Rhododendron Microshoot Culture as a Source of Phenolic Antioxidants for Biomedicine
Abstract
1. Introduction
2. Results
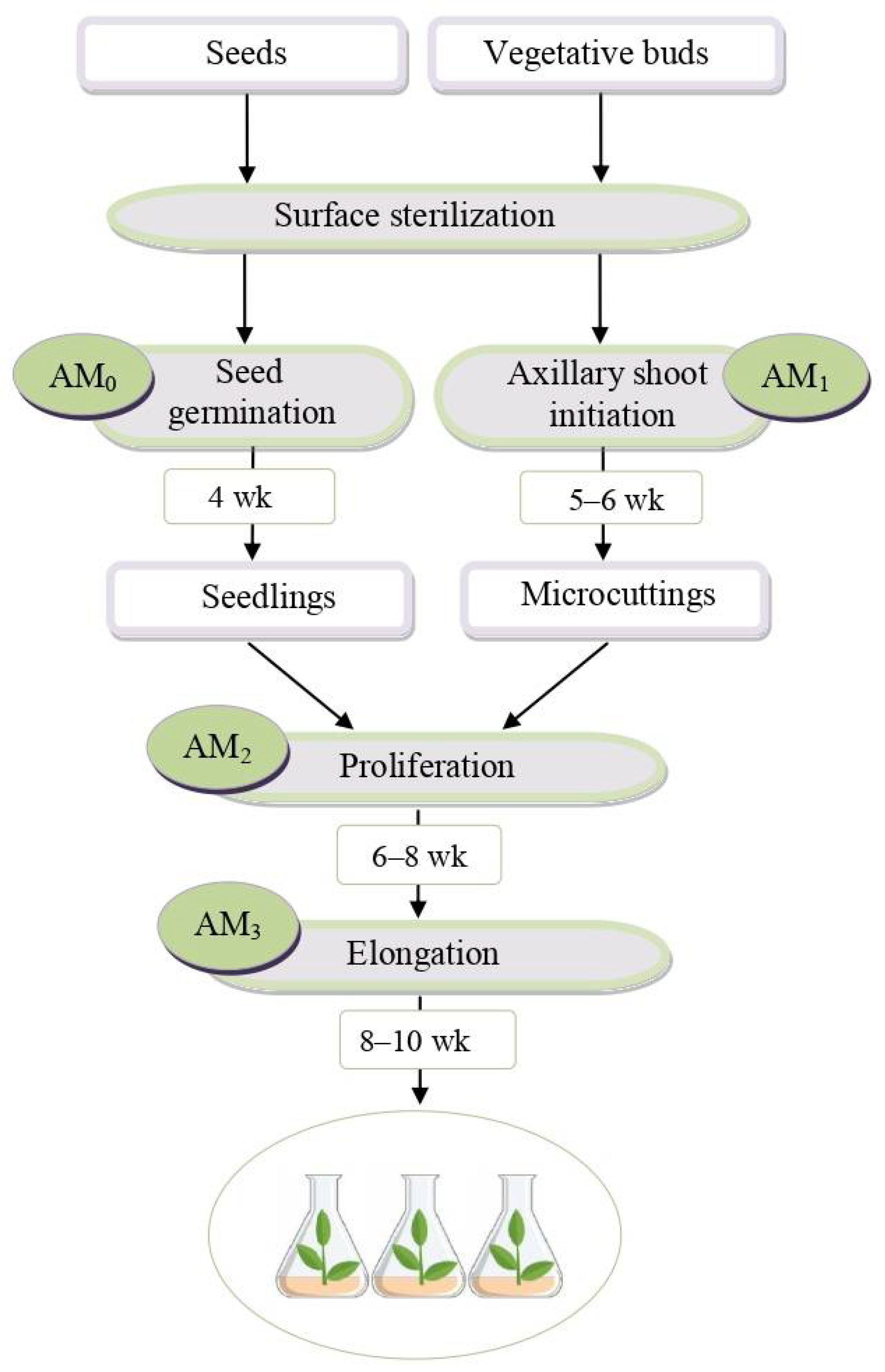
2.1. In Vitro Shoot Initiation, Proliferation, and Elongation of Rhododendron Microshoots
2.2. Morphophysiological Characteristics of Rhododendron Microshoots
2.3. The Photosynthetic Pigment Content in Rhododendron Microshoots
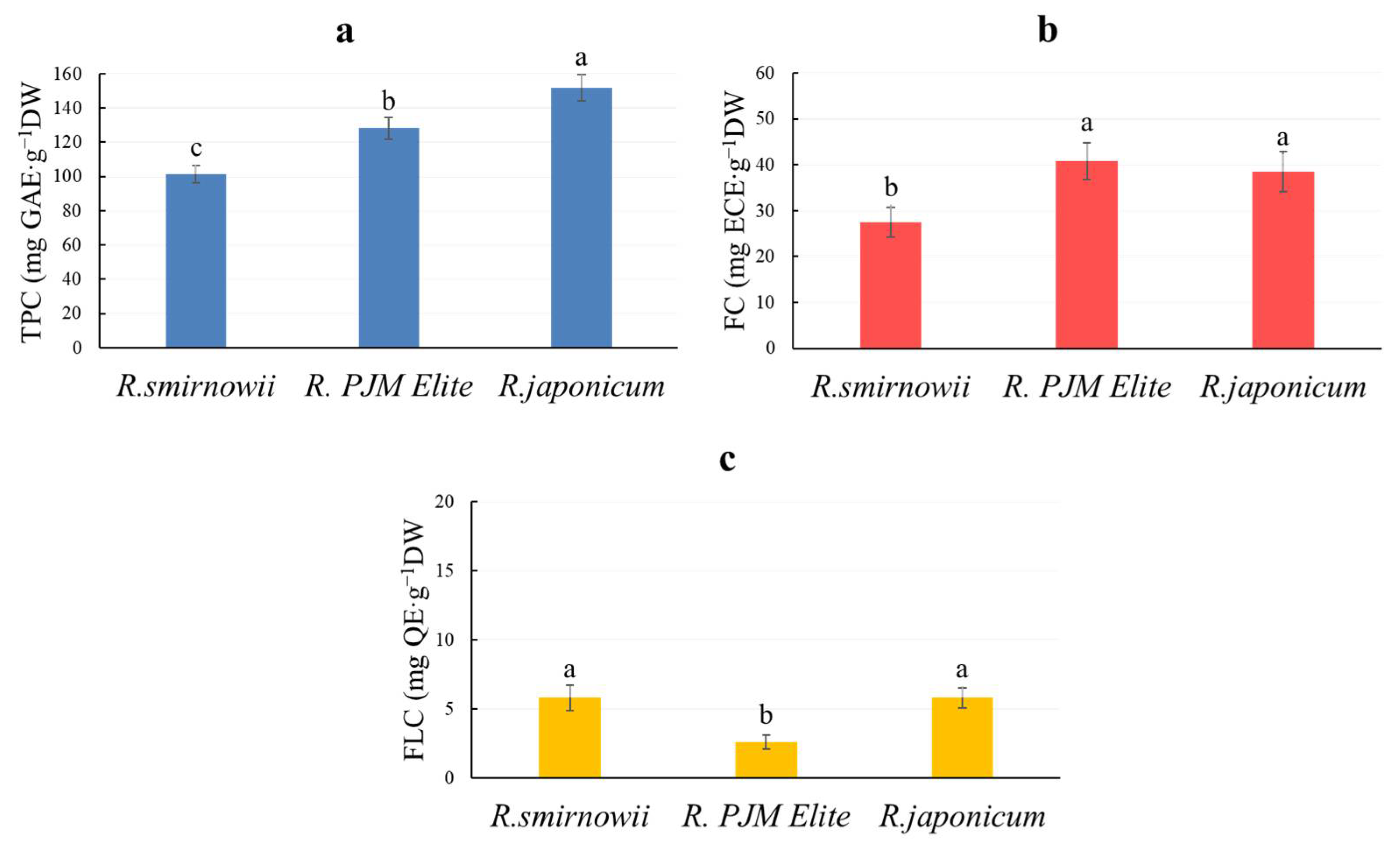
2.4. The Phenolic Compound Content in Rhododendron Microshoots
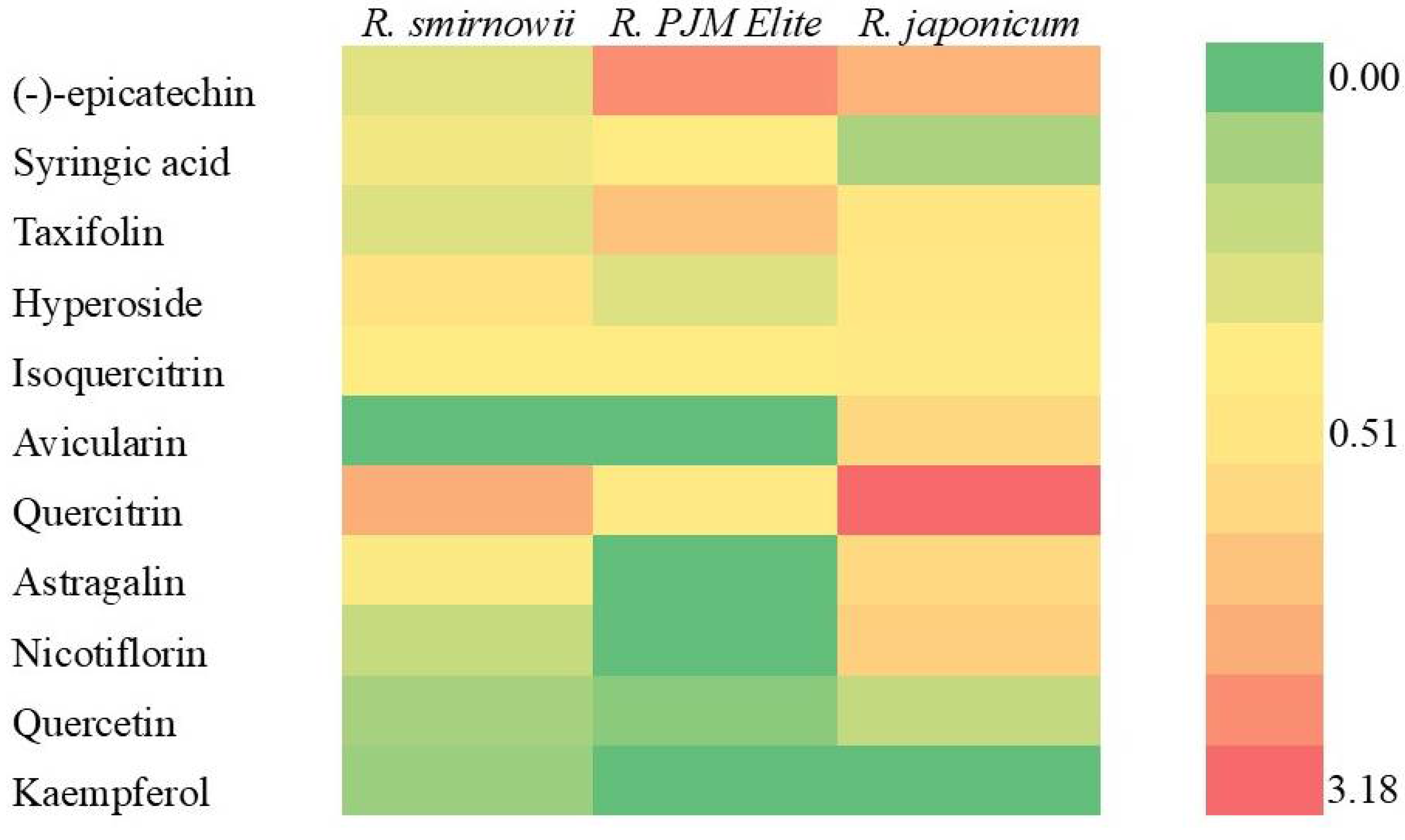
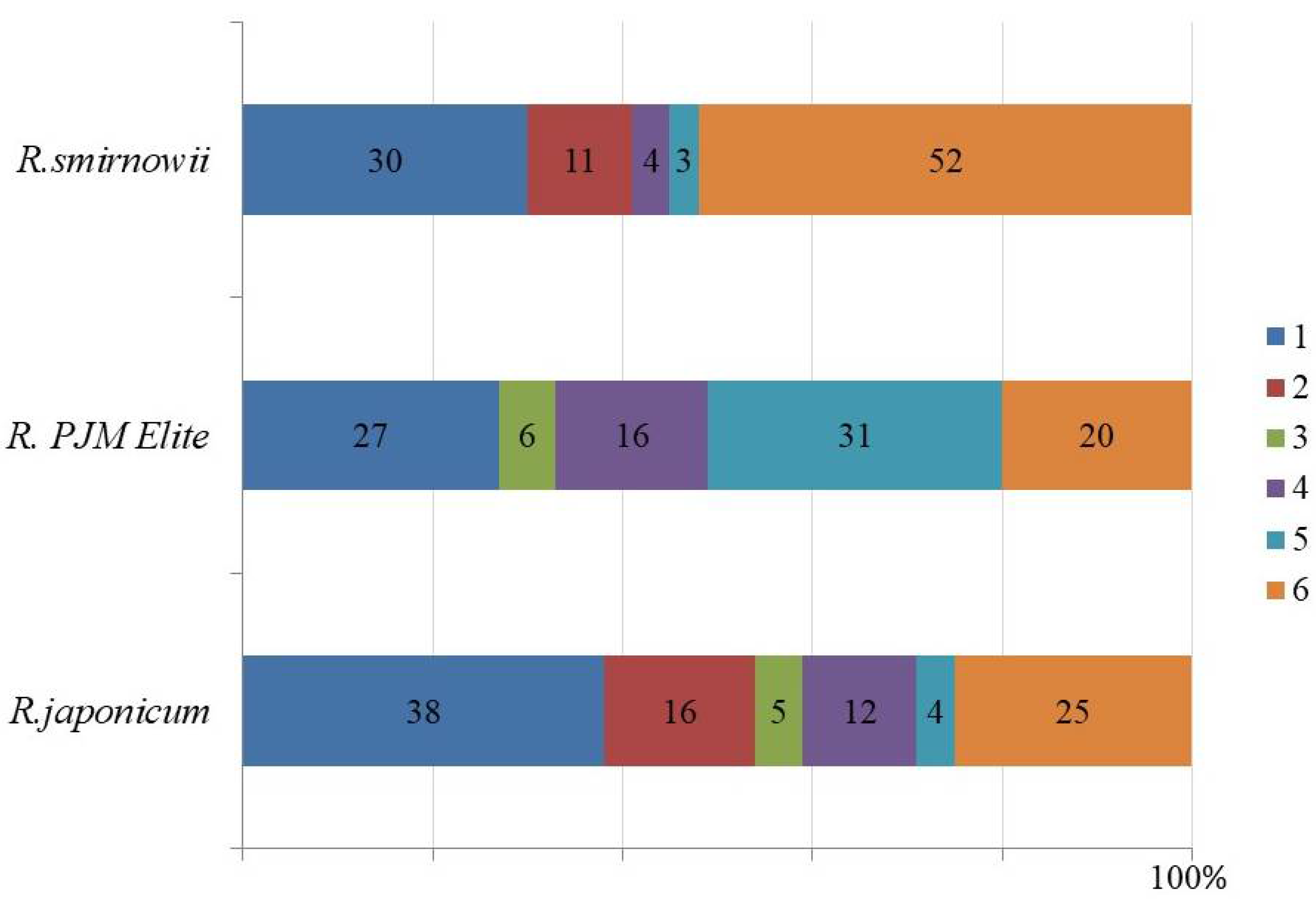
2.5. Phenolic Profile of Rhododendron Microshoots
2.6. Antiradical Activity of Rhododendron Microshoot Extracts in the DPPH-Radical System
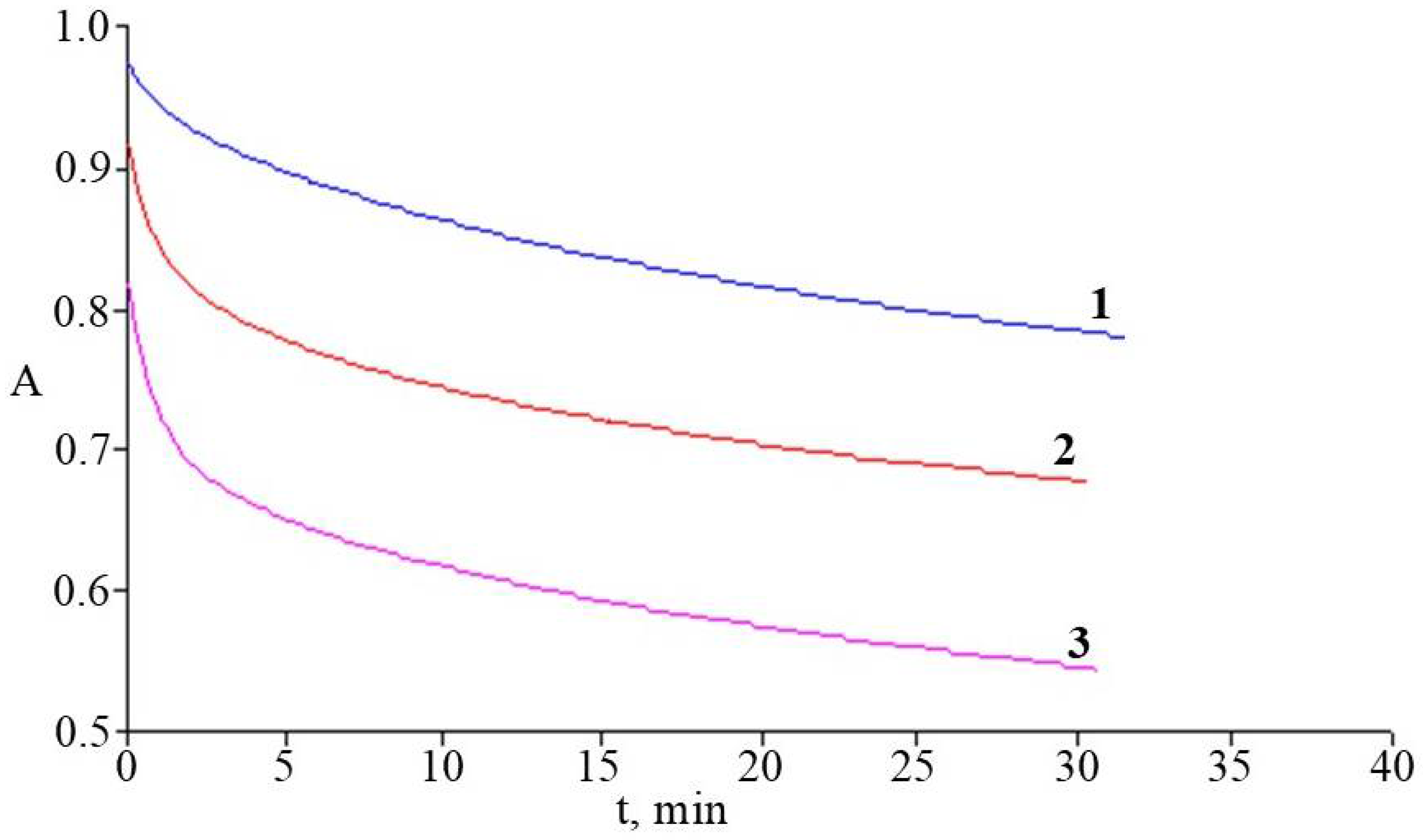
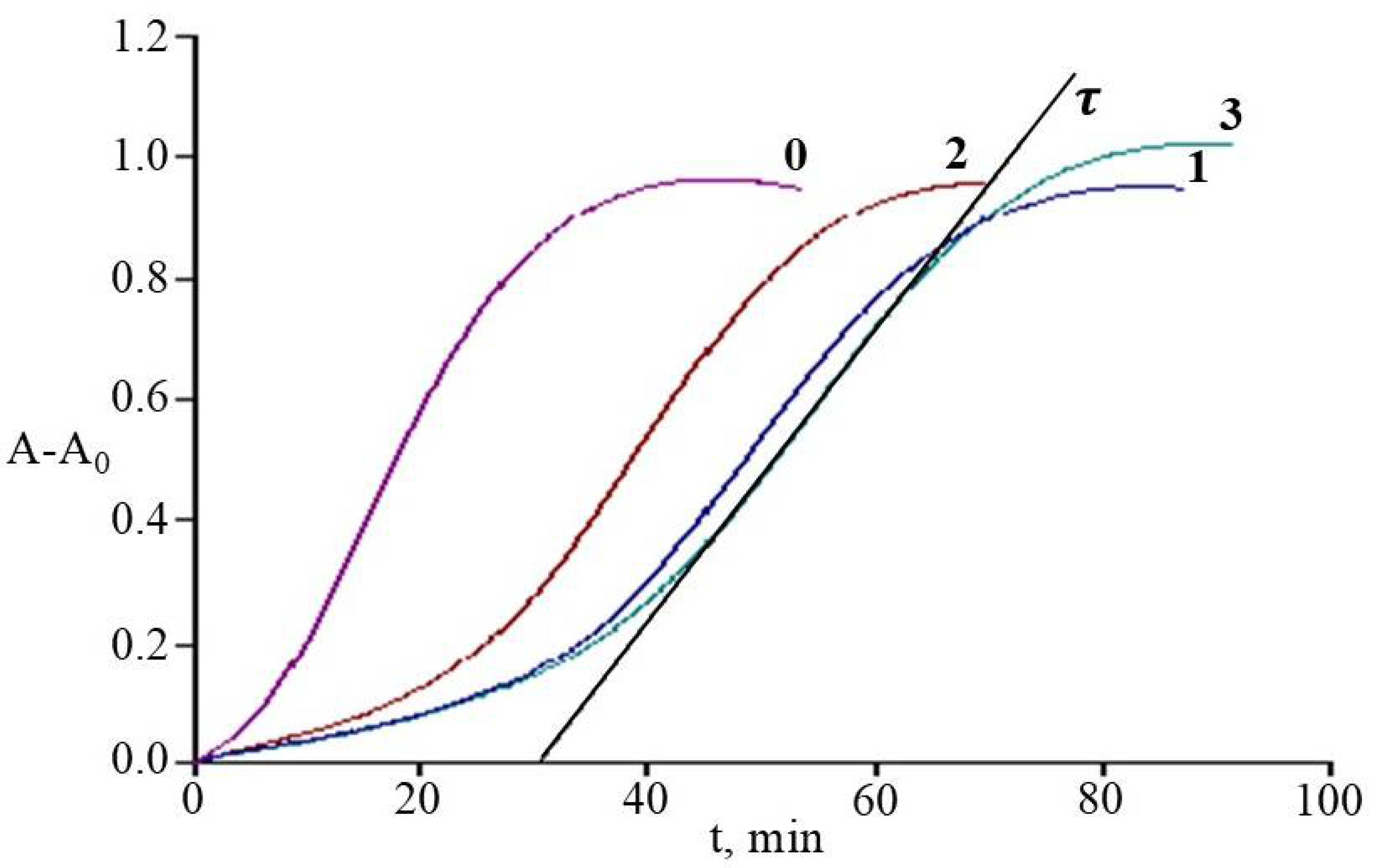
2.7. Antioxidant Activity of Rhododendrons’ Extracts in the System of Initiated Liposome Oxidation
3. Discussion
3.1. In Vitro Shoot Initiation, Proliferation, and Elongation of Rhododendron Microshoots
3.2. Morphophysiological Characteristics of Rhododendrons Microshoots
3.3. The Photosynthetic Pigment Content in Rhododendron Microshoots
3.4. The Phenolic Compounds Content in Rhododendron Microshoots
3.5. Phenolic Profile of Rhododendron Microshoots
3.6. Antiradical Activity of Rhododendron Microshoot Extracts in the DPPH-Radical System
3.7. Antioxidant Activity of Rhododendrons’ Extracts in the System of Initiated Liposome Oxidation
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
4.2. In Vitro Culture Initiation
4.3. In Vitro Shoot Proliferation
4.4. In Vitro Shoot Elongation
4.5. Morphometric Analysis
4.6. Determination of Water Content
4.7. Determination of Chlorophyll a and b Content
4.8. Determination of Different Phenolic Compounds Classes Total Content
4.9. Analysis of Individual Phenolic Compounds Using High Performance Liquid Chromatography (HPLC)
4.10. Determination of the Antiradical Activity of In Vitro Rhododendron Microshoots’ Extracts in the 1,1-Diphenyl-2-picrylhydrazyl Radical System
4.11. Determination of the Antioxidant Activity of In Vitro Rhododendron Microshoots’ Extracts in the System of Initiated Liposome Oxidation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCs | Phenolic compound |
| AOA | Antioxidant activity |
| BAS | Biologically active substances |
| AM | Anderson’s medium |
| ARA | Antiradical activity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| AO | Antioxidant |
| AAPH | 2,2′-azobis-(amidinopropane)-dihydrochloride |
| PCh | Phosphatidylcholine |
| DC | Diene conjugates |
References
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Tijjani, H.; Zangoma, M.H.; Mohammed, Z.S.; Obidola, S.M.; Egbuna, C.; Abdulai, S.I. Polyphenols: Classifications, Biosynthesis and Bioactivities. In Functional Foods and Nutraceuticals; Egbuna, C., Dable Tupas, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 389–414. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Rana, B.; Chahal, K. Phenolic Compounds Under Stress. In Plant Metabolites Under Environmental Stress, 1st ed.; Hithamani, G., Naveen, J., Pushpalatha, H.G., Eds.; Apple Academic Press: New York, NY, USA, 2023; pp. 203–218. ISBN 9781003304869. [Google Scholar]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharm. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A comprehensive review of the classification, sources, biosynthesis, and biological properties of hydroxybenzoic and hydroxycinnamic acids. Phytochem. Rev. 2023, 24, 1061–1090. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Simón, J.; Casado-Andrés, M.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Martínez-Chantar, M.L. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients 2020, 12, 3517. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef]
- Mishra, C.B.; Pandey, P.; Sharma, R.D.; Malik, M.Z.; Mongre, R.K.; Lynn, A.M.; Prasad, R.; Jeon, R.; Prakash, A. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: An integrated computational approach. Brief. Bioinform. 2021, 22, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Schwery, O.; Onstein, R.E. As old as the mountains: The radiations of the Ericaceae. New Phytol. 2015, 207, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Wang, Z.H.; Su, X.Y.; Xu, X.T.; Lyu, L.; Liu, Y.P.; Dimitrov, D.; Kennedy, J.D.; Wang, Q.G.; Tang, Z.Y.; et al. Global patterns of Rhododendron diversity: The role of evolutionary time and diversification rates. Glob. Ecol. Biogeogr. 2018, 27, 913–924. [Google Scholar] [CrossRef]
- Lipscomb, M.V.; Nilsen, E.T. Environmental and physiological factors influencing the natural distribution of evergreen and deciduous Ericaceous shrubs on northeast and southwest slopes of the southern Appalachian mountains. I Irradiance Tolerance. Amer. J. Bot. 1990, 77, 108–115. [Google Scholar] [CrossRef]
- Basnett, S.; Ganesan, R. A comprehensive review on the taxonomy, ecology, reproductive biology, economic importance and conservation status of Indian Himalayan Rhododendrons. Bot. Rev. 2022, 88, 505–544. [Google Scholar] [CrossRef]
- Krebs, S. Rhododendron. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Volume 11, pp. 673–718. [Google Scholar]
- Kondratovics, R.; Kondratovics, U. Introduction and breeding of Rhododendron in Latvia. Proc. Latv. Acad. Sci. Sect. B 2017, 71, 248–252. [Google Scholar] [CrossRef]
- Choudhary, S.; Thakur, S.; Majeed, A.; Bhardwaj, P. Adaptability of Rhododendrons in high altitude habitats. J. For. Res. 2021, 32, 449–460. [Google Scholar] [CrossRef]
- Biswas, S.; Mukhopadhyay, M. Adaptation Strategies of Rhododendron Under Environmental Stress. In Plant Metabolites Under Environmental Stress, 3rd ed.; Desai, N.M., Patil, M., Pawar, U.R., Eds.; Apple Academic Press: New York, NY, USA, 2023; pp. 45–60. [Google Scholar]
- Prakash, D.; Upadhyay, G.; Singh, B.; Dhakarey, R.; Kumar, S.; Singh, K. Free-radical scavenging activities of Himalayan rhododendrons. Cur. Sci. 2007, 92, 526–531. [Google Scholar]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan Dalya Tea, a New “Old” Probable Adaptogenic Drug: Metabolic Characterization and Bioactivity Potentials of Rhododendronadamsii Leaves. Antioxidants 2021, 10, 863. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yan, H.M.; Zhu, Y.X.; Zhang, H.P.; Chai, L.S.; Li, L.; Wang, X.J.; Liu, Y.B.; Li, Y. New lignans, sesquiterpenes and other constituents from twigs and leaves of Rhododendron micranthum. Fitoterapia 2019, 135, 15–21. [Google Scholar] [CrossRef]
- Liu, J.Y.; Guo, P.J.; Wang, X.L.; Chen, H.M.; Chen, L.J.; Sang, Y.L.; Hao, Y.J.; Lu, J. Study on phytochemical and pharmacological activities of four Rhododendron plants endemic to Northeast China. J. Agric. Food Res. 2022, 7, 100255. [Google Scholar] [CrossRef]
- Hussain, A.; Azam, S.; Maqsood, R.; Anwar, R.; Akash, M.S.H.; Hussain, H.; Wang, D.; Imran, M.; Kotwica-Mojzych, K.; Khan, S.; et al. Chemistry, biosynthesis, and theranostics of antioxidant flavonoids and polyphenolics of genus Rhododendron: An overview. Naunyn-Schmiedeb. Arch. Pharmacol. 2025, 398, 1171–1214. [Google Scholar] [CrossRef] [PubMed]
- Chernonosov, A.A.; Karpova, E.A.; Karakulov, A.V. Metabolomic profiling of three Rhododendron species from Eastern Siberia by liquid chromatography with high-resolution mass spectrometry. S. Afr. J. Bot. 2023, 157, 622–634. [Google Scholar] [CrossRef]
- Vengryte, M.; Raudone, L. Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential. of Labrador Tea Species in the Northern Hemisphere. Plants 2024, 13, 901. [Google Scholar] [CrossRef]
- Mok, S.Y.; Lee, S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase. Food Chem. 2013, 136, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Sendri, N.; Swati, K.; Devidas, S.B.; Bhandari, P. Identification and Quantification of Anthocyanins, Flavonoids, and Phenolic Acids in Flowers of Rhododendron arboreum and Evaluation of Their Antioxidant Potential. J. Sep. Sci. 2022, 45, 2555–2565. [Google Scholar] [CrossRef]
- Łyko, L.; Olech, M.; Nowak, R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules 2022, 27, 827. [Google Scholar] [CrossRef]
- Takahashi, H.; Hirata, S.; Minami, H.; Fukuyama, Y. Triterpene and flavanon glycoside from Rhododendron simsii. Phytochemistry 2001, 56, 875–879. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, H.X.; Wang, C.M. Isolation, modification and cytotoxic evaluation of flavonoids from. J. Pharm. Pharmacol. 2012, 1, 1785–1792. [Google Scholar] [CrossRef]
- Lamichhane, J.; Bhattarai, K.; Shrivastava, A.K.; Shrestha, T.M.; Jain, S.C. Chemical constituents of Rhododendron lepidotum from Nepal Himalayas. Chem. Nat. Compd. 2014, 50, 767–769. [Google Scholar] [CrossRef]
- Rateb, M.E.; Hassan, H.M.; Arafa, E.-S.A.; Jaspars, M.; Ebel, R. Decorosides A and B, cytotoxic flavonoid glycosides from the leaves of Rhododendron decorum. Nat. Prod. Commun. 2014, 9, 11–14. [Google Scholar] [CrossRef]
- Grimbs, A.; Shrestha, A.; Rezk, A.S.D.; Grimbs, S.; Hakeem Said, I.; Schepker, H.; Hütt, M.T.; Albach, D.C.; Brix, K.; Kuhnert, N.; et al. Bioactivity in rhododendron: A systemic analysis of antimicrobial and cytotoxic activities and their phylogenetic and phytochemical origins. Front. Plant. Sci. 2017, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, J.; Chen, Z. Effects of total flavone from Rhododendron simsii Planch. flower on postischemic cardiac dysfunction and cardiac remodeling in rats. Evid.-Based Integr. Med. 2017, 2017, 5389272. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Tsai, S.J.; Wang, C.M.; Jhan, Y.L.; Ho, C.T.; Chou, C.H. Cinnamtannin D1 from Rhododendron formosanum Induces Autophagy via the Inhibition of Akt/mTOR and Activation of ERK1/2 in Non-Small-Cell Lung Carcinoma Cells. J. Agric. Food Chem. 2015, 63, 10407–10417. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Szopa, A.; Ekiert, H.M.; Luczkiewicz, M. Bioreactor-Grown Shoot Cultures for the Secondary Metabolite Production. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 187–247. [Google Scholar]
- Eeckhaut, T.; Janssens, K.; De Keyser, Е.; De Riek, J. Micropropagation of Rhododendron. In Protocols for In Vitro Propagation of Ornamental Plants. Methods in Molecular Biology; Jain, S., Ochatt, S., Eds.; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ. Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Molkanova, O.; Shirnina, I.; Mitrofanova, I. Conservation and micropropagation of rare and endemic species in genepool collections of the Russian Federation. J. Biotechnol. 2018, 280, S83–S88. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Zhang, C.; Wang, Z. In vitro shoot culture of Rhododendron fortunei: An important plant for bioactive phytochemicals. Ind. Crops Prod. 2018, 126, 459–465. [Google Scholar] [CrossRef]
- Novikova, T.I.; Asbaganov, S.V.; Ambros, E.V.; Zaytseva, Y.G. TDZ-induced axillary shoot proliferation of Rhododendron mucronulatumTurcz and assessment of clonal fidelity using DNA-based markers and flow cytometry. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 307–317. [Google Scholar] [CrossRef]
- Zaytseva, Y.; Petruk, A.; Novikova, T. Thidiazuron and LED Lighting Enhance Taxifolin and Rutin Production in Rhododendron mucronulatum Turcz. Microshoot Cult. J. Plant Growth Regul. 2023, 42, 2933–2942. [Google Scholar] [CrossRef]
- Elmongy, M.S.; Wang, X.; Zhou, H.; Xia, Y. Humic acid and auxins induced metabolic changes and differential gene expression during adventitious root development in Azalea microshoots. Hortic. Sci. 2020, 55, 926–935. [Google Scholar] [CrossRef]
- Sazhina, N.N.; Antipova, A.S.; Semenova, M.G.; Palmina, N.P. Initiated Oxidation of Phosphatidylcholine Liposomes with Some Functional Nutraceuticals. Russ. J. Bioorg. Chem. 2019, 45, 34–41. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Ma, Q.; Jia, X.; Zhang, M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020, 284, 110065. [Google Scholar] [CrossRef]
- Van Beek, T.A. Chemical analysis of Gingo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–35. [Google Scholar] [CrossRef]
- Yuriev, D.V.; Eller, K.I.; Arzamascev, A.P. Analysis of flavonol glycosides in preparations and dietary supplements based on Gingo biloba extract. Farmaciya 2003, 2, 7–9. [Google Scholar]
- Volkov, V.A.; Misin, V.M. Kinetics of reactions of antioxidants from some food and medicinal plants with the stable radical 2,2-diphenyl-1-picrylhydrazyl. Kinet. Catal. 2015, 56, 43–48. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Biney, E.; Nkoom, M.; Darkwah, W.K.; Puplampu, J. High-Performance Liquid Chromatography Analysis and Antioxidant Activities of Extract of Azadirachta Indica (Neem) Leaves. Pharmacogn. Res. 2021, 12, 29–34. [Google Scholar] [CrossRef]
- Faroux, J.M.; Ureta, M.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. An overview of peroxidation reactions using liposomes as model systems and analytical methods as monitoring tools. Colloids Surf. B Biointerfaces 2020, 195, 111254. [Google Scholar] [CrossRef]
- Duche, G.; Sanderson, J.M. The Chemical Reactivity of Membrane Lipids. Chem. Rev. 2024, 124, 3284–3330. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Dinkova-Kostova, A.T.; György, Z.; Mirmazloum, I.; Aneva, I.Y.; Georgiev, M.I. Rhodiola rosea L.: From golden root to green cell factories. Phytochem. Rev. 2016, 15, 515–536. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Granica, S.; Klimek-Szczykutowicz, M.; Kubica, P.; Warzecha, A.; Jafernik, K.; Ekiert, H. Schisandra rubriflora Plant Material and in Vitro Microshoot Cultures as Rich Sources of Natural Phenolic Antioxidants. Antioxidants 2020, 9, 488. [Google Scholar] [CrossRef]
- Singh, P.R.; Singh, L.J. In vitro propagation for improvement of medicinal plants: A review. J. Pharmacogn. Phytochem. 2021, 10, 1484–1489. [Google Scholar]
- Arora, K.; Rai, M.K.; Sharma, A.K. Tissue Culture Mediated Biotechnological Interventions in Medicinal Trees: Recent Progress. Plant Cell Tissue Organ Cult. 2022, 150, 267–287. [Google Scholar] [CrossRef]
- Duta-Cornescu, G.; Constantin, N.; Pojoga, D.-M.; Nicuta, D.; Simon-Gruita, A. Somaclonal variation—Advantage or disadvantage in micropropagation of the medicinal plants. Int. J. Mol. Sci. 2023, 24, 838. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Debnath, S.C.; Arigundam, U. In Vitro Propagation Strategies of Medicinally Important Berry Crop, Lingonberry (Vaccinium vitis-idaea L.). Agronomy 2020, 10, 744. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Gerald, L.T.S.; da Silva, J.A.T. Micropropagation in the Twenty-First Century. In Micropropagation in the Twenty-First Century, in Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 17–46. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Shen, J.-R.; Allakhverdiev, S.I. Natural and artificial photosynthesis: Fundamentals, progress, and challenges. Photosynth. Res. 2022, 154, 229–231. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, J.; Yang, H.; Xu, L.; Wang, Q.; Sack, L.; Wu, X.; Hou, J.; He, N. Variation in Leaf Chlorophyll Concentration from Tropical to Cold-Temperate Forests: Association with Gross Primary Productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Kono, M.; Terashima, I. Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J. Photochem. Photobiol. B Biol. 2014, 137, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Zheng, B. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.V.; Nosov, A.V.; Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Kulichenko, I.E.; Nosov, A.M. Advanced biotechnologies: Collections of plant cell cultures as a basis for development and production of medicinal preparations. Russ. J. Plant Physiol. 2021, 68, 385–400. [Google Scholar] [CrossRef]
- Nechaeva, T.L.; Nikolaeva, T.N.; Zagoskina, N.V. Salicylic and hydroxybenzoic acids affect the accumulation of phenolic compounds in tea-plant cultures in vitro. Biol. Bull. 2020, 47, 374–380. [Google Scholar] [CrossRef]
- Ricco, M.; Bari, M.; Catalano, A.; López, P.; Dobrecky, C.; Teves, S.; Posadaz, A.; Becher, M.; Ricco, R.; Wagner, M.; et al. Dynamics of polyphenol biosynthesis by calli cultures, suspension cultures and wild specimens of the medicinal plant Ligariacuneifolia (Ruiz & Pav.) Tiegh. (Loranthaceae). Analysis of their biological activity. Plants 2021, 10, 1713. [Google Scholar] [CrossRef]
- Zaprometov, M.N.; Nikolaeva, T.N. Chloroplasts Isolated from Kidney Bean Leaves Are Capable of Phenolic Compound Biosynthesis. Russ. J. Plant Physiol. 2003, 50, 623–626. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Sroka, Z. Antioxidative and antiradical properties of plant phenolics. Z. Naturforsch. C J. Biosci. 2005, 60, 833–843. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Wianowska, D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures—Considerations on Myricetin, Kaempferol and Quercetin. Int. J. Mol. Sci. 2023, 24, 10070. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V.; Skibsted, L.H. pH dependent antioxidant activity of lettuce (L. sativa) and synergism with added phenolic antioxidants. Food Chem. 2016, 190, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.C. A revised tissue culture medium for shoot multiplication of Rhododendron. J. Am. Soc. Hortic. Sci. 1984, 109, 343–347. [Google Scholar] [CrossRef]
- Tomilova, S.V.; Kochkin, D.V.; Tyurina, T.M.; Glagoleva, E.S.; Labunskaya, E.A.; Galishev, B.A.; Nosov, A.M. Growth and biosynthetic profiles of callus and suspension cell cultures of two rare foxglove species, Digitalis grandiflora Mill. and D. ciliate Trautv. Plant Cell Tissue Organ Cult. 2022, 149, 213–224. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Pryadekhina, E.V.; Lapshin, P.V.; Yur’eva, N.O.; Goldenkova-Pavlova, I.V. Morphophysiological and biochemical characteristics of potato plants with various expression rates of the Δ12 acyl-lipiddesaturase gene. Biol. Bull. 2014, 41, 126–132. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophyll a and b of leaf extract in different solvent. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for determining the total content of phenolic compounds in plant extracts with Folin–Denis reagent and Folin–Ciocalteu reagent: Modification and comparison. Russ. J. Bioorg. Chem. 2022, 48, 1519–1525. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Ossipov, V.; Zubova, M.; Nechaeva, T.; Zagoskina, N.; Salminen, J.P. The regulating effect of light on the content of flavan-3-ols and derivatives of hydroxybenzoic acids in the callus culture of the tea plant, Camellia sinensis L. Biochem. Syst. Ecol. 2022, 101, 104383. [Google Scholar] [CrossRef]
- Karpova, E.A.; Kostikova, V.A.; Khramova, E.P.; Shaldaeva, T.M.; Vasil’eva, О.Y.; Mazurkova, N.A.; Filippova, E.I.; Mazurkov, O.Y.; Makarevich, Е.V. Roots of Rosa majalis Herrm. as a source of antioxidants and anti-influenza agents. Rend. Fis. Acc. Lincei 2024, 35, 97–108. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thomas, A.H.; Catalá, Á.; Vignoni, M. Soybean phosphatidylcholine liposomes as model membranes to study lipid peroxidation photoinduced by pterin. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 139–145. [Google Scholar] [CrossRef] [PubMed]

| Species | Shoot Fresh Weight, mg | Water Content, % | Shoot Length, mm | Number of Internodes | Branching Frequency, % |
|---|---|---|---|---|---|
| R. smirnowii | 7.1 ± 0.5 b | 92.3 ± 0.2 b | 16.3 ± 1.1 c | 4.3 ± 0.1 b | - |
| R. PJM Elite | 13.1 ± 1.8 a | 93.1 ± 0.7 a | 31.1 ± 1.5 a | 9.2 ± 0.3 a | 3.1 ± 0.5 b |
| R. japonicum | 13.5 ± 1.9 a | 93.4 ± 0.5 a | 27.7 ± 1.2 b | 8.9 ± 0.5 a | 25.0 ± 0.7 a |
| Species | Chlorophyll | |||
|---|---|---|---|---|
| a | b | a + b | a/b Ratio | |
| R. smirnowii | 0.25 ± 0.011 b | 0.11 ± 0.004 b | 0.36 ± 0.018 b | 2.2 ± 0.098 a |
| R. PJM Elite | 0.35 ± 0.022 a | 0.19 ± 0.007 a | 0.54 ± 0.028 a | 1.8 ± 0.065 b |
| R. japonicum | 0.15 ± 0.006 c | 0.08 ± 0.003 c | 0.23 ± 0.012 c | 1.8 ± 0.061 b |
| Species | I, % | IC50, μgdw/mL | ARATr, μmolTr/mgdw |
|---|---|---|---|
| R. smirnowii | 38.9 ± 1.7 c | 41.6 ± 3.7 a | 0.474 ± 0.024 c |
| R. PJM Elite | 44.5 ± 2.2 b | 37.3 ± 2.6 b | 0.548 ± 0.027 b |
| R. japonicum | 61.6 ± 3.1 a | 28.8 ± 1.8 c | 0.665 ± 0.039 a |
| Species | τ(C), y = anx + b, | AOATr ± SD, μmolTr/mgdw |
|---|---|---|
| R. smirnowii | y = 1933.3x + 4.6; R2 = 0.998 | 0.179 ± 0.011 |
| R. PJM Elite | y = 1322.2x + 5.7; R2 = 0.993 | 0.155 ± 0.014 |
| R. japonicum | y = 2160.6x + 4.8; R2 = 0.997 | 0.206 ± 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanskaya, V.M.; Vasilyeva, O.G.; Khramova, E.P.; Sazhina, N.N.; Goncharuk, E.A.; Nechaeva, T.L.; Zubova, M.Y.; Aksenova, M.A.; Lapshin, P.V.; Zagoskina, N.V. Rhododendron Microshoot Culture as a Source of Phenolic Antioxidants for Biomedicine. Molecules 2025, 30, 2949. https://doi.org/10.3390/molecules30142949
Katanskaya VM, Vasilyeva OG, Khramova EP, Sazhina NN, Goncharuk EA, Nechaeva TL, Zubova MY, Aksenova MA, Lapshin PV, Zagoskina NV. Rhododendron Microshoot Culture as a Source of Phenolic Antioxidants for Biomedicine. Molecules. 2025; 30(14):2949. https://doi.org/10.3390/molecules30142949
Chicago/Turabian StyleKatanskaya, Vera M., Olga G. Vasilyeva, Elena P. Khramova, Natalia N. Sazhina, Evgenia A. Goncharuk, Tatiana L. Nechaeva, Maria Y. Zubova, Maria A. Aksenova, Petr V. Lapshin, and Natalia V. Zagoskina. 2025. "Rhododendron Microshoot Culture as a Source of Phenolic Antioxidants for Biomedicine" Molecules 30, no. 14: 2949. https://doi.org/10.3390/molecules30142949
APA StyleKatanskaya, V. M., Vasilyeva, O. G., Khramova, E. P., Sazhina, N. N., Goncharuk, E. A., Nechaeva, T. L., Zubova, M. Y., Aksenova, M. A., Lapshin, P. V., & Zagoskina, N. V. (2025). Rhododendron Microshoot Culture as a Source of Phenolic Antioxidants for Biomedicine. Molecules, 30(14), 2949. https://doi.org/10.3390/molecules30142949







