Iron-Based Metal–Organic Frameworks for the Removal of Different Organic and Inorganic Arsenic Species from Water: Kinetic and Adsorption Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Kinetics of Different Arsenicals
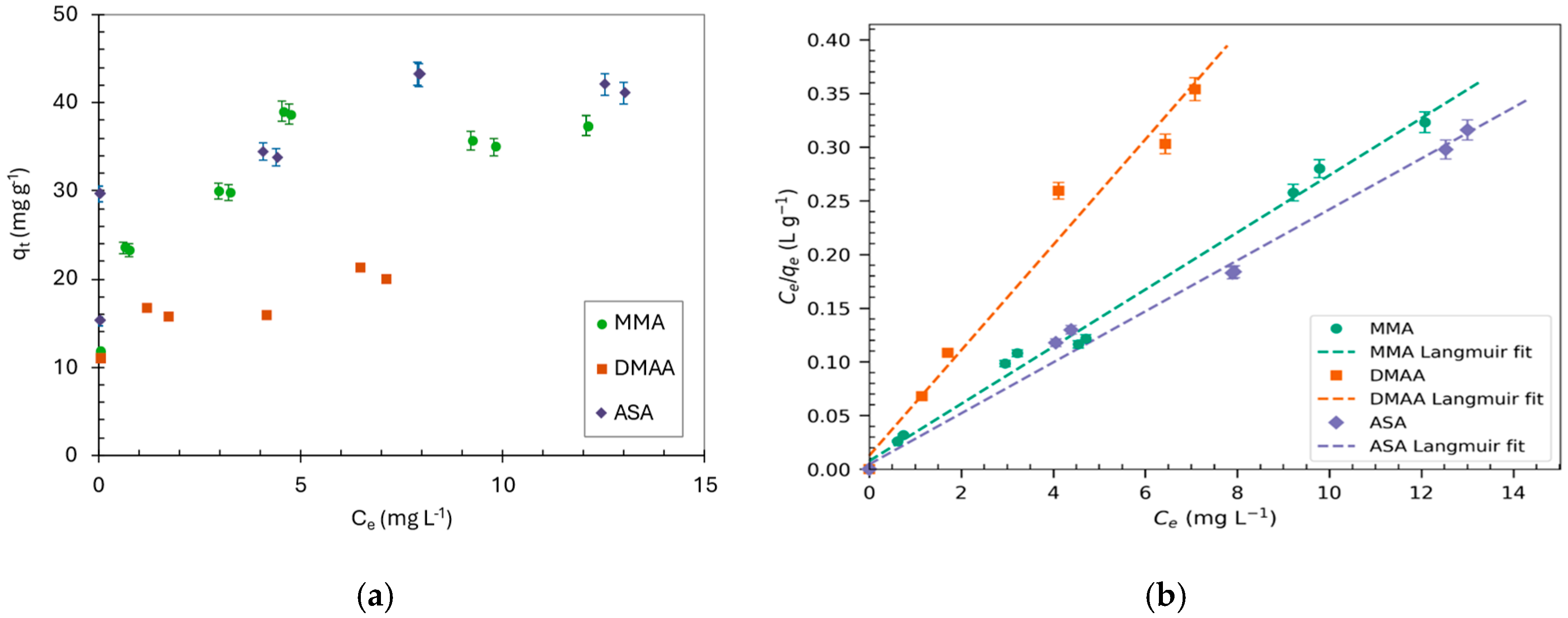
2.2. Adsorption Isotherms of Arsenic Species
2.3. The Effect of pH
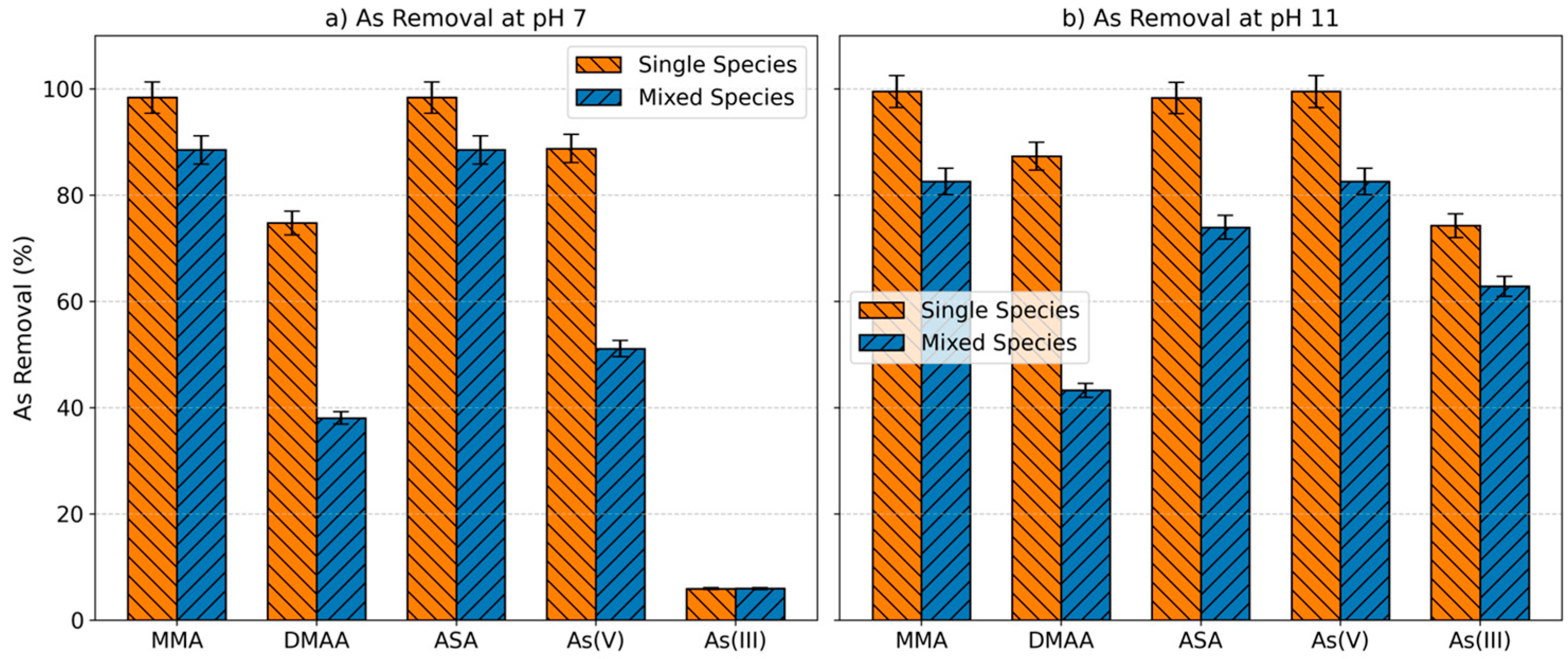
2.4. Competition of the Arsenic Species for the Adsorbent
2.5. Competing Ions in the Adsorption of Arsenic Species by Fe-BTC MOFs
2.6. Elution with Methanol: Desorption and Reuse
3. Materials and Methods
3.1. Chemicals
3.2. Batch Adsorption Experiments
3.3. Desorption and Reusability Experiments
3.4. Chromatographic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisbie, S.H.; Mitchell, E.J. Arsenic in drinking water: An analysis of global drinking water regulations and recommendations for updates to protect public health. PLoS ONE 2022, 17, e0263505. [Google Scholar] [CrossRef]
- García, M.; Chang, Y.; Lee, J. Memory Impairment and Intelligence Retardation with Exposure to Fluoride and Arsenic. Sci. Insights 2019, 32, 116–124. [Google Scholar] [CrossRef]
- Martinez, V.D.; Vucic, E.A.; Becker-Santos, D.D.; Gil, L.; Lam, W.L. Arsenic exposure and the induction of human cancers. J. Toxicol. 2011, 2011, 431287. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Rehman, Y.; Katsoyiannis, I.A.; Kokkinos, E.; Zouboulis, A.I. Arsenic Exposure via Contaminated Water and Food Sources. Water 2022, 14, 1884. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Jin, W.; Hu, Q.; Zhao, Y. Adsorption behavior of arsenicals on MIL-101(Fe): The role of arsenic chemical structures. J. Colloid Interface Sci. 2019, 554, 692–704. [Google Scholar] [CrossRef]
- Bencko, V.; Foong, F.Y.L. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann. Agric. Environ. Med. 2017, 24, 312–316. [Google Scholar] [CrossRef]
- Bednar, A.J.; Garbarino, J.R.; Ranville, J.F.; Wildeman, T.R. Presence of Organoarsenicals Used in Cotton Production in Agricultural Water and Soil of the Southern United States. J. Agric. Food Chem. 2002, 50, 7340–7344. [Google Scholar] [CrossRef]
- Mojiri, A.; Razmi, E.; KarimiDermani, B.; Rezania, S.; Kasmuri, N.; Vakili, M.; Farraji, H. Adsorption methods for arsenic removal in water bodies: A critical evaluation of effectiveness and limitations. Front. Water 2024, 6, 1301648. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, H. Birnessite (δ-MnO2) mediated degradation of organoarsenic feed additive p-arsanilic acid. Environ. Sci. Technol. 2015, 49, 3473–3481. [Google Scholar] [CrossRef]
- Almousa, M.; Al-tayyem, B.H.; Ali, M.E.A.; Lim, Y.H.; Alshami, A. Enhanced arsenic adsorption in groundwater using Zirconium MOF-modified granular activated carbon. ACS EST Water 2025, 5, 4678–4691. [Google Scholar] [CrossRef]
- Ahmad, A.; Rutten, S.; de Waal, L.; Vollaard, P.; van Genuchten, C.; Bruning, H.; Cornelissen, E.; van der Wal, A. Mechanisms ofarsenate removal and membrane fouling in ferric based coprecipitation–low pressure membrane filtration systems. Sep. Purif. Technol. 2020, 241, 116644. [Google Scholar] [CrossRef]
- Zakhar, R.; Derco, J.; Čacho, F. An overview of main arsenic removal technologies. Acta Chim. Slov. 2018, 11, 107–113. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Singh, S.; Karwadiya, J.; Srivastava, S.; Patra, P.K.; Venugopalan, V. Potential of indigenous plant species for phytoremediation of arsenic contaminated water and soil. Ecol. Eng. 2022, 175, 106476. [Google Scholar] [CrossRef]
- Samimi, M.; Zakeri, M.; Alobaid, F.; Aghel, B. A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials 2022, 13, 60. [Google Scholar] [CrossRef]
- Mohan, B.; Kamboj, A.; Virender; Singh, K.; Priyanka; Singh, G.; Pombeiro, A.J.; Ren, P. Metal-organic frameworks (MOFs) materials for pesticides, heavy metals, and drugs removal: Environmental safety. Sep. Purif. Technol. 2023, 310, 123175. [Google Scholar] [CrossRef]
- Rasheed, T.; Hassan, A.A.; Bilal, M.; Hussain, T.; Rizwan, K. Metal-organic frameworks based adsorbents: A review from. removal perspective of various environmental contaminants from wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef]
- Ding, W.-Q.; Labiadh, L.; Xu, L.; Li, X.-Y.; Chen, C.; Fu, M.-L.; Yuan, B. Current advances in the detection and removal of organic arsenic by metal-organic frameworks. Chemosphere 2023, 339, 139687. [Google Scholar] [CrossRef]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Nguyen, D.V.; Jeong, G.; Asghar, N.; Jang, A. Critical. evaluation of hybrid metal–organic framework composites for efficient treatment of arsenic–contaminated solutions by adsorption and membrane–separation process. Chem. Eng. J. 2023, 461, 141789. [Google Scholar] [CrossRef]
- Berardozzi, E.; Tuninetti, J.S.; Einschlag, F.S.G.; Azzaroni, O.; Ceolín, M.; Rafti, M. Comparison of Arsenate Adsorption from Neutral pH Aqueous Solutions Using Two Different Iron-Trimesate Porous Solids: Kinetics, Equilibrium Isotherms, and Synchrotron X-Ray Absorption Experiments. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1185–1194. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Tang, L.; Luo, L.; Zeng, G. Iron Containing Metal–Organic Frameworks: Structure, Synthesis, and Applications in Environmental Remediation. ACS Appl. Mater. Interfaces 2017, 9, 20255–20275. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Malhotra, M.; Kaur, B.; Soni, V.; Patial, S.; Sharma, K.; Kumar, R.; Singh, P.; Thakur, S.; Pham, P.V.; Ahamad, T.; et al. Fe-based MOFs as promising adsorbents and photocatalysts for re-use water contained arsenic: Strategies and challenges. Chemosphere 2024, 357, 141786. [Google Scholar] [CrossRef]
- Jun, J.W.; Tong, M.; Jung, B.K.; Hasan, Z.; Zhong, C.; Jhung, S.H. Effect of Central Metal Ions of Analogous Metal–Organic Frameworks on Adsorption of Organoarsenic Compounds from Water: Plausible Mechanism of Adsorption and Water Purification. Chem. Eur. J. 2015, 21, 347–354. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2014, 5, 5261–5268. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, B.; Liu, J.; Hu, Y.; Cheng, H. Adsorption behavior and mechanism of modified Fe-based metal–organic framework for different kinds of arsenic pollutants. J. Colloid Interface Sci. 2024, 654, 426–436. [Google Scholar] [CrossRef]
- Phanthasri, J.; Khamdahsag, P.; Jutaporn, P.; Sorachoti, K.; Wantala, K.; Tanboonchuy, V. Enhancement of arsenite removal using manganese oxide coupled with iron (III) trimesic. Appl. Surf. Sci. 2018, 427, 545–552. [Google Scholar] [CrossRef]
- Azri, A.; Ben Amar, M.; Walha, K.; Fontàs, C.; Conde-González, J.E.; Salvadó, V.; Peña-Méndez, E.M. New Insights on Iron-Trimesate MOFs for Inorganic As(III) and As(V) Adsorption from Aqueous Media. Nanomaterials 2024, 15, 36. [Google Scholar] [CrossRef]
- Alam, E. Metal–organic frameworks (MOFs) for arsenic remediation: A brief overview of recent progress. RSC Adv. 2025, 15, 20281. [Google Scholar] [CrossRef] [PubMed]
- Conde-González, J.E.; Peña-Méndez, E.M.; Melián-Fernández, A.M.; Havel, J.; Salvadó, V. Synthesis, performance and mechanism of nanoporous Fe-(1,3,5-tricarboxylic acid) metal-organic framework in the removal of anionic dyes from water. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100541. [Google Scholar] [CrossRef]
- Cai, J.; Mao, X.; Song, W.-G. Adsorption behavior and structure transformation of mesoporous metal–organic frameworks towards arsenates and organic pollutants in aqueous solution. Mater. Chem. Front. 2018, 2, 1389–1396. [Google Scholar] [CrossRef]
- Li, X.; Lachmanski, L.; Safi, S.; Sene, S.; Serre, C.; Grenèche, J.M.; Zhang, J.; Gref, R. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci. Rep. 2017, 7, 13142. [Google Scholar] [CrossRef]
- Pena-Mendez, E.; Mawale, R.; Conde-Gonzalez, J.; Socas-Rodriguez, B.; Havel, J.; Ruiz-Perez, C. Metal organic framework composite, nano-Fe3O4@Fe-(benzene-1,3,5-tricarboxylic acid), for solid phase extraction of blood lipid regulators from water. Talanta 2020, 207, 120275. [Google Scholar] [CrossRef]
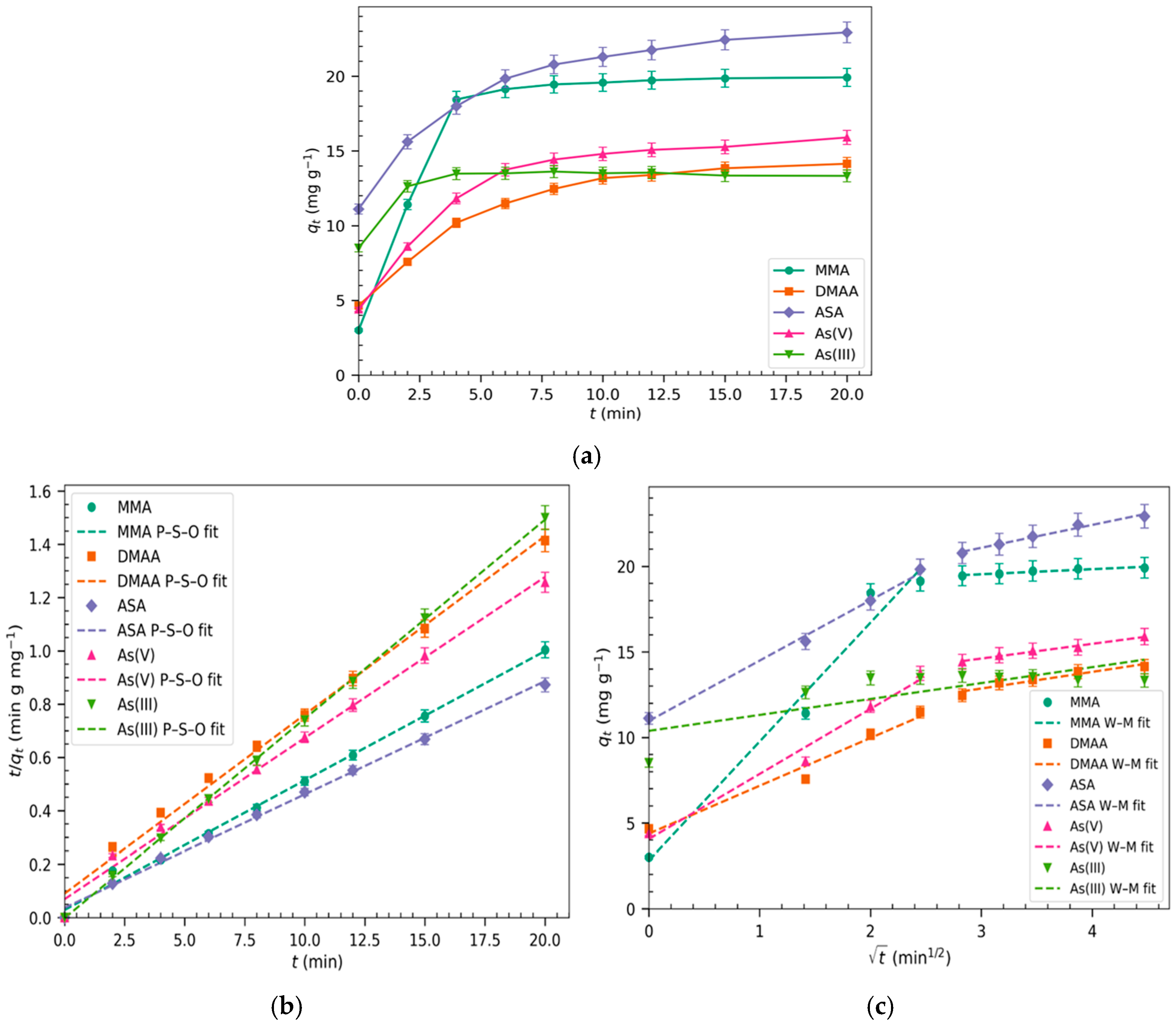



| Model | Equation | MMA | DMAA | ASA | As(V) | As(III) | |
|---|---|---|---|---|---|---|---|
| PFO | qe (mg g−1) | 6.95 | 9.29 | 10.11 | 8.70 | 0.672 | |
| K1 (min−1) | 0.223 | 0.080 | 0.122 | 0.138 | 0.092 | ||
| R2 | 0.841 | 0.895 | 0.968 | 0.934 | 0.198 | ||
| PSO | qe (mg g−1) | 20.6 | 14.9 | 23.53 | 16.6 | 13.4 | |
| k2 (g mg−1 min−1) | 0.082 | 0.050 | 4.94 × 10 −4 | 0.053 | −2.93 | ||
| R2 | 0.996 | 0.992 | 0.995 | 0.994 | 0.999 | ||
| Elovich | α (mg g−1 min−1) | 32.8 | 30.7 | 125.32 | 29.3 | 1.07 × 104 | |
| M1 | |||||||
| β (g mg−1) | 0.047 | 0.114 | 0.092 | 0.084 | 0.339 | ||
| R2 | 0.961 | 0.998 | 0.98 | 0.995 | 0.583 | ||
| Elovich | α′ (mg g−1 min−1) | 7.97 × 106 | 612 × 103 | 3734.79 | 4.98 × 104 | ||
| M2 | |||||||
| β′ (g mg−1) | 0.807 | 0.244 | 0.179 | 0.280 | |||
| R2 | 0.955 | 0.954 | 0.993 | 0.983 | |||
| W-M | KWM1 (mg g−1 min−1/2) | 6.95 | 2.79 | 3.53 | 3.80 | 0.932 | |
| M1 | |||||||
| DWM1 | 2.810 | 4.379 | 10.96 | 4.085 | 10.41 | ||
| R2 | 0.970 | 0.972 | 0.996 | 0.981 | 0.592 | ||
| W-M | KWM2 (mg g−1 min−1/2) | 0.295 | 0.975 | 1.338 | 0.862 | ||
| M2 | |||||||
| DWM2 | 18.66 | 9.934 | 17.07 | 12.04 | |||
| R2 | 0.926 | 0.924 | 0.982 | 0.987 |
| Model | Equation | MMA | DMAA | ASA | |
|---|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 37.6 | 25.9 | 42.2 | |
| KL (L mg−1) | 3.50 | 4.69 | 5.15 | ||
| R2 | 0.994 | 0.966 | 0.993 | ||
| SS | 0.001 | 0.033 | 0.001 | ||
| Freundlich | n | 6.1 | 5.7 | 3.4 | |
| KF (L g−1) | 25.7 | 21.1 | 21.5 | ||
| R2 | 0.811 | 0.389 | 0.663 | ||
| Temkin | A (L mg−1) B (J mol−1) | 7.11 × 10−25 0.069 | 7.21 × 10−10 0.032 | 3.87 × 10−20 0.040 | |
| R2 | 0.777 | 0.357 | 0.761 | ||
| Redlich–Peterson | βRP KRP (g L−1) | 0.033 0.039 | 0.033 0.047 | 0.033 0.037 | |
| R2 | 0.991 | 0.935 | 0.975 | ||
| SS | 0.012 | 0.067 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azri, A.; Walha, K.; Fontàs, C.; Conde-González, J.-E.; Peña-Méndez, E.M.; Seubert, A.; Salvadó, V. Iron-Based Metal–Organic Frameworks for the Removal of Different Organic and Inorganic Arsenic Species from Water: Kinetic and Adsorption Studies. Molecules 2025, 30, 4198. https://doi.org/10.3390/molecules30214198
Azri A, Walha K, Fontàs C, Conde-González J-E, Peña-Méndez EM, Seubert A, Salvadó V. Iron-Based Metal–Organic Frameworks for the Removal of Different Organic and Inorganic Arsenic Species from Water: Kinetic and Adsorption Studies. Molecules. 2025; 30(21):4198. https://doi.org/10.3390/molecules30214198
Chicago/Turabian StyleAzri, Afef, Khaled Walha, Claudia Fontàs, José-Elias Conde-González, Eladia M. Peña-Méndez, Andreas Seubert, and Victoria Salvadó. 2025. "Iron-Based Metal–Organic Frameworks for the Removal of Different Organic and Inorganic Arsenic Species from Water: Kinetic and Adsorption Studies" Molecules 30, no. 21: 4198. https://doi.org/10.3390/molecules30214198
APA StyleAzri, A., Walha, K., Fontàs, C., Conde-González, J.-E., Peña-Méndez, E. M., Seubert, A., & Salvadó, V. (2025). Iron-Based Metal–Organic Frameworks for the Removal of Different Organic and Inorganic Arsenic Species from Water: Kinetic and Adsorption Studies. Molecules, 30(21), 4198. https://doi.org/10.3390/molecules30214198










