Effect of Torrefaction Condensate on the Growth and Exopolysaccharide Production of Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Results and Discussion
2.1. TC Yield
2.2. TC Characterization
2.3. Growth of C. reinhardtii
2.4. Biomass and EPS Yield
2.5. Biochemical Analyses of Biomass
2.5.1. Total and Free Amino Acid Analysis
2.5.2. Lipid and Fatty Acid Analysis
2.6. Structural Characterization of the Exopolysaccharides
2.6.1. Molecular Weight of EPS
2.6.2. Fourier Transform Infrared (FTIR) Analysis
2.7. Biochemical Analysis of EPS
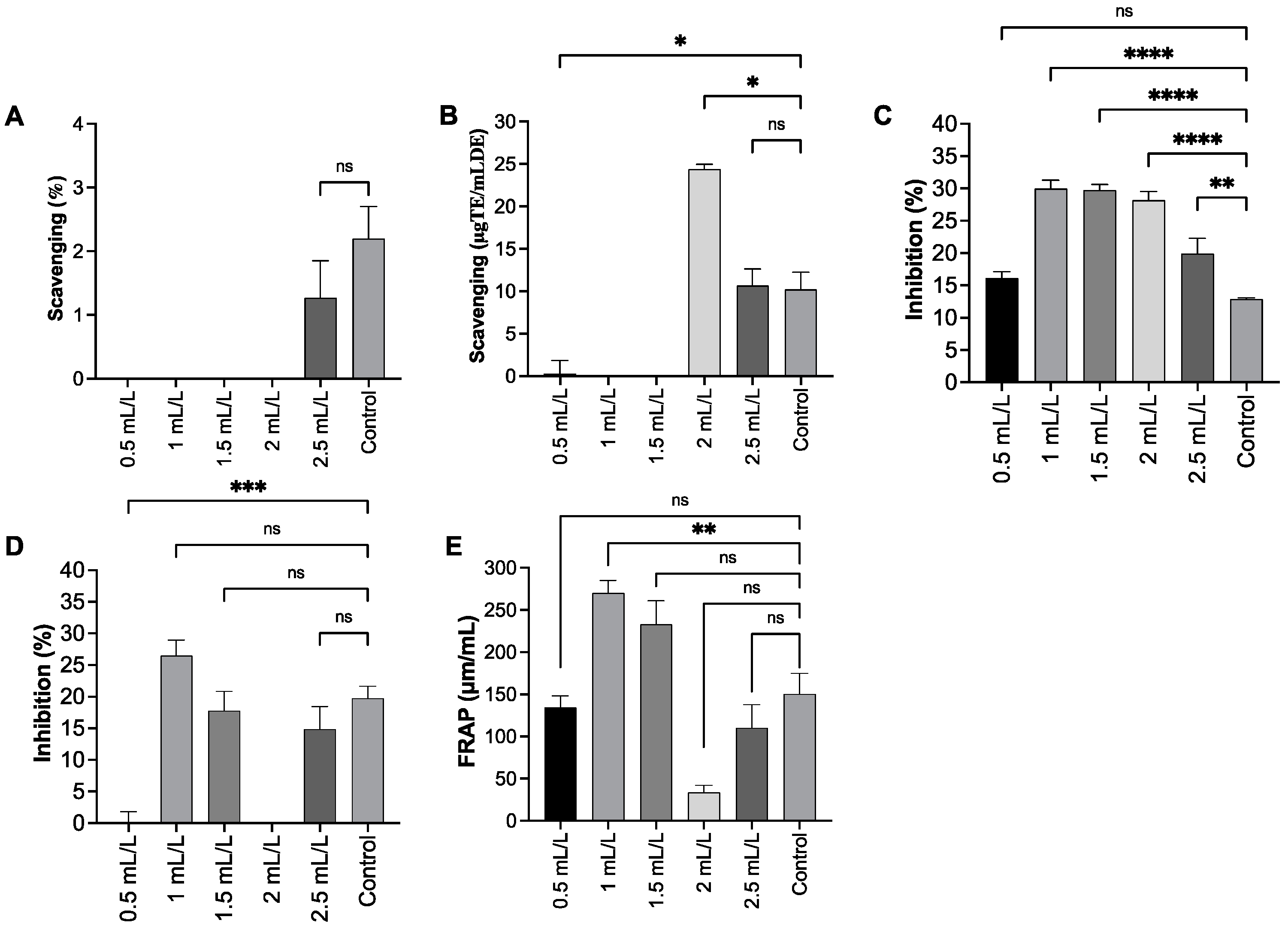
2.8. Antioxidant Capacity of EPS
3. Materials and Methods
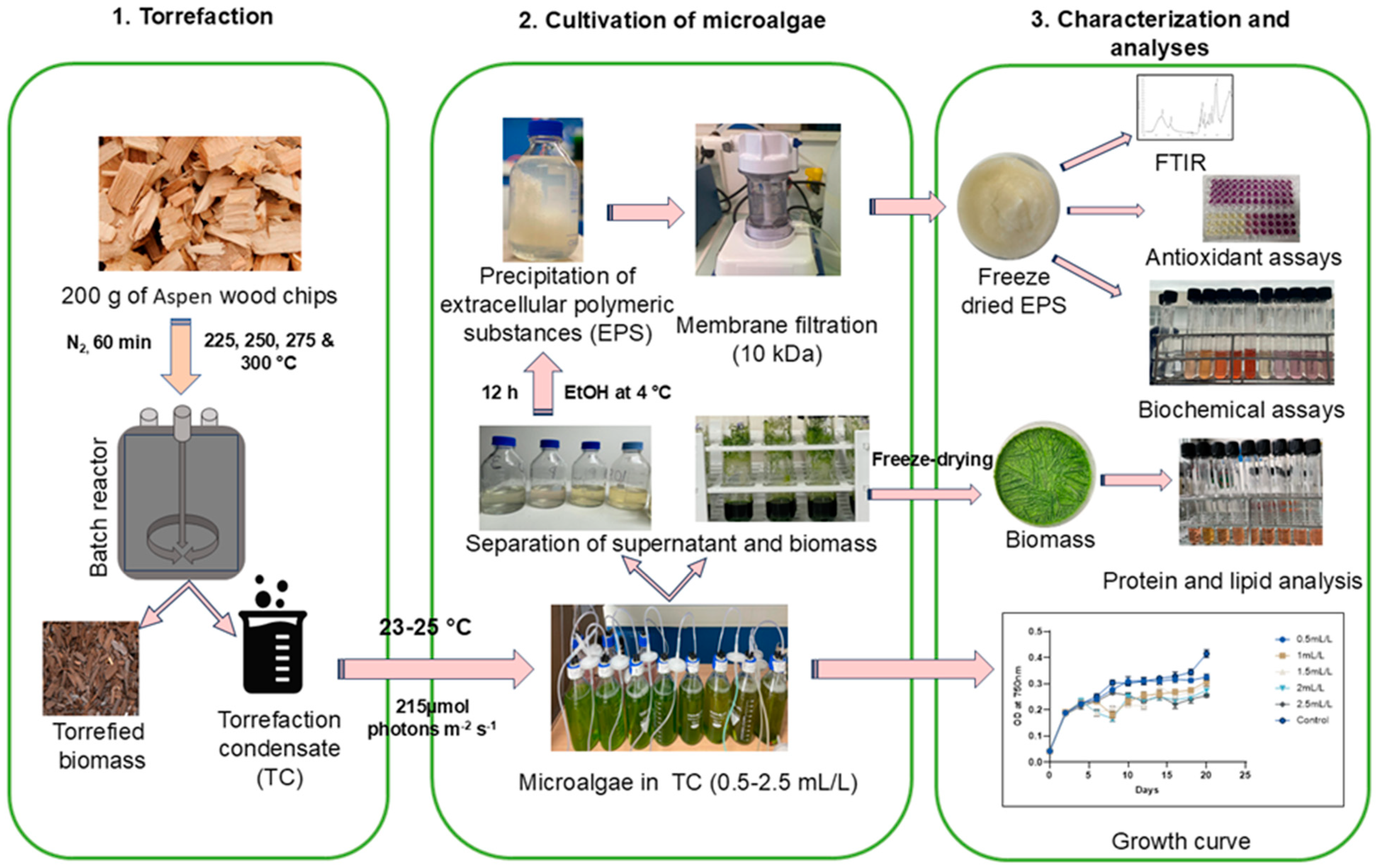
3.1. Torrefaction of Biomass
3.2. Characterization of TC
3.2.1. GC-MS Analysis of TC
3.2.2. HPLC Analysis of TC
3.3. Cultivation of Microalgae
3.4. Recovery of Biomass and Extraction of EPS
3.5. Biochemical Analyses of C. reinhardtii
3.5.1. Protein and Amino Acid Analysis
3.5.2. Lipid and Fatty Acid Analysis
3.6. Structural Characterization of EPS
3.6.1. Size-Exclusion Chromatography of EPS
3.6.2. Fourier Transform Infrared (FTIR) Analysis
3.7. Biochemical Analyses of EPS
3.7.1. Total Sugar Estimation
3.7.2. Estimation of Uronic Acid and Neutral Sugar
3.7.3. Protein Estimation
3.8. Determination of Antioxidant Capacity of EPS
3.8.1. DPPH Radical Scavenging Activity Assay
3.8.2. ABTS Free Radical Scavenging Activity Assay
3.8.3. SOD Scavenging Activity Assay
3.8.4. Hydroxyl Radical Scavenging Activity
3.8.5. FRAP Assay
3.9. Statistical Analyses
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TC | Torrefaction condensate |
| EPS | Exopolysaccharide |
| OD | Optical density |
| DCW | Dry cell weight |
| PCD | Programmed cell death |
References
- Publications Office of the European Union. European Bioeconomy Policy-KI0122230ENN; Publications Office of the European Union: Luxembourg City, Luxembourg, 2022. [Google Scholar]
- Doddapaneni, T.R.K.C.; Kikas, T. Advanced Applications of Torrefied Biomass: A Perspective View. Energies 2023, 16, 1635. [Google Scholar] [CrossRef]
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent Advances of Thermochemical Conversion Processes for Biorefinery. Bioresour. Technol. 2022, 343, 126109. [Google Scholar] [CrossRef]
- Klinger, J.; Klemetsrud, B.; Bar-Ziv, E.; Shonnard, D. Temperature Dependence of Aspen Torrefaction Kinetics. J. Anal. Appl. Pyrolysis 2014, 110, 424–429. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Klinger, J.; Bar-Ziv, E.; Shonnard, D. Kinetic Study of Aspen during Torrefaction. J. Anal. Appl. Pyrolysis 2013, 104, 146–152. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Ikubanni, P.P.; Emmanuel, S.S.; Fajobi, M.O.; Nwachukwu, P.; Adesibikan, A.A.; Odusote, J.K.; Adeyemi, E.O.; Abioye, O.M.; Okolie, J.A. A Comprehensive Review on the Similarity and Disparity of Torrefied Biomass and Coal Properties. Renew. Sustain. Energy Rev. 2024, 199, 114502. [Google Scholar] [CrossRef]
- Lê Thành, K.; Commandré, J.-M.; Valette, J.; Volle, G.; Meyer, M. Detailed Identification and Quantification of the Condensable Species Released during Torrefaction of Lignocellulosic Biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar] [CrossRef]
- Nocquet, T.; Dupont, C.; Commandre, J.-M.; Grateau, M.; Thiery, S.; Salvador, S. Volatile Species Release during Torrefaction of Wood and Its Macromolecular Constituents: Part 1—Experimental Study. Energy 2014, 72, 180–187. [Google Scholar] [CrossRef]
- Roy, B.; Kleine-Möllhoff, P.; Dalibard, A. Superheated Steam Torrefaction of Biomass Residues with Valorisation of Platform Chemicals—Part 1: Ecological Assessment. Sustainability 2022, 14, 1212. [Google Scholar] [CrossRef]
- Fagernäs, L.; Kuoppala, E.; Arpiainen, V. Composition, Utilization and Economic Assessment of Torrefaction Condensates. Energy Fuels 2015, 29, 3134–3142. [Google Scholar] [CrossRef]
- Peter, A.P.; Yew, G.Y.; Tang, D.Y.Y.; Koyande, A.K.; Chew, K.W.; Show, P.L. Microalgae’s Prospects in Attaining Sustainable Economic and Environmental Development. J. Biotechnol. 2022, 357, 18–27. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, Q.; Tang, C.; Li, S.; Li, Z.; Chen, C.; Zhu, L. Microalgae Cultivation with Recycled Harvesting Water Achieved Economic and Sustainable Production of Biomass and Lipid: Feasibility Assessment and Inhibitory Factors Analysis. Bioresour. Technol. 2024, 394, 130276. [Google Scholar] [CrossRef] [PubMed]
- Mollo, L.; Drigo, F.; Moglie, M.; Norici, A. Screening for Tolerance to Natural Phenols of Different Algal Species: Toward the Phycoremediation of Olive Mill Wastewater. Algal Res. 2023, 75, 103256. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Bafana, A. Characterization and Optimization of Production of Exopolysaccharide from Chlamydomonas reinhardtii. Carbohydr. Polym. 2013, 95, 746–752. [Google Scholar] [CrossRef]
- Koçer, A.T.; İnan, B.; Kaptan Usul, S.; Özçimen, D.; Yılmaz, M.T.; Işıldak, İ. Exopolysaccharides from Microalgae: Production, Characterization, Optimization and Techno-Economic Assessment. Braz. J. Microbiol. 2021, 52, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chi, Z.; Rover, M.; Brown, R.; Jarboe, L.; Wen, Z. Microalgae Fermentation of Acetic Acid-rich Pyrolytic Bio-oil: Reducing Bio-oil Toxicity by Alkali Treatment. Environ. Prog. Sustain. Energy 2013, 32, 955–961. [Google Scholar] [CrossRef]
- Weeks, D.P. Chapter 13—Genetic Transformation of Chlamydomonas Nuclear, Chloroplast, and Mitochondrial Genomes. In The Chlamydomonas Sourcebook, 3rd ed.; Goodenough, U., Ed.; Academic Press: Oxford, UK, 2023; pp. 325–343. ISBN 978-0-12-822457-1. [Google Scholar]
- Li, C.; Li, P.; Fu, H.; Chen, J.; Ye, M.; Zhai, S.; Hu, F.; Zhang, C.; Ge, Y.; Fortin, C. A Comparative Study of the Accumulation and Detoxification of Copper and Zinc in Chlamydomonas reinhardtii: The Role of Extracellular Polymeric Substances. Sci. Total Environ. 2023, 871, 161995. [Google Scholar] [CrossRef]
- Otto, B.; Beuchel, C.; Liers, C.; Reisser, W.; Harms, H.; Schlosser, D. Laccase-like Enzyme Activities from Chlorophycean Green Algae with Potential for Bioconversion of Phenolic Pollutants. FEMS Microbiol. Lett. 2015, 362, fnv072. [Google Scholar] [CrossRef] [PubMed]
- Bellido-Pedraza, C.M.; Torres, M.J.; Llamas, A. The Microalgae Chlamydomonas for Bioremediation and Bioproduct Production. Cells 2024, 13, 1137. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Chi, Z.; Rover, M.; Johnston, P.; Brown, R.; Jarboe, L.; Wen, Z. Utilization of Acetic Acid-Rich Pyrolytic Bio-Oil by Microalga Chlamydomonas reinhardtii: Reducing Bio-Oil Toxicity and Enhancing Algal Toxicity Tolerance. Bioresour. Technol. 2013, 133, 500–506. [Google Scholar] [CrossRef]
- Gul, S.; Ramzan, N.; Hanif, M.A.; Bano, S. Kinetic, Volatile Release Modeling and Optimization of Torrefaction. J. Anal. Appl. Pyrolysis 2017, 128, 44–53. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Praveenkumar, R.; Tolvanen, H.; Palmroth, M.R.T.; Konttinen, J.; Rintala, J. Anaerobic Batch Conversion of Pine Wood Torrefaction Condensate. Bioresour. Technol. 2017, 225, 299–307. [Google Scholar] [CrossRef]
- Macedo, L.A.; Silveira, E.A.; Rousset, P.; Valette, J.; Commandré, J.-M. Synergistic Effect of Biomass Potassium Content and Oxidative Atmosphere: Impact on Torrefaction Severity and Released Condensables. Energy 2022, 254, 124472. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. Torrefaction of Wood Part 2. Analysis of Products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H.; Pittman, C.U., Jr. Current Trends in the Production and Applications of Torrefied Wood/Biomass—A Review. BioResources 2015, 10, 8812–8858. [Google Scholar] [CrossRef]
- Bi, D.; Li, B.; Liu, S.; Yi, W.; Jiang, M.; Lin, Z. Influence of Pyrolysis and Torrefaction Pretreatment Temperature on the Pyrolysis Product Distribution. BioResources 2018, 14, 1185–1197. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Likozar, B. Wet Torrefaction of Biomass Waste into Levulinic Acid and High-Quality Hydrochar Using H-Beta Zeolite Catalyst. J. Clean. Prod. 2024, 449, 141735. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent Advances in Hydrothermal Carbonisation: From Tailored Carbon Materials and Biochemicals to Applications and Bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; Liu, D.; Xie, X.; Li, B. Effect of Biomass Particle Size on the Torrefaction Characteristics in a Fixed-Bed Reactor. Energies 2023, 16, 1104. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhu, Y.; Bai, Y.; Wang, Y. Acetic Acid-Induced Programmed Cell Death and Release of Volatile Organic Compounds in Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2012, 51, 175–184. [Google Scholar] [CrossRef]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Z.; Yan, Z.; Zhou, G.; Zhang, W.; Wang, Y.; Li, X. Defense Pathways of Chlamydomonas Reinhardtii under Silver Nanoparticle Stress: Extracellular Biosorption, Internalization and Antioxidant Genes. Chemosphere 2022, 291, 132764. [Google Scholar] [CrossRef]
- Wase, N.; Black, P.N.; Stanley, B.A.; DiRusso, C.C. Integrated Quantitative Analysis of Nitrogen Stress Response in Chlamydomonas reinhardtii Using Metabolite and Protein Profiling. J. Proteome Res. 2014, 13, 1373–1396. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-E.; Koh, H.G.; Kang, N.K.; Suh, W.I.; Jeong, B.; Lee, B.; Chang, Y.K. Isolation, Phenotypic Characterization and Genome Wide Analysis of a Chlamydomonas reinhardtii Strain Naturally Modified under Laboratory Conditions: Towards Enhanced Microalgal Biomass and Lipid Production for Biofuels. Biotechnol. Biofuels 2017, 10, 308. [Google Scholar] [CrossRef]
- Cunha, E.; Sousa, V.; Geada, P.; Teixeira, J.A.; Vicente, A.A.; Dias, O. Systems Biology’s Role in Leveraging Microalgal Biomass Potential: Current Status and Future Perspectives. Algal Res. 2023, 69, 102963. [Google Scholar] [CrossRef]
- Borjas Esqueda, A.; Gardarin, C.; Laroche, C. Exploring the Diversity of Red Microalgae for Exopolysaccharide Production. Mar. Drugs 2022, 20, 246. [Google Scholar] [CrossRef]
- Kamble, P.; Cheriyamundath, S.; Lopus, M.; Sirisha, V.L. Chemical Characteristics, Antioxidant and Anticancer Potential of Sulfated Polysaccharides from Chlamydomonas reinhardtii. J. Appl. Phycol. 2018, 30, 1641–1653. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Hari, A.; Rooni, V.; Veerabagu, U.; Sarker, S.; Konist, A.; Kikas, T. Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production. Polymers 2025, 17, 1808. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Sueoka, N. Mitotic Replication of Deoxyribonucleic Acid in Chlamydomonas reinhardii. Proc. Natl. Acad. Sci. USA 1960, 46, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Phélippé, M.; Gonçalves, O.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the Polysaccharides Chemical Diversity of the Cyanobacteria Arthrospira platensis. Algal Res. 2019, 38, 101426. [Google Scholar] [CrossRef]
- Adler, I.; Kotta, J.; Robal, M.; Humayun, S.; Vene, K.; Tuvikene, R. Valorization of Baltic Sea Farmed Blue Mussels: Chemical Profiling and Prebiotic Potential for Nutraceutical and Functional Food Development. Food Chem. X 2024, 23, 101736. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, A.D.; Sooäär, A.; Ahmed, T.A.E.; Rjabovs, V.; Hincke, M.T.; Tuvikene, R. Isolation, Structural Characterization and Biological Activities of Polysaccharides from Chondrus crispus. Food Hydrocoll. 2024, 154, 110131. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.-C. Colorimetric Determination of Neutral Sugars by a Resorcinol Sulfuric Acid Micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, H.; Bi, Y.; Fan, X. Preparation and Antioxidant Activity of Selenium Nanoparticles Decorated by Polysaccharides from Sargassum fusiforme. J. Food Sci. 2021, 86, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Improved Pyrogallol Autoxidation Method: A Reliable and Cheap Superoxide-Scavenging Assay Suitable for All Antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
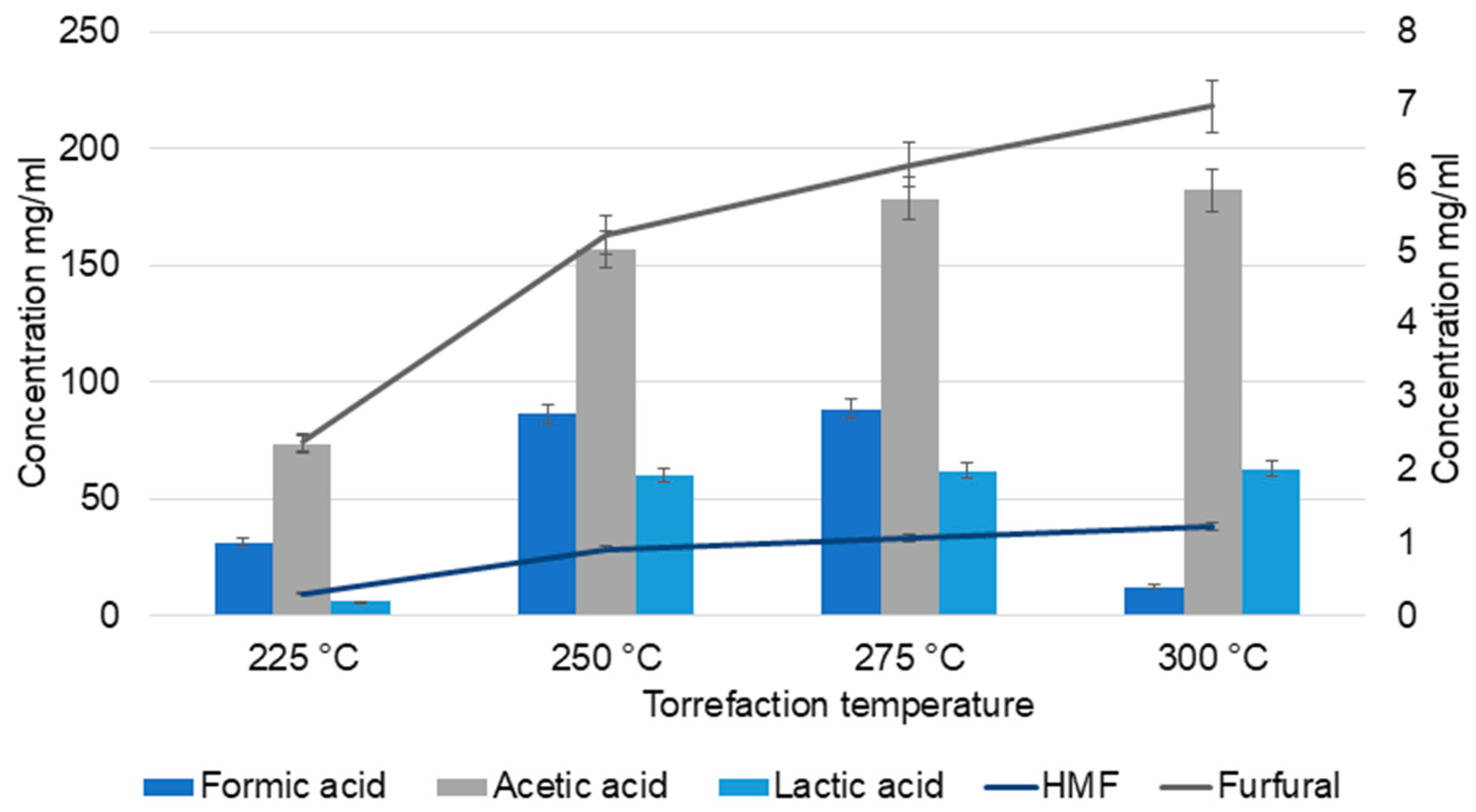

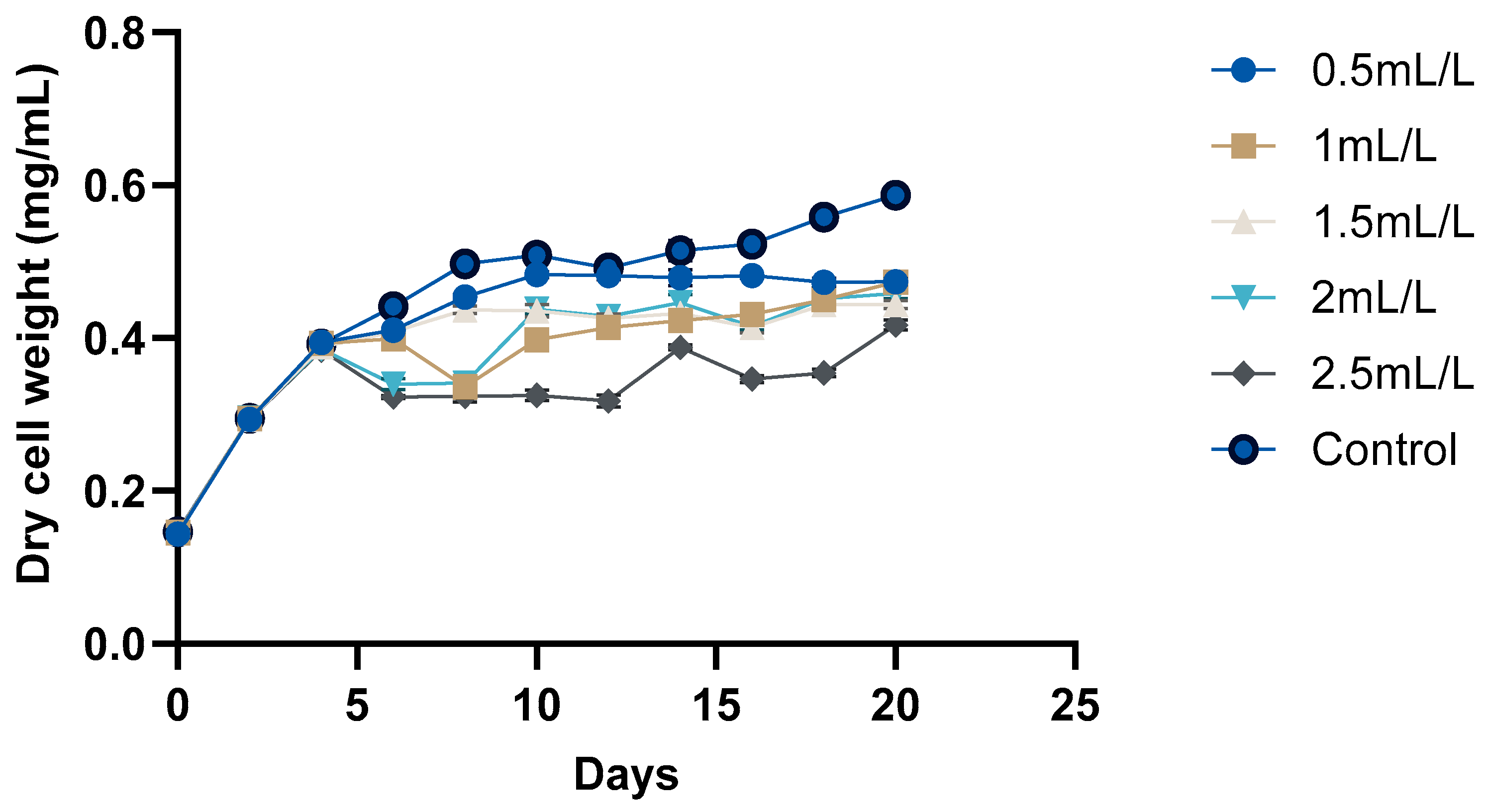
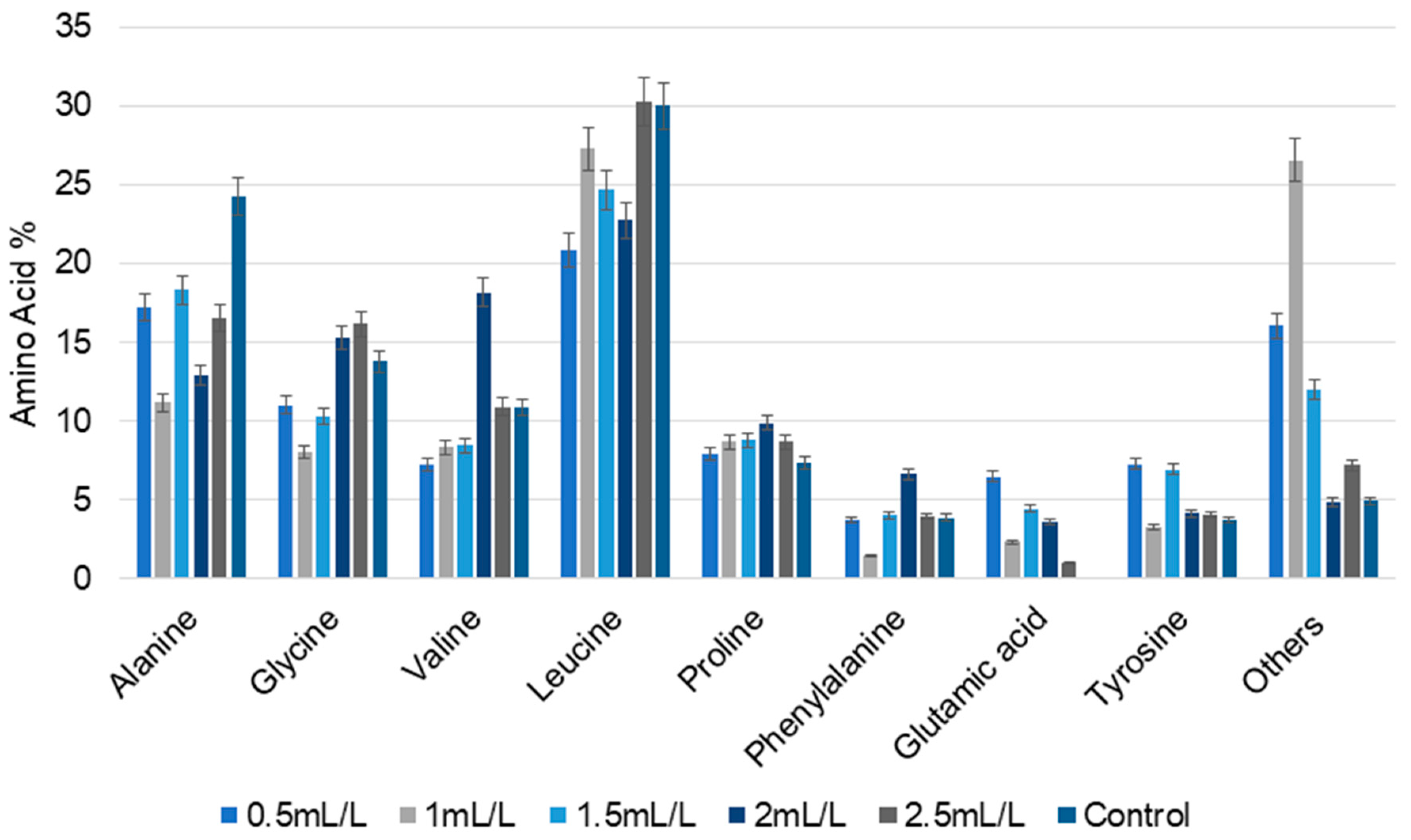
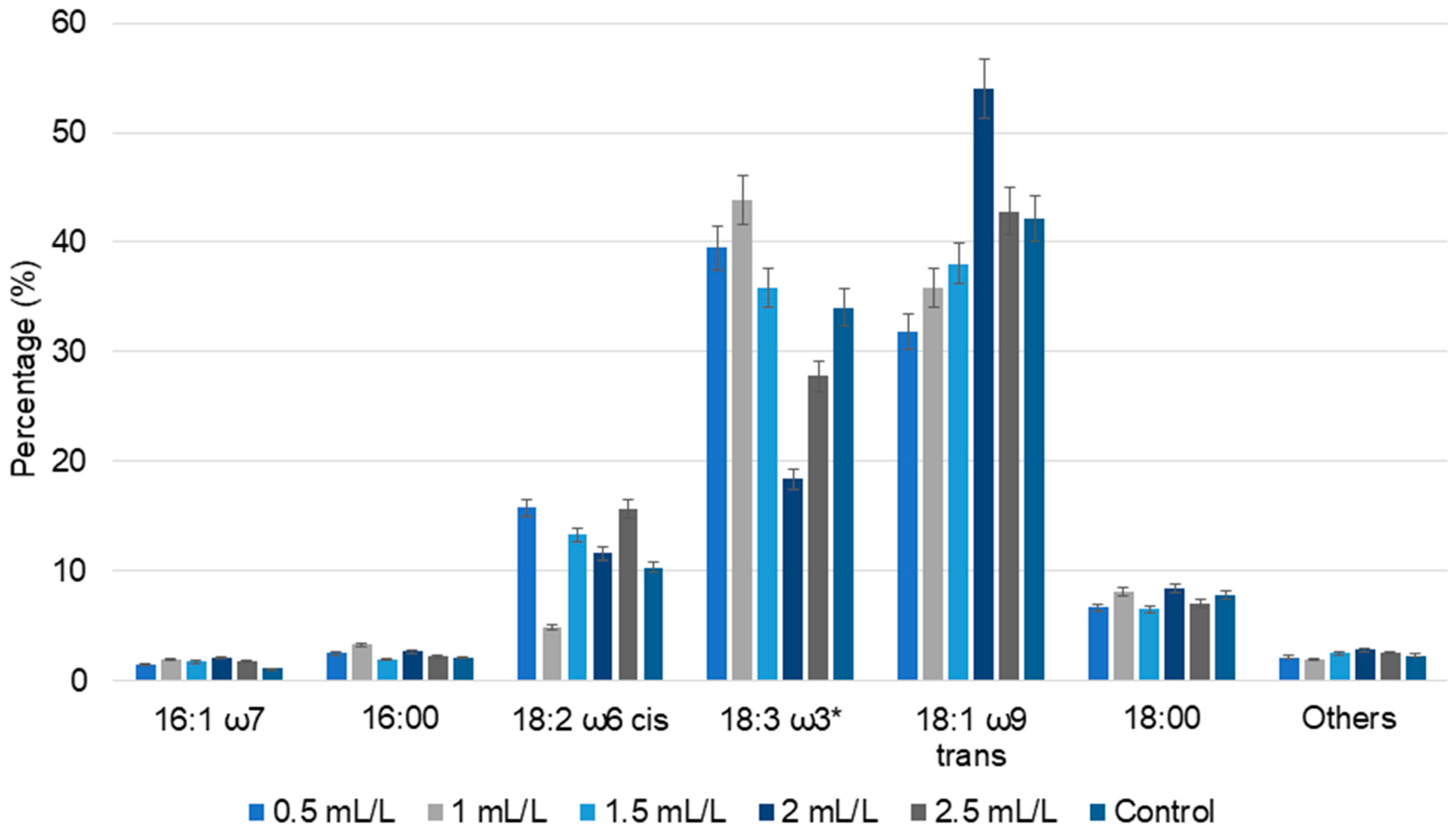

| No | Temperature (°C) | Biomass (g) | TC Yield (mL) | pH |
|---|---|---|---|---|
| 1 | 225 | 172.23 | 17 ± 2 | 2.7 |
| 2 | 250 | 164.56 | 24 ± 2 | 2.4 |
| 3 | 275 | 134.24 | 28 ± 2 | 2.1 |
| 4 | 300 | 68 | 50 ± 5 | 2.2 |
| No | Group 1 | Composition of Torrefaction Condensate | GC-MS % Area | |||
|---|---|---|---|---|---|---|
| Compound | 225 °C | 250 °C | 275 °C | 300 °C | ||
| 1 | IO | Carbon dioxide | 10.38 | 6.65 | 2.66 | 2.30 |
| 2 | Es | Acetic acid, methyl ester | - | 7.49 | 5.00 | 1.95 |
| 3 | Ac | Acetic acid | 41.86 | 35.95 | 30.82 | 29.99 |
| 4 | K | 1-hydroxy-2-butanone | - | - | 3.09 | - |
| 5 | K | 1-hydroxy-2-propanone | - | 6.72 | 6.95 | 7.75 |
| 6 | Fu | Furfural | 4.53 | 8.72 | 5.61 | 5.36 |
| 7 | Ac | Benzoic acid | 5.84 | - | - | - |
| 8 | K | 1-(2,4,6-trihydroxy-3-methylphenyl)-1-butanone | - | 2.95 | - | - |
| 9 | Es | 2-butoxyethyl acetate | - | 4.90 | - | - |
| 10 | Am | N-methyl-1,3-Propanediamine | - | 9.97 | - | 8.61 |
| 11 | Di | 1,2-ethanediol | - | 2.75 | - | 0.68 |
| 12 | Fu | 2-furanmethanol | - | - | 1.16 | 2.49 |
| 13 | K | 1-(acetyloxy)-2-propanone | - | - | 1.05 | 1.18 |
| 14 | Fu | (S)-2-(furan-2-yl)-2-methoxyethanol | - | 6.25 | 3.60 | - |
| 15 | Fu | tetrahydro-2,5-dimethoxy-furan | - | 3.57 | 4.27 | 0.45 |
| 16 | Ace | 1,1-dimethoxy-heptane | - | 1.30 | 1.22 | - |
| 17 | Ph | Phenol | - | - | 1.61 | 2.51 |
| 18 | Ace | Hexanal dimethyl acetal | - | - | 1.11 | - |
| 19 | Alc | Cyclobutanol | - | - | 13.21 | - |
| 20 | Ph | 2,6-dimethoxy-phenol | - | 2.78 | 4.23 | 6.25 |
| 21 | Ac | 3,5-dimethoxy-4-hydroxyphenylacetc acid | - | - | 4.22 | 4.56 |
| 22 | Ph | 1-(4-hydroxy-3,5-dimethxyphenyl)-ethanone | - | - | 1.27 | 1.83 |
| 23 | Ph | 2,6-dimethoxy-4-(2-propenyl)-phenol | - | - | 1.87 | 3.21 |
| 24 | Ben | 4-hydroxy-3,5-dimetoxy-benzaldehyde | - | - | 1.21 | 0.96 |
| 25 | Ben | 4-ethylbiphenyl | - | - | 1.92 | - |
| 26 | Ph | 5-tert-butylpyrogallol | - | - | 1.29 | - |
| 27 | Ad | Glyceraldehyde | - | - | 0.31 | - |
| 28 | Ace | Octanal dimethyl acetal | - | - | 1.47 | - |
| 29 | K | 2,3-butanedione | - | - | - | 0.22 |
| 30 | Pyr | 2,4-dihydroxypyridine | - | - | - | 1.41 |
| 31 | Th | N-methylthio-formamide | - | - | - | 0.48 |
| 32 | Fu | 5-methyl-2-furancarboxaldehyde | - | - | - | - |
| 33 | Es | 2-butoxy-1-methylethyl butanoate | - | - | - | 0.86 |
| 34 | K | 3-methyl-1,2-cyclopentanedione | - | - | - | 1.66 |
| 35 | Ph | 2-methoxy-phenol | - | - | - | 0.95 |
| 36 | Ph | Creosol | - | - | - | 0.68 |
| 37 | Ph | 4-ethyl-2-methoxy-phenol | - | - | - | 0.72 |
| 38 | Ben | 4-hydroxy-3-methoxy- benzoic acid | - | - | - | 2.83 |
| 39 | Ph | 2-methoxy-4-(1-propenyl)-phenol | - | - | - | 0.93 |
| 40 | K | 1-(2,6-dihydroxy-4-methoxyphenyl)-ethanone | - | - | - | 2.34 |
| 41 | Ph | Homovanillyl alcohol | - | - | - | 0.75 |
| 42 | Ben | N-butyl-benzenesulfonamide | - | - | - | 2.05 |
| 43 | Ac | Propanoic acid | - | - | - | 0.28 |
| 44 | K | 4-hydroxy-2-butanone | - | - | - | 2.49 |
| 45 | K | 2-pentanone | - | - | - | 1.26 |
| No | Name | Free Amino Acid % | |||||
|---|---|---|---|---|---|---|---|
| 0.5 mL/L | 1 mL/L | 1.5 mL/L | 2 mL/L | 2.5 mL/L | Control | ||
| 1 | Alanine | 19.13 | 17.45 | 17.52 | 15.29 | 18.26 | 5.01 |
| 2 | Glycine | 5.79 | 5.75 | 5.39 | 4.59 | 3.64 | 1.60 |
| 3 | Beta-Alanine | 0.34 | 0.28 | 0.23 | 0.15 | 12.36 | 4.05 |
| 4 | Valine | 7.06 | 6.93 | 6.73 | 6.82 | 4.79 | 1.21 |
| 5 | Leucine | 21.92 | 21.86 | 21.00 | 22.41 | 8.95 | 2.65 |
| 6 | Isoleucine | 7.04 | 7.01 | 6.50 | 7.29 | 4.71 | 1.17 |
| 7 | Proline | 13.81 | 12.43 | 12.58 | 14.44 | 16.70 | 4.87 |
| 8 | Methionine | 0.88 | 1.40 | 1.49 | 1.70 | 0.59 | 0.20 |
| 9 | Serine | 3.63 | 3.73 | 3.08 | 3.28 | 1.32 | 0.90 |
| 10 | Threonine | 2.12 | 3.34 | 3.06 | 3.28 | 1.82 | 0.80 |
| 11 | Phenylalanine | 2.32 | 2.44 | 2.38 | 2.78 | 0.71 | 0.15 |
| 12 | Aspartic acid | 1.30 | 1.60 | 0.84 | 1.65 | 0.21 | 0.02 |
| 13 | Cysteine | 0.09 | 0.16 | 0.52 | 0.17 | 2.43 | 0.89 |
| 14 | Glutamic acid | 3.90 | 4.55 | 1.60 | 4.73 | 3.82 | 0.88 |
| 15 | Lysine | 2.21 | 1.54 | 0.79 | 1.10 | 1.11 | 0.07 |
| 16 | Tyrosine | 5.18 | 5.79 | 5.32 | 7.18 | 2.09 | 0.30 |
| 17 | Tryptophan | 0.99 | 2.09 | 2.03 | 0.00 | 1.36 | 0.18 |
| 18 | Cystine | 0.83 | 1.03 | 8.47 | 2.45 | 14.41 | 75.02 |
| EPS Samples [% Mass (g/100 g EPS)] | Total Carbohydrate (%) | Neutral Sugars (%) | Uronic Acids (%) | Protein (%) |
|---|---|---|---|---|
| Control | 91.68 | 85.42 | 14.58 | 5.89 |
| 0.5 mL/L | 90.40 | 85.94 | 14.06 | 7.33 |
| 1 mL/L | 78.32 | 83.70 | 16.30 | 9.49 |
| 1.5 mL/L | 74.25 | 84.37 | 15.63 | 11.31 |
| 2 mL/L | 80.55 | 83.76 | 16.24 | 7.21 |
| 2.5 mL/L | 88.18 | 84.02 | 15.98 | 11.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekharan Nair, S.; Premarathna, A.D.; Hari, A.; Gardarin, C.; Laroche, C.; Tuvikene, R.; Geetha Bai, R.; Kikas, T. Effect of Torrefaction Condensate on the Growth and Exopolysaccharide Production of Chlamydomonas reinhardtii. Molecules 2025, 30, 4313. https://doi.org/10.3390/molecules30214313
Chandrasekharan Nair S, Premarathna AD, Hari A, Gardarin C, Laroche C, Tuvikene R, Geetha Bai R, Kikas T. Effect of Torrefaction Condensate on the Growth and Exopolysaccharide Production of Chlamydomonas reinhardtii. Molecules. 2025; 30(21):4313. https://doi.org/10.3390/molecules30214313
Chicago/Turabian StyleChandrasekharan Nair, Salini, Amal D. Premarathna, Anjana Hari, Christine Gardarin, Céline Laroche, Rando Tuvikene, Renu Geetha Bai, and Timo Kikas. 2025. "Effect of Torrefaction Condensate on the Growth and Exopolysaccharide Production of Chlamydomonas reinhardtii" Molecules 30, no. 21: 4313. https://doi.org/10.3390/molecules30214313
APA StyleChandrasekharan Nair, S., Premarathna, A. D., Hari, A., Gardarin, C., Laroche, C., Tuvikene, R., Geetha Bai, R., & Kikas, T. (2025). Effect of Torrefaction Condensate on the Growth and Exopolysaccharide Production of Chlamydomonas reinhardtii. Molecules, 30(21), 4313. https://doi.org/10.3390/molecules30214313









