Natural Products Targeting BCR-ABL: A Plant-Based Approach to Chronic Myeloid Leukemia Treatment
Abstract
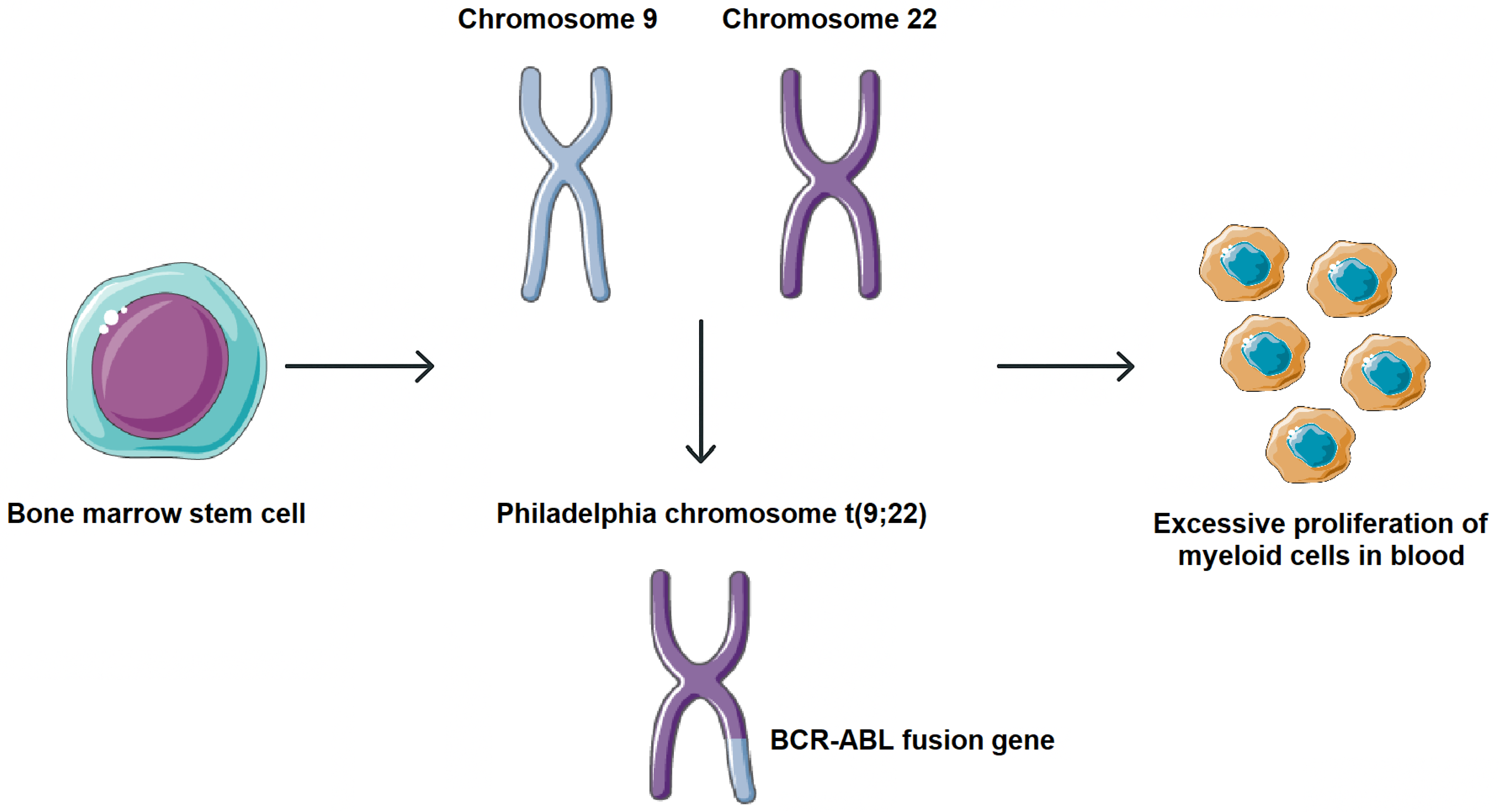
1. Introduction
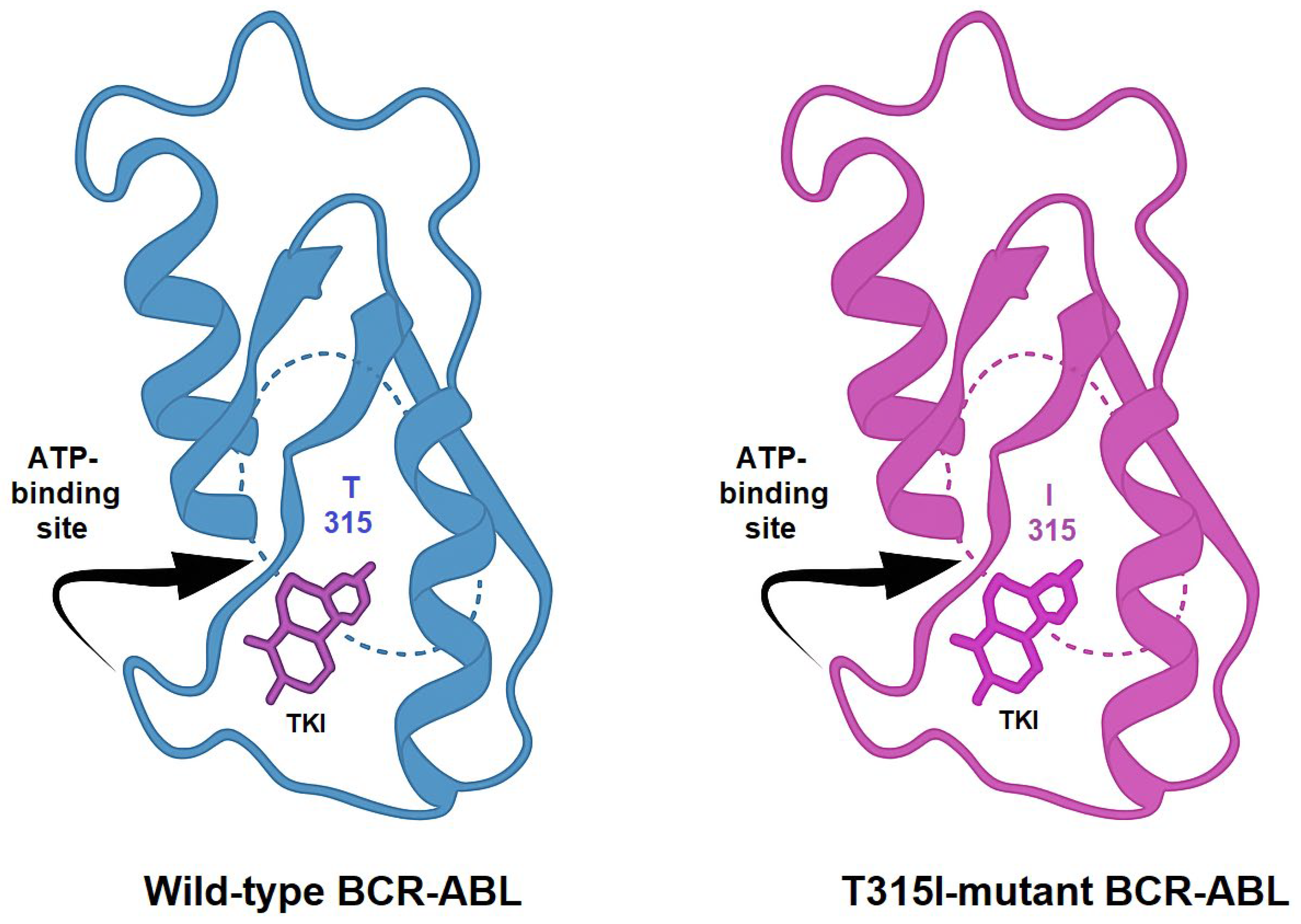
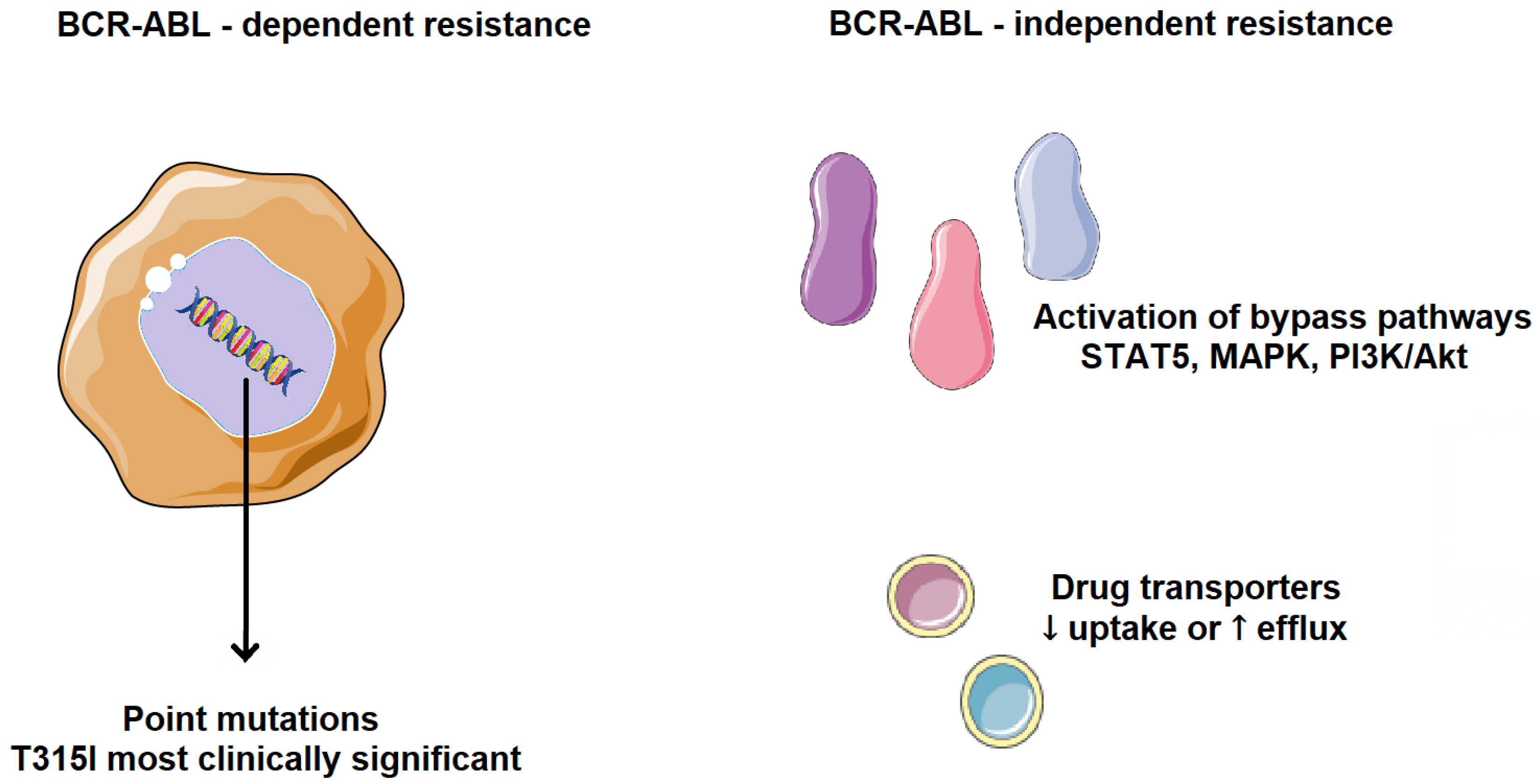
2. Tumor Resistance to Therapy
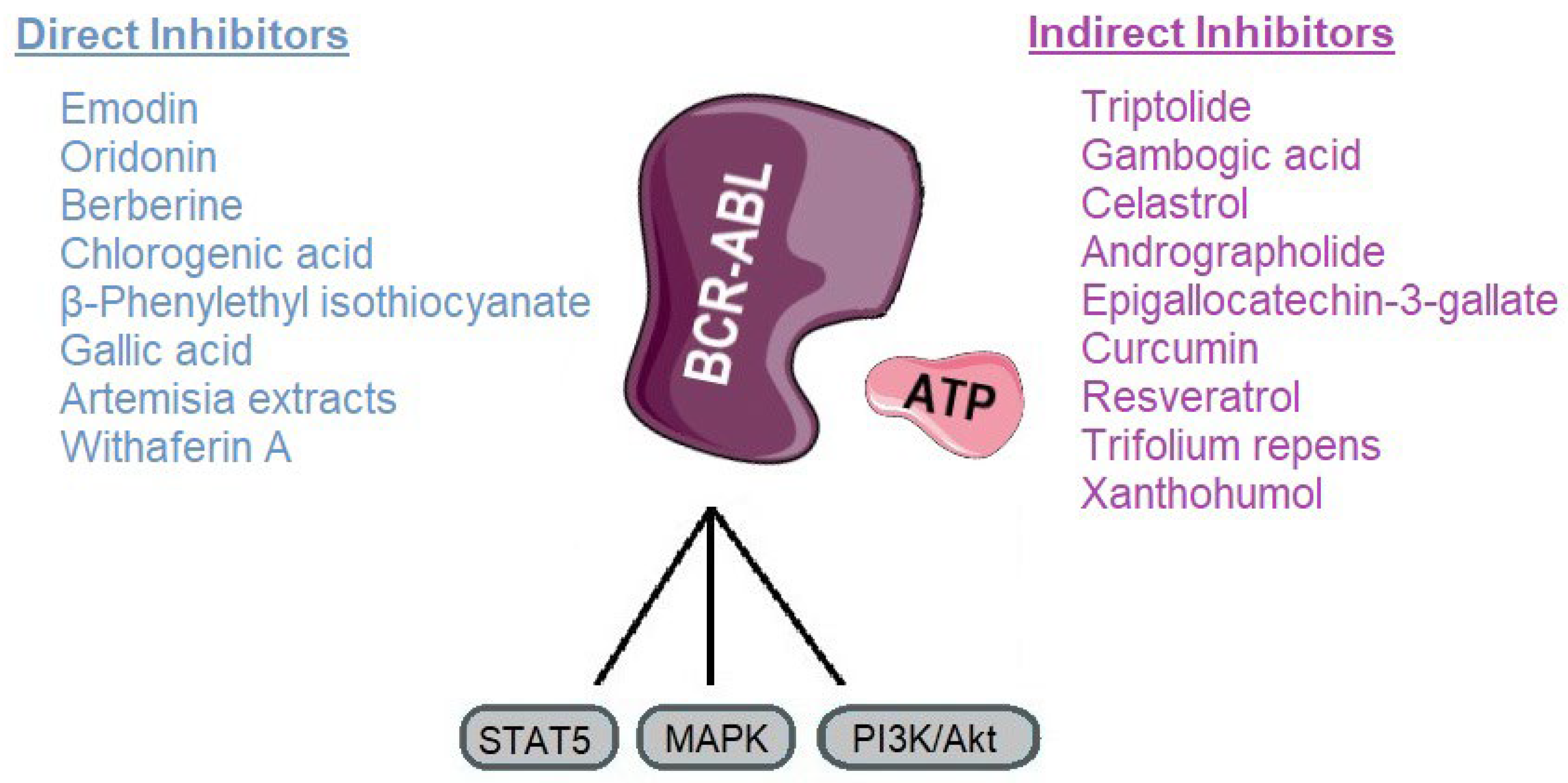
3. Direct BCR-ABL Kinase Inhibitors
3.1. Emodin
3.2. Oridonin
3.3. Berberine
3.4. Chlorogenic Acid
3.5. β-Phenylethyl Isothiocyanate
3.6. Gallic Acid
3.7. Artemisia Extracts
3.8. Withaferin A
4. Compounds Promoting BCR-ABL Degradation or Downregulation/Modulators of BCR-ABL Signaling
4.1. Triptolide
4.2. Gambogic Acid
4.3. Celastrol
4.4. Andrographolide
4.5. Epigallocatechin-3-Gallate
4.6. Curcumin
4.7. Resveratrol
4.8. Trifolium Repens
4.9. Xanthohumol
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| AZT | 3′-azido-3′-deoxythymidine |
| BCR-ABL | Breakpoint Cluster Region-Abelson murine leukemia viral oncogene homolog 1 |
| CML | Chronic Myeloid Leukemia |
| COX-2 | Cyclooxygenase-2 |
| CPK | Creatine Phosphokinase |
| EGCG | Epigallocatechin-3-gallate |
| EGR1 | Early Growth Response protein-1 |
| JAK | Janus Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear Factor-kappa B |
| PI3K | Phosphoinositide 3-kinase |
| PTEN | Phosphatase and Tensin homolog |
| ROS | Reactive Oxygen Species |
| STAMP | Specifically Target the ABL Myristoyl Pocket |
| STAT | Signal Transducer and Activator of Transcription |
| TKI | Tyrosine Kinase Inhibitor |
| VEGF | Vascular Endothelial Growth Factor |
References
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia. JAMA 2025, 333, 1618. [Google Scholar] [CrossRef]
- Eden, R.E.; Coviello, J.M. Chronic Myelogenous Leukemia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531459 (accessed on 4 September 2025).
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, Present, and Future of Bcr-Abl Inhibitors: From Chemical Development to Clinical Efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [CrossRef]
- Pechlivani, L.; Ntemou, N.; Pantazi, D.; Alivertis, D.; Skobridis, K.; Tselepis, A.D. Synthesis of Novel Nilotinib Analogues and Biological Evaluation of Their Antiplatelet Activity and Functionality towards Cancer Cell Proliferation In Vitro. Pharmaceuticals 2024, 17, 349. [Google Scholar] [CrossRef]
- Jayavel, S.; Subramanian, M.; Kesavan, P.K.; Jayavel, S. Current and Future of Targeted Therapies against BCR::ABL Kinases. J. Egypt. Natl. Cancer Inst. 2025, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Shammas, T.; Peiris, M.N.; Meyer, A.N.; Donoghue, D.J. BCR-ABL: The Molecular Mastermind behind Chronic Myeloid Leukemia. Cytokine Growth Factor Rev. 2025, 83, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Malik, S.; Hassan, S.; Eşkazan, A.E. Novel BCR-ABL1 Tyrosine Kinase Inhibitors in the Treatment of Chronic Myeloid Leukemia. Expert Rev. Hematol. 2021, 14, 975–978. [Google Scholar] [CrossRef]
- Özmen, D.; Alpaydın, D.D.; Saldoğan, M.A.; Eşkazan, A.E. A Safety Review of Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia. Expert Opin. Drug Saf. 2024, 23, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 14672. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Y.; Li, C.; Xue, Y.; Ba, X. Targeting the DNA Damage Response for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15907. [Google Scholar] [CrossRef]
- El-Tanani, M.; Nsairat, H.; Matalka, I.I.; Lee, Y.F.; Rizzo, M.; Aljabali, A.A.; Mishra, V.; Mishra, Y.; Hromić-Jahjefendić, A.; Tambuwala, M.M. The Impact of the BCR-ABL Oncogene in the Pathology and Treatment of Chronic Myeloid Leukemia. Pathol.-Res. Pract. 2024, 254, 155161. [Google Scholar] [CrossRef]
- Patel, A.B.; O’Hare, T.; Deininger, M.W. Mechanisms of Resistance to ABL Kinase Inhibition in Chronic Myeloid Leukemia and the Development of Next Generation ABL Kinase Inhibitors. Hematol./Oncol. Clin. N. Am. 2017, 31, 589–612. [Google Scholar] [CrossRef]
- Ali, M.A.M. Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: An Evolving Paradigm of Molecularly Targeted Therapy. Mol. Diagn. Ther. 2016, 20, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, L.; Martelli, M.; Cavo, M.; Soverini, S. Mechanisms of Disease Progression and Resistance to Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukemia: An Update. Int. J. Mol. Sci. 2019, 20, 6141. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Colarossi, S.; Gnani, A.; Rosti, G.; Castagnetti, F.; Poerio, A.; Iacobucci, I.; Amabile, M.; Abruzzese, E.; Orlandi, E.; et al. Contribution of ABL Kinase Domain Mutations to Imatinib Resistance in Different Subsets of Philadelphia-Positive Patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin. Cancer Res. 2006, 12, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, B.; Shahab, S.; Shamsi, T.S. T315I—A Gatekeeper Point Mutation and Its Impact on the Prognosis of Chronic Myeloid Leukemia. Adv. Lab. Med./Av. Med. Lab. 2024, 5, 412–417. [Google Scholar] [CrossRef]
- Massimino, M.; Stella, S.; Tirrò, E.; Pennisi, M.S.; Vitale, S.R.; Puma, A.; Romano, C.; Di Gregorio, S.; Tomarchio, C.; Di Raimondo, F.; et al. ABL1-Directed Inhibitors for CML: Efficacy, Resistance and Future Perspectives. Anticancer Res. 2020, 40, 2457–2465. [Google Scholar] [CrossRef]
- Mandalà, M.; Romano, E. (Eds.) Mechanisms of Drug Resistance in Cancer Therapy; Springer: Cham, Switzerland, 2018; ISBN 9783030105068. [Google Scholar]
- Wang, Z.; Wang, X.; Wang, Z.; Feng, Y.; Jia, Y.; Jiang, L.; Xia, Y.; Cao, J.; Liu, Y. Comparison of Hepatotoxicity Associated With New BCR-ABL Tyrosine Kinase Inhibitors vs Imatinib Among Patients With Chronic Myeloid Leukemia. JAMA Netw. Open 2021, 4, e2120165. [Google Scholar] [CrossRef]
- Yu, Z.; Lei, Z.; Yao, X.; Wang, H.; Zhang, M.; Hou, Z.; Li, Y.; Zhao, Y.; Li, H.; Liu, D.; et al. Potential Drug-Drug Interaction of Olverembatinib (HQP1351) Using Physiologically Based Pharmacokinetic Models. Front. Pharmacol. 2022, 13, 1065130. [Google Scholar] [CrossRef]
- Kantarjian, H.; Zhai, Y.; Oehler, V.G.; Jamy, O.; Koller, P.B.; Haddad, F.G.; Sasaki, K.; Jabbour, E.J. Olverembatinib in Chronic Myeloid Leukemia—Review of Historical Development, Current Status, and Future Research. Cancer 2025, 131, e35832. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Qin, Y.; Li, W.; Xu, N.; Liu, B.; Zhang, Y.; Meng, L.; Zhu, H.; Du, X.; et al. Olverembatinib (HQP1351), a Well-Tolerated and Effective Tyrosine Kinase Inhibitor for Patients with T315I-Mutated Chronic Myeloid Leukemia: Results of an Open-Label, Multicenter Phase 1/2 Trial. J. Hematol. Oncol. 2022, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Saikia, T.; Kim, D.-W.; Alvarado, Y.; Nicolini, F.E.; Rathnam, K.; Khattry, N.; Punatar, S.; Apperley, J.F.; Charbonnier, A.; et al. Efficacy and Safety of Vodobatinib in Patients (Pts) with Chronic Phase Philadelphia Positive Chronic Myeloid Leukemia (Ph+ CML): A Sub Group Analysis By Lines of Tyrosine Kinase Inhibitor (TKI) Therapy. Blood 2022, 140, 205–207. [Google Scholar] [CrossRef]
- Vodobatinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vodobatinib (accessed on 18 October 2025).
- Turkina, A.; Vinogradova, O.; Lomaia, E.; Shatokhina, E.; Shukhov, O.; Chelysheva, E.; Shikhbabaeva, D.; Nemchenko, I.; Petrova, A.; Bykova, A.; et al. Phase-1 Study of Vamotinib (PF-114), a 3rd Generation BCR::ABL1 Tyrosine Kinase-Inhibitor, in Chronic Myeloid Leukaemia. Ann. Hematol. 2025, 104, 2707–2715. [Google Scholar] [CrossRef]
- Vamotinib (PF-114). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vamotinib (accessed on 18 October 2025).
- Hoch, M.; Huth, F.; Manley, P.W.; Loisios-Konstantinidis, I.; Combes, F.P.; Li, Y.F.; Fu, Y.; Sy, S.K.B.; Obourn, V.; Chakraborty, A.; et al. Clinical Pharmacology of Asciminib: A Review. Clin. Pharmacokinet. 2024, 63, 1513–1528. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, H.-Z.; Zhang, C.; Zhou, C.-H. Comprehensive Insights into Emodin Compounds Research in Medicinal Chemistry. Bioorg. Med. Chem. 2025, 128, 118262. [Google Scholar] [CrossRef]
- Min, H.; Niu, M.; Zhang, W.; Yan, J.; Li, J.; Tan, X.; Li, B.; Su, M.; Di, B.; Yan, F. Emodin Reverses Leukemia Multidrug Resistance by Competitive Inhibition and Downregulation of P-Glycoprotein. PLoS ONE 2017, 12, e0187971. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-G.; Zhong, L.; Liu, Y.-L.; Shi, X.-J.; Shi, L.-Q.; Zeng, L.; Liu, B.-Z. Emodin Exerts an Antiapoptotic Effect on Human Chronic Myelocytic Leukemia K562 Cell Lines by Targeting the PTEN/PI3K-AKT Signaling Pathway and Deleting BCR-ABL. Integr. Cancer Ther. 2017, 16, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Chen, B.; Chen, C.; Qiu, B.; Zheng, Z.; Zheng, J.; Liu, T.; Wang, W.; Hu, J. Inhibition of 32Dp210 Cells Harboring T315I Mutation by a Novel Derivative of Emodin Correlates with Down-Regulation of BCR-ABL and Its Downstream Signaling Pathways. J. Cancer Res. Clin. Oncol. 2015, 141, 283–293. [Google Scholar] [CrossRef]
- Li, B.-J.; Liu, T.-B.; Wang, W.-F.; Lin, M.-H.; Hu, J.-D. Effect of A Novel Emodin Derivative on Chronic Myelogenous Leukemia K562 Cells and Imatinib-Resistant K562/G01 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 1–7. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Sun, G.-B.; Wang, Y.-J.; Yan, F. Emodin Inhibits Resistance to Imatinib by Downregulation of Bcr-Abl and STAT5 and Allosteric Inhibition in Chronic Myeloid Leukemia Cells. Biol. Pharm. Bull. 2020, 43, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, F.; Yuan, L.; Zhao, C.; Chen, C. Emodin and AZT Synergistically Inhibit the Proliferation and Induce the Apoptosis of Leukemia K562 Cells through the EGR1 and the Wnt/Β-catenin Pathway. Oncol. Rep. 2019, 43, 260–269. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, X.; Han, C.; Niu, X.; Han, L.; Yu, H.; Pan, G.; Fu, Z. Comprehensive Investigation on the Metabolism of Emodin Both in Vivo and in Vitro. J. Pharm. Biomed. Anal. 2023, 223, 115122. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Li, B.; Geng, X. Advances in the Mechanism of Emodin-Induced Hepatotoxicity. Heliyon 2024, 10, e33631. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Khan, N.; Ali, A.; Akram, H.; Zafar, N.; Imran, K.; Khan, T.; Khan, K.; Armaghan, M.; Palma-Morales, M.; et al. Oridonin from Rabdosia Rubescens: An Emerging Potential in Cancer Therapy—A Comprehensive Review. Food Sci. Nutr. 2024, 12, 3046–3067. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Dong, B.; Zhao, P.; Zhou, H.; Qu, L. Oridonin Triggers Chaperon-Mediated Proteasomal Degradation of BCR-ABL in Leukemia. Sci. Rep. 2017, 7, 41525. [Google Scholar] [CrossRef]
- Shan, Q.-Q.; Guo, Y.; Gong, Y.-P.; Lin, J.; Wang, Y.-S. Anti-Leukemia Effect and Mechanism of Oridonin on Imatinib-Sensitive and Imatinib-Resistant K562 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Zhou, J.; Shen, Q. Oridonin and Its Derivatives for Cancer Treatment and Overcoming Therapeutic Resistance. Genes Dis. 2021, 8, 448–462. [Google Scholar] [CrossRef]
- Guo, Y.; Shan, Q.; Gong, Y.; Lin, J.; Yang, X.; Zhou, R. Oridonin in Combination with Imatinib Exerts Synergetic Anti-Leukemia Effect in Ph+ Acute Lymphoblastic Leukemia Cells in Vitro by Inhibiting Activation of LYN/MTOR Signaling Pathway. Cancer Biol. Ther. 2012, 13, 1244–1254. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Zhang, L.; Gao, C.; Li, F.; Li, X.; Ke, Y.; Liu, H.-M.; Hu, Z.; Wei, L.; et al. Differentiation of Imatinib -Resistant Chronic Myeloid Leukemia Cells with BCR-ABL-T315I Mutation Induced by Jiyuan Oridonin A. J. Cancer 2023, 14, 1182–1194. [Google Scholar] [CrossRef]
- Xu, J.; Wold, E.; Ding, Y.; Shen, Q.; Zhou, J. Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection. Molecules 2018, 23, 474. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, J.; Zhang, T.; Ma, B.; Cui, S.; Chen, D.; He, Z. Pharmacokinetic Behaviors and Oral Bioavailability of Oridonin in Rat Plasma. Acta Pharmacol. Sin. 2006, 27, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dai, M.; Nai, J.; Zhu, L.; Sheng, H. Solubility and Bioavailability Enhancement of Oridonin: A Review. Molecules 2020, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, L.; Lin, C.; Deng, D.; Li, Y.; Huang, M.; Wang, Y.; Ling, K.; Wang, H.; Chen, Q.; et al. Enhancing Cancer Therapy: Advanced Nanovehicle Delivery Systems for Oridonin. Front. Pharmacol. 2024, 15, 1476739. [Google Scholar] [CrossRef]
- Elf, S.E. “All Our Wisdom Is Stored in the Trees”—Degrading BCR-ABL with Berberis Vulgaris. Clin. Cancer Res. 2020, 26, 3899–3900. [Google Scholar] [CrossRef]
- Jin, L.; Liao, H.-J.; Zhang, M.-Y.; Liu, Q.-Y.; Wang, Y.-F. Effect of Berberine on the Differentiation and Apoptosis of K562 Cell Line. J. Chin. Med. Mater. 2009, 32, 384–388. [Google Scholar]
- Yin, Z.; Huang, G.; Gu, C.; Liu, Y.; Yang, J.; Fei, J. Discovery of Berberine That Targetedly Induces Autophagic Degradation of Both BCR-ABL and BCR-ABL T315I through Recruiting LRSAM1 for Overcoming Imatinib Resistance. Clin. Cancer Res. 2020, 26, 4040–4053. [Google Scholar] [CrossRef]
- Mazandaranian, M.R.; Dana, P.M.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B. Effects of Berberine on Leukemia with a Focus on Its Molecular Targets. Anticancer Agents Med. Chem. 2022, 22, 2766–2774. [Google Scholar] [CrossRef]
- Song, C.; Li, D.; Zhang, J.; Zhao, X. Berberine Hydrochloride Alleviates Imatinib Mesylate—Induced Cardiotoxicity through the Inhibition of Nrf2-Dependent Ferroptosis. Food Funct. 2023, 14, 1087–1098. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, Q.; Jin, M.; Jiang, S.; Shang, P.; Dong, X.; Li, L. Research Progress on Pharmacological Effects and Bioavailability of Berberine. Naunyn.-Schmiedeberg’s. Arch. Pharmacol. 2024, 397, 8485–8514. [Google Scholar] [CrossRef]
- Solnier, J.; Zhang, Y.; Kuo, Y.; Du, M.; Roh, K.; Gahler, R.; Wood, S.; Chang, C. Characterization and Pharmacokinetic Assessment of a New Berberine Formulation with Enhanced Absorption In Vitro and in Human Volunteers. Pharmaceutics 2023, 15, 2567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Asif, A.; Situ, C.; Wang, J.; Hao, H. Multiple Target and Regulatory Pathways of Berberine. Phytomedicine 2025, 146, 157030. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.; Biswas, T.; Roy, K.C.; Mandal, S.; Mandal, C.; Pal, B.C.; Bhattacharya, S.; Rakshit, S.; Bhattacharya, D.K.; Chaudhuri, U.; et al. Chlorogenic Acid Inhibits Bcr-Abl Tyrosine Kinase and Triggers P38 Mitogen-Activated Protein Kinase–Dependent Apoptosis in Chronic Myelogenous Leukemic Cells. Blood 2004, 104, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in Chlorogenic Acid-Induced Apoptosis of Bcr-Abl+ CML Cells. Biochem. Pharmacol. 2010, 80, 1662–1675. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, T.; Pei, Q.; Liu, S.; Yuan, H. Pharmacokinetics and Tissue Distribution Study of Chlorogenic Acid from Lonicerae Japonicae Flos Following Oral Administrations in Rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 979414. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of Chlorogenic Acids Following Acute Ingestion of Coffee by Humans with an Ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Adeyemo-Salami, O.A.; Afolabi, D.A.; Amuzat, A.A.; Adekanye, J.O.; Oladokun, O.O. Effect of Acute Exposure of Swiss Mice to Chlorogenic Acid. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Trachootham, D.; Lu, W.; Carew, J.; Giles, F.J.; Keating, M.J.; Arlinghaus, R.B.; Huang, P. Effective Killing of Gleevec-Resistant CML Cells with T315I Mutation by a Natural Compound PEITC through Redox-Mediated Mechanism. Leukemia 2008, 22, 1191–1199. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Wang, J.; Fang, Q.; Chai, Q. Phenethyl Isothiocyanate Inhibits Growth of Human Chronic Myeloid Leukemia K562 Cells via Reactive Oxygen Species Generation and Caspases. Mol. Med. Rep. 2014, 10, 543–549. [Google Scholar] [CrossRef]
- Lawson, A.P.; Long, M.J.C.; Coffey, R.T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Naturally Occurring Isothiocyanates Exert Anticancer Effects by Inhibiting Deubiquitinating Enzymes. Cancer Res. 2015, 75, 5130–5142. [Google Scholar] [CrossRef]
- Roy, M.; Sarkar, R.; Mukherjee, A.; Mukherjee, S. Inhibition of Crosstalk between Bcr-Abl and PKC Signaling by PEITC, Augments Imatinib Sensitivity in Chronic Myelogenous Leukemia Cells. Chem.-Biol. Interact. 2015, 242, 195–201. [Google Scholar] [CrossRef]
- Ji, Y.; Kuo, Y.; Morris, M.E. Pharmacokinetics of Dietary Phenethyl Isothiocyanate in Rats. Pharm. Res. 2005, 22, 1658–1666. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M.M. Phenylethyl Isothiocyanate: A Bioactive Agent for Gastrointestinal Health. Molecules 2022, 27, 794. [Google Scholar] [CrossRef] [PubMed]
- Lam-Ubol, A.; Sukhaboon, J.; Rasio, W.; Tupwongse, P.; Tangshewinsirikul, T.; Trachootham, D. Nutri-PEITC Jelly Significantly Improves Progression-Free Survival and Quality of Life in Patients with Advanced Oral and Oropharyngeal Cancer: A Blinded Randomized Placebo-Controlled Trial. Int. J. Mol. Sci. 2023, 24, 7824. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan Reddy, T.; Bharat Reddy, D.; Aparna, A.; Arunasree, K.M.; Gupta, G.; Achari, C.; Reddy, G.V.; Lakshmipathi, V.; Subramanyam, A.; Reddanna, P. Anti-Leukemic Effects of Gallic Acid on Human Leukemia K562 Cells: Downregulation of COX-2, Inhibition of BCR/ABL Kinase and NF-ΚB Inactivation. Toxicol. Vitr. 2012, 26, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, L. Gallic Acid Downregulates Matrix Metalloproteinase-2 (MMP-2) and MMP-9 in Human Leukemia Cells with Expressed Bcr/Abl. Mol. Nutr. Food Res. 2012, 56, 1398–1412. [Google Scholar] [CrossRef]
- Raghi, K.R.; Sherin, D.R.; Saumya, M.J.; Arun, P.S.; Sobha, V.N.; Manojkumar, T.K. Computational Study of Molecular Electrostatic Potential, Docking and Dynamics Simulations of Gallic Acid Derivatives as ABL Inhibitors. Comput. Biol. Chem. 2018, 74, 239–246. [Google Scholar] [CrossRef]
- Xiang, W.; Sng, C.; Lam, Y.-H.; Kok, Z.-H.; Linn, Y.-C.; Neo, S.-Y.; Siew, Y.-Y.; Singh, D.; Koh, H.-L.; Chuah, C. Gallic Acid Enhances the Efficacy of BCR::ABL1 Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia through Inhibition of Mitochondrial Respiration and Modulation of Oncogenic Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 7958. [Google Scholar] [CrossRef]
- Narumi, K.; Sonoda, J.-I.; Shiotani, K.; Shigeru, M.; Shibata, M.; Kawachi, A.; Tomishige, E.; Sato, K.; Motoya, T. Simultaneous Detection of Green Tea Catechins and Gallic Acid in Human Serum after Ingestion of Green Tea Tablets Using Ion-Pair High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B 2014, 945–946, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary Gallic Acid as an Antioxidant: A Review of Its Food Industry Applications, Health Benefits, Bioavailability, Nano-Delivery Systems, and Drug Interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef]
- Chen, P.; Zou, F.; Liu, W. Recent Advancement in Prevention against Hepatotoxicity, Molecular Mechanisms, and Bioavailability of Gallic Acid, a Natural Phenolic Compound: Challenges and Perspectives. Front. Pharmacol. 2025, 16, 1549526. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Diseases: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Truzzi, F.; Valerii, M.C.; Tibaldi, C.; Zhang, Y.; Abduazizova, V.; Spisni, E.; Dinelli, G. Are Supplements Safe? Effects of Gallic and Ferulic Acids on In Vitro Cell Models. Nutrients 2020, 12, 1591. [Google Scholar] [CrossRef]
- Chi, H.; Ly, B. Artemisia Vulgaris Inhibits BCR/ABL and Promotes Apoptosis in Chronic Myeloid Leukemia Cells. Biomed. Rep. 2022, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-J.; Wang, W.-Q.; Wu, G.-D.; Lee, J.; Li, A. Artesunate Inhibits Angiogenesis and Downregulates Vascular Endothelial Growth Factor Expression in Chronic Myeloid Leukemia K562 Cells. Vasc. Pharmacol. 2007, 47, 131–138. [Google Scholar] [CrossRef]
- Thanh Chi, H.; Kim Ly, B.T. Effects of Imatinib and Artemisia Vulgaris Extracts in Combination on Leukemia Cell Proliferation. Res. J. Pharm. Technol. 2023, 16, 5416–5420. [Google Scholar] [CrossRef]
- Tarning, J.; Hanboonkunupakarn, B.; Hoglund, R.M.; Chotivanich, K.; Mukaka, M.; Pukrittayakamee, S.; Day, N.P.J.; White, N.J.; Dondorp, A.M.; Jittamala, P. Safety and Pharmacokinetic Properties of a New Formulation of Parenteral Artesunate in Healthy Thai Volunteers. Malar. J. 2024, 23, 296. [Google Scholar] [CrossRef]
- Abanyie, F.; Acharya, S.D.; Leavy, I.; Bowe, M.; Tan, K.R. Safety and Effectiveness of Intravenous Artesunate for Treatment of Severe Malaria in the United States—April 2019 Through December 2020. Clin. Infect. Dis. 2021, 73, 1965–1972. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Artesunate; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Frasat, T.; Tulain, U.R.; Erum, A.; Saleem, U.; Sohail, M.F.; Kausar, R. Aloe Vera and Artemisia Vulgaris Hydrogels: Exploring the Toxic Effects of Structural Transformation of the Biocompatible Materials. Drug Dev. Ind. Pharm. 2021, 47, 1753–1763. [Google Scholar] [CrossRef]
- Bhandari, S.; Sharma, J.; Rizal, S.; Yi, Y.-J.; Manandhar, G. Artemisia Vulgaris Extract Causes Precocious Acrosome Reaction and Viability Loss but Low Rate of Membrane Damage in Mouse Spermatozoa. J. Anim. Sci. Technol. 2021, 63, 58–68. [Google Scholar] [CrossRef]
- Famurewa Oluwatoyin, O.A.U. Improving Treatment Outcomes in Chronic Myeloid Leukaemia Patients Using Imatinib and Artesunate Combination Therapy. Available online: https://clinicaltrials.gov/study/NCT07022743?intr=Artesunate&rank=9&utm_source=chatgpt.com (accessed on 15 September 2025).
- Malik, V.; Radhakrishnan, N.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Computational Identification of BCR-ABL Oncogenic Signaling as a Candidate Target of Withaferin A and Withanone. Biomolecules 2022, 12, 212. [Google Scholar] [CrossRef]
- Xing, Z.; Su, A.; Mi, L.; Zhang, Y.; He, T.; Qiu, Y.; Wei, T.; Li, Z.; Zhu, J.; Wu, W. Withaferin A: A Dietary Supplement with Promising Potential as an Anti-Tumor Therapeutic for Cancer Treatment—Pharmacology and Mechanisms. Drug Des. Dev. Ther. 2023, 17, 2909–2929. [Google Scholar] [CrossRef]
- Gupta, S.K.; Jadhav, S.; Gohil, D.; Panigrahi, G.C.; Kaushal, R.K.; Gandhi, K.; Patil, A.; Chavan, P.; Gota, V. Safety, Toxicity and Pharmacokinetic Assessment of Oral Withaferin-A in Mice. Toxicol. Rep. 2022, 9, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jin, Y.; Cheng, C.; Zhang, H.; Zou, W.; Zheng, Q.; Lu, Z.; Chen, Q.; Lai, Y.; Pan, J. Triptolide Inhibits Bcr-Abl Transcription and Induces Apoptosis in STI571-Resistant Chronic Myelogenous Leukemia Cells Harboring T315I Mutation. Clin. Cancer Res. 2009, 15, 1686–1697. [Google Scholar] [CrossRef]
- Wen, S.-Q.; Ma, L.-M.; Lu, Y.-J.; Bai, B. Triptolide Inhibits Proliferation and Induces Apoptosis of Imatinib Resistant K562/G01 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 1148–1152. [Google Scholar] [CrossRef]
- Xu, F.; Shi, X.; Li, S.; Cui, J.; Lu, Z.; Jin, Y.; Lin, Y.; Pang, J.; Pan, J. Design, Synthesis, and Biological Evaluation of Novel Water-Soluble Triptolide Derivatives: Antineoplastic Activity against Imatinib-Resistant CML Cells Bearing T315I Mutant Bcr-Abl. Bioorg. Med. Chem. 2010, 18, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Ge, S.; Liu, S.-B. The Roles of TPL in Hematological Malignancies. Hematology 2023, 28, 2231765. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.H.; Schober, W.D.; Chen, W.; Konopleva, M.; Cortes, J.; Kantarjian, H.M.; Andreeff, M.; Carter, B.Z. Triptolide Induces Cell Death Independent of Cellular Responses to Imatinib in Blast Crisis Chronic Myelogenous Leukemia Cells Including Quiescent CD34+ Primitive Progenitor Cells. Mol. Cancer Ther. 2009, 8, 2509–2516. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praça, F.G.; Kravicz, M.; Bentley, M.V.L.B. Therapeutic Applications and Delivery Systems for Triptolide. Drug Deliv. Transl. Res. 2020, 10, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Xu, D.; Yue, S.; Yan, C.; Liu, W.; Fu, R.; Ma, W.; Tang, Y. Recent Advances in the Pharmacological Applications and Liver Toxicity of Triptolide. Chem.-Biol. Interact. 2023, 382, 110651. [Google Scholar] [CrossRef]
- Song, J.; He, G.-N.; Dai, L. A Comprehensive Review on Celastrol, Triptolide and Triptonide: Insights on Their Pharmacological Activity, Toxicity, Combination Therapy, New Dosage Form and Novel Drug Delivery Routes. Biomed. Pharmacother. 2023, 162, 114705. [Google Scholar] [CrossRef]
- Zeng, L.-S.; Yang, P.; Qin, Y.-Y.; He, W.-H.; Cao, L. Pharmacological Activity and Clinical Progress of Triptolide and Its Derivatives LLDT-8, PG490-88Na, and Minnelide: A Narrative Review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10181–10203. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Li, X.; Lan, X.; Zhao, C.; Liu, S.; Huang, H.; Liu, N.; Liao, S.; Song, W.; et al. Gambogic Acid Induces Apoptosis in Imatinib-Resistant Chronic Myeloid Leukemia Cells via Inducing Proteasome Inhibition and Caspase-Dependent Bcr-Abl Downregulation. Clin. Cancer Res. 2014, 20, 151–163. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.; Zhang, Q.; Xu, J.; Ouyang, J. Gambogic Acid Induces Death of K562 Cells through Autophagy and Apoptosis Mechanisms. Leuk. Lymphoma 2015, 56, 2953–2958. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, Z.; Yang, Y.; Du, Y.; Cui, S.; Zhang, Y.; Tong, Y.; Song, Z.; Zeng, H.; Zou, Q.; et al. Development of a Safety and Efficacy Nanoemulsion Delivery System Encapsulated Gambogic Acid for Acute Myeloid Leukemia in Vitro and in Vivo. Eur. J. Pharm. Sci. 2018, 125, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Ganugula, R.; Kumar, N.; Kaur, G.; Pellois, J.-P.; Garg, P.; Kumar, M.N.V.R. Next-Generation Noncompetitive Nanosystems Based on Gambogic Acid: In Silico Identification of Transferrin Receptor Binding Sites, Regulatory Shelf Stability, and Their Preliminary Safety in Healthy Rodents. ACS Appl. Bio Mater. 2019, 2, 3540–3550. [Google Scholar] [CrossRef]
- Hatami, E.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Gambogic Acid: A Shining Natural Compound to Nanomedicine for Cancer Therapeutics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188381. [Google Scholar] [CrossRef]
- Lu, Z.; Jin, Y.; Qiu, L.; Lai, Y.; Pan, J. Celastrol, a Novel HSP90 Inhibitor, Depletes Bcr–Abl and Induces Apoptosis in Imatinib-Resistant Chronic Myelogenous Leukemia Cells Harboring T315I Mutation. Cancer Lett. 2010, 290, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, H.; Luo, P.; Jia, L.; Hou, M.; Huang, J.; Gao, L.; Zhang, Q.; Guan, Y.; Bao, H.; et al. Celastrol Induces DNA Damage and Cell Death in BCR-ABL T315I-Mutant CML by Targeting YY1 and HMCES. Phytomedicine 2024, 134, 155937. [Google Scholar] [CrossRef]
- Davenport, A.; Frezza, M.; Shen, M.; Ge, Y.; Huo, C.; Chan, T.H.; Dou, Q.P. Celastrol and an EGCG Pro-Drug Exhibit Potent Chemosensitizing Activity in Human Leukemia Cells. Int. J. Mol. Med. 2010, 25, 465–470. [Google Scholar] [CrossRef]
- Amir Yusri, M.A.; Sekar, M.; Wong, L.S.; Gan, S.H.; Ravi, S.; Subramaniyan, V.; Mat Rani, N.N.I.; Chidambaram, K.; Begum, M.Y.; Ramar, M.; et al. Celastrol: A Potential Natural Lead Molecule for New Drug Design, Development and Therapy for Memory Impairment. Drug Des. Dev. Ther. 2023, 17, 1079–1096. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Duan, X.; Zhao, G.; Zhang, M. Celastrol: A Promising Agent Fighting against Cardiovascular Diseases. Antioxidants 2022, 11, 1597. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming Its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Li, X.; Lu, J.; Wang, M. Recent Advances in Drug Delivery of Celastrol for Enhancing Efficiency and Reducing the Toxicity. Front. Pharmacol. 2024, 15, 1137289. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Paik, A.; Onyeabor, F.; Ding, B.; Prabhu, S.; Wang, J. Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method. Molecules 2020, 25, 3422. [Google Scholar] [CrossRef]
- Liao, H.-C.; Chou, Y.-J.; Lin, C.-C.; Liu, S.-H.; Oswita, A.; Huang, Y.-L.; Wang, Y.-L.; Syu, J.-L.; Sun, C.-M.; Leu, C.-M.; et al. Andrographolide and Its Potent Derivative Exhibit Anticancer Effects against Imatinib-Resistant Chronic Myeloid Leukemia Cells by Downregulating the Bcr-Abl Oncoprotein. Biochem. Pharmacol. 2019, 163, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, C.-H.; Liang, F.-P.; Chen, P.-F.; Kuo, C.-D.; Alam, M.M.; Maiti, B.; Hung, S.-K.; Chi, C.-W.; Sun, C.-M.; et al. Andrographolide Downregulates the V-Src and Bcr-Abl Oncoproteins and Induces Hsp90 Cleavage in the ROS-Dependent Suppression of Cancer Malignancy. Biochem. Pharmacol. 2014, 87, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Hsu, Y.-L.; Liu, S.-H.; Liao, H.-C.; Lee, P.-X.; Lin, C.-H.; Lo, L.-C.; Fu, S.-L. Development of a Bifunctional Andrographolide-Based Chemical Probe for Pharmacological Study. PLoS ONE 2016, 11, e0152770. [Google Scholar] [CrossRef]
- Pandey, G.; Rao, C. Andrographolide: Its Pharmacology, Natural Bioavailability and Current Approaches to Increase Its Content in Andrographispaniculata. Int. J. Complement. Altern. Med. 2018, 11, 1. [Google Scholar] [CrossRef]
- Songvut, P.; Suriyo, T.; Panomvana, D.; Rangkadilok, N.; Satayavivad, J. A Comprehensive Review on Disposition Kinetics and Dosage of Oral Administration of Andrographis Paniculata, an Alternative Herbal Medicine, in Co-Treatment of Coronavirus Disease. Front. Pharmacol. 2022, 13, 952660. [Google Scholar] [CrossRef]
- Songvut, P.; Akanimanee, J.; Suriyo, T.; Pholphana, N.; Rangkadilok, N.; Panomvana, D.; Puranajoti, P.; Satayavivad, J. Non-Linear Oral Bioavailability and Clinical Pharmacokinetics of High-Dose Andrographis Paniculata Ethanolic Extract: Relevant Dosage Implications for COVID-19 Treatment. Pharm. Biol. 2025, 63, 42–52. [Google Scholar] [CrossRef]
- Songvut, P.; Boonyarattanasoonthorn, T.; Nuengchamnong, N.; Junsai, T.; Kongratanapasert, T.; Supannapan, K.; Khemawoot, P. Enhancing Oral Bioavailability of Andrographolide Using Solubilizing Agents and Bioenhancer: Comparative Pharmacokinetics of Andrographis Paniculata Formulations in Beagle Dogs. Pharm. Biol. 2024, 62, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Songvut, P.; Rangkadilok, N.; Pholphana, N.; Suriyo, T.; Panomvana, D.; Puranajoti, P.; Akanimanee, J.; Satayavivad, J. Comparative Pharmacokinetics and Safety Evaluation of High Dosage Regimens of Andrographis Paniculata Aqueous Extract after Single and Multiple Oral Administration in Healthy Participants. Front. Pharmacol. 2023, 14, 1230401. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A Review of Its Pharmacology, Pharmacokinetics, Toxicity and Clinical Trials and Pharmaceutical Researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Yun, M.; Choo, E.; Kim, S.; Jeong, M.; Jung, D.; Lee, H.; Kim, E.; Kato, N.; Kim, B.; et al. A Derivative of Epigallocatechin-3-gallate Induces Apoptosis via SHP-1-mediated Suppression of BCR-ABL and STAT3 Signalling in Chronic Myelogenous Leukaemia. Br. J. Pharmacol. 2015, 172, 3565–3578. [Google Scholar] [CrossRef]
- Huang, Y.; Kumazoe, M.; Bae, J.; Yamada, S.; Takai, M.; Hidaka, S.; Yamashita, S.; Kim, Y.; Won, Y.; Murata, M.; et al. Green Tea Polyphenol Epigallocatechin-O-Gallate Induces Cell Death by Acid Sphingomyelinase Activation in Chronic Myeloid Leukemia Cells. Oncol. Rep. 2015, 34, 1162–1168. [Google Scholar] [CrossRef]
- Morang, S.; Bisht, M.; Upadhyay, V.; Thapliyal, S.; Handu, S. S1P Signaling Genes as Prominent Drivers of BCR-ABL1-Independent Imatinib Resistance and Six Herbal Compounds as Potential Drugs for Chronic Myeloid Leukemia. OMICS A J. Integr. Biol. 2024, 28, 367–376. [Google Scholar] [CrossRef]
- Vitkeviciene, A.; Baksiene, S.; Borutinskaite, V.; Navakauskiene, R. Epigallocatechin-3-Gallate and BIX-01294 Have Different Impact on Epigenetics and Senescence Modulation in Acute and Chronic Myeloid Leukemia Cells. Eur. J. Pharmacol. 2018, 838, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Goker, B.; Caliskan, C.; Onur Caglar, H.; Kayabasi, C.; Balci, T.; Erbaykent Tepedelen, B.; Aygunes, D.; Yilmaz Susluer, S.; Mutlu, Z.; Selvi Gunel, N.; et al. Synergistic Effect of Ponatinib and Epigallocatechin-3-Gallate Induces Apoptosis in Chronic Myeloid Leukemia Cells through Altering Expressions of Cell Cycle Regulatory Genes. J. BUON 2014, 19, 992–998. [Google Scholar]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (−)-Epigallocatechin-3-gallate Induces Cell Apoptosis in Chronic Myeloid Leukaemia by Regulating Bcr/Abl-mediated P38-MAPK/JNK and JAK 2/STAT 3/AKT Signalling Pathways. Clin. Exp. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef]
- Andreu Fernández, V.; Almeida Toledano, L.; Pizarro Lozano, N.; Navarro Tapia, E.; Gómez Roig, M.D.; De la Torre Fornell, R.; García Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Ferrari, E.; Naponelli, V. Catechins and Human Health: Breakthroughs from Clinical Trials. Molecules 2025, 30, 3128. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Isomura, T.; Suzuki, S.; Origasa, H.; Hosono, A.; Suzuki, M.; Sawada, T.; Terao, S.; Muto, Y.; Koga, T. Liver-Related Safety Assessment of Green Tea Extracts in Humans: A Systematic Review of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2016, 70, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.B.; Pow-Sang, J.; Spiess, P.E.; Park, J.; Salup, R.; Williams, C.R.; Parnes, H.; Schell, M.J. Randomized, Placebo-Controlled Trial Evaluating the Safety of One-Year Administration of Green Tea Catechins. Oncotarget 2016, 7, 70794–70802. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, Y.; Cui, G.-H.; Gu, J.-X.; Hu, D.; Chen, W.-K.; Li, X.-G. Effect of Curcumin on STAT5 Signaling Pathway in Primary CML Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2004, 12, 572–576. [Google Scholar] [PubMed]
- William, B.M.; Goodrich, A.; Peng, C.; Li, S. Curcumin Inhibits Proliferation and Induces Apoptosis of Leukemic Cells Expressing Wild-Type or T315I-BCR-ABL and Prolongs Survival of Mice with Acute Lymphoblastic Leukemia. Hematology 2008, 13, 333–343. [Google Scholar] [CrossRef]
- Feriotto, G.; Marchetti, P.; Rondanin, R.; Tagliati, F.; Aguzzi, S.; Beninati, S.; Casciano, F.; Tabolacci, C.; Mischiati, C. Cytotoxicity of Isoxazole Curcumin Analogs on Chronic Myeloid Leukemia-Derived K562 Cell Lines Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2023, 24, 2356. [Google Scholar] [CrossRef]
- Mutlu Altundağ, E.; Yılmaz, A.M.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Synergistic Induction of Apoptosis by Quercetin and Curcumin in Chronic Myeloid Leukemia (K562) Cells. Nutr. Cancer 2018, 70, 97–108. [Google Scholar] [CrossRef]
- Park, J.; Bae, E.; Yoon, S.-S.; Kim, B.K. Combined Treatment of STI571 (Glivec) and Curcumin Synergistically Suppresses the Growth of K562 Cells Via Inhibition of Bcr-Abl Pathway. Blood 2007, 110, 4537. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Shan, Q.; He, G.; Lin, J.; Gong, Y. Curcumin Potentiates the Anti-Leukemia Effects of Imatinib by Downregulation of the AKT/MTOR Pathway and BCR/ABL Gene Expression in Ph+ Acute Lymphoblastic Leukemia. Int. J. Biochem. Cell Biol. 2015, 65, 1–11. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Khosravi, M.A.; Seifert, R. Clinical Trials on Curcumin in Relation to Its Bioavailability and Effect on Malignant Diseases: Critical Analysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3477–3491. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Kothaplly, S.; Alukapally, S.; Nagula, N.; Maddela, R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Adv. Ther. 2022, 39, 2128–2138. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ghalaut, V.S.; Sangwan, L.; Dahiya, K.; Ghalaut, P.; Dhankhar, R.; Saharan, R. Effect of Imatinib Therapy with and without Turmeric Powder on Nitric Oxide Levels in Chronic Myeloid Leukemia. J. Oncol. Pharm. Pract. 2012, 18, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Grosso, S.; Jacquel, A.; Belhacene, N.; Colosetti, P.; Cassuto, J.; Auberger, P. Imatinib Mesylate-resistant Human Chronic Myelogenous Leukemia Cell Lines Exhibit High Sensitivity to the Phytoalexin Resveratrol. FASEB J. 2008, 22, 1894–1904. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Ma, L.; Bai, X.; Li, X.; Zhao, M.; Sui, T. Resveratrol Inhibits STAT5 Activation through the Induction of SHP-1 and SHP-2 Tyrosine Phosphatases in Chronic Myelogenous Leukemia Cells. Anticancer Drugs 2018, 29, 646–651. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, M.; Xu, C.; Duan, L.; Dong, Y.; Luo, Y.; Niu, T.; Lu, C. Resveratrol Induces Apoptosis of Human Chronic Myelogenous Leukemia Cells in Vitro through P38 and JNK-Regulated H2AX Phosphorylation. Acta Pharmacol. Sin. 2015, 36, 353–361. [Google Scholar] [CrossRef]
- Banerjee Mustafi, S.; Chakraborty, P.K.; Raha, S. Modulation of Akt and ERK1/2 Pathways by Resveratrol in Chronic Myelogenous Leukemia (CML) Cells Results in the Downregulation of Hsp70. PLoS ONE 2010, 5, e8719. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, T.; Madihally, S.V. Targeted Cancer Treatment Using a Combination of SiRNA-Liposomes and Resveratrol-Electrospun Fibers in Co-Cultures. Int. J. Pharm. 2019, 569, 118599. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.H. Inhibition of Human Chronic Myelogenous Leukemia K562 Cell Growth Following Combination Treatment with Resveratrol and Imatinib Mesylate. Genet. Mol. Res. 2015, 14, 6413–6418. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and Safety Study of Resveratrol 500 Mg Tablets in Healthy Male and Female Volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Dikmetas, D.N.; Yenipazar, H.; Can Karaca, A. Recent Advances in Encapsulation of Resveratrol for Enhanced Delivery. Food Chem. 2024, 460, 140475. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante de Freitas, P.G.; Rodrigues Arruda, B.; Araújo Mendes, M.G.; Barroso de Freitas, J.V.; da Silva, M.E.; Sampaio, T.L.; Petrilli, R.; Eloy, J.O. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In Vitro and In Vivo. Cancers 2023, 15, 2802. [Google Scholar] [CrossRef]
- Montalesi, E.; Cracco, P.; Acconcia, F.; Fiocchetti, M.; Iucci, G.; Battocchio, C.; Orlandini, E.; Ciccone, L.; Nencetti, S.; Muzzi, M.; et al. Resveratrol Analogs and Prodrugs Differently Affect the Survival of Breast Cancer Cells Impairing Estrogen/Estrogen Receptor α/Neuroglobin Pathway. Int. J. Mol. Sci. 2023, 24, 2148. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics 2025, 17, 134. [Google Scholar] [CrossRef]
- Ruparelia, K.C.; Zeka, K.; Beresford, K.J.M.; Wilsher, N.E.; Potter, G.A.; Androutsopoulos, V.P.; Brucoli, F.; Arroo, R.R.J. CYP1-Activation and Anticancer Properties of Synthetic Methoxylated Resveratrol Analogues. Molecules 2024, 29, 423. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Chen, P.; Yin, D.; Zhang, H.; Qiu, X.; Zou, S.; Li, W. Pharmacokinetic Evaluation of Two Oral Resveratrol Formulations in a Randomized, Open-Label, Crossover Study in Healthy Fasting Subjects. Sci. Rep. 2025, 15, 24515. [Google Scholar] [CrossRef] [PubMed]
- Sarno, F.; Pepe, G.; Termolino, P.; Carafa, V.; Massaro, C.; Merciai, F.; Campiglia, P.; Nebbioso, A.; Altucci, L. Trifolium Repens Blocks Proliferation in Chronic Myelogenous Leukemia via the BCR-ABL/STAT5 Pathway. Cells 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Ngangom, L.; Venugopal, D.; Pandey, N. Investigation of Trifolium Repens L. from the Indian Himalayan Region as a Phyto-Therapeutic Agent. Nat. Prod. Res. 2024, 38, 4468–4478. [Google Scholar] [CrossRef]
- Tundis, R.; Marrelli, M.; Conforti, F.; Tenuta, M.; Bonesi, M.; Menichini, F.; Loizzo, M. Trifolium Pratense and T. Repens (Leguminosae): Edible Flower Extracts as Functional Ingredients. Foods 2015, 4, 338–348. [Google Scholar] [CrossRef]
- Ahmad, S.; Zeb, A. Phytochemical Profile and Pharmacological Properties of Trifolium Repens. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 20200015. [Google Scholar] [CrossRef]
- Tjeerdsma, A.M.; van Hunsel, F.P.A.M.; van de Koppel, S.; Ekhart, C.; Vitalone, A.; Woerdenbag, H.J. Analysis of Safety Concerns on Herbal Products with Assumed Phytoestrogenic Activity. Pharmaceuticals 2023, 16, 1137. [Google Scholar] [CrossRef]
- Lu, X.; Geng, J.; Zhang, J.; Miao, J.; Liu, M. Xanthohumol, a Prenylated Flavonoid from Hops, Induces Caspase-Dependent Degradation of Oncoprotein BCR-ABL in K562 Cells. Antioxidants 2019, 8, 402. [Google Scholar] [CrossRef]
- Monteghirfo, S.; Tosetti, F.; Ambrosini, C.; Stigliani, S.; Pozzi, S.; Frassoni, F.; Fassina, G.; Soverini, S.; Albini, A.; Ferrari, N. Antileukemia Effects of Xanthohumol in Bcr/Abl-Transformed Cells Involve Nuclear Factor-ΚB and P53 Modulation. Mol. Cancer Ther. 2008, 7, 2692–2702. [Google Scholar] [CrossRef]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human Pharmacokinetics of Xanthohumol, an Antihyperglycemic Flavonoid from Hops. Mol. Nutr. Food Res. 2014, 58, 248–255. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Bohiltea, R.-E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.F.; Frank, J.; Venturelli, S.; Egert, S. Bioavailability and Cardiometabolic Effects of Xanthohumol: Evidence from Animal and Human Studies. Mol. Nutr. Food Res. 2022, 66, 2100831. [Google Scholar] [CrossRef]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro, R. Curcumin Inhibits in Vitro and in Vivo Chronic Myelogenous Leukemia Cells Growth: A Possible Role for Exosomal Disposal of MiR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef]
- Rojas, A.; González, I.; Morales, M.A. Natural Products and Cancer: The Urgent Need to Bridge the Gap between Preclinical and Clinical Research. World J. Gastrointest. Oncol. 2025, 17, 100484. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Pan, T.; Jia, N. Plant Natural Compounds in the Cancer Treatment: A Systematic Bibliometric Analysis. Heliyon 2024, 10, e34462. [Google Scholar] [CrossRef] [PubMed]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Miutescu, E.; Hermenean, A. The Anti-Leukemic Activity of Natural Compounds. Molecules 2021, 26, 2709. [Google Scholar] [CrossRef]
- Mir, M.; Banik, B.K. Sustainable Healing: Natural Compounds Facilitating the Future Cancer Treatment. World Dev. Sustain. 2025, 6, 100215. [Google Scholar] [CrossRef]
- Gahtori, R.; Tripathi, A.H.; Kumari, A.; Negi, N.; Paliwal, A.; Tripathi, P.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Anticancer Plant-Derivatives: Deciphering Their Oncopreventive and Therapeutic Potential in Molecular Terms. Futur. J. Pharm. Sci. 2023, 9, 14. [Google Scholar] [CrossRef]
- Moriello, C.; De Rosa, C.; D’Angelo, S.; Pasquale, P. Polyphenols and Chronic Myeloid Leukemia: Emerging Therapeutic Opportunities. Hemato 2025, 6, 28. [Google Scholar] [CrossRef]

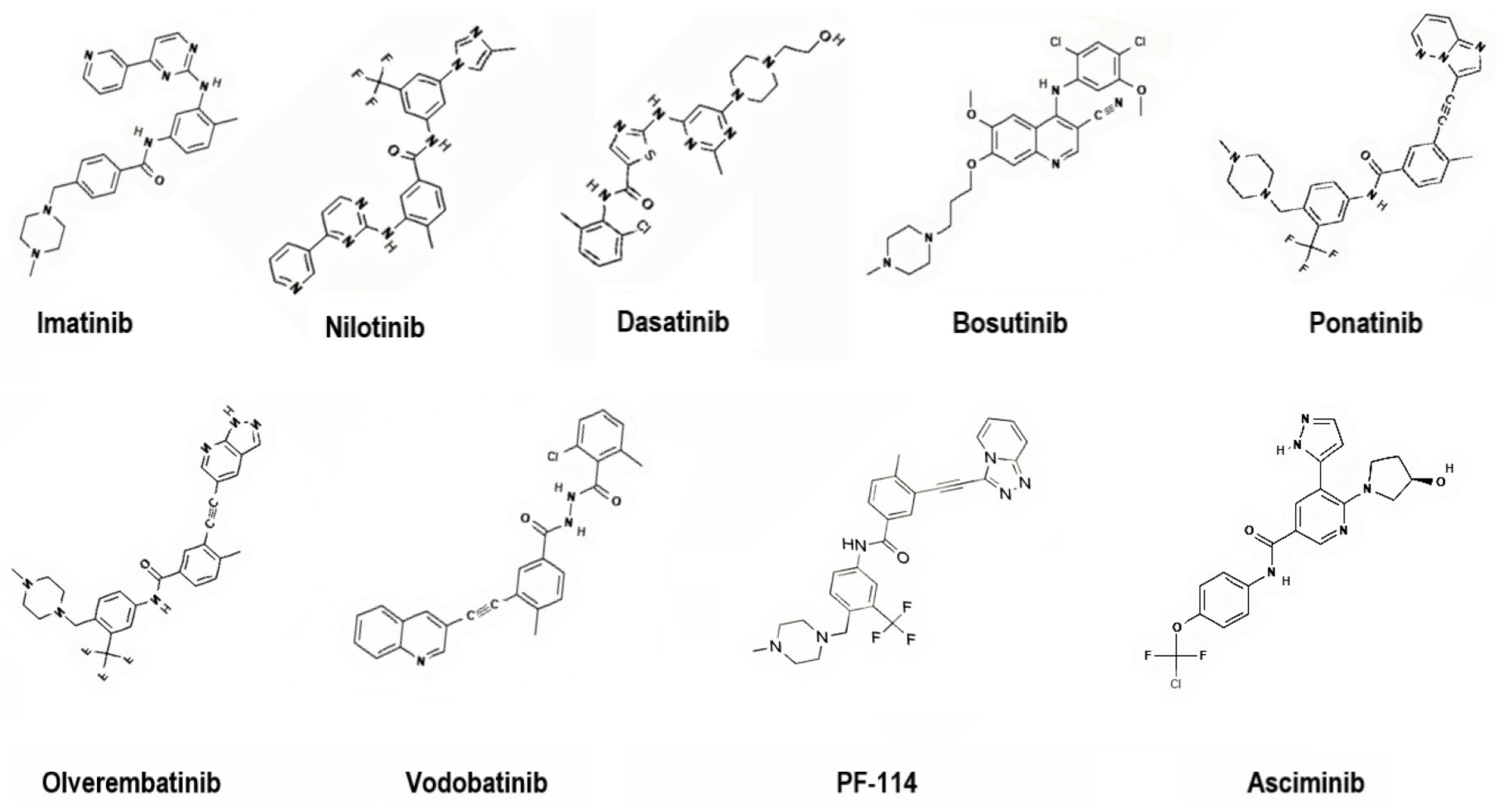

| TKI | Potency Scale | Bioavailability/ Absorption | Apparent Half-Life | Major Toxicities/Safety Concerns |
|---|---|---|---|---|
| Imatinib (NDA #021335) | Low/ moderate | High | ~18 h | Edema, hepatotoxicity, cytopenias, gastrointestinal disturbances |
| Nilotinib (NDA #022068) | Moderate | Moderate | ~15–17 h | QT prolongation, metabolic disturbances, hepatotoxicity |
| Dasatinib (NDA #021986) | High | Low/ moderate | ~3–5 h | Pleural effusion, cytopenias, bleeding, QT prolongation |
| Bosutinib (NDA #203341) | High | Moderate | ~22.5 h | Diarrhea, hepatotoxicity, cytopenias |
| Ponatinib (NDA #203469) | Very high | Moderate | ~24 h | Arterial occlusive events, pancreatitis, hepatotoxicity, hypertension |
| Olverembatinib [21,22,23] | High | Moderate | ~32.7 h | Cardiovascular events, hypertension, pericardial effusion, cytopenias, elevated CPK, thrombocytopenia |
| Vodobatinib [24,25] | Moderate/ high | Moderate | Not well characterized | Safety profile in trials is favorable to date (less off-target toxicity) |
| PF-114 [26,27] | High | Moderate | ~13.5 h | Skin hyperpigmentation, proteinuria, elevated liver enzymes, hypertriglyceridemia |
| Asciminib (NDA #215358) [28] | High/ very high | High | ~7 to 15 h | Fatigue, nausea, headache, diarrhea, elevated liver enzymes, thrombocytopenia, neutropenia, rare QT prolongation |
| Compound | Classification | Main Mechanism of Action | Key Molecular Targets |
|---|---|---|---|
| Emodin | Direct inhibitor | Allosteric inhibition and downregulation of BCR-ABL expression. | BCR-ABL, STAT5 |
| Oridonin | Direct inhibitor | Promotes proteasome-mediated degradation of BCR-ABL. | BCR-ABL, HSF1-HSP70 axis |
| Berberine | Direct inhibitor | Autophagy-dependent degradation of wild-type and T315I BCR-ABL. | BCR-ABL, LRSAM1, ubiquitin pathway |
| Chlorogenic acid | Direct inhibitor | Inhibits BCR-ABL kinase activity, induces apoptosis via ROS. | BCR-ABL, p38 MAPK |
| β-Phenylethyl isothiocyanate | Direct inhibitor | Generates ROS, disrupts PKC-BCR-ABL crosstalk, induces apoptosis. | BCR-ABL, PKC, ROS pathway |
| Gallic acid | Direct inhibitor | Inhibits BCR-ABL phosphorylation, downregulates COX-2, NF-κB. | BCR-ABL, NF-κB, COX-2 |
| Artemisia extracts | Direct inhibitor | Reduces BCR-ABL expression and promotes apoptosis. | BCR-ABL, VEGF signaling |
| Withaferin A | Direct inhibitor | Predicted binding to BCR-ABL, suppresses oncogenic signaling. | BCR-ABL, JAK/STAT, PI3K/Akt |
| Triptolide | Indirect inhibitor | Inhibits BCR-ABL transcription, induces apoptosis in resistant cells. | BCR-ABL mRNA, NF-κB |
| Gambogic acid | Indirect inhibitor | Proteasome inhibition leading to BCR-ABL downregulation. | BCR-ABL, caspases |
| Celastrol | Indirect inhibitor | Disrupts HSP90 function, destabilizing BCR-ABL. | HSP90, BCR-ABL |
| Andrographolide | Indirect inhibitor | Induces ROS, HSP90 cleavage, reduces BCR-ABL signaling. | HSP90, ROS pathway |
| Epigallocatechin-3-gallate | Indirect inhibitor | Activates SHP-1 phosphatase, suppresses BCR-ABL/STAT3. | BCR-ABL, STAT3, SHP-1 |
| Curcumin | Indirect inhibitor | Inhibits STAT5 and exosomal miRNA signaling, induces apoptosis | STAT5, NF-κB, miR-21 |
| Resveratrol | Indirect inhibitor | Suppresses STAT5, Akt/ERK pathways, downregulates HSP70. | STAT5, PI3K/Akt, ERK1/2, HSP70 |
| Trifolium repens | Indirect inhibitor | Suppresses BCR-ABL/STAT5 signaling, inhibits proliferation. | BCR-ABL/STAT5 pathway |
| Xanthohumol | Indirect inhibitor | Induces caspase-dependent degradation of BCR-ABL, suppresses NF-κB and p53 signaling. | BCR-ABL, NF-κB, p53, autophagy pathway |
| Compound | Bioavailability | Toxicity Profile |
|---|---|---|
| Emodin | Poor oral bioavailability due to limited absorption and rapid metabolism. | Generally safe at low doses. High doses associated with hepatotoxicity and nephrotoxicity in preclinical studies. |
| Oridonin | Low solubility and poor pharmacokinetics. Derivatives are being developed to improve bioavailability. | It can cause hepatotoxicity and gastrointestinal toxicity at higher doses. |
| Berberine | Very low oral bioavailability (<1%). Affected by P-glycoprotein efflux. | Safe at moderate doses. Gastrointestinal discomfort and potential hepatotoxicity at high doses. |
| Chlorogenic acid | Limited stability and variable oral absorption. | Considered safe. Excessive intake may cause gastrointestinal effects. |
| β-Phenylethyl isothiocyanate | Moderate bioavailability from dietary sources. | Safe at nutritional levels. High doses may cause gastrointestinal irritation. |
| Gallic acid | Low oral bioavailability due to rapid metabolism. | Safe at dietary levels. Possible oxidative stress at high doses. |
| Artemisia extracts | Variable bioavailability. Artesunate more stable. | Safe at therapeutic doses. Possible hepatotoxicity and neurotoxicity at high levels. |
| Withaferin A | Low oral bioavailability. Limited pharmacokinetic data. | Safe up to 2000 mg/kg in mice. Higher doses may cause hepatotoxic or reproductive effects. |
| Triptolide | Very low oral bioavailability. | Narrow therapeutic window. Hepatotoxicity, nephrotoxicity, and reproductive toxicity reported. |
| Gambogic acid | Poor solubility and low oral absorption. | Selective cytotoxicity toward tumor cells. Hepatotoxicity and gastrointestinal effects at higher doses. |
| Celastrol | Limited oral bioavailability. Highly lipophilic. | Hepatotoxicity, nephrotoxicity, and reproductive toxicity in preclinical models. |
| Andrographolide | Low oral bioavailability. | Generally safe at clinical doses. High doses are linked to hepatotoxicity and reproductive toxicity. |
| Epigallocatechin-3-gallate | Poor oral bioavailability due to rapid metabolism. | Safe in dietary amounts. Hepatotoxicity observed in concentrated supplements. |
| Curcumin | Extremely low oral bioavailability (<1%). Enhanced by piperine or formulations. | Very safe. Mild gastrointestinal or hepatotoxic effects at high doses. |
| Resveratrol | Very low oral bioavailability. Rapid metabolism. | Safe at moderate doses. Gastrointestinal upset at high doses. |
| Trifolium repens | Limited bioavailability data. Similar to other isoflavonoids. | Considered safe at dietary levels. Possible estrogenic effects with excessive use. |
| Xanthohumol | Absorbed after oral administration. Overall bioavailability is relatively low. | Appears well-tolerated in humans. No significant adverse effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pechlivani, L.; Giannakis, A.; Sioka, C.; Alexiou, G.A.; Kyritsis, A.P. Natural Products Targeting BCR-ABL: A Plant-Based Approach to Chronic Myeloid Leukemia Treatment. Molecules 2025, 30, 4160. https://doi.org/10.3390/molecules30214160
Pechlivani L, Giannakis A, Sioka C, Alexiou GA, Kyritsis AP. Natural Products Targeting BCR-ABL: A Plant-Based Approach to Chronic Myeloid Leukemia Treatment. Molecules. 2025; 30(21):4160. https://doi.org/10.3390/molecules30214160
Chicago/Turabian StylePechlivani, Louisa, Alexandros Giannakis, Chrissa Sioka, Georgios A. Alexiou, and Athanassios P. Kyritsis. 2025. "Natural Products Targeting BCR-ABL: A Plant-Based Approach to Chronic Myeloid Leukemia Treatment" Molecules 30, no. 21: 4160. https://doi.org/10.3390/molecules30214160
APA StylePechlivani, L., Giannakis, A., Sioka, C., Alexiou, G. A., & Kyritsis, A. P. (2025). Natural Products Targeting BCR-ABL: A Plant-Based Approach to Chronic Myeloid Leukemia Treatment. Molecules, 30(21), 4160. https://doi.org/10.3390/molecules30214160







