Abstract
This study aimed to investigate the phytochemical constituents of C. odorata leaves and stems and to evaluate their antioxidant, total phenol, α-glucosidase, and antibacterial activities. Furthermore, liquid chromatography-high-resolution mass spectrometry (LC–HRMS)-based metabolite profiling combined with principal component analysis (PCA) was applied to correlate metabolite composition with functional activities, providing comprehensive insights into the metabolomic diversity and bioactive differentiation between plant parts. The plant materials were extracted using 70% and 100% ethanol for 24 h. The leaf extract of ethanol 70% (EtOH 70) exhibited the highest antioxidant activity (IC50 of 223.33 ± 9.20 µg/mL) and total phenolic content (113.15 mg GAE/g), while the stem EtOH 70% extract showed the strongest antidiabetic activity through α-glucosidase inhibitory activity (78.57%). Although appearing less potent, all extracts showed dose-dependent inhibitory activity, such as Staphylococcus aureus (highest value at 9.31 mm), Escherichia coli (highest value at 9.92 mm), and Salmonella typhimurium (highest value at 9.00 mm). Comparing the plant parts, leaf extracts generally showed more potent activity than stem extracts, particularly evident against E. coli (e.g., Leaf EtOH 70% at 5 mg/mL: 9.92 mm vs. Stem EtOH 70%: 7.97 mm). LC-HRMS analysis revealed the presence of phenolics, flavonoids, amino acids, organic acids, and alkaloids. Furthermore, the result indicates that C. odorata is a rich source of bioactive compounds with significant antioxidant, α-glucosidase inhibitory, and antibacterial potency. The findings advance existing knowledge beyond earlier phytochemical or single-activity studies, offering a more holistic understanding of C. odorata’s therapeutic potential and its relevance for natural product development.
1. Introduction
Chromolaena odorata, commonly known as devil weed, is a highly invasive plant that poses major ecological and agricultural challenges in tropical regions. Its rapid spread suppresses native flora, alters soil microbial communities, and reduces crop productivity through strong allelopathic effects that inhibit the germination and growth of tropical crops such as mungbean, chilli, and common beans [1,2]. The invasion of C. odorata disrupts ecosystem balance by altering soil nutrient dynamics and biodiversity, posing difficulties for sustainable land management [3,4]. However, its biomass has been used for soil fertility restoration, suggesting that appropriate management strategies could balance its ecological threats with potential agronomic benefits [5].
Despite its invasive nature, C. odorata is extensively utilized in traditional medicine systems such as Ayurveda, Siddha, and Unani for treating various ailments, including wounds, fever, malaria, and stomach disorders [4,6,7,8]. The leaves of C. odorata are commonly crushed to extract juice, which is applied to skin wounds for its haemostatic and anti-inflammatory properties [7,9]. Its rich phytochemical composition, including flavonoids, terpenoids, and phenolic compounds, accounts for its reported antimicrobial, anti-inflammatory, and antioxidant activities [8,10]. In several regions of West Africa, the plant is also valued for improving soil fertility and as a fallow shrub in slash-and-burn agriculture [4,6].
While antioxidant and antidiabetic activities of C. odorata have been previously reported, most studies have focused solely on the leaves [11,12,13], often with limited metabolite characterization. There remains a lack of comprehensive comparative analyses of different plant parts and their bioactivities. Therefore, this study aims to evaluate the phytochemical constituents and compare the antioxidant, antidiabetic, and antibacterial properties of the stem and leaf extracts of C. odorata. By integrating LC-HRMS-based global metabolite profiling and PCA, this work provides novel insights into the metabolomic diversity and bioactive differentiation between plant parts. The findings advance existing knowledge beyond earlier phytochemical or single-activity studies, offering a more holistic understanding of C. odorata’s therapeutic potential and its relevance for natural product development.
2. Results and Discussion
2.1. Yield of Extraction
Several factors, such as environmental conditions, harvest timing, and biomass composition, influence the yield of extraction from biomass. Biomass yield is significantly affected by precipitation, particularly during the growing season. For instance, May precipitation was found to increase biomass yield, while rainfall in other months had no effect [14]. The timing of biomass harvest plays a crucial role in yield. Harvesting in late summer to early fall or late spring is recommended to maximize yield and minimize moisture content, while winter harvests tend to yield the lowest biomass [15]. The type of biomass, such as switchgrass or native polyculture, and the presence of specific plant species (e.g., forbs) can influence yield. For example, forb cover was a better predictor of biomass yield than warm-season grass in some locations [14]. The presence of extractives in biomass affects its fuel properties. Extractive-free samples showed a significant decrease in ash content, fixed carbon content, and calorific value, indicating that unextracted biomass has better fuel properties [16].
In this study (Table 1), the extraction of C. odorata leaves using 70% ethanol had the highest yield of 15.83%, followed by the extraction of C. odorata stems using 70% ethanol at 13.53%. The greater yield observed with 70% ethanol compared to 100% ethanol can be attributed to several factors, such as azeotropic behavior and solvent properties. The unique interaction between ethanol and water at specific concentrations can enhance process efficiency [17]. The presence of water in ethanol can alter the solvent properties, such as polarity and viscosity, which can affect the solubility and reactivity of substances [18]. This suggests that a mixture of ethanol and water (70% ethanol) might have more favorable solvent properties compared to pure ethanol. Furthermore, water can improve the solubility of substrates and intermediates, facilitating better conversion rates [19].

Table 1.
The extraction yield of C. odorata leaf and stem by the maceration method using ethanol 70% and ethanol 100%.
2.2. Antioxidant Activity
The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay is a widely used method to evaluate the antioxidant activity of various compounds and extracts. This method is based on the reduction in the DPPH radical, which changes color from violet to yellow as an antioxidant neutralizes it [20]. The assay helps in understanding the relationship between the chemical structure of compounds and their antioxidant activity. The DPPH assay is straightforward and does not require complex instrumentation. It can be adapted for various types of samples, including plant extracts, food products, and synthetic compounds [21]. The assay may not distinguish between different antioxidant mechanisms, such as electron transfer and hydrogen atom transfer [22].
Table 2 shows the antioxidant activity of C. odorata leaf and stem extracts using the DPPH test. The results obtained showed that the 70% EtOH leaf extract had the highest antioxidant activity, with an IC50 value of 223.33 µg/mL, followed by the 100% EtOH leaf extract at 458.99 µg/mL. Meanwhile, C. odorata stem extract had lower antioxidant activity than the leaf extract because its IC50 value was greater than 1500 µg/mL. In addition, ascorbic acid as positive control had IC50 value of 14.31 µg/mL. Several studies have shown that the IC50 value of antioxidants using the DPPH method of several extracts has very good antioxidant activity, such as the chloroform extract of C. odorata leaves was found to be 310 µg/mL [12], the methanol root extract was reported as 191.68 µg/mL [23], while the n-butanol fraction of C. odorata leaves had an IC50 value of 33.54 µg/mL [24]. Differences in IC50 values from the literature are likely to be due to differences in solvents and extraction methods used [25]. Studies also show that plant origin and harvest conditions can also influence the results of biological activity tests on a plant [26]. While specific data on the DPPH activity of C. odorata stem extracts is limited, studies on similar plant parts suggest that the stems also possess significant antioxidant properties due to the presence of bioactive compounds such as phenolics and flavonoids [27]. The high antioxidant activity of C. odorata extracts is attributed to their rich phenolic and flavonoid content. These compounds are known for their ability to donate hydrogen atoms or electrons, neutralizing free radicals and preventing oxidative stress [28]. The presence of specific flavonoids such as odoratenin, isosakuranetin, and subscandenin in the methanol extract of C. odorata leaves further enhances its antioxidant potential [29].

Table 2.
Antioxidant activity of C. odorata extracts assessed via DPPH assay.
The beta-carotene bleaching assay is a widely used method for evaluating the antioxidant activity of various substances. This assay measures the ability of antioxidants to prevent the oxidative degradation of beta-carotene in the presence of peroxyl radicals [30]. The assay involves spectrophotometric measurement of beta-carotene concentration changes in a beta-carotene/peroxyl radical system, both with and without the antioxidant. The assay applies to natural extracts, food samples, and commercial antioxidants, providing robust criteria for comparing antioxidant and prooxidant activities [31].
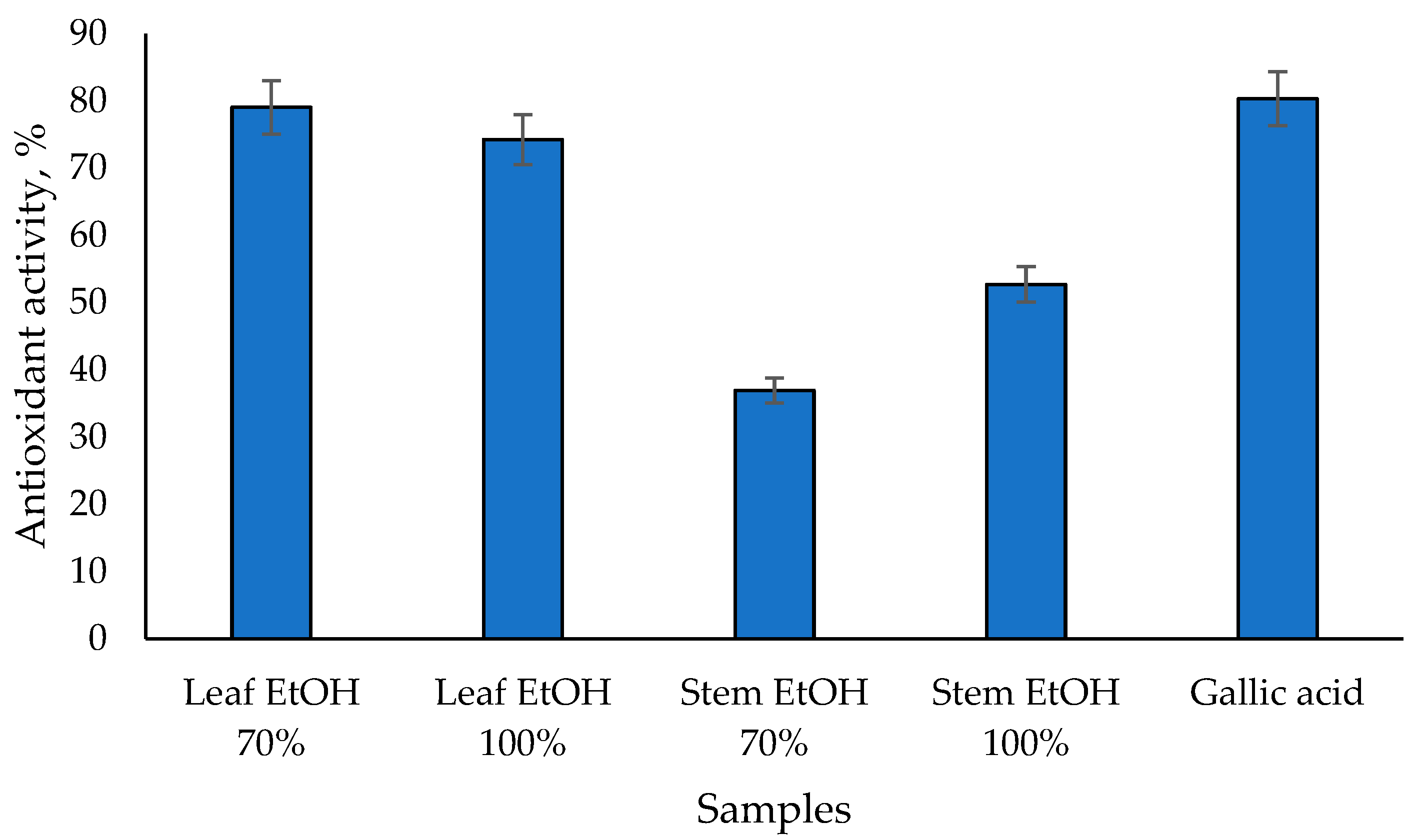
The results of the antioxidant activity test of C. odorata leaf and stem extracts using the beta carotene bleaching assay are shown in Figure 1. The 70% EtOH leaf extract has the highest antioxidant activity of 79.05%, followed by the 100% EtOH leaf extract at 74.26%. 100% EtOH and 70% EtOH stem extracts have lower antioxidant activities of 52.72% and 36.96%, respectively. On the other hand, gallic acid as a positive control has the highest value of 80.33%. These results are linear with the antioxidant activity test using the DPPH assay, in which C. odorata leaf extract has a higher antioxidant activity than its stem extract. A previous study showed that the ethanol extract of C. odorata leaves demonstrated potent antioxidant activity using the beta-carotene bleaching assay, with an IC50 value of ≤50 µg/mL [32]. Meanwhile, the ethanol extract of C. odorata stems showed vigorous antioxidant activity with an IC50 value ranging from 50 to 100 µg/mL [32]. The antioxidant activity observed in the beta-carotene bleaching assay is likely due to the high content of phenolics and flavonoids in the extracts. The total phenolic content and total flavonoid content of C. odorata extracts varied significantly, with the leaf extracts showing higher values compared to the stem extracts [32].
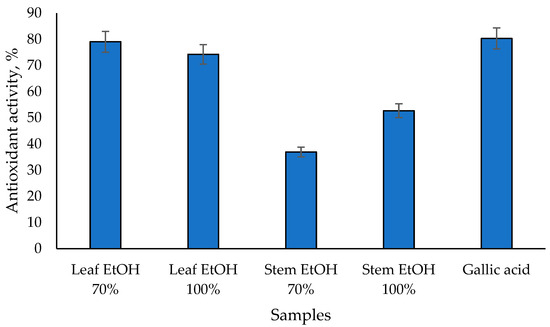
Figure 1.
Antioxidant activity of C. odorata extracts assessed via β-carotene bleaching assay at 1000 µg/mL.
Bioactive compounds such as rutin and coumarin are also observed from the LC-HRMS analysis of C. odorata (Table 3). Rutin has been shown to have potent free radical scavenging effects in multiple in vitro assays, including DPPH free radical scavenging, lipid peroxidation, and reducing power assays [33]; meanwhile, coumarins exhibit antioxidant activity primarily through free radical scavenging. Both compounds contribute to various health benefits through their antioxidant mechanisms, making them valuable in medicinal and therapeutic applications.

Table 3.
The constituents of C. odorata leaf & stem were analyzed using LC-HRMS analysis.
2.3. α-Glucosidase Inhibitory Activity
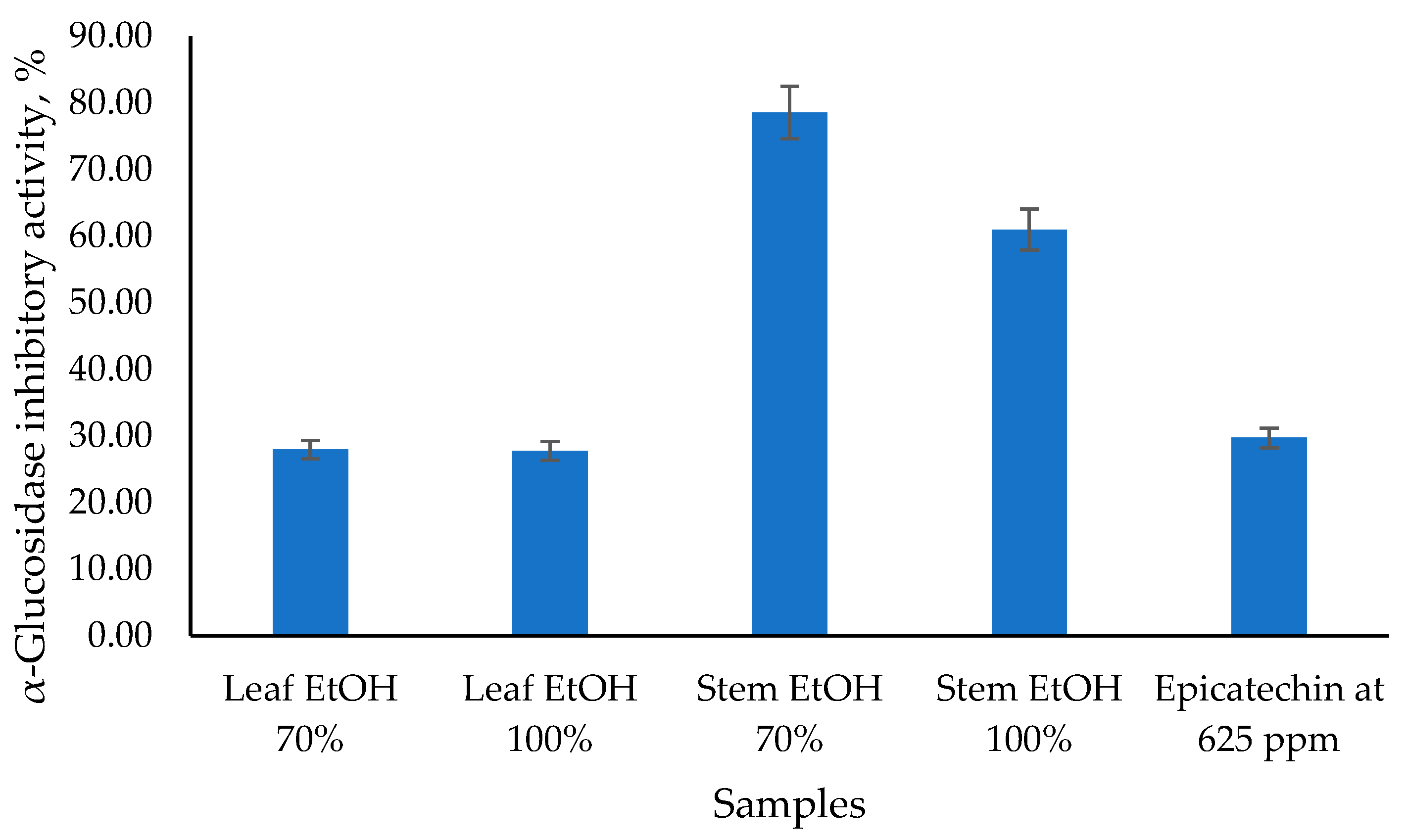
The antidiabetic potential of C. odorata has been explored through its α-glucosidase inhibitory activity, as presented in Figure 2, which is a key mechanism in managing diabetes by slowing down carbohydrate digestion and glucose absorption. The results obtained showed that the 70% EtOH stem extract had the highest α-glucosidase inhibitory activity value of 78.57%, followed by the 100% EtOH stem extract at 60.98%. Meanwhile, 70% EtOH and 100% EtOH leaf extracts had similar α-glucosidase inhibitory activity values of 27.97% and 27.77%, respectively. Epicatechin (EC), as a standard, has α-glucosidase inhibitory activity values of 29.74%. Epicatechin has been identified as an α-glucosidase inhibitor, which can help manage postprandial hyperglycemia by reducing the breakdown of carbohydrates into glucose. EC acts as a mixed-type inhibitor, meaning it can bind to both the enzyme and the enzyme-substrate complex, altering the enzyme’s activity [34]. This type of inhibition is beneficial as it can provide a more comprehensive reduction in enzyme activity. Epicatechin (EC), as a standard, has α-glucosidase inhibitory activity values of 29.74%.
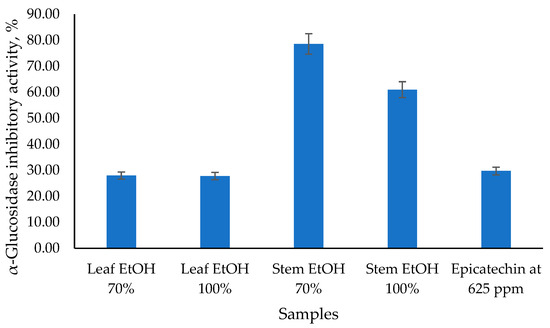
Figure 2.
The α-glucosidase inhibitory activity of C. odorata leaf and stem using the α-glucosidase inhibitory assay at 1000 µg/mL.
Previous study showed that the methanolic leaf extract of C. odorata contains several flavonoids and phenolic acids. Among these, isosakuranetin exhibited significant α-glucosidase inhibitory activity with an IC50 of 55.99 ± 1.25 μg/mL. Other flavonoids such as 4-O-methylsakuranetin, odoratin, ombuin, and rhamnetin showed weaker inhibitory activities [35]. In diabetic-induced rats, the ethanolic extract of C. odorata leaves significantly reduced blood glucose levels and increased insulin levels. This suggests that the extract not only inhibits α-glucosidase but also promotes beta-cell regeneration in the pancreas [36].
Additionally, the extract upregulated the expression of genes involved in glucose metabolism and oxidative stress response, indicating a multifaceted approach to managing diabetes [13]. Another study showed that the ethyl acetate fraction of the flower extract demonstrated potent α-glucosidase inhibitory activity with an IC50 of 53.87 ± 0.42 μg/mL, which is comparable to the activity observed in leaf extracts [37]. The ethanolic extract of C. odorata leaves was found to upregulate the expression of genes involved in glucose metabolism (Glut2 and glucokinase) and oxidative stress response (Nrf2), while downregulating Keap1, which is a negative regulator of Nrf2. This indicates a multifaceted approach to managing diabetes, including enhancing glucose uptake and reducing oxidative stress [13].
From the LC-HRMS analysis, leaf and steam extract of C. odorata had rutin and coumarin (Table 3). These two compounds may play a role in the antidiabetic activity of C. odorata. Rutin helps regulate blood glucose levels by enhancing tissue glucose uptake, reducing carbohydrate absorption in the intestines, preserving islet cell function, and boosting insulin secretion [38]; meanwhile coumarin administration significantly reduces plasma glucose levels and glycosylated hemoglobin (HbA1c) in diabetic rats [39].
2.4. Antibacterial Activity
The disc diffusion assay revealed that both leaf and stem extracts of C. odorata, prepared using either 70% or 100% ethanol, exhibited measurable inhibitory activity against all three tested bacterial pathogens: S. aureus, E. coli, and S. typhimurium (Table 4). A clear concentration-dependent effect was consistently observed for nearly all active extracts, where the mean zone of inhibition decreased significantly (p < 0.05) as the extract concentration was reduced from 5 mg/mL to 1.25 mg/mL. For example, leaf extracts (both solvents) against S. aureus showed significant stepwise decreases (e.g., Leaf EtOH 70%: 9.31 mm → 8.05 mm → 7.23 mm). This trend highlights the dose-responsive nature of the antibacterial compounds present in the extracts, indicating that higher concentrations yield a greater antimicrobial effect. These results support previous studies that reported that devil weed has anti-pathogenic bacterial activity [40].

Table 4.
The antibacterial activity of C. odorata leaf and stem using the disc diffusion method.
While demonstrating activity, the potency of the C. odorata extracts was markedly lower than the positive control antibiotic amoxicillin (10 µg), which consistently produced large inhibition zones averaging around 30 mm. Comparing the plant parts, leaf extracts generally showed more potent activity than stem extracts, particularly evident against E. coli (e.g., Leaf EtOH 70% at 5 mg/mL: 9.92 mm vs. Stem EtOH 70%: 7.97 mm). The effect of solvent concentration was variable and bacteria-dependent; 100% EtOH sometimes yielded slightly larger zones (e.g., Leaf against S. aureus at 2.5 mg/mL: 8.94 mm vs. 8.05 mm for 70%). In comparison, 70% EtOH was sometimes more effective (e.g., Leaf against E. coli at 5 mg/mL: 9.92 mm vs. 9.02 mm for 100%). S. typhimurium appeared less susceptible overall, with many extracts showing minimal activity (~6 mm) at lower concentrations.
In line with the results of this study, previous research reported that C. odorata leaf extract effectively inhibited all strains of S. suis bacteria with an MIC of 3.9–62.5 mg/mL [41]. These results confirm the presence of antibacterial principles in C. odorata leaves and stems, validating its potential for further investigation, but highlight that its crude extracts are significantly less potent than conventional antibiotics like amoxicillin against these specific pathogens. Referring to the results of metabolite content evaluation, several compounds have antibacterial activity, including chlorinated-quinoline derivatives. Sambavekar et al. [42] synthesized quinoline derivatives that showed antibacterial activity against S. aureus (100 μg/mL) equivalent to the activity of ampicillin (10 μg/mL). In addition, chlorogenic acid also showed potent antibacterial activity. Chlorogenic acid (CGA) induces bacterial apoptosis-like death in E. coli by depleting intracellular reactive oxygen species (ROS), leading to membrane depolarization and DNA fragmentation [43]. Quinic acid also showed similar activity (MIC 80–120 μg/mL) [44,45]. In addition, flavonoid compounds (rutin, luteolin, and genistein) have also been reported to possess antibacterial activity with varying effectiveness [46,47]. However, further purification or fractionation of the extract appears to be necessary to obtain sufficient extract for good therapeutic effectiveness.
2.5. Total Phenolic Content
The Folin–Ciocalteu (FC) assay is a widely used method for determining the total phenolic content (TPC) in various samples, including plant extracts, foods, and beverages. This method is based on the reduction in the Folin–Ciocalteu reagent by phenolic compounds, resulting in a blue complex that can be measured spectrophotometrically. The FC reagent reacts with phenolic compounds in an alkaline medium to form a blue complex, which is measured at 765 nm [48]. Gallic acid is commonly used as a standard, and results are expressed as gallic acid equivalents (GAE) [49].
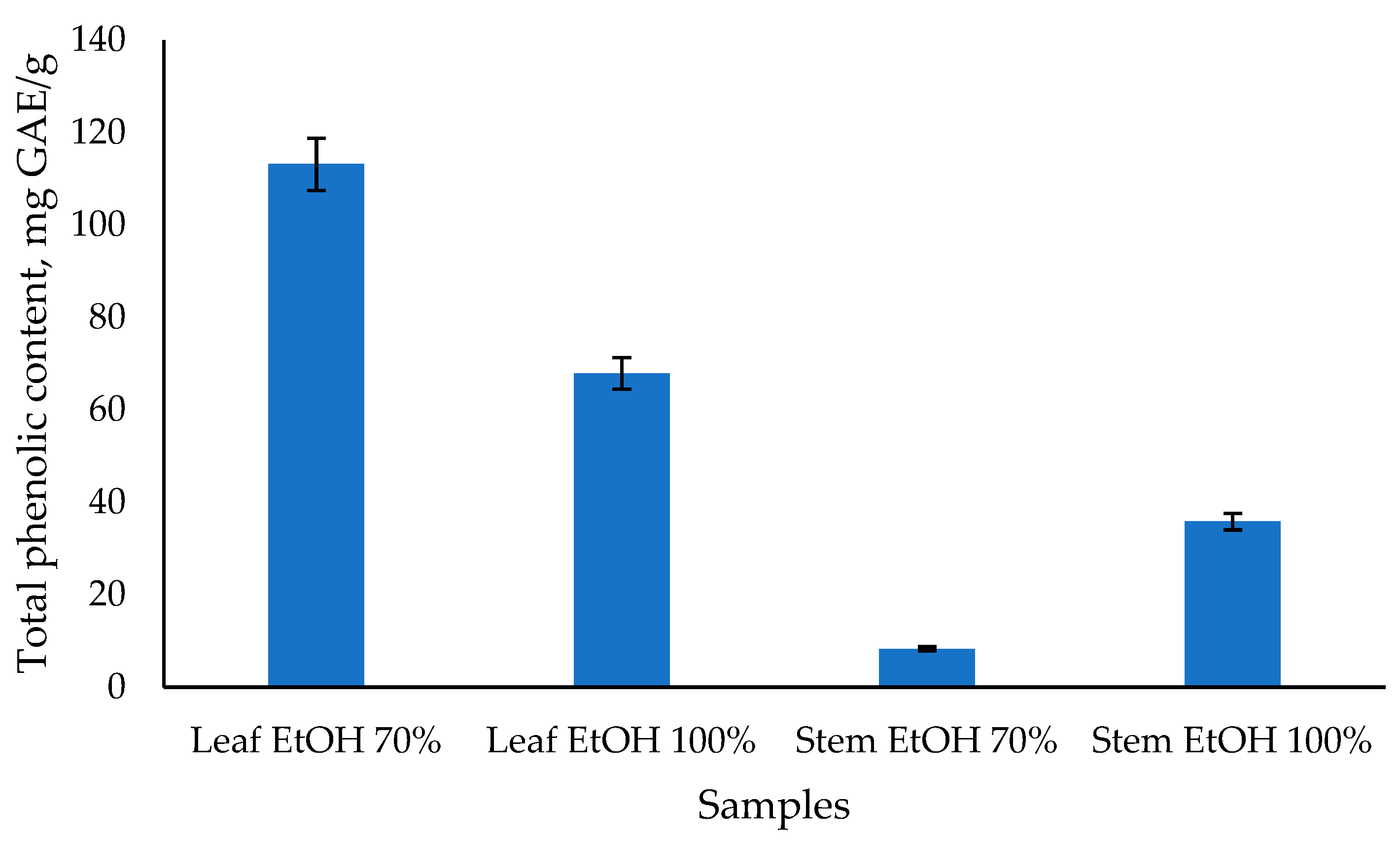
From Figure 3, it can be seen that the total phenolic content of C. odorata leaf extract with 70% EtOH has the highest value, followed by 100% EtOH leaf extract at 113.15 and 67.91 mg GAE/g, respectively. Meanwhile, the stem extract of C. odorata has a lower total phenolic content value of 35.85 and 8.33 mg GAE/g, respectively, using 100% EtOH and 70% EtOH solvents. Previous study reported that the highest TPC was found in the aqueous leaf extract of C. odorata after 24 h, with a value of 198.02 ± 3.96 mg GAE per gram of extract [50]. Another study reported the TPC of chloroform extract of C. odorata leaves to be 242.2 mg/g, which was significantly higher compared to the reference standard gallic acid [12]. The results of the TPC analysis are also linear with those of the antioxidant activity test, indicating that the C. odorata leaf extract has a higher value than the stem extract. Several studies found a strong positive correlation between total phenolic content and antioxidant capacity. For instance, one study reported significant correlations using various assays such as ABTS, CUPRAC, FRAP, and DPPH, with correlation coefficients ranging from 0.78 to 0.94 [51]. Another study found a high correlation (R2 = 0.814) between total phenolic content and antioxidant capacity measured by DPPH-RSA [52]. Overall, while there is a general trend that higher total phenolic content is associated with greater antioxidant capacity, the strength of this relationship can vary based on the specific plant material, extraction methods, and assays used. This indicates that while phenolics are major contributors to antioxidant activity, other factors and compounds may also play significant roles.
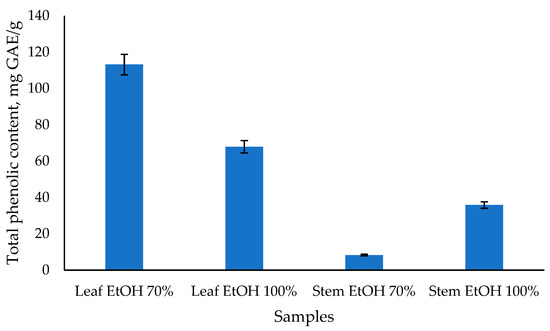
Figure 3.
The total phenolic content of C. odorata leaf and stem was determined using the Folin-Ciocalteau method.
2.6. FTIR Spectra
The leaf and stem extracts of C. odorata were subjected to FTIR analysis to identify the functional groups of compounds contained in the extracts. FTIR spectroscopy is a powerful analytical technique used to identify functional groups in plant extracts. The method relies on the absorption of infrared radiation by molecular vibrations, which provides a unique spectral fingerprint for different compounds. Moreover, FTIR can help in screening plant extracts for potential medicinal compounds [53].
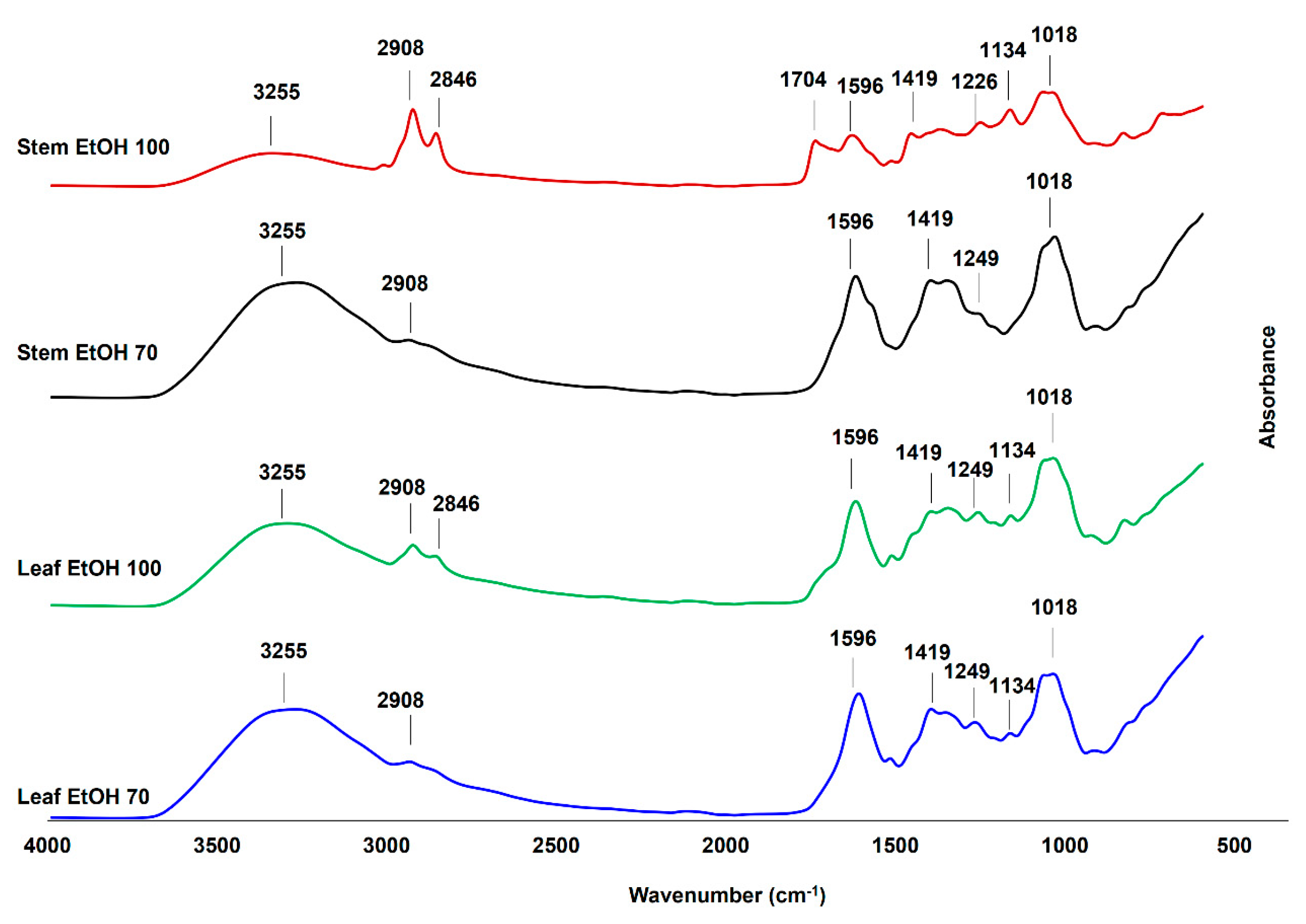
The FTIR spectra of C. odorata leaf and stem extracts obtained using 70% and 100% ethanol (Figure 4) revealed the presence of various functional groups, indicating a rich composition of bioactive compounds. Key absorption bands were observed in the region of 4000–600 cm−1, corresponding to different molecular vibrations and functional group characteristics. In this study, the identification of FTIR vibration from C. odorata stem and leaf extracts is based on previous studies [53,54]. All extracts exhibited a broad peak around 3255 cm−1, indicative of O–H stretching vibrations commonly associated with alcohols, phenols, and carboxylic acids. The peaks at 2908 cm−1 and 2846 cm−1 corresponded to the asymmetric and symmetric vibrations of aliphatic –CH, –CH2, and –CH3 groups. These bands were more prominent in the EtOH 100% extracts (especially in the stem), implying the presence of lipophilic compounds such as fatty acids and terpenoids, which were better extracted in absolute ethanol [55]. The absorption at 1704 cm−1 in the stem EtOH 100% extract suggested the presence of carbonyl groups (C=O), which could be from esters, aldehydes, ketones, or carboxylic acids. This peak was either weak or absent in other extracts, highlighting that 100% ethanol might be more effective in extracting such carbonyl-containing compounds from the stem. The bands at 1596 cm−1 and 1419 cm−1 were attributed to the vibration of aromatic ring C=C stretching and C–C skeletal vibrations, respectively. These could be characteristic of flavonoids and other aromatic compounds, which were consistently present across all extracts, indicating their abundance in both leaf and stem. The bands at 1134 cm−1 and 1018 cm−1, present in all extracts, were attributed to the C–O stretching vibration of polysaccharides or secondary alcohols. In addition, the bending vibration of aliphatic –CH, –CH2, and –CH3 was observed at around 1226 cm−1 and 1249 cm−1. Therefore, the FTIR analysis confirmed the presence of various functional groups in C. odorata extracts, including hydroxyl, carbonyl, aliphatic, and aromatic compounds that might be from phenolic compounds, organic acids, amino acids and flavonoid compounds. These results were consistent with the result of LC-HRMS metabolites analysis presented in Table 3. These findings support the phytochemical complexity of the plant and its potential pharmacological properties.
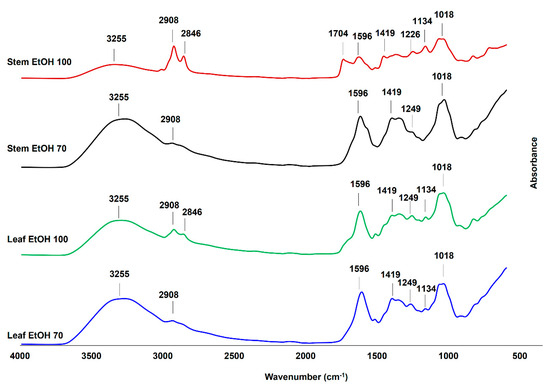
Figure 4.
The Fourier transform infrared (FTIR) spectra of leaf and stem extracts of C. odorata, extracted using 100% ethanol (EtOH 100) and 70% ethanol (EtOH 70), were measured at a wavenumber range of 4000–600 cm−1.
2.7. LC-HRMS and PCA
Table 3 summarizes major phytochemical constituents in C. odorata leaf and stem extracts. Various compounds, including primary metabolites (amino acids, nucleobases, sugars, etc.), secondary metabolites (phenolics, flavonoids, and terpenoids), and lipid/steroid derivatives, were identified in the extracts. Polar compounds such as amino acids, sugar, and sugar derivatives (e.g., glycosides) were extracted due to the relatively high polarity of extracting solvents (i.e., 70% and 100% ethanol). Rutin, a flavonoid glycoside, was more abundant in the 70% ethanolic lead extract, compared with other extracts. The compound has been reported to directly scavenge reactive oxygen species (ROS), inhibit lipid peroxidation, and boost endogenous antioxidants in cells and animal models [56,57]. Other detected flavonoid glycosides include 5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-bis[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-one and 5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 6-O-(6-deoxyhexopyranosyl)hexopyranoside.
Phenolic compounds, including flavonoids and coumarins, were also more abundant in the 70% ethanolic leaf extract. This finding is in agreement with results from TPC measurement, in which the leaf extract had the highest phenolic content (113 mg GAE/g extract). Chlorogenic acid and its isomer, neochlorogenic acid, are some antioxidant polyphenols detected in the extract, which are also often found in coffee and tea. Flavonoids detected in the C. odorata leaf and stem extracts include rutin, luteolin, isorhamnetin, genistein, hispidulin, padmatin, glycitein, and 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6-dimethoxy-4H-chromen-4-one. Many of these compounds have been reported to exhibit antioxidant and anti-inflammatory activity in vitro and in vivo [58,59,60,61]. Coumarin and its derivative, scrophulein, also exhibit several biological activities, including antioxidant, antimicrobial, anti-inflammatory, neuroprotective, antidiabetic, anticonvulsant, and antiproliferative properties [62]. The relatively high abundance of these phenolic compounds likely contributes to the DPPH radical scavenging activity of the 70% ethanolic leaf extract.
In addition to being excellent antioxidants, several phenolic compounds possess moderate to potent antimicrobial activities and can act synergistically with synthetic antibiotics or natural compounds to fight against microbes. Luteolin has been reported to inhibit several methicillin-resistant S. aureus (MRSA) isolates with an MIC of 62.5 µg/mL and fluoroquinolone-resistant Enterococcus with an MIC of 16 µg/mL [63,64]. A combination of luteolin with ampicillin, oxacillin, and gentamicin can reduce bacterial counts below the detectable limit after 24 h, when tested against MRSA [63]. Other phenolics, including rutin, isorhamnetin, genistein, and hispidulin, have also demonstrated moderate antibacterial activity (MIC > 100 µg/mL) and are more active against Gram-positive bacteria, such as S. aureus, MRSA, and Streptococcus [65,66,67,68]. The presence of these phenolic compounds in C. odorata extracts might explain the more potent antibacterial activity against S. aureus, as discussed in the previous section.
Several phytochemical constituents of C odorata leaf and stem extracts have previously shown antidiabetic activity. For example, trehalose has been studied in both animals and humans for the prevention and treatment of diabetes [69]. Daily intake of trehalose helped lower postprandial blood glucose levels, potentially due to improved insulin sensitivity and glucose tolerance. Clinical trials have shown that chlorogenic acid can reduce fasting blood glucose levels in individuals with impaired glucose tolerance [70]. Several studies using rodent models reported antidiabetic activity of rutin, luteolin, L-isoleucine, isorhamnetin, genistein, nootkatone, and coumarin derivatives. Rutin lowered glucose levels via multiple potential mechanisms, including the inhibition of carbohydrate-digesting enzymes and modulation of gut microbiota [71]. Similarly, luteolin also inhibits the enzymes (α-glucosidase and DPP-4) and enhances insulin secretion by protecting the pancreatic cells [72]. Improved insulin sensitivity and pancreatic β-cell survival were also found in studies using isorhamnetin, genistein, hispidulin, and nootkatone [73,74,75]. In contrast, L-isoleucine’s antidiabetic effect is independent of insulin. It decreases blood glucose by increasing glucose uptake by skeletal muscle [76].
C. odorata leaf and stem ethanolic extracts contain various phytochemicals that exert biological activities such as antioxidant, anti-inflammatory, antidiabetic, and antimicrobial. The abundance of the compounds was affected by the part of the plant and the composition of the extracting solvents (i.e., polar compounds and phenolics mainly were extracted from leaves using 70% ethanol). Understanding the relationship between extraction conditions, phytochemical constituents, and their biological activity can provide directions for phytochemical-targeted extraction for various medicinal applications.
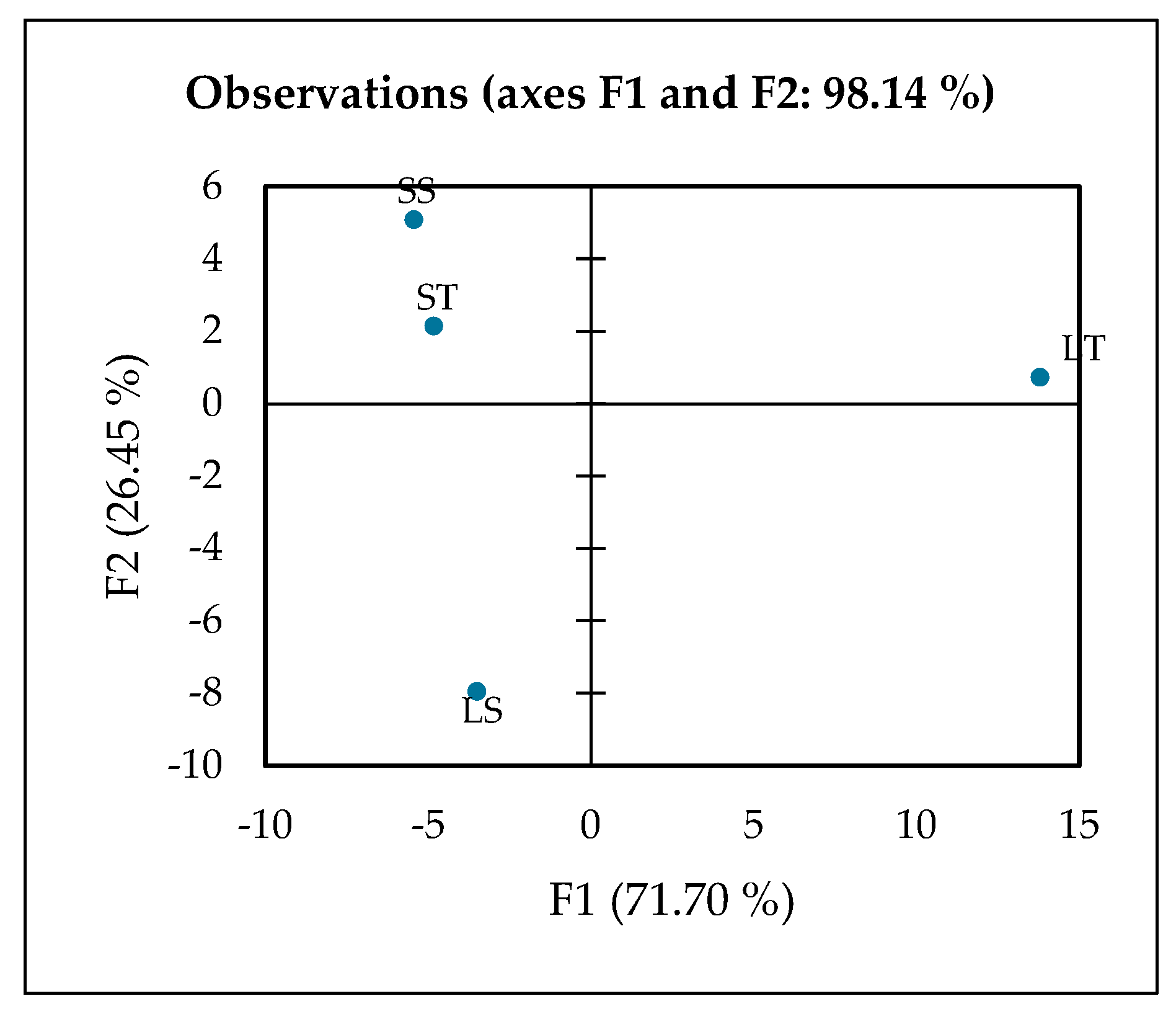
The contribution of extraction using 70% or 100% ethanol in leaf or stem samples was further evaluated using XLSTAT. XLSTAT is a practical tool for performing PCA, offering a user-friendly interface and comprehensive analysis capabilities. The first two principal components (F1 and F2) accounted for a cumulative variance of 98.14%, indicating that the two-dimensional PCA plot provides an almost complete representation of the overall variability within the dataset. The PCA revealed that F1 (71.70%) serves as the primary axis of separation, explaining the majority of the total variance and therefore representing the most important source of metabolic differences among the extracts (Figure 5). Along this axis, the leaf 70% ethanol extract (LT) is clearly distinguished from all other groups, occupying a far-positive position on the F1 score plot. This distinct clustering suggests that LT possesses a unique metabolic profile, heavily influenced by the solvent polarity and the tissue type, resulting in a selective enrichment of phenolic metabolites.
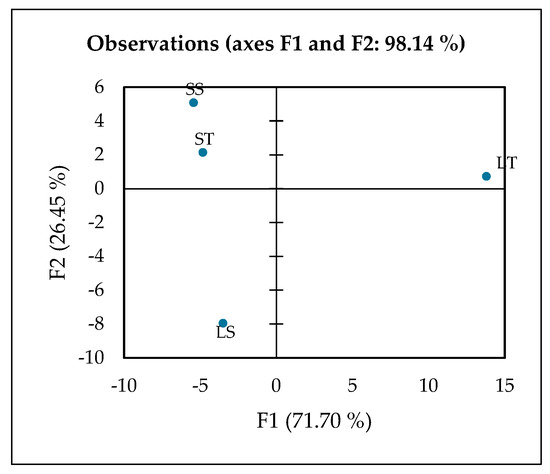
Figure 5.
PCA plot observed by XLSTAT analysis, LT (Leaf 70% ethanol extract), LS (Leaf 100% ethanol extract), ST (Stem 70% ethanol extract), and SS (Stem 100% ethanol extract).
The variables driving F1 are dominated by phenolics and flavonoids, including hesperetin, gallic acid, luteolin, rutin, salicylic acid, and phenylglyoxylic acid. These compounds are well-known secondary metabolites in plants, acting as defense molecules and providing strong antioxidant capacity [77]. For instance, gallic acid and rutin are potent radical scavengers that contribute to oxidative stress protection [78,79], while luteolin and hesperetin possess anti-inflammatory and vasoprotective properties [80,81]. Salicylic acid, beyond its role as a plant signaling molecule, is the natural precursor to aspirin, linking LT to potential anti-inflammatory benefits [82]. The simultaneous presence of multiple bioactive phenolics highlights the chemical richness of LT and suggests a synergistic contribution to its biological potency [83].
The metabolic interpretation of this pattern indicates that LT is the most phenolic-rich and functionally potent extract among the tested samples. The strong positive loadings of these phenolic and flavonoid compounds on F1 confirm that phenolic composition is the dominant factor separating LT from the stem extracts and from the 100% ethanol leaf extract (LS). This observation also demonstrates that 70% ethanol is an effective solvent system for recovering phenolic compounds from leaves, likely due to the balance between water and ethanol that maximizes the extraction of mid-polarity phytochemicals [84,85]. Consequently, LT not only serves as a chemically distinct sample but also as a promising candidate for functional food or nutraceutical applications aimed at delivering antioxidant and anti-inflammatory benefits [86,87].
F2 (26.45%) represents the secondary axis of separation, which distinguishes the leaf 100% ethanol extract (LS) from the stem-derived extracts (ST and SS). LS is strongly correlated with compounds such as adenine, guanine, L-isoleucine, L-phenylalanine, α,α-trehalose, N-acetylvaline, and amines, reflecting enrichment in primary metabolites including amino acids, nucleotides, and sugars. These molecules play crucial roles in plant energy metabolism, protein biosynthesis, and stress response [88,89]. The pattern suggests that absolute ethanol extraction favors the extraction of lower-polarity or nitrogen-containing compounds rather than polyphenols, consistent with reports that solvent polarity critically influences metabolite recovery [84,85]. Thus, although LS is less enriched in phenolics compared to LT, it provides a distinct biochemical profile centered on primary metabolism, making it metabolically complementary to phenolic-rich extracts. The positioning of LS along F2 underscores the importance of solvent strength in shaping extract composition.
Stem 70% ethanol extract (ST) is located closer to the central area of the PCA plot with a slight contribution from F3, indicating more subtle metabolic differentiation. Compounds most associated with ST include sphinganine, N,N-diethyldodecanamide, and N-benzylformamide, which are primarily lipid derivatives and amides. These metabolites are characteristic of membrane structure, signaling lipids, and nitrogen metabolism, reflecting tissue-specific biochemistry of stems compared to leaves [90,91]. The ST profile, therefore, emphasizes the structural and metabolic functions of stem tissue, which typically contains more lipids and amine derivatives than leaves, further highlighting tissue-driven metabolic variation.
Stem 100% ethanol extract (SS) occupies an intermediate position between LT and LS on the PCA score plot, suggesting a mixed metabolic profile. It shares some F1-driven phenolics with LT and exhibits specific F2-associated amino acids and sugars similar to those of LS. This intermediate positioning implies that SS contains both polyphenols and primary metabolites, reflecting a balanced extraction outcome rather than a specialized profile. Such hybrid compositions have been observed in metabolomics studies when solvents extract a broad polarity range of compounds [92]. Functionally, SS may represent a bridge extract, capture characteristics of both leaf and stem tissues, and demonstrate how solvent choice and plant part interact to determine final metabolite composition.
The 2D PCA effectively summarizes the main patterns and relationships in the data, allowing clear visualization of sample clustering and differentiation based on their underlying metabolic profiles. The distinct clustering observed in the PCA score plot indicates a strong influence of both tissue type and solvent polarity on metabolite extraction. The 70% ethanol leaf extract (LT) was clearly separated from the aqueous and stem extracts along the first principal component, suggesting a selective enrichment of ethanol-soluble metabolites such as phenolics, flavonoids, and other semi-polar secondary compounds. In contrast, the aqueous leaf (LS) and stem (ST and SS) extracts clustered together, reflecting a predominance of hydrophilic primary metabolites like sugars, amino acids, and organic acids. This separation highlights preferential solvent–metabolite interactions, where the intermediate polarity of ethanol facilitates broader metabolite recovery compared to water alone. Biologically, this distinction implies that the ethanol extract may possess enhanced bioactive potential, supporting its relevance for functional food, nutraceutical, or antioxidant applications.
3. Materials and Methods
3.1. Materials and Chemicals
C. odorata leaves and stems were taken from the Gunungkidul area, Yogyakarta Province, Indonesia, in April 2021. The voucher specimen was identified and deposited in the Research Center for Food Technology and Processing (PRTPP BRIN), Yogyakarta, Indonesia. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), alpha-glucosidase enzyme, ascorbic acid, gallic acid, and Folin–Ciocalteau reagent were purchased from Sigma-Aldrich (Tokyo, Japan).
3.2. Preparation of C. odorata Extracts
Dried C. odorata leaf and stem were macerated using 70% and 100% ethanol (EtOH, 1:10 w/v)—the extraction process was conducted for 24 h at 27 °C. After filtration and evaporation, the extracts of C. odorata leaf and stem were stored at 4 °C until further analysis. The flowchart of experiments was presented in Figure S1.
3.3. Antioxidant Assay
3.3.1. DPPH Assay
The DPPH assay was conducted according to a prior study with slight modification [93]. Briefly, the sample was reacted with DPPH (1.01 mM) for 30 min at room temperature in the dark condition. The absorbance of the final solution was measured using an ELISA reader (Thermo Scientific, Waltham, MA, USA) at 517 nm. Ascorbic acid was used as the positive control. The antioxidant assay was conducted in triplicate. The antioxidant activity of C. odorata extracts was calculated using the following Equation (1):
where A0 and A1 are absorbances of the DPPH solution in the absence and presence of the extract, respectively.
3.3.2. β-Carotene-Bleaching Assay
The antioxidant action of C. odorata extracts was evaluated using the β-carotene-linoleate test according to a previous study [94]. Briefly, β-carotene reagent (consisting of 0.2 mg of β-carotene, 1 mL of linoleic acid, 20 mg of Tween 40, and 5 mL of distilled water) was reacted with the extract (1000 µg/mL in methanol). The mixture was incubated at 50 °C for 120 min, and the absorbance was measured at 470 nm using an ELISA reader (Thermo Scientific, Waltham, MA, USA) at 0 and 120 min. Ascorbic acid (1000 µg/mL) was used as the positive control. The antioxidant activity was calculated using the following Equation:
where As0 and As120 are the absorbance of β-carotene in the presence of extract at 0 min and 120 min, respectively, while Ab0 and Ab120 is the absorbance of β-carotene without the extract at 0 min and 120 min, respectively.
3.4. α-Glucosidase Inhibitory Assay
The antidiabetic activity of C. odorata extract was performed according to a prior study [93]. Briefly, the samples (20 μL, 1000 ppm, in 20% dimethyl sulfoxide) was reacted with α-glucosidase enzyme (40 μL). After 5 min of incubation at 37 °C, 4-nitrophenyl α-D glucopyranoside (40 μL, 3 mM) in a phosphate buffer solution at pH 7 was added to the solution, and the incubation continued for 20 min at 37 °C. Sodium carbonate (0.2 M) was added to stop the reaction, and the absorbance of the final solution was measured at 405 nm using an ELISA reader (Thermo Scientific, USA).
3.5. Total Phenolic Content (TPC) Analysis
The total phenolic content of C. odorata extract was evaluated using the Folin–Ciocalteu reagent [94]. Briefly, the samples (10 μL, 1000 ppm) was reacted with Folin–Ciocalteu reagent (50 μL), Na2CO3 (20%, 150 μL), and water (790 μL). After 2 h of incubation at room temperature, the absorbance of the final solution was measured at 765 nm using an ELISA reader (Thermo Scientific, USA). The analysis was conducted in triplicate.
3.6. Antibacterial Study
The ability of C. odorata extracts to inhibit or kill the bacteria was tested using the agar disc diffusion method [94]. The extracts were tested against S. aureus, E. coli, and S. typhimurium. Concisely, extract (1000 ppm) was put into a circular disk with a diameter of 6 mm. The disks were placed onto the MHA medium plate containing 108 CFU/mL bacteria (equivalent to a 0.5 Mc Farland standard). A six mm-diameter amoxicillin disc (positive control, 10 µg in a disc) and sterile distilled water (negative control) were also placed onto the medium plate. The plates were incubated for 24 h at 37 °C. The diameter of inhibition zones was then measured and used to assess the bacterial activity of the samples and control. Measurement results were reported as the average of three independent observations.
3.7. FTIR Study
All extracts of C. odorata were evaluated for their functional group using FTIR analysis according to a previous research [95]. FTIR spectra were recorded at a resolution of 4 cm with 32 scans across the 4000–500 cm−1 range.
3.8. LC-HRMS Study
The metabolite constituents of C. odorata extracts were evaluated using LC-HRMS (Thermo Scientific™ Vanquish™ UHPLC Binary Pump and Orbitrap high-resolution mass spectrometry; Thermo Scientific, Waltham, MA, USA) according to the literature [96]. Analysis was performed using an Accucore Phenyl-hexyl column (100 mm × 2.1 mm × 2.6 μm) (Thermo Scientific, Waltham, MA, USA) maintained at 40 °C. A total of 5 μL of samples was injected and eluted using mobile phase consisted of water (A) and methanol (B) both containing 0.1% formic acid with the flow rate of 0.30 mL/min. A gradient elution technique was used programmed as follows: 0–5 min (5% B), 5–16 min (5% B to 90% B), 16–20 min (90% B), and 20–25 min (90% B to 5% B). The sheath gas flow rate in LC-HRMS instrument was set at 32 arbitrary unit (AU) with auxiliary gas flow rate of 8 AU. The compounds were detected in both positive and negative ionization modes with scan range of 66.7–1000 m/z and resolution of 70,000 for MS1 and 17,500 for MS2. The capillary temperature was set at 320 °C during the analysis.
3.9. Statistical Analysis
The biological activity of C. odorata extract was statistically examined using one-way analysis of variance (ANOVA) followed by Duncan’s test [93]. PCA was performed utilizing XLSTAT Student 2025.1.3.1431 Software (Lumivero, France) [97].
4. Conclusions
This study provides comprehensive evidence that Chromolaena odorata possesses a wide range of pharmacologically active compounds contributing to its significant antidiabetic, antioxidant, and antibacterial activities. The 70% ethanol leaf extract demonstrated the most substantial antioxidant potential, correlating with its high phenolic content. In comparison, the 70% ethanol stem extract showed superior α-glucosidase inhibitory activity, indicating promising antidiabetic effects. Although the pathogenic antibacterial activity appears to be less potent, there is still a dose-dependent relationship to the inhibition of S. aureus, E. coli, and S. typhimurium that requires further research or fractionation. The LC-HRMS analysis has facilitated the identification of numerous bioactive compounds, including phenolics, flavonoids, alkaloids, and organic acids, providing a deeper understanding of the chemical profile of C. odorata. The findings justify the traditional use of C. odorata and indicate it is a promising candidate for developing plant-based treatments to lower blood sugar, particularly for conditions related to oxidative stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30214314/s1, Figure S1: Flow chart about the experimental process.
Author Contributions
Conceptualization, A.W.I.; methodology, A.W.I.; validation, A.W.I., M.F.F.A., A.W.; formal analysis, A.W.I., M.F.F.A., A.W., T.W., E.N., N.A.F., R.N.A.; writing—original draft preparation, A.W.I.; writing—review and editing, A.W., S., T.W., E.N., N.A.F., R.N.A.; supervision, A.W.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors thank BRIN Indonesia for the research facilities through E-Layanan Sains BRIN.
Conflicts of Interest
The authors declare that there are no conflicts in relation to this work.
References
- Sahid, I.; Yusoff, N. Allelopathic Effects of Chromolaena odorata (L.) King and Robinson and Mikania Micrantha H.B.K. on Three Selected Weed Species. Aust. J. Crop Sci. 2014, 8, 1024–1028. [Google Scholar]
- Rizali, A.; Hadi, M.S.; Pudjianto, P.; Buchori, D. A New Trophic Interaction between Invasive Weed, Its Biological Control Agent, and Local Insects: A Case Study of Chromolaena odorata. Biodivers. J. Biol. Divers. 2019, 20, 1006–1011. [Google Scholar] [CrossRef]
- Juru, V.N.; Ndam, L.M.; Tatah, B.N.; Fonge, B.A. Rhizospheric Soil Chemical Properties and Microbial Response to a Gradient of Chromolaena odorata (L) Invasion in the Mount Cameroon Region. PLoS ONE 2024, 19, e0312199. [Google Scholar] [CrossRef] [PubMed]
- Otabor, J.I.; Egbon, I.; Toews, M.D.; Uyi, O. The Double-Edged Sword: Local Perspectives on the Spread, Impact, Management, and Uses of the Invasive Chromolaena odorata in Southern Nigeria. Sustainability 2025, 17, 3514. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J.S. Ecological Insights and Environmental Threats of Invasive Alien Plant Chromolaena odorata: Prospects for Sustainable Management. Weed Biol. Manag. 2024, 24, 15–37. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O. Weed or Not a Weed? Density, Perceptions and Management of Chromolaena odorata (Asteraceae) in West Africa: Voices from Ghana. Weed Res. 2020, 60, 406–414. [Google Scholar] [CrossRef]
- Kanase, V.; Shaikh, S. A Pharmacognostic and Pharmacological Review on Chromolaena odorata (Siam Weed). Asian J. Pharm. Clin. Res. 2018, 11, 34–38. [Google Scholar] [CrossRef]
- Omokhua, A.G.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) in Sub-Saharan Africa: A Synthesis and Review of Its Medicinal Potential. J. Ethnopharmacol. 2016, 183, 112–122. [Google Scholar] [CrossRef]
- Pitakpawasutthi, Y.; Palanuvej, C.; Ruangrungsi, N. Microscopic Leaf Constant Numbers of Chromolaena odorata in Thailand. Pharmacogn. J. 2018, 10, s95–s99. [Google Scholar] [CrossRef]
- Zahara, M. Description of Chromolaena odorata L. R.M King and H. Robinson as Medicinal Plant: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 506, 012022. [Google Scholar] [CrossRef]
- Emani, L.; Ravada, S.; Meka, B.; Garaga, M.; Golakoti, T. A New Flavanone from the Leaves of Chromolaena odorata. Nat. Prod. Commun. 2015, 10, 1555–1559. [Google Scholar] [CrossRef]
- Srinivasa Rao, K.; Chaudhury, P.K.; Pradhan, A. Evaluation of Anti-Oxidant Activities and Total Phenolic Content of Chromolaena odorata. Food Chem. Toxicol. 2010, 48, 729–732. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Adewumi, N.A.; Iwaloye, O. Antidiabetic Potential of Chromolaena odorata Leave Extract and Its Effect on Nrf2/Keap1 Antioxidant Pathway in the Liver of Diabetic-Induced Wistar Rats. Adv. Tradit. Med. 2023, 23, 513–523. [Google Scholar] [CrossRef]
- Jungers, J.M.; Fargione, J.E.; Sheaffer, C.C.; Wyse, D.L.; Lehman, C. Energy Potential of Biomass from Conservation Grasslands in Minnesota, USA. PLoS ONE 2013, 8, e61209. [Google Scholar] [CrossRef]
- Gamble, J.D.; Jungers, J.M.; Wyse, D.L.; Johnson, G.A.; Lamb, J.A.; Sheaffer, C.C. Harvest Date Effects on Biomass Yield, Moisture Content, Mineral Concentration, and Mineral Export in Switchgrass and Native Polycultures Managed for Bioenergy. BioEnergy Res. 2015, 8, 740–749. [Google Scholar] [CrossRef]
- Kumar, R.; Chandrashekar, N.; Prasad, N.R.R.; Tailor, R. Effect of Extractive Content on Fuelwood Characteristics of Certain Woody and Non-Woody Biomass. Curr. Sci. 2020, 118, 966–969. [Google Scholar] [CrossRef]
- Kumar Dohare, R.; Singh, A.; Jain, P.; Singh, K.; Upadhyaya, S.; Agrawal, M. Simulated Heat Integration Study of Reactive Distillation Column for Ethanol Synthesis. Iran. J. Chem. Chem. Eng. 2019, 38, 183–191. [Google Scholar] [CrossRef]
- Qu, Y.; Harte, F.M.; Elias, R.J.; Coupland, J.N. Effect of Ethanol on the Solubilization of Hydrophobic Molecules by Sodium Caseinate. Food Hydrocoll. 2018, 77, 454–459. [Google Scholar] [CrossRef]
- Matsushika, A.; Sawayama, S. Characterization of a Recombinant Flocculent Saccharomyces cerevisiae Strain That Co-Ferments Glucose and Xylose: I. Influence of the Ratio of Glucose/Xylose on Ethanol Production. Appl. Biochem. Biotechnol. 2013, 169, 712–721. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Chandrasekar, D.; Madhusudhana, K.; Ramakrishna, S.; Diwan, P. V Determination of DPPH Free Radical Scavenging Activity by Reversed-Phase HPLC: A Sensitive Screening Method for Polyherbal Formulations. J. Pharm. Biomed. Anal. 2006, 40, 460–464. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Omonije, O.O.; Saidu, A.N.; Muhammad, H.L. Antioxidant and Hypolipidemic Effects of Methanolic Root Extract of Chromolaena odorata in Alloxan-Induced Diabetic Rats. Iran. J. Toxicol. 2020, 14, 63–70. [Google Scholar] [CrossRef]
- Tahir, K.A.; Miskad, U.A.; Djawad, K.; Sartini, S.; Djide, N.; Khaerani, K.; Indrisari, M. Evaluation of Antioxidant Activity of Botto-Botto Leaf Fraction (Chromolaena odorata L.) Using Dpph and Abts Methods. Open Access Maced. J. Med. Sci. 2021, 9, 183–188. [Google Scholar] [CrossRef]
- Widayanti, A.; Jufri, M.; Surini, S.; Ellya, B. Antioxidant Activity of the Active Fraction of Mangosteen Rind Extract (Garcinia mangostana). Int. J. Appl. Pharm. 2024, 16, 145–148. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A.; Taleb, M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco). Biointerface Res. Appl. Chem. 2022, 12, 8441–8452. [Google Scholar] [CrossRef]
- Mamyrbékova-Békro, J.A.; Konan, K.M.; Békro, Y.A.; Djié Bi, M.G.; Zomi Bi, T.J.; Mambo, V.; Boua, B.B. Phytocompounds of the Extracts of Four Medicinal Plants of Côte d’ivoire and Assessment of Their Potential Antioxidant by Thin Layer Chromatography. Eur. J. Sci. Res. 2008, 24, 219–228. [Google Scholar]
- Budha Magar, A.; Shrestha, D.; Pakka, S.; Sharma, K.R. Phytochemistry, Biological, and Toxicity Study on Aqueous and Methanol Extracts of Chromolaena odorata. Sci. World J. 2023, 2023, 6689271. [Google Scholar] [CrossRef]
- Putri, D.A.; Fatmawati, S. A New Flavanone as a Potent Antioxidant Isolated from Chromolaena odorata L. Leaves. Evid. Based Complement. Alternat. Med. 2019, 2019, 1453612. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Depletion/Protection of β-Carotene in Estimating Antioxidant Activity by β-Carotene Bleaching Assay. J. Food Sci. Technol. 2015, 52, 7321–7328. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application to Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- Hardianti, B.; Amin, A.; Lallo, S.; Hertati, A. Phytochemical Composition by GC–MS, Invitro Antioxidant, Insilico Chemical Active Compound of Chromolaena odorata L. Weed Extract Targeting EGFR as Anti Lung Cancer. Res. J. Pharm. Technol. 2024, 17, 6020–6031. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In Vitro Antioxidant Properties of Rutin. LWT—Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Gong, T.; Yang, X.; Bai, F.; Li, D.; Zhao, T.; Zhang, J.; Sun, L.; Guo, Y. Young Apple Polyphenols as Natural α-Glucosidase Inhibitors: In Vitro and in Silico Studies. Bioorg. Chem. 2020, 96, 103625. [Google Scholar] [CrossRef]
- Giang, P.M.; Huong, D.T.V.; Thao, V.M.; Thuy, T.T.T.; Trang, V.M. Flavonoids from the Leaves of Chromolaena odorata and Their α-Glucosidase Inhibitory Activity. Pharm. Chem. J. 2024, 57, 1621–1626. [Google Scholar] [CrossRef]
- Yusuf, H.; Yusni, Y.; Meutia, F.; Fahriani, M. Pharmacological Evaluation of Antidiabetic Activity of Chromolaena odorata Leaves Extract in Streptozotocin-Induced Rats. Syst. Rev. Pharm. 2020, 11, 772–778. [Google Scholar] [CrossRef]
- Tran, C.L.; Chong, K.T.D.; Do, V.M.; Huynh, V.T.; Nguyen, T.A.L. In Vitro Investigations of Chemical Composition, Antibacterial, Antioxidant, Antidiabetic, and Anti-Inflammatory Activities of Chromolaena odorata Flower Extracts. Songklanakarin J. Sci. Technol. 2024, 46, 522–530. [Google Scholar]
- Ghorbani, A. Mechanisms of Antidiabetic Effects of Flavonoid Rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Pari, L.; Rajarajeswari, N. Efficacy of Coumarin on Hepatic Key Enzymes of Glucose Metabolism in Chemical Induced Type 2 Diabetic Rats. Chem. Biol. Interact. 2009, 181, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Rajkumar, J.; Seyed, M.A. Phytochemical Screening, Free Radical Scavenging and Antimicrobial Potential of Chromolaena odorata Leaf Extracts against Pathogenic Bacterium in Wound Infections—A Multispectrum Perspective. Biocatal. Agric. Biotechnol. 2018, 15, 103–112. [Google Scholar] [CrossRef]
- Phetburom, N.; Chopjitt, P.; Dulyasucharit, R.; Nontunha, N.; Daenprakhom, K.; Ongarj, P.; Hatachote, S.; Srichaijaroonpong, S.; Hatrongjit, R.; Kerdsin, A.; et al. Antimicrobial Activity of Chromolaena odorata Crude Extracts against Streptococcus suis. Microb. Pathog. 2025, 206, 107799. [Google Scholar] [CrossRef]
- Sambavekar, P.P.; Aitawade, M.M.; Patil, D.R.; Kolekar, G.B.; Deshmukh, M.B.; Anbhule, P.V. In-Silico, in-Vitro Antibacterial Activity and Toxicity Profile of New Quinoline Derivatives. Indian J. Chem.—Sect. B Org. Med. Chem. 2013, 52, 1521–1526. [Google Scholar]
- Lee, B.; Lee, D.G. Depletion of Reactive Oxygen Species Induced by Chlorogenic Acid Triggers Apoptosis-like Death in Escherichia coli. Free Radic. Res. 2018, 52, 605–615. [Google Scholar] [CrossRef]
- Bajaj, H.; Kaushal, S.; Kaur, V.; Panwar, H.; Sharma, P.; Jangra, R. Isolation of Quinic Acid from Dropped Citrus Reticulata Blanco Fruits: Its Derivatization, Antibacterial Potential, Docking Studies, and ADMET Profiling. Front. Chem. 2024, 12, 1372560. [Google Scholar] [CrossRef]
- Ercan, L.; Dogru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, G.; Wei, X.; Zhou, H.; Li, C.; Luo, Z.; Ou, D.; Yang, J.; Song, X. Antibacterial Activity and Mechanism of Luteolin Isolated from Lophatherum gracile Brongn. against Multidrug-Resistant Escherichia coli. Front. Pharmacol. 2024, 15, 1430564. [Google Scholar] [CrossRef]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Wȩgrzyn, G. Differential Antibacterial Activity of Genistein Arising from Global Inhibition of DNA, RNA and Protein Synthesis in Some Bacterial Strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef]
- Dominguez-López, I.; Pérez, M.; Lamuela-Raventós, R.M. Total (Poly)Phenol Analysis by the Folin-Ciocalteu Assay as an Anti-Inflammatory Biomarker in Biological Samples. Crit. Rev. Food Sci. Nutr. 2024, 64, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.A.; Noviana, E.; Ash Shiddiq, M.A.F.; Wardani, N.K.; Windarsih, A.; Indrasyah, F.S.; Fakhrudin, N.; Indrianingsih, A.W.; Henry, C.S. Paper-Based Device for Phenolic Content Determination in Tea Extracts. Phytochem. Anal. 2025, 36, 1094–1104. [Google Scholar] [CrossRef]
- Sirinthipaporn, A.; Jiraungkoorskul, K.; Jiraungkoorskul, W. Artemia Salina Lethality and Histopathological Studies of Siam Weed, Chromolaena odorata. J. Nat. Remedies 2016, 16, 131–136. [Google Scholar] [CrossRef]
- Kut, K.; Tama, A.; Furdak, P.; Bartosz, G.; Sadowska-Bartosz, I. Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices. Processes 2024, 12, 166. [Google Scholar] [CrossRef]
- Sharmin, H.; Nazma, S.; Mohiduzzaman, M.; Cadi, P.B. Antioxidant Capacity and Total Phenol Content of Commonly Consumed Selected Vegetables of Bangladesh. Malays. J. Nutr. 2011, 17, 377–383. [Google Scholar]
- Hamza, Z.M.; Kadhim, S.A.; Hussain, H.H. Study of Spectra Physical for Some Samples of Medical Herbal by FTIR-ATR Spectroscopy. J. Pharm. Negat. Results 2022, 13, 49–55. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Hossain, M.A.M.; Johan, M.R.; Ali, M.E.; Fadzilah, N.A. Application of Near- and Mid-Infrared Spectroscopy Combined with Chemometrics for Discrimination and Authentication of Herbal Products: A Review. J. Appl. Pharm. Sci. 2019, 9, 137–147. [Google Scholar] [CrossRef]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A Glimpse into the Extraction Methods of Active Compounds from Plants. Crit. Rev. Anal. Chem. 2022, 52, 667–696. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Bani Melhim, S.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Chunmei, Z.; Shuai, W. Molecular Mechanisms of Neuroprotective Effect of Rutin. Front. Pharmacol. 2025, 16, 1599167. [Google Scholar] [CrossRef]
- Huang, L.; Kim, M.-Y.; Cho, J.Y. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 2136. [Google Scholar] [CrossRef]
- Mijiti, N.; Someya, A.; Nagaoka, I. Effects of Isoflavone Derivatives on the Production of Inflammatory Cytokines by Synovial Cells. Exp. Ther. Med. 2021, 22, 1300. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.-K.; Kang, O.-H.; Seo, Y.-S.; Zhou, T.; Lee, Y.-S.; Han, S.-H.; Mun, S.-H.; Kong, R.; Song, H.-J.; Shin, D.-W.; et al. Luteolin Potentiates the Effects of Aminoglycoside and β-Lactam Antibiotics against Methicillin-Resistant Staphylococcus aureus in Vitro. Exp. Ther. Med. 2016, 11, 2597–2601. [Google Scholar] [CrossRef]
- Morimoto, Y.; Aiba, Y.; Miyanaga, K.; Hishinuma, T.; Cui, L.; Baba, T.; Hiramatsu, K. CID12261165, a Flavonoid Compound as Antibacterial Agents against Quinolone-Resistant Staphylococcus aureus. Sci. Rep. 2023, 13, 1725. [Google Scholar] [CrossRef]
- Suarez, A.F.L.; Tirador, A.D.G.; Villorente, Z.M.; Bagarinao, C.F.; Sollesta, J.V.N.; Dumancas, G.G.; Sun, Z.; Zhan, Z.Q.; Saludes, J.P.; Dalisay, D.S. The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula Biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus. Molecules 2021, 26, 1688. [Google Scholar] [CrossRef]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen Genistein as an Anti-Staphylococcal Agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Yi, L.; Bai, Y.; Chen, X.; Wang, W.; Zhang, C.; Shang, Z.; Zhang, Z.; Li, J.; Cao, M.; Zhu, Z.; et al. Synergistic Effects and Mechanisms of Action of Rutin with Conventional Antibiotics Against Escherichia coli. Int. J. Mol. Sci. 2024, 25, 13684. [Google Scholar] [CrossRef] [PubMed]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition. Molecules 2025, 30, 2300. [Google Scholar] [CrossRef]
- Yoshizane, C.; Mizote, A.; Arai, C.; Arai, N.; Ogawa, R.; Endo, S.; Mitsuzumi, H.; Ushio, S. Daily Consumption of One Teaspoon of Trehalose Can Help Maintain Glucose Homeostasis: A Double-Blind, Randomized Controlled Trial Conducted in Healthy Volunteers. Nutr. J. 2020, 19, 68. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Cai, C.; Cheng, W.; Shi, T.; Liao, Y.; Zhou, M.; Liao, Z. Rutin Alleviates Colon Lesions and Regulates Gut Microbiota in Diabetic Mice. Sci. Rep. 2023, 13, 4897. [Google Scholar] [CrossRef]
- Chang, X.-Q.; Yue, R.-S. Therapeutic Potential of Luteolin for Diabetes Mellitus and Its Complications. Chin. J. Integr. Med. 2025, 31, 566–576. [Google Scholar] [CrossRef]
- Abbasi, E.; Khodadadi, I. Antidiabetic Effects of Genistein: Mechanism of Action. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, L.; Chen, M.; Li, R.; Yu, Y.; Qiao, L.; Liu, J.; Zhang, X.; Zhang, Y.; Zhang, Y.; et al. Nootkatone Alleviates Type 2 Diabetes in Db/Db Mice Through AMPK Activation and ERK Inhibition: An Integrated In Vitro and In Vivo Study. Molecules 2025, 30, 2111. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Alkhalidy, H.; Luo, J.; Moomaw, E.; Neilson, A.P.; Liu, D. Flavone Hispidulin Stimulates Glucagon-Like Peptide-1 Secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2020, 64, e1900978. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a Blood Glucose-Lowering Amino Acid, Increases Glucose Uptake in Rat Skeletal Muscle in the Absence of Increases in AMP-Activated Protein Kinase Activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Keramat, M.; Niakousari, M.; Golmakani, M.-T. Comparing the Antioxidant Activity of Gallic Acid and Its Alkyl Esters in Emulsion Gel and Non-Gelled Emulsion. Food Chem. 2023, 407, 135078. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting Luteolin: An Updated Review on Its Anticancer Potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How Do Plants Achieve Immunity? Defence without Specialized Immune Cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics--the Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.F.; Rodrigues, J.A.; Caldana, C.; Schmidt, R.; van Dongen, J.T.; Thomas-Oates, J.; António, C. Mass Spectrometry-Based Plant Metabolomics: Metabolite Responses to Abiotic Stress. Mass Spectrom. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems Level Studies of Mammalian Metabolomes: The Roles of Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Markham, J.E.; Lynch, D.V.; Napier, J.A.; Dunn, T.M.; Cahoon, E.B. Plant Sphingolipids: Function Follows Form. Curr. Opin. Plant Biol. 2013, 16, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yu, Y.; Jo, Y.; Han, J.H.; Xue, Y.; Cho, M.; Bae, S.-J.; Ryu, D.; Park, W.; Ha, K.-T.; et al. Impact of Extraction Techniques on Phytochemical Composition and Bioactivity of Natural Product Mixtures. Front. Pharmacol. 2025, 16, 1615338. [Google Scholar] [CrossRef]
- Windarsih, A.; Indrianingsih, A.W.; Suryani, R.; Wiyono, T.; Noviana, E.; Darsih, C.; Rahayu, E.; Pangestuti, R.; Asari, S.M.; Pratiwi, S.I.; et al. Evaluation of the Antioxidant, Antidiabetic, and Antibacterial Activity of Curcuma domestica, Zingiber officinale, Alpinia galanga, Curcuma xanthorrhiza, and Kaempferia galanga Concurrent with Its Metabolite’s Constituent Analysis. Chem. Biodivers. 2025, 22, e00645. [Google Scholar] [CrossRef] [PubMed]
- Indrianingsih, A.W.; Styaningrum, P.; Suratno; Windarsih, A.; Suryani, R.; Noviana, E.; Itoh, K. The Effect of Extraction Method on Biological Activity and Phytochemical Content of Artocarpus heterophyllus (Jackfruit) Leaves Extract Concurrent with Its Principal Component Analysis. Process Biochem. 2024, 143, 135–147. [Google Scholar] [CrossRef]
- Windarsih, A.; Ahla, M.F.F.; Indrianingsih, A.W.; Suratno; Noviana, E.; Bhattacharjya, D.K.; Sulistyowaty, M.I. In Vitro Evaluation of Antioxidant, Antibacterial, and Antidiabetes of Billygoat Weed Leaves, Stem, and Flower (Ageratum conyzoides L.) Concurrent with Its Phytochemical Constituents. Waste Biomass Valorization 2025, 16, 4711–4725. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Hayati, S.N.; Rosyida, V.T.; Apriyana, W.; Darsih, C.; Nisa, K.; Suryani, A.E.; Wiyono, T.; Windarsih, A.; Handayani, S. Improving the Functional Benefits of Powdered Ginger Beverage through the Incorporation of Ganoderma lucidum. Bioact. Carbohydrates Diet. Fibre 2025, 34, 100482. [Google Scholar] [CrossRef]
- Yulianti, Y.; Andarwulan, N.; Adawiyah, D.R.; Herawati, D.; Indrasti, D.; Wanita, Y.P. Phenolic Content and α-Glucosidase Inhibition of Tubruk-Brew Kalosi Coffee Processed by Different Post-Harvest Processing. Coffee Sci. 2025, 20, e202333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).