Abstract
Mitigating climate change through the reduction of atmospheric CO2 emissions remains a critical global priority. Solid adsorbents, particularly shales, have become promising options for CO2 storage due to their favorable structural and chemical properties. In this study, a solid sorbent was developed by pyrolyzing shale at 800 °C under a nitrogen (N2) atmospheric condition, yielding spent shale. The key physicochemical properties influencing CO2 sorption were characterized using X-ray diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM), Brunauer–Emmett–Teller (BET) surface area analysis, and Temperature-Programmed Desorption (TPD). Mineralogical analysis revealed the presence of quartz, feldspars, clays, and carbonate minerals. The spent shale exhibited surface areas of 30–34 m2/g and pore diameters ranging from 3 to 10 nm. TPD results confirmed the presence of active adsorption sites, with a maximum CO2 sorption capacity of about 1.62 mmol/g—surpassing several commercial sorbents. Adsorption behavior was best described by the Sips and Toth isotherm models (R2 > 0.99), indicating multilayer and heterogeneous adsorption processes. Kinetic modeling using both pseudo-first-order and pseudo-second-order equations revealed that CO2 uptake was governed by both diffusion and chemisorption mechanisms. These findings positioned spent shale as a low-cost, efficient sorbent for CO2 storage, promoting circular resource utilization and advancing sustainable carbon management strategies. This novel shale-derived material offers a competitive pathway for carbon capture, storage, and sequestration applications.
1. Introduction
Carbon dioxide (CO2) stands out as the most prevalent greenhouse gas (GHG) acknowledged for its pivotal role in driving climate change and global warming [1]. Various nations, including the USA, China, and Malaysia, are currently advocating for the reduction in GHG emissions to protect the global climate. In January 2022, atmospheric carbon dioxide levels reached a peak of 418.19 ppm, a significant increase from 403 ppm in 2016 [2]. The increase in the rate of CO2 emission to the environment as a result of the combustion of fossil fuels (due to the high percentage of CO2 present in shale) has raised more concern about global climate change as well [3]. In response to this critical issue, it is imperative to implement effective strategies and develop innovative, cost-efficient technologies suitable for the CO2’s sequestration and utilization. The adoption of CCUS (Carbon Capture, Utilization, and Storage) techniques has become widespread. CCUS involves injecting CO2 into stable geological formations, such as shale, using predominantly sorption techniques to ensure its permanent retention in the geological formation [4].
The sorption of CO2 in shale holds significant promise for substantially reducing costs, enhancing safety, and prolonging the storage of CO2 for extended periods [5]. In addition, the low permeability, low porosity, and some of the minerals present in shale can adsorb large amounts of ions, water, natural gas, or other substances, making it an effective adsorbent material [6,7,8]. In the last few decades, several studies have shown that Shale formation possesses a significant storage capacity for CO2 under simulated conditions, and this has helped in minimizing the storage cost, which makes it an economically viable option compared to other solid adsorbents [9,10]. Zardari et al. [11] confirmed that CO2 sorption in shale occurs through both physical and chemical adsorption mechanisms. In physical sorption, van der Waals forces attract some gas molecules to the shale surface [12,13]. The CO2-quadrupole moment also aids its strong interaction with the electric field gradient of shale, hence, enhancing its adsorption as CO2 has the highest quadrupole moment among various gases, followed by CO (carbon monoxide), N2 (nitrogen), H2 (hydrogen), and CH4 (methane) [14].
However, several reports have enlightened the utilization of spent shales as solid sorbents in the last few years [15,16] based on their petrochemical characteristics. Spent shales, on the other hand, are end-products or solid residues of oil shale obtained through the oil-shale retorting process. These solid residues are quite available because they are usually discarded or transported back to the milling site, where they are dumped [17], hence causing more environmental pollution. Furthermore, the sorption potential of spent shales, as reports have shown that spent shales possess a larger surface area than raw shale, including a more microporous nature that enables the capturing of CO2 molecules, and retention of sorption-supporting minerals, to mention a few [18,19]. These properties are the prominent characteristics of a sustainable sorbent, especially for CO2 storage. Wang et al. [20] stated that the surface area and pore structure formed within the spent shale during pyrolysis have a major impact on its reaction with gas as a solid sorbent, while Wang, et al. [21] conducted an adsorption measurement on pyrolyzed coals (semicoke) and discovered that the surface area, pore diameters, as well as adsorption potential of these semicokes increased effectively with pyrolytic temperature during the pyrolysis process. Furthermore, Han et al. [17] summarized the potentiality of spent shale as a sorbent material based on the nature and characteristics of its pore structures, with a significant outcome, and Bai et al. [22] reported that the internal structures of spent shale can affect the efficient separation of gases during a sorption process, which will result in adequate recovery of pure gases. Alaloul et al. [23] performed a comprehensive review and confirmed that oil shale waste can be utilized for different purposes, and Bayaidah et al. [24] utilized spent shale in concrete production and stated that the chemical composition of spent shale aids its utilization for this purpose.
Thus far, most of these studies have shown that there has been more focus on the surface characteristics of spent shale and its utilization for other purposes, different from CO2 sorption. Invariably, there is a gap in knowledge on the CO2 sorption capacity of spent shale, which includes the effect that the pyrolytic temperatures can have on the sorption capacity of the spent shale or the influence of the pore variation after the pyrolysis on its sorption performance. Hence, understanding their geological origin, processing pathways, and physicochemical characteristics is essential for assessing their suitability as potential solid sorbents.
Therefore, this study aims to investigate the sorption capacity of some spent shales, obtained from hydrocarbon-bearing Marcellus shale, as effective solid sorbents for CO2 storage. Marcellus shales were employed as reports have proved that after their hydrocarbon production, numerous pores are available in the spent shales generated, which can serve as CO2 storage sites [25,26]. Thus, exploring their potential as an alternative to commercially available sorbents. In so doing, the physicochemical characteristics and the CO2 sorption analyses were investigated. The CO2 adsorption experiments were performed at 8 MPa to replicate subsurface reservoir conditions typical of geological storage (5–10 MPa), rather than atmospheric post-combustion capture. This pressure range reflects realistic conditions encountered in deep formations and allows a more accurate assessment of CO2 -geological formations interactions for long-term storage applications [27]. To validate the results obtained, some isotherm and kinetic models were employed. Finally, the results were compared with the raw shale adsorption behavior.
The significance of this research lies in its contribution to sustainable development by providing an alternative use for spent shales, thus reducing waste and promoting resource efficiency (supporting United Nations Sustainable Development Goal (UN-SDG): 12 Responsible Consumption and Clean Energy).
2. Results and Discussion
2.1. Morphology Characterization
2.1.1. Pore Distribution
Understanding the relationship between porous structures and adsorption aids in the calculation of gas in situ; thus, the characteristics of pores, such as pore shapes and distribution of pore sizes, help in determining the interconnectivity between pores and the sorption behaviors of shale.
Surface areas are commonly reported as BET surface areas obtained by applying the theory of Brunauer, Emmett, and Teller to nitrogen adsorption isotherms measured at 77 K, as nitrogen at 77 K is considered to be a standard adsorbate for surface area and pore size analysis. This is a standard procedure that allows for comparisons among different materials and with benchmark materials from the literature [28]. The pyrolyzed samples, S3-PY and S6-PY, possess larger surface area, pore sizes, and pore volume than the samples S3 and S6, as shown in Table 1. A notable increase in the pore size of S3-PY was observed compared to S3. This could be attributed to the decomposition of kerogen because kerogen holds a significant amount of micropores in shale, as reported by Gonciaruk et al. [29]. Thermal stress and kerogen pyrolysis can result in the development of more mesopores, and more pores and fractures can connect to form micro-fractures [30,31]. The kerogen type of sample S3 may have easily pyrolyzed during the pyrolysis process, thereby making S3-PY have more extensive pore merging, leading to a higher average pore size compared to the others. It can also be observed that the quantity of N2 adsorbed by the pyrolyzed samples is greater than the amount adsorbed by the non-pyrolyzed samples. The increased N2 uptake after pyrolysis indicates that the shale adsorption capacity is strongly related to the factors that can produce numerous pores [28]. The presence of abundant mesopores and micropores and a larger surface area in adsorbents results in a greater interactive energy between adsorbate and adsorbent. Therefore, the types of pores and surface areas can result in increased adsorption performance.

Table 1.
The BET characterization values of the raw and pyrolyzed samples.
The isotherm curves for the studied samples were described as Type II isotherm curves with an H2 hysteresis loop, as shown in Figure 1a–d. This description is in accordance with the IUPAC and BET classification. The Type II isotherm curves revealed complete monolayer adsorption up to the relative pressure of approximately 0.4 before multilayer adsorption occurs. Neck-like and wide-body pores or ink bottle-like pores obtained from the hysteresis loop confirmed the meso-macroporous nature of the samples. These features have been reported to be favorable for CO2 sequestration and storage, as the size of the pores present on the samples can adsorb more CO2 (33 nm) physically [32].
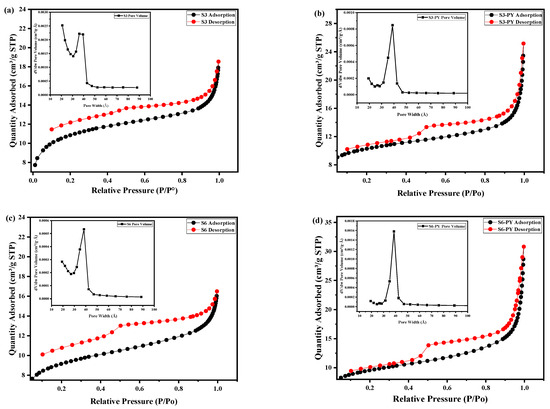
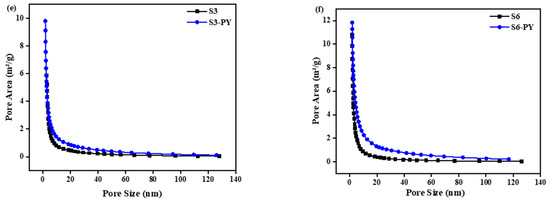
Figure 1.
N2 adsorption/desorption curves and the corresponding pore size distributions (insets) of (a) S3, (b) S3-PY, (c) S6, (d) S6-PY; and BJH pore size distribution of (e) S3/S3-PY, (f) S6/S6-PY.
The steepness of the capillary condensation steps suggested the presence of mesopores, which was further confirmed by the pore size distribution in the inset of Figure 1a–d.
The insets of Figure 1a–d show the pore volume distribution of the samples in relation to the pore width. Samples S3, S3PY, S6, and S6-PY exhibit pore width distribution in the range of 15–50 Å (1.5–5 nm), indicating the inner width of the pores. In the raw S3 and pyrolyzed samples, a pore filling was observed between 29 and 47 Å (2.9–4.7 nm) with a relatively lower peak pore volume, whereas S3-PY and S6-PY samples displayed a pore filling at 31–46 Å (3.1–4.6 nm), reflecting a more uniform pore structure. These apparent peaks observed around 3–4 nm in the inset graph of the pore volume of all samples show an artifact associated with cavitation and pore-blocking effects during desorption. The pore volume significantly increased in the pyrolysed samples as a result of a greater concentration of uniform pore size. Thus, pyrolysis of shale samples results in the development of a uniform pore network, concentrating on mesopores, which would contribute significantly to enhanced adsorption capability. The BJH pore size distribution analysis, as shown in Figure 1e,f, reveals the porosity in both raw and pyrolyzed samples, with the pyrolyzed samples having a larger area of pores. It further shows that the mesopore development becomes dominant after pyrolysis, reflecting structural reorganization and mineral decomposition at elevated temperature [33].
2.1.2. Mineral Contents
The XRD spectra of the shale samples S3, S6, S3-PY, and S6-PY indicate the presence of silicate minerals, dominantly quartz, feldspar, clay minerals, and carbonates, as shown in Figure 2. The intensity of the clay minerals present in the pyrolyzed samples reduces due to the heat of pyrolysis, eliminating some of their absorbed water. The presence of minerals such as quartz, feldspar, clay minerals, and carbonates in the samples influences the sorption capacity of shale due to the CEC (cation exchange capacity) of some of the minerals, specifically the clay minerals, as well as the inner sphere complexation that aids a direct bonding of the molecules to the surface of the minerals during chemical sorption [10]. Likewise, calcites are known to have a high affinity for CO2, with the molecular size of CO2 occupying several spaces, and also the energy of Ca-O, C-C, C-O, and O-O pair interaction of Lennard-Jones ranging from 0 to 1.43 × 10−2, 3.08 × 10−4, and 5.21 × 10−5 KJnm6mol−1, respectively, indicates an easier bond of CO2 on oxygen bonded with calcium molecules in Calcite [34].
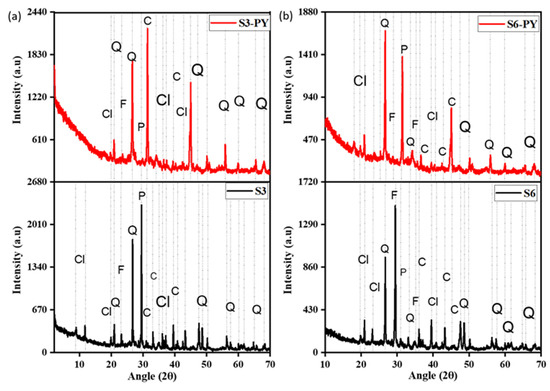
Figure 2.
The mineralogical content of (a) S3 and (b) S6 before and after pyrolysis, Cl = clay, Q = quartz, F = feldspar, P = pyrite, and C = carbonate minerals, respectively.
Furthermore, the abundance of feldspar and quartz in all samples reflects their stability at higher temperatures after the pyrolysis process, and these minerals have also been reported to influence the sorption behavior of shales [35,36,37]. The reduction in the peaks of most clay minerals is due to dehydroxylation at about 100 °C and deformation above 450–500 °C, e.g., kaolinite. However, some clay minerals, such as illite, deform at temperatures greater than 900 °C, and the internal structures of montmorillonite, kaolinite, and illite play a vital role in the sorption of CO2 on shales [9,38]. In addition to the mineral composition, the FTIR spectra indicate the presence of O-H, Si-O, and C-O functional groups in both raw and spent shales, as shown in Figure 3.
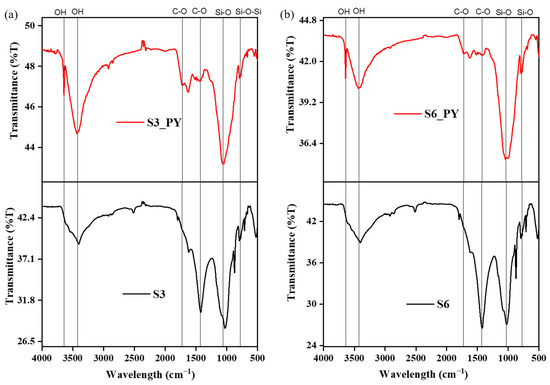
Figure 3.
The variation in the functional groups of (a) S3 and (b) S6 before and after pyrolysis.
These functional groups confirm the presence of the silicate and carbonate minerals obtained from the XRD, as some silicate minerals, such as kaolinite, illite, and other clay minerals, possess some absorbed moisture, which brought about the O-H group at 3000–3600 cm−1 [39,40]. Also, some silicate minerals such as feldspar and quartz with Si-O bonds appear at about 400–1100 cm−1 [41], and some carbonate bonds appear at 700–1400 cm−1 [42]. Reports have shown that these functional groups play vital roles in the adsorption behavior of any samples they are found in, as they can adsorb or desorb ions and molecules physically or chemically [43,44].
2.1.3. Surface Morphology
A comparison of FESEM images obtained before and after pyrolysis revealed significant changes in the pore morphology of the studied sample. Before pyrolysis, the images of the raw samples (Figure 4a,c) show the presence of various pore sizes due to the low porosity of an undisturbed sample. The pyrolysis of the samples created a pore network that increases the porosity of the samples due to the release of moisture and volatiles at elevated temperatures (Figure 4b,d).
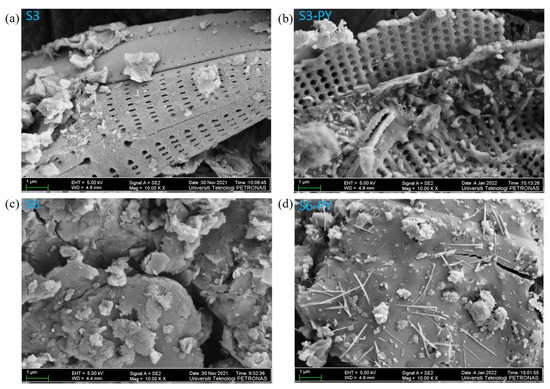
Figure 4.
FESEM images of (a) S3, (b) S3-PY, (c) S6, (d) S6-PY at 1 μm resolution and 10k magnification ratio.
In addition, the pyrolyzed samples reveal the presence of both intra-and intergranular pore structures, which are preserved in the mineral particles of feldspar and quartz, and also intercrystalline pores associated with the space generated in the clay minerals during dehydroxylation. Figure 4a also reveals the striae formation of silica with visible nanopores in sample S3. The distinct pore size, as well as the number of pores, increased in samples S3-PY (Figure 4b) due to the pyrolysis process. Furthermore, illite fibers were observed on the authigenic quartz and feldspar in sample S6-PY (Figure 4d), aligning with the sheet-like and hexagonal structures of illite identified in sample S6 (Figure 4c). The weight percent (wt.%) of each elemental composite in the studied samples was measured through the FESEM-EDX, as illustrated in Table 2.

Table 2.
Elemental composition (wt.%) of raw and pyrolyzed S3 and S6 obtained from FESEM-EDX.
Based on the EDX analysis, all samples contain a large amount of carbon, oxygen, silicon, and calcium, with some amount of aluminum, magnesium, potassium, iron, and sulfur, which are the main elements found in the silicate and carbonate minerals, as shown in Figure 5 and Figure 6. The carbon content of S3-PY is nearly identical to that of S3, indicating minimal change upon pyrolysis. In contrast, S6 exhibits a higher carbon content than S6-PY, and the observed decrease in carbon content after pyrolysis can be attributed to the loss of hydrocarbons present in the shale oil [45]. The amount of calcium in the pyrolyzed samples increased, which describes the abundance of calcite after the pyrolysis, as it is one of the minerals that are stable at higher temperatures [46]. Although these elements confirm the presence of the minerals observed from the XRD but the variation in the wt.% before and after pyrolysis is based on the thermal stability of each mineral [47]. In addition, the FESEM micrographs reveal some pieces of evidence that support the pore structures and mineralogy of the studied samples. Thus, they are good indications that the samples are porous and have significant sites for gas sorption.
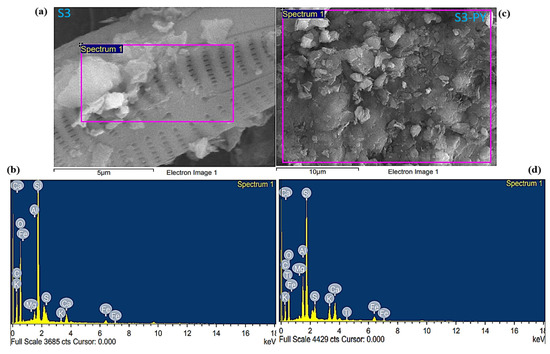
Figure 5.
FESEM-EDX of (a,b) S3 (c,d) S3-PY.
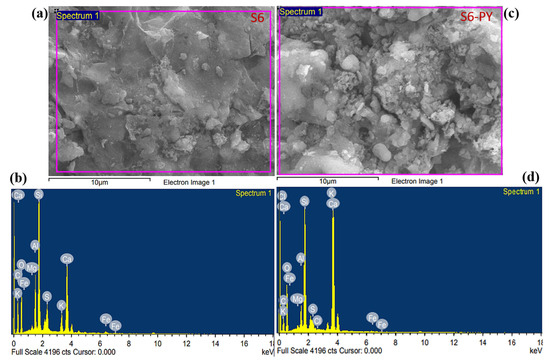
Figure 6.
FESEM-EDX of (a,b) S6 (c,d) S6-PY.
2.2. CO2 Sorption Measurements
The CO2 sorption capacity of both raw and pyrolyzed samples was measured through the Temperature Programmed Desorption (TPD) analysis and volumetric technique. The TPD techniques involve measuring the acidic/basic strength of the spent shale for sorption performance. This technique was employed primarily to probe the nature of CO2- shale surface interactions and to assess the strength of the adsorption sites. The procedure involves heating the samples under N2 gas at 28 °C, followed by the injection of CO2 until the temperature reaches 75 °C, and is held for 30 min to obtain the sorption measurement, then being completed by injecting Helium gas up to 500 °C for the next 30 min to desorb any form of chemisorbed CO2. The results showed that all samples obtained their maximum desorption peaks at around 450–500 °C (i.e., the highest temperature at which the chemically adsorbed CO2 in the spent shale completely desorbs [48,49]. As shown in Figure 7, the desorption spectrum (red line) indicates how the CO2 is being desorbed with increasing temperature until it attains its maximum peak, where it begins to fall. This same point aligns with the maximum temperature for chemisorbed CO2 (green line), which indicates that the temperature required for the desorption of CO2 was quite high as a result of the higher interaction of the CO2 with the active sites, thereby making it more difficult to desorb [49]. Invariably, this determines the bond strength between them as chemisorption, which can be attributed primarily to the presence of reactive clay mineral surfaces and carbonate decomposition products. Figure 7 shows that all studied samples have great adsorption sites for CO2, as well as a great affinity for CO2, and support the chemisorption process.
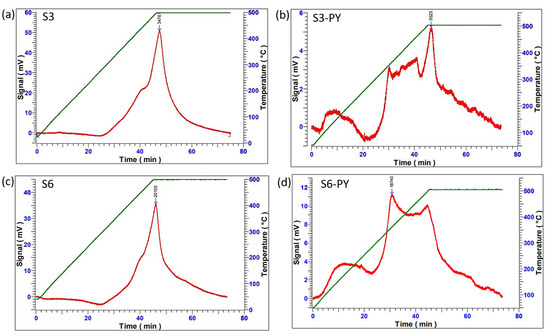
Figure 7.
The CO2 sorption/desorption process on (a) S3, (b) S3-PY, (c) S6, (d) S6-PY.
In addition to the TPD analysis, the results obtained through the volumetric technique showed that the sorption of CO2 on all studied samples increases relatively with pressure at an isothermal condition (See Figure 8). Also, the amount of CO2 sorbed varied with samples as the maximum amount of CO2 sorbed on raw samples S3 and S6 is 1.31 mmol/g and 1.45 mmol/g, while the pyrolyzed samples S3-PY and S6-PY obtained 1.62 mmol/g and 1.58 mmol/g, respectively (See Figure 8a). This indicates that the amount of CO2 adsorbed increased after pyrolysis. Furthermore, the results obtained from the volumetric techniques showed that the studied samples followed the same pattern under the same operating conditions, which indicates that the pyrolyzed shale samples also possess the force of attraction to sorb the CO2 molecules, similar to the raw shale samples. In addition, the persistence and transformation of clay minerals due to the pyrolysis treatment directly influence surface area and adsorption sites, which correlate with the observed uptake values of the pyrolyzed samples under the same operating conditions. In addition, the coexistence of mesopores and micropores is complementary because the micropores aid the sorption capacity by providing strong sites, while the mesopores improve gas transport and diffusion, ensuring that sorption sites remain accessible under high-pressure conditions. These explain why the spent shale exhibits high sorption capacity. Therefore, under relative geological conditions, spent shales showed effective sorption performance, making them suitable for industrial applications.
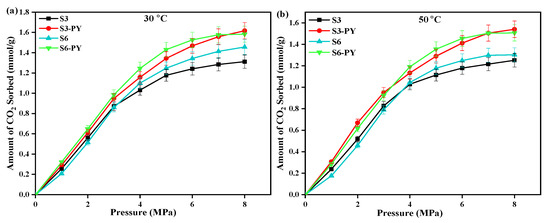
Figure 8.
Amount of CO2 sorbed and error bar points on raw and pyrolyzed samples S3 and S6 at (a) 30 °C, (b) 50 °C, and up to 8 MPa pressure.
2.3. Sorption Equilibrium Modeling
The CO2 sorption experiments for raw and pyrolyzed samples S3 and S6 were modulated with Langmuir, Freundlich, Sips, and Toth models as shown in Figure 9 and Figure 10. The values of each parameter obtained from the plots are illustrated in Table 3 and Table 4. The estimated values/parameters given in Table 3, Table 4, Table 5 and Table 6 were obtained using a non-linear regression analysis with 95% confidence intervals (CI). The experimental data showed a good match with all models; i.e., the sorption behaviors of all studied samples are best fitted with the Freundlich, Langmuir, Sips, and Toth models, with their R-squared value greater than 0.96.
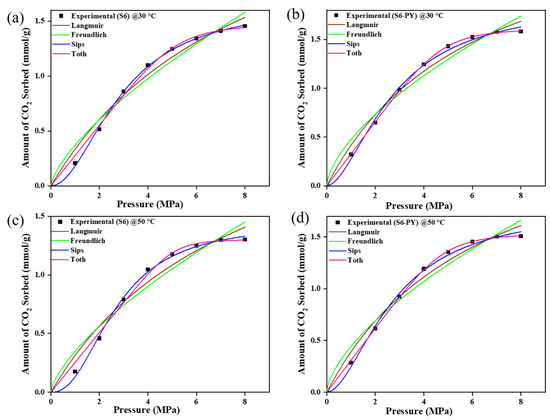
Figure 9.
The Isotherm curve-fit of samples S3 and S3-PY data at 30 °C (a,b) and 50 °C (c,d).
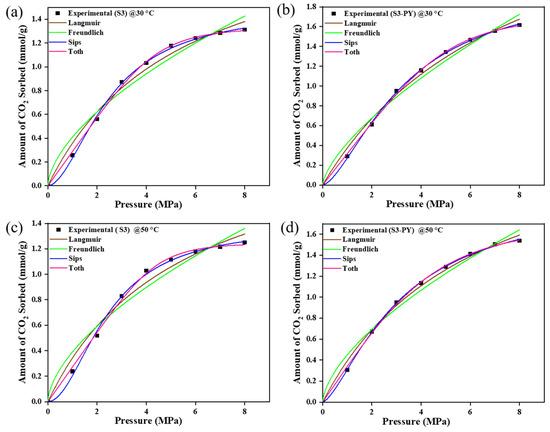
Figure 10.
The Isotherm curve-fit of samples S6 and S6-PY data at 30 °C (a,b) and 50 °C (c,d).

Table 3.
Isotherm model parameters’ values obtained at 30 °C.

Table 4.
Isotherm model parameters’ values obtained at 50 °C.

Table 5.
Kinetic model parameters’ values obtained at 30 °C.

Table 6.
Kinetic model parameters’ values obtained at 50 °C.
However, the pyrolysed samples showed greater agreement with the Sips and Toth model (R2 > 0.99), with the n-values of Sips exceeding 1 and being less than 1 for the Toth model.
The equilibrium models showed that the sorption process is supported by a heterogeneous phenomenon, as they conformed best to both the Sips and Toth models. This is because the Sips and Toth models best define a heterogeneous adsorption whereby the molecules of the adsorbate disperse throughout the surface of the adsorbent.
For the Sips model, the n-values were consistently greater than 1, indicating a heterogeneous adsorption surface with consistent adsorption behavior and the presence of adsorption sites created by pyrolysis. In contrast, the Toth model yielded n-values less than 1, suggesting a broader distribution of adsorption energies and highlighting the non-ideal, heterogeneous nature of the surface after pyrolysis. These results collectively imply that the structural modifications induced by pyrolysis enhance the heterogeneity of pore formation, thereby improving the samples’ capacity to adsorb gas molecules at varying energy sites. Additionally, the amount of CO2 sorbed increased in the range of 0.02–0.1 mmol/g for the Toth model and 0.2–0.4 mmol/g for the Sips model, which implies that the spent shale has the potential to attain increased sorption capacity above the studied temperature and pressure. This further confirms the viability of spent shale absorbents.
The strong agreement with both models demonstrates the applicability of the Sips and Toth isotherms for describing the adsorption characteristics of pyrolysed materials, contrasting with the fits observed for the raw samples. This confirms the agreement of the spent shale supporting heterogeneous adsorption. The heterogeneity of the materials has been confirmed by the morphological display of the FESEM images.
Similarly, as shown in Table 3 and Table 4, the n-values of the Freundlich model are less than 1, which supports favorable and physical sorption. This is in agreement with the type of sorption that has been reported to be favorable for CO2 sequestration on several shale samples [50,51]. Finally, a monolayer form of sorption is confirmed by the Langmuir model.
2.4. Kinetic Studies
The rate of sorption for the raw and pyrolyzed S3 and S6 at temperatures of 30 °C, 50 °C, and up to 8 MPa pressure is shown in Figure 11. The results revealed that the rates of sorption are reflections of the influence of the morphology, mineralogy, and the impact of pyrolysis. The information about the rate of adsorption was obtained from the curve fitting of the CO2 sorption results to the pseudo-first-order and pseudo-second-order models, with the parameters listed in Table 5 and Table 6. Thus far, the adsorption of CO2 on shale has been reported to be more diffusion-driven than chemisorption. However, there is a possibility of both scenarios occurring depending on the sorption behavior of the materials involved. These have been revealed from the sorption experimental results and the equilibrium model curves.
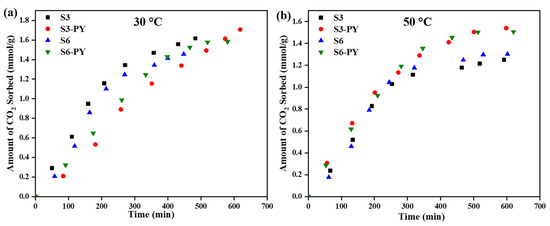
Figure 11.
Rate of CO2 sorption on raw and pyrolyzed S3 and S6 at (a) 30 °C, (b) 50 °C.
Based on the R2- values obtained for pseudo-first order and pseudo-second order being greater than 0.95 (see Table 5 and Table 6), it indicates that the rate of adsorption is driven by both diffusion (first-order) and chemical (second-order) processes. When this occurs, it means both models support the adsorption kinetics of the material, as pseudo-first order supports a diffusion phenomenon, while pseudo-second order supports a chemical phenomenon. This phenomenon is due to the minerals present in the shale samples, which contain some functional groups that can aid the chemical reaction of the shale with CO2 [52], along with the physisorption through the porous media. The models further validate the enhancement of the CO2 sorption capacity of shale by the pyrolysis method.
Table 7 presents a comparative analysis of the CO2 adsorption performance of the studied spent shale with other geological materials tested under similar pressure conditions (6–9 MPa) but at varying temperatures. The results indicate that the spent shale generally exhibits higher CO2 uptake than raw shale and coal samples evaluated at comparable pressures and slightly different thermal conditions. In some instances, its adsorption capacity was comparable to that of the reference materials; however, the superior performance of the spent shale can be attributed to the structural and surface modifications induced by pyrolysis. The high-temperature treatment enhanced pore development exposed additional active sites, and increased surface heterogeneity, all of which contributed to greater CO2 affinity. These findings demonstrate that thermal activation through pyrolysis significantly improves the sorption potential of shale for high-pressure CO2 storage applications.

Table 7.
Comparison of the sorption capacity of the shale sample within 6–8 MPa at different temperatures.
Beyond adsorption capacity, spent shale offers several practical advantages over conventional adsorbents. As a by-product of oil shale processing, it is readily available at low or negligible cost, providing a highly cost-effective option for large-scale CO2 capture. Its mineral-rich framework imparts good mechanical strength, reducing the risk of structural collapse under high-pressure storage conditions. In addition, the predominantly inorganic composition enhances hydrothermal stability, ensuring that adsorption performance is maintained even under moist or thermally fluctuating environments. These attributes make spent shale not only a technically viable adsorbent but also a sustainable and scalable material for carbon capture applications.
3. Materials and Methods
3.1. Materials
Two Devonian shale samples were selected from the Marcellus Formation, USA, labeled S3 and S6 with high Total Organic Content (TOC) of 16.2% and 17.9%, respectively. Nitrogen (N2) gas was utilized for the regeneration, purging, and pyrolysis processes, while high-purity CO2 and helium (He) gases were used for the sorption process. A mortar grinder for sample pulverization and a heating tube furnace for the pyrolysis procedure were used respectively.
3.2. Sample Preparation
The shale samples were first cleaned and oven-dried at 100 °C for 4 h to remove volatile impurities. The dried material was then crushed into a fine powder using a mortar grinder and sieved to obtain particles within the 0.118–0.250 mm range. To preserve consistency prior to pyrolysis and adsorption measurements, the sieved samples were stored in airtight bags under controlled conditions. The pyrolysis of the powdered samples was carried out in a tube furnace (PROTHERM 5) using nitrogen gas (99.995% purity). The equipment set-up gradually increases the temperature from room temperature to 800 °C and holds for 4 h with a 5 °C/min ramping rate and 2 mL/min gas purging, then automatically cools to room temperature after the pyrolysis is completed. The pyrolysis temperature of 800 °C was selected based on reported studies that indicated that shale heated to ~800 °C undergoes extensive volatile escape, carbonate decomposition, and pore structure development [30,45], hence, resulting in enhanced adsorption capacity compared to lower temperatures. The char generated after the pyrolysis is referred to as a spent shale, which was used in this study for structural analysis and adsorption capacity measurement. These char samples were labeled S3-PY and S6-PY, respectively.
3.3. Sample Characterization
The porous classification of the raw and spent shale samples was examined through the BET/N2 adsorption/desorption techniques using the ASAP 2020 equipment (Micromeritics, Norcross, GA, USA) at the boiling point of N2 (−195.8 °C). The degassing of the samples was performed in a vacuum at 90 °C for an hour, followed by the injection of helium at 150 °C for 2 h to desorb the N2. The BET surface area was obtained from the BET multipoint data. Also, the amount of N2 sorbed at the relative pressure (P/P0 ≈ 1) was calculated, and the distribution of pore sizes and volume was determined through the Barrett-Joyner-Halenda (BJH) method.
Crystallographic phase identification and morphological structures of the samples were measured under XRD using the Malvern Panalytical equipment (Malvern, UK, with Cu Kα (λ = 0.1540 nm) radiation, ranging 2–70° 2θ angle, and a SUPRA 55VP FESEM (Field Emission Scanning Electron Microscopy, ZEISS, Oberkochen, Germany), respectively, before and after pyrolysis. Additional identification of the elements present in the raw and spent shale was examined through Energy-dispersive X-ray Spectroscopy (EDX) attached to the FESEM equipment. The nature of functional groups on the surface of the materials was determined through Fourier Transform Infrared (FTIR) Spectra using a Perkin Elmer spectrometer (Model: Frontier 01, Shelton, CT, USA) within the range of 4000–500 cm−1.
The Temperature Programmed Desorption (TPD) technique was utilized under the TPDRO model 1100 equipment (Thermo Fisher Scientific, Waltham, MA, USA) to measure the acidic/basic strength of the spent shale for sorption by identifying and characterizing the active sites present on the surface under the influence of temperature. Samples were heated under N2 gas at 28 °C, followed by the injection of CO2 until the temperature reached 75 °C, and held for 30 min to obtain the sorption measurement. Then TPD analysis was completed by injecting the Helium gas up to 500 °C for the next 30 min to desorb any form of chemisorbed CO2. The TPD results provide qualitative and semi-quantitative insights into the binding behavior of CO2 on shale surfaces.
3.4. Sorption Measurement
The volumetric sorption technique is one of the most prominent methods for evaluating the sorption capacity of a material. In this study, CO2 sorption measurements were performed at temperatures of 30 °C and 50 °C, and pressures of up to 8 MPa. The approach is to simulate a reservoir condition. The pressure was increased by 1 MPa per section to obtain an equilibrium until the maximum pressure was reached. The amount of gas sorbed at each section was calculated using the ideal gas law with a substantial compressibility factor. The Peng-Robinson Equation of State was further employed to determine the compressibility factors at interval pressures.
Equation (1) is an expression for the equilibrium pressure attained at each section, where Pi and Pf are the initial gas pressure injected into the sorbent and the final pressure obtained at equilibrium, respectively. The amount of gas sorbed at each section is expressed by Equations (2) and (3), where Z is the compressibility factor measured by the initial and final pressure at each section. R denotes the gas constant, and T refers to temperature, which is also constant throughout a certain section [59]. At 50 °C and pressures above 73.8 bar, CO2 is in the supercritical state; thus, the isotherm data represent supercritical adsorption behavior. The experimental apparatus used for these measurements has been previously validated for high-pressure and supercritical CO2 adsorption studies, ensuring accuracy and reliability. These measurements provide quantitative adsorption capacities relevant for geological storage applications.
3.5. Isotherm Models
Some two- and three-parameter isotherm models were employed to validate the experimental results of this study. The equilibrium parameters essential to explain the sorption process were obtained using the non-linear regression (OriginPro 2022). This helps to estimate the values of the parameters by minimizing the sum of squared residuals, and the quality of the fit was assessed based on the coefficient of determination (R2) and the root mean square error (RMSE). This approach ensures robust and reproducible parameter estimation.
3.5.1. Langmuir Model
This is one of the most common isotherm models employed for gas-solids sorption. It assumes that both sorbate and sorbent behave in an ideal manner at isothermal conditions, whereby the sorbates are distributed homogeneously on the surface of a sorbent, thereby forming a monolayer type of sorption [60,61]. It also presumes an equilibrium between the rate at which gas sorbs on the surface and the rate at which the solid desorbs the gas afterward [38,62]. The equilibrium Langmuir isotherm can be expressed as:
The qm and kL are the two parameters of the Langmuir model, where qm denotes the maximum sorption capacity, while kL is the constant.
3.5.2. Freundlich Model
The Freundlich model is one of the oldest known empirical models with two parameters, frequently employed for the sorption of gases [63]. It assumes the sorbates and sorbent interaction are in a heterogeneous system where the sorption is not limited to a monolayer formation, i.e., a formation of multilayer sorption is possible [64]. It best describes a non-ideal and reversible type of sorption. In addition, it describes the active site distribution, surface heterogeneity, and the energy of sorption of each site [65,66]. It can be expressed in a non-linear form in Equation (5), whereby the adsorption intensity (1/nF) represents the energy distribution and measures the heterogeneity of adsorbent sites. At 0 < 1/nF < 1, sorption is considered favorable, and when 1/nF > 1, the sorption process is unfavorable, and irreversible when = 1. While kF is the sorption potential of a sorbent.
3.5.3. Sips Model
The Sips isotherm model is an empirical three-parameter model also known as a hybrid isotherm of the Langmuir and the Freundlich models [67]. This isotherm model is presented to better predict the heterogeneity of the sorption surfaces and to overcome the inadequacies of the Freundlich isotherm’s increasing sorbate concentration [68]. It tends toward the Freundlich isotherm when the concentration of the sorbate is low and tends to predict the Langmuir isotherm when the concentration is high, forming a monolayer sorption phenomenon [69]. Equation (6) expresses the Sips model form.
The maximum sorption capacity, qm, constant, KS, and the Sips exponential, 1/nS, are the three parameters of the model.
3.5.4. Toth Model
The Toth model is an empirical isotherm model designed to improve the fitness of the Langmuir model to experimental results at relatively high pressures. It describes heterogeneous sorption systems, and it is appropriate for both low and high pressures or concentration ranges [60,70]. It describes a variety of systems with consistent sub-monolayer coverage, with its equilibrium expression linearized in Equation (7).
The qm, KT, and nT represent the maximum sorption capacity, the Toth model constant, and the Toth isotherm exponential, respectively. Where the Toth exponential, nT, denotes the surface heterogeneity, usually less than or equal to unity (0 < 1/nT < 1).
3.6. Kinetic Model
This model describes the sorption mechanism of an adsorbate molecule on the surface of a sorbent under the influence of the sorption equilibrium time [71]. The kinetic study is essential for the sorption process because it evaluates the sorption capacity of the sorbent, the total mass transfer of the sorbate on the sorbent, the pathway of the reaction, and the sorption mechanism [72]. In this study, the pseudo-first-order and pseudo-second-order reactions were employed to best explain the CO2 sorption mechanism and equilibrium rate in correlation with the studied data.
3.6.1. Pseudo-First-Order Model
This is the first-order rate of adsorption suggested by Lagergren [73] to describe a liquid-solid sorption phase [74]. It assumes that the rate of sorption of a sorbate is an expression of the difference between the saturation concentration and the amount of uptake with time. This model is expressed in Equation (8).
qt and qe denote the uptake at a time “t” and equilibrium, respectively, and k1 is the rate constant.
3.6.2. Pseudo-Second-Order Model
The pseudo-second order, on the other hand, assumes that the kinetic rate of sorption could not be limited to chemisorption, i.e., a covalent bond or electrons shared between the sorbate and the sorbent [74]. It applies to all sorption processes that involve internal particle diffusion and external film diffusion, where the total rate of adsorption is assumed [75]. Equation (9) best expresses the pseudo-second order
qt and qe denote the uptake at a time “t” and equilibrium, respectively, while k2 is the rate constant.
4. Conclusions
This study demonstrates the potential of pyrolyzed shale as an effective solid sorbent for CO2 capture. Thermal treatment at 800 °C under a nitrogen atmospheric condition significantly enhanced the shale’s physicochemical properties, including surface area and pore structure, which are critical for adsorption performance. Mineralogical analysis revealed the presence of quartz, feldspars, clays, and carbonate minerals, while TPD analysis confirmed the availability of active sites conducive to CO2 sorption. The spent shale achieved a notable CO2 sorption capacity of 1.62 mmol/g, outperforming several commercial sorbents. Adsorption isotherm modeling, particularly using the Sips and Toth models, indicated multilayer and heterogeneous adsorption behavior, while kinetic studies revealed that both diffusion and chemisorption processes governed the sorption mechanism. These findings position spent shale as a promising, low-cost, and sustainable sorbent for CO2 capture and sequestration. Beyond its technical viability, the reuse of spent shale for environmental remediation supports circular economy principles and resource valorisation. The selectivity of pyrolyzed shale toward CO2 under mixed-gas conditions has not yet been addressed in this study, owing to the fact that in actual flue gas, CO2 competes with N2, O2, and water vapor.
Beyond laboratory-scale adsorption, challenges remain in shaping, mechanical strength, and regeneration energy requirements that influence industrial viability. Future research should focus on process optimization, regeneration performance, and scale-up potential to further establish spent shale as a viable option for industrial carbon management applications.
Author Contributions
Conceptualization, A.I.B., H.T.G. and E.P.; methodology, A.I.B., H.T.G. and J.Y.Y.; software, E.P., A.O.B. and H.S.; validation, E.P., H.S. and H.T.G.; formal analysis, A.I.B. and J.Y.Y.; investigation, A.I.B., H.T.G. and J.Y.Y.; resources, H.T.G., H.S. and A.O.B.; data curation, A.I.B., J.Y.Y. and A.O.B.; writing—A.I.B. and J.Y.Y.; writing—review and editing, H.T.G., E.P. and H.S.; visualization, A.I.B., J.Y.Y. and A.O.B.; supervision, H.T.G. and H.S.; project administration, H.T.G. and E.P.; funding acquisition, H.T.G. and A.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Yayasan Universiti Teknologi PETRONAS (YUTP) Research Grant Scheme under grant numbers YUTP-FRG/015LC0-489 and YUTP-PRG/015PBC059, awarded to Haylay Tsegab Gebretsadik and Abdullateef Oluwagbemiga Balogun, respectively. Additional financial support from the Centre for Graduate Studies, Universiti Teknologi PETRONAS (CGS-UTP), is also gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to express their sincere gratitude to Abdurrasheed I. Yerima for his valuable and constructive review of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett-Joyner-Halenda |
| CCUS | Carbon Capture Uptake and Sequestration |
| EDX | Energy-dispersive X-ray Spectroscopy |
| EoS | Equation of State |
| FESEM | Field Emission Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared |
| GHG | Greenhouse Gases |
| IUPAC | International Union of Pure and Applied Chemistry |
| MPa | MegaPascal |
| SDG | Sustainable Development Goal |
| TOC | Total Organic Content |
| TPD | Temperature-Programmed Desorption |
| UN | United Nation |
| XRD | X-ray Diffraction |
| RMSE | Root Mean Square Error |
References
- Bernard, R.; Tzamourani, P.; Weber, M. Climate Change and Individual Behavior; SSRN: Rochester, NY, USA, 2022. [Google Scholar]
- Edouard, M.N.; Okere, C.J.; Ejike, C.; Dong, P.; Suliman, M.A. Comparative numerical study on the co-optimization of CO2 storage and utilization in EOR, EGR, and EWR: Implications for CCUS project development. Appl. Energy 2023, 347, 121448. [Google Scholar] [CrossRef]
- Fu, H.; Song, K.; Zhang, Z.; Han, X.; Yang, E.; Zhao, Y.; Song, Q.; Liang, L. Application of CO2 flooding method in heterogeneous shale oil reservoirs with nano-confinement effect: Enhanced oil recovery and CO2 sequestration. Geoenergy Sci. Eng. 2025, 255, 214098. [Google Scholar] [CrossRef]
- Mudoi, M.P.; Prusty, B.K. Controlling parameters of CH4 and CO2 adsorption on shale—A review. Arab. J. Geosci. 2022, 15, 526. [Google Scholar] [CrossRef]
- Bashir, A.; Ali, M.; Patil, S.; Aljawad, M.S.; Mahmoud, M.; Al-Shehri, D.; Hoteit, H.; Kamal, M.S. Comprehensive review of CO2 geological storage: Exploring principles, mechanisms, and prospects. Earth-Sci. Rev. 2024, 249, 104672. [Google Scholar] [CrossRef]
- Mansouri, S.; Majdoubi, H.; Haddaji, Y.; Tamraoui, Y.; El Achaby, M.; Manoun, B.; Abourriche, A.; Hannache, H.; Oumam, M. Optimization Studies of Porous Carbon Preparation from Oil Shale Using Response Surface Methodology and Its Application for Phenol Adsorption. Chem. Res. Chin. Univ. 2020, 36, 1339–1347. [Google Scholar] [CrossRef]
- Oumam, M.; Abourriche, A.; Mansouri, S.; Mouiya, M.; Benhammou, A.; Abouliatim, Y.; Hafiane, Y.E.; Hannache, H.; Birot, M.; Pailler, R. Comparison of chemical and physical activation processes at obtaining adsorbents from Moroccan oil shale. Oil Shale 2020, 37, 139–157. [Google Scholar] [CrossRef]
- Balogun, A.I.; Abdulkareem, F.A.; Irfan, S.A.; Padmanabhan, E. The Effective Process Parameters of CO2 Adsorption on Peninsular Malaysia Shale Samples. In Proceedings of the SPE/AAPG/SEG Asia Pacific Unconventional Resources Technology Conference, Virtual, 16–18 November 2021. [Google Scholar]
- Du, X.; Guang, W.; Cheng, Y.; Hou, Z.; Liu, Z.; Yin, H.; Huo, L.; Lei, R.; Shu, C. Thermodynamics analysis of the adsorption of CH4 and CO2 on montmorillonite. Appl. Clay Sci. 2020, 192, 105631. [Google Scholar] [CrossRef]
- Du, X.; Pang, D.; Cheng, Y.; Zhao, Y.; Hou, Z.; Liu, Z.; Wu, T.; Shu, C. Adsorption of CH4, N2, CO2, and their mixture on montmorillonite with implications for enhanced hydrocarbon extraction by gas injection. Appl. Clay Sci. 2021, 210, 106160. [Google Scholar] [CrossRef]
- Zardari, Z.H.; Mohshim, D.F.; Sarmadivaleh, M.; Md Yusof, M.A.; Aftab, A. Comprehensive review of CO2 adsorption on shale formations: Exploring widely adopted isothermal models and calculation techniques. ACS Omega 2024, 9, 50078–50096. [Google Scholar] [CrossRef]
- Lee, G.; Ahmed, I.; Jhung, S.H. CO2 adsorption using functionalized metal–organic frameworks under low pressure: Contribution of functional groups, excluding amines, to adsorption. Chem. Eng. J. 2024, 481, 148440. [Google Scholar] [CrossRef]
- Weldekidan, H.; Patel, H.; Mohanty, A.; Misra, M. Synthesis of porous and activated carbon from lemon peel waste for CO2 adsorption. Carbon Capture Sci. Technol. 2024, 10, 100149. [Google Scholar] [CrossRef]
- Chen, S.; Wu, W.; Niu, Z.; Kong, D.; Li, W.; Tang, Z.; Zhang, D. High adsorption selectivity of activated carbon and carbon molecular sieve boosting CO2/N2 and CH4/N2 separation. Chin. J. Chem. Eng. 2024, 67, 282–297. [Google Scholar]
- Avid, B.; Purevsuren, B.; Dugarjav, J. Pyrolysis and thermogravimetric investigation of the Mongolian Khoot oil shale. Oil Shale 2000, 17, 241–251. [Google Scholar] [CrossRef]
- Lin, L.; Lai, D.; Guo, E.; Zhang, C.; Xu, G. Oil shale pyrolysis in indirectly heated fixed bed with metallic plates of heating enhancement. Fuel 2016, 163, 48–55. [Google Scholar] [CrossRef]
- Han, X.; Kulaots, I.; Jiang, X.; Suuberg, E.M. Review of oil shale semicoke and its combustion utilization. Fuel 2014, 126, 143–161. [Google Scholar] [CrossRef]
- Balogun, A.I.; Padmanabhan, E.; Abdulkareem, F.A.; Gebretsadik, H.T.; Wilfred, C.D.; Soleimani, H.; Viswanathan, P.M.; Wee, B.S.; Yusuf, J.Y. Optimization of CO2 Sorption onto Spent Shale with Diethylenetriamine (DETA) and Ethylenediamine (EDA). Materials 2022, 15, 8293. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Han, X.; Tong, J. Effect of retorting temperature on product yield and characteristics of non-condensable gases and shale oil obtained by retorting Huadian oil shales. Fuel Process. Technol. 2014, 121, 9–15. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Guo, Q.; Geng, Q.; Yan, Y.; Hu, X. The further activation and functionalization of semicoke for CO2 capture from flue gases. RSC Adv. 2018, 8, 35521–35527. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y.; Guo, M. Evaluation of the porous structure of Huadian oil shale during pyrolysis using multiple approaches. Fuel 2017, 187, 1–8. [Google Scholar] [CrossRef]
- Alaloul, W.S.; Al Salaheen, M.; Malkawi, A.B.; Alzubi, K.; Al-Sabaeei, A.M.; Musarat, M.A. Utilizing of oil shale ash as a construction material: A systematic review. Constr. Build. Mater. 2021, 299, 123844. [Google Scholar] [CrossRef]
- Bayaidah, R.H.; Habashneh, A.A.O.; Al-Ma’aitah, S.H.; Alfahajin, M.S.; Al-Kheetan, M.J.; Jweihan, Y.S.; Alrwashdeh, S.S.; Al-Hamaiedeh, H.; Ghaffar, S.H. Utilisation of raw oil shale as fine aggregate to replace natural sand in concrete: Microstructure, surface chemistry and macro properties. Results Eng. 2023, 19, 101265. [Google Scholar] [CrossRef]
- Yildirim, L.T.O.; Wang, J. Assessment of Total and Contingent CO2 Storage Resources in the Marcellus Shale. Geoenergy Sci. Eng. 2025, 247, 213669. [Google Scholar] [CrossRef]
- Saputra, W.; Kirati, W.; Hughes, D.; Patzek, T.W. Forecast of economic gas production in the Marcellus Shale. AAPG Bull. 2024, 108, 15–40. [Google Scholar] [CrossRef]
- Chang, Y.; Yao, Y.; Wang, L.; Zhang, K. High-Pressure adsorption of supercritical methane and carbon dioxide on Coal: Analysis of adsorbed phase density. Chem. Eng. J. 2024, 487, 150483. [Google Scholar] [CrossRef]
- Garvie, L.A.; Trif, L.; Cotto-Figueroa, D.; Asphaug, E.; Hoover, C.G. High surface area and interconnected nanoporosity of clay-rich astromaterials. Sci. Rep. 2024, 14, 10358. [Google Scholar] [CrossRef]
- Gonciaruk, A.; Hall, M.R.; Fay, M.W.; Parmenter, C.D.; Vane, C.H.; Khlobystov, A.N.; Ripepi, N. Kerogen nanoscale structure and CO2 adsorption in shale micropores. Sci. Rep. 2021, 11, 3920. [Google Scholar] [CrossRef]
- Lei, J.; Pan, B.; Guo, Y.; Fan, Y.; Xue, L.; Deng, S.; Zhang, L.; Ruhan, A. A comprehensive analysis of the pyrolysis effects on oil shale pore structures at multiscale using different measurement methods. Energy 2021, 227, 120359. [Google Scholar] [CrossRef]
- Liu, B.-S.; Yao, C.-J.; Qi, J.-L.; Liu, Y.-Q.; Xu, L.; Hou, J.-X. Experimental investigation on pyrolysis products and pore structure characteristics of organic-rich shale heated by supercritical carbon dioxide. Pet. Sci. 2024, 21, 2393–2406. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, H.; Zhu, X.; Li, Z.; Liu, J. Characterization of shale pore structure by successive pretreatments and its significance. Fuel 2020, 269, 117412. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Lu, C.; Zou, J.; Ruan, M.; Yin, Y.; Qing, M.; Song, Q. Enhancement of catalytic activity in NH3-SCR reaction by promoting dispersibility of CuCe/TiO2-ZrO2 with ultrasonic treatment. Ultrason. Sonochem. 2021, 72, 105466. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Franco, L.F.; Castier, M.; Economou, I.G. Molecular dynamics simulation of n-alkanes and CO2 confined by calcite nanopores. Energy Fuels 2018, 32, 1934–1941. [Google Scholar] [CrossRef]
- McConville, C.J.; Lee, W.E. Microstructural development on firing illite and smectite clays compared with that in kaolinite. J. Am. Ceram. Soc. 2005, 88, 2267–2276. [Google Scholar] [CrossRef]
- Šliaupa, S.; Lozovskis, S.; Lazauskienė, J.; Šliaupienė, R. Petrophysical and mechanical properties of the lower Silurian perspective oil/gas shales of Lithuania. J. Nat. Gas Sci. Eng. 2020, 79, 103336. [Google Scholar] [CrossRef]
- Mahmoud, M.; Hussein, I.; Carchini, G.; Shawabkeh, R.; Eliebid, M.; Al-Marri, M.J. Effect of rock mineralogy on Hot-CO2 injection for enhanced gas recovery. J. Nat. Gas Sci. Eng. 2019, 72, 103030. [Google Scholar] [CrossRef]
- Abdulkareem, F.A.; Radman, A.; Faugere, G.; Sathiavelu, S.; Irfan, S.A.; Padmanabhan, E. Petro-physical properties of Marcellus shale samples and their impact on CO2 adsorption: Equilibrium, kinetics, and empirical modeling study. J. Nat. Gas Sci. Eng. 2020, 81, 103423. [Google Scholar] [CrossRef]
- Farmer, V. Transverse and longitudinal crystal modes associated with OH stretching vibrations in single crystals of kaolinite and dickite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 927–930. [Google Scholar] [CrossRef]
- Jozanikohan, G.; Abarghooei, M.N. The Fourier transform infrared spectroscopy (FTIR) analysis for the clay mineralogy studies in a clastic reservoir. J. Pet. Explor. Prod. Technol. 2022, 12, 2093–2106. [Google Scholar] [CrossRef]
- Nana, A.; Tchummegne, I.K.; Tome, S.; Adesina, A.; Alomayri, T.; Singla, R.; Kaze, R.C.; Kamseu, E.; Kumar, S.; Leonelli, C. Comparison of feldspar and meta-halloysite geopolymers by alkaline and acidic activation. Constr. Build. Mater. 2024, 424, 135953. [Google Scholar] [CrossRef]
- Kim, Y.; Caumon, M.-C.; Barres, O.; Sall, A.; Cauzid, J. Identification and composition of carbonate minerals of the calcite structure by Raman and infrared spectroscopies using portable devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119980. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR spectroscopic and thermal study of waterborne polyurethane-acrylate leather coatings using tartaric acid as an ionomer. e-Polymers 2016, 16, 463–474. [Google Scholar] [CrossRef]
- Lu, C.; Ma, L.; Guo, J.; Xiao, S.; Zheng, Y.; Yin, C. Effect of acidizing treatment on microstructures and mechanical properties of shale. Nat. Gas Ind. B 2020, 7, 254–261. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Zhang, L.; Zhang, C.; Wang, T.; Zhang, H. The effect of temperature on pyrolysis products during oil shale thermal decomposition. Sci. Rep. 2025, 15, 26135. [Google Scholar] [CrossRef] [PubMed]
- Karunadasa, K.S.; Manoratne, C.; Pitawala, H.; Rajapakse, R. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; Elzinga, E.J.; Ramlogan, M.; Rouff, A.A.; Alleoni, L.R.F. Calcium enhances adsorption and thermal stability of organic compounds on soil minerals. Chem. Geol. 2021, 559, 119804. [Google Scholar] [CrossRef]
- Barrie, P.J. Analysis of temperature programmed desorption (TPD) data for the characterisation of catalysts containing a distribution of adsorption sites. Phys. Chem. Chem. Phys. 2008, 10, 1688–1696. [Google Scholar] [CrossRef]
- Rakić, V.; Damjanović, L. Temperature-programmed desorption (TPD) methods. In Calorimetry and Thermal Methods in Catalysis; Springer: Berlin/Heidelberg, Germany, 2013; pp. 131–174. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Iddphonce, R.; Wang, J.; Zhao, L. Review of CO2 injection techniques for enhanced shale gas recovery: Prospect and challenges. J. Nat. Gas Sci. Eng. 2020, 77, 103240. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, X.; Wang, Z.; Fu, F.; Li, W. Effective adsorption of phenol from aqueous solutions on activated semi-coke. J. Mater. Sci. 2015, 50, 4200–4208. [Google Scholar] [CrossRef]
- Hu, K.; Herdegen, V.; Mischo, H. Carbon dioxide adsorption to 40 MPa on extracted shale from Sichuan Basin, southwestern China. Fuel 2022, 318, 123666. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Wolf, K.-H.; Bruining, H. Sorption of CH4 and CO2 on a carboniferous shale from Belgium using a manometric setup. Int. J. Coal Geol. 2014, 128, 153–161. [Google Scholar] [CrossRef]
- Weniger, P.; Kalkreuth, W.; Busch, A.; Krooss, B.M. High-pressure methane and carbon dioxide sorption on coal and shale samples from the Paraná Basin, Brazil. Int. J. Coal Geol. 2010, 84, 190–205. [Google Scholar] [CrossRef]
- Han, S.; Wang, S.; Guo, C.; Sang, S.; Xu, A.; Gao, W.; Zhou, P. Distribution of the adsorbed density of supercritical CO2 onto the anthracite and its implication for CO2 geologic storage in deep coal. Geoenergy Sci. Eng. 2024, 234, 212624. [Google Scholar] [CrossRef]
- de Oliveira, S.B.; Rocha, H.V.; Rodrigues, C.F.; Lemos de Sousa, M.J.; Tassinari, C.C. Defining CO2 Geological Storage Capacity in Unmineable Coal Seams Through Adsorption Data in 3D: Case Study of the Chico Lomã Deposit, Southern Brazil. Sustainability 2025, 17, 2856. [Google Scholar] [CrossRef]
- Abunowara, M.; Bustam, M.A.; Sufian, S.; Babar, M.; Eldemerdash, U.; Mukhtar, A.; Ullah, S.; Assiri, M.A.; Al-Sehemi, A.G.; Lam, S.S. High pressure CO2 adsorption onto Malaysian Mukah-Balingian coals: Adsorption isotherms, thermodynamic and kinetic investigations. Environ. Res. 2023, 218, 114905. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, H.M.; Shariff, A.M.; Johari, K. 3-Dimethylaminopropylamine (DMAPA) mixed with glycine (GLY) as an absorbent for carbon dioxide capture and subsequent utilization. Sep. Purif. Technol. 2019, 222, 297–308. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010–2020). Sci. Total Environ. 2021, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, J.; Li, H.; Jin, B.; Li, Z.; Zhang, T.; Zhu, X. Mechanistic insights into ball milling enhanced montmorillonite modification with tetramethylammonium for adsorption of gaseous toluene. Chemosphere 2022, 296, 133962. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Sun, W.; Selim, H.M. Fate and transport of molybdenum in soils: Kinetic modeling. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 164, pp. 51–92. [Google Scholar]
- Walsh, K.; Mayer, S.; Rehmann, D.; Hofmann, T.; Glas, K. Equilibrium data and its analysis with the Freundlich model in the adsorption of arsenic (V) on granular ferric hydroxide. Sep. Purif. Technol. 2020, 243, 116704. [Google Scholar] [CrossRef]
- Qiu, H.; Vijver, M.G.; He, E.; Peijnenburg, W.J. Predicting copper toxicity to different earthworm species using a multicomponent Freundlich model. Environ. Sci. Technol. 2013, 47, 4796–4803. [Google Scholar] [CrossRef]
- Ng, C.; Losso, J.N.; Marshall, W.E.; Rao, R.M. Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bioresour. Technol. 2002, 85, 131–135. [Google Scholar] [CrossRef]
- Tzabar, N.; ter Brake, H. Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 2016, 22, 901–914. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gomez-Granados, F.; Giraldo, L.; Moreno-Pirajan, J.C. Application of the Sips model to the calculation of maximum adsorption capacity and immersion enthalpy of phenol aqueous solutions on activated carbons. Eur. J. Chem. 2017, 8, 112–118. [Google Scholar] [CrossRef]
- Melouki, R.; Ouadah, A.; Llewellyn, P.L. The CO2 adsorption behavior study on activated carbon synthesized from olive waste. J. CO2 Util. 2020, 42, 101292. [Google Scholar] [CrossRef]
- Özkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2006, 129, 158–163. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Kazakov, A.; Dryer, F.L. An updated comprehensive kinetic model of hydrogen combustion. Int. J. Chem. Kinet. 2004, 36, 566–575. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.; Law, C.K. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Baharudin, L.; Severinsen, I.; Yip, A.C.; Golovko, V.B.; Watson, M.J. Kinetics and constraints of CO oxidation over hexameric copper nanocluster catalyst supported on carboxyl-functionalised MWCNT at high temperatures. Chem. Eng. J. 2020, 389, 124399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).