culture Control |
|---|---|---|---|---|---|
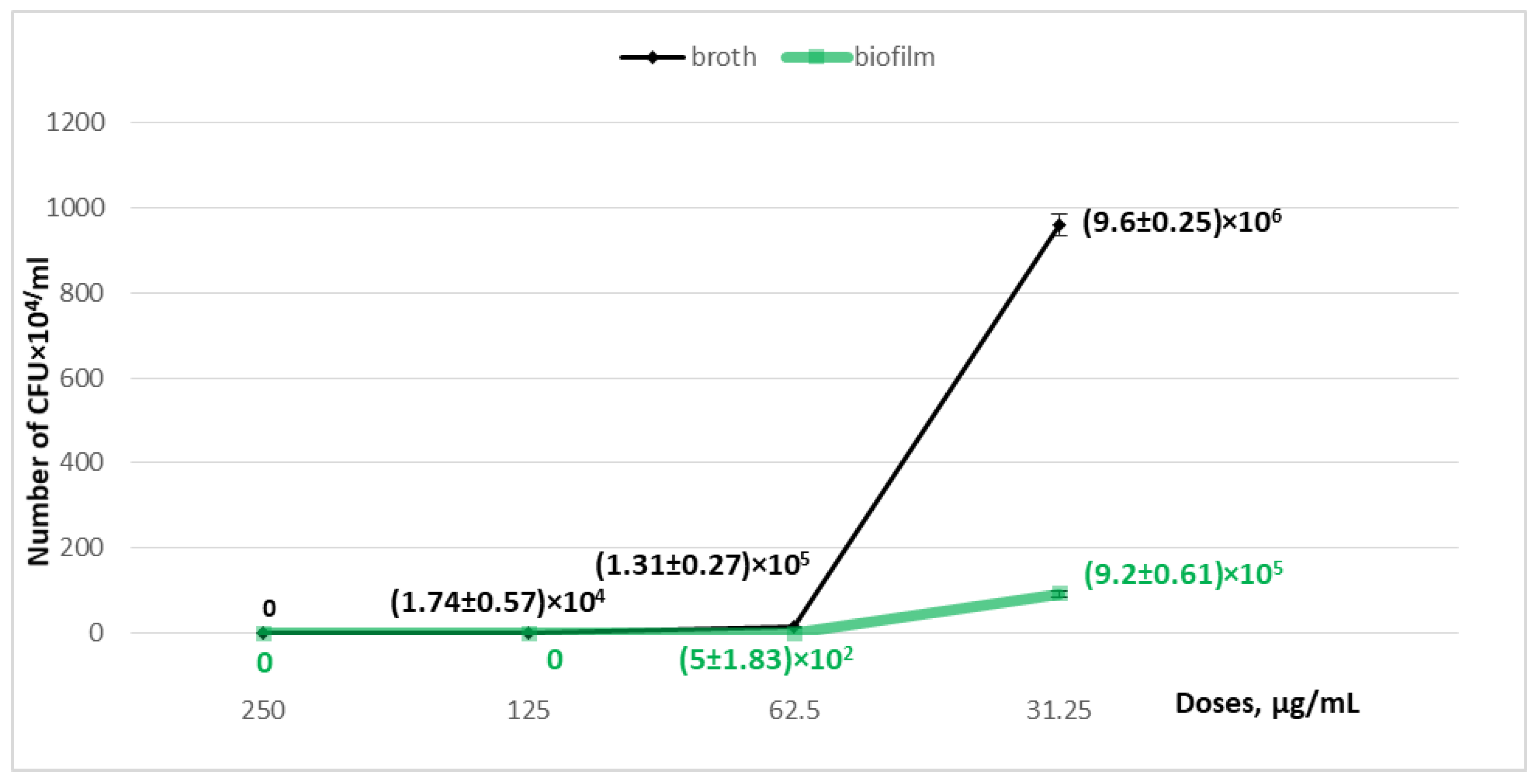
| Growth in the broth, CFU/mL | 0 | (1.74 ± 0.57) × 104 * | (1.31 ± 0.27) × 105 * | (9.6 ± 0.25) × 106 * | (1.38 ± 0.12) × 107 |
| biofilm formation, CFU/mL | 0 | 0 | (5.0 ± 1.83) × 102 * | (9.2 ± 0.61) × 105 * | (2.9 ± 0.2) × 106 |
| Doses (M ± m), µg/mL | 250 | 125 | 62.5 | 31.25 | Culture Control |
|---|---|---|---|---|---|
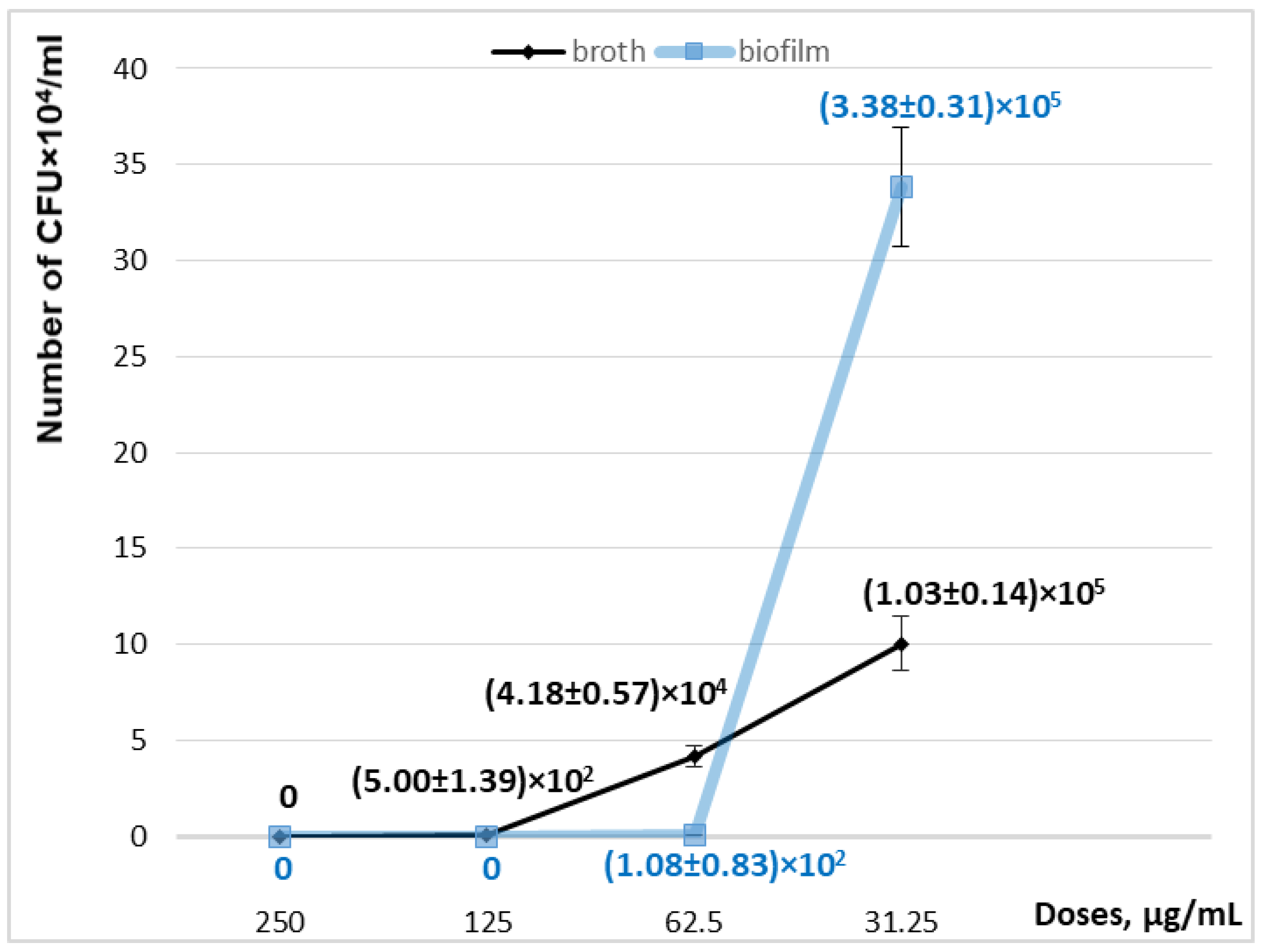
| Growth in the broth, CFU/mL | 0 | (5.0 ± 1.39) × 102 * | (4.18 ± 0.57) × 104 * | (1.03 ± 0.14) × 105 * | (1.38 ± 0.12) × 107 |
| biofilm formation, CFU/mL | 0 | 0 | (1.08 ± 0.83) × 102 | (3.38 ± 0.31) × 105 * | (2.9 ± 0.2) × 106 |
| Com-pound | DS * | Interacting Residues ** | Hydrogen Bonds (Ligand Group) | Location of The Ligand Moieties *** |
|---|---|---|---|---|
| 1 | −123.5 | Tyr77, Tyr149, Val199, Gly145, Gly146, Leu78 | Tyr77 (OH), Ser143 (OMe), Val199 (quinoline nitrogen) | Quinoline on adenine, quinuclidine on ribose, vinyl on pyrophosphate |
| 4d | −173.6 | Tyr149, Tyr77, Gly146, Leu78, Ser143, Gly145, Arg310, Gly79, Asn83 | Ser143 (OMe), Tyr149 (Me2CHOH), Arg310 (triazole N-3, weak) | Quinoline on adenine, quinuclidine and vinyl on pyrophosphate, triazole on ribose |
| 11 | −185.7 | Tyr149, Gly146, Tyr77, Arg310, Asn83, Leu78, Gly145, Ser143, Gly79, Ile84 | Ser143 (OMe), Tyr149 (propargyloxy), Arg310 (triazole N-3, weak; propargyloxy, weak) | Quinoline on adenine, quinuclidine and vinyl on pyrophosphate, triazole on ribose, propargyloxy apart from FAD |
| 12 | −191.0 | Tyr149, Tyr77, Leu78, Gly146, Gly145, Ser143, Asn83 | Ser82 (propargyloxy), Ser143 (triazole N-3) | Quinoline on adenine, quinuclidine on ribose, triazole near pyrophosphate, propargyloxy on ribitol |
| 23 | −199.2 | Tyr77, Tyr149, Leu78, Ser82, Gly146, Gly145, Gly79, Asn83, Pro141 | Gly79 (triazole N-2; triazole N-3, weak), Gly81 (triazole N-3), Ser82 (propargyloxy) | Quinoline on adenine, quinuclidine on ribose, triazole near pyrophosphate, propargyloxy on ribitol, pyrrolidine near isoalloxazine |
| 27 | −216.3 | Tyr77, Tyr149, Ser82, Asn83, Leu78, Gly145, Pro141, Gly146, Ser143 | Ser82 (propargyloxy), Ser143 (triazole N-3) | Quinoline on adenine, quinuclidine on ribose, triazole near pyrophosphate, propargyloxy on ribitol, pyrrolidine near isoalloxazine |
| 26 | −207.7 | Tyr77, Tyr149, Leu78, Ser82, Gly146, Pro141, Gly145, Gly79 | Gly79 (triazole N-2, N-3), Gly81 (triazole N-3), Ser82 (propargyloxy) | Quinoline on adenine, quinuclidine on ribose, triazole near pyrophosphate, propargyloxy on ribitol, azocane on isoalloxazine |
| 30 | −226.4 | Tyr77, Tyr149, Asn83, Ser82, Leu78, Gly145, Pro141, Gly146, Ser143 | Ser82 (propargyloxy), Ser143 (triazole N-3) | Quinoline on adenine, quinuclidine on ribose, triazole near pyrophosphate, propargyloxy on ribitol, azocane on isoalloxazine |
| FAD | −315.5 | Ser82, Pro141, Tyr149, Tyr77, Ile140, Val199, Gly146, Arg225, Asn83, Asn80, Ser143, Leu78, Ile84, Gly79, Gly145 | Tyr77 (ribose OH), Gly79(pyrophosphate), Asn80(pyrophosphate), Gly81 (pyrophosphate), Ser82 (pyrophosphate; ribitol OH), Asn83 (pyrophpsphate), Pro141 (ribitol OH), Ser143(pyrophosphate), Tyr149 (ribose OH, weak), Gly153 (isoalloxazine N-3; isoalloxazine carbonyl at C-2), Val199 (adenine NH2; adenine N-1), Arg225 (isoalloxazine N-5; isoalloxazine carbonyl at C-4) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukusheva, G.K.; Toigambekova, N.N.; Savelyev, V.A.; Khlebnikov, A.I.; Burova, L.G.; Afanaseva, S.D.; Nurkenov, O.A.; Kishkentayeva, A.S.; Olzhabayeva, A.S.; Gatilov, Y.V.; et al. Synthesis, Antibacterial Properties and Molecular Docking Studies of Nitrogen Substituted 9-(((4X-But-2-ynyloxy)methyl)-1,2,3-triazolyl)–Cinchona Alkaloid Conjugates. Molecules 2025, 30, 4352. https://doi.org/10.3390/molecules30224352
Mukusheva GK, Toigambekova NN, Savelyev VA, Khlebnikov AI, Burova LG, Afanaseva SD, Nurkenov OA, Kishkentayeva AS, Olzhabayeva AS, Gatilov YV, et al. Synthesis, Antibacterial Properties and Molecular Docking Studies of Nitrogen Substituted 9-(((4X-But-2-ynyloxy)methyl)-1,2,3-triazolyl)–Cinchona Alkaloid Conjugates. Molecules. 2025; 30(22):4352. https://doi.org/10.3390/molecules30224352
Chicago/Turabian StyleMukusheva, Gulim K., Nurizat N. Toigambekova, Victor A. Savelyev, Andrey I. Khlebnikov, Liubov G. Burova, Sofiia D. Afanaseva, Oralgazy A. Nurkenov, Anarkul S. Kishkentayeva, Aikerim S. Olzhabayeva, Yurii V. Gatilov, and et al. 2025. "Synthesis, Antibacterial Properties and Molecular Docking Studies of Nitrogen Substituted 9-(((4X-But-2-ynyloxy)methyl)-1,2,3-triazolyl)–Cinchona Alkaloid Conjugates" Molecules 30, no. 22: 4352. https://doi.org/10.3390/molecules30224352
APA StyleMukusheva, G. K., Toigambekova, N. N., Savelyev, V. A., Khlebnikov, A. I., Burova, L. G., Afanaseva, S. D., Nurkenov, O. A., Kishkentayeva, A. S., Olzhabayeva, A. S., Gatilov, Y. V., Seidakhmetova, R. B., Evstropov, A. N., & Shults, E. E. (2025). Synthesis, Antibacterial Properties and Molecular Docking Studies of Nitrogen Substituted 9-(((4X-But-2-ynyloxy)methyl)-1,2,3-triazolyl)–Cinchona Alkaloid Conjugates. Molecules, 30(22), 4352. https://doi.org/10.3390/molecules30224352








