Cancer Chemopreventive Potential of Claoxylon longifolium Grown in Southern Thailand: A Bioassay-Guided Isolation of Vicenin 1 as the Active Compound and In Silico Studies on Related C-Glycosyl Flavones
Abstract
1. Introduction
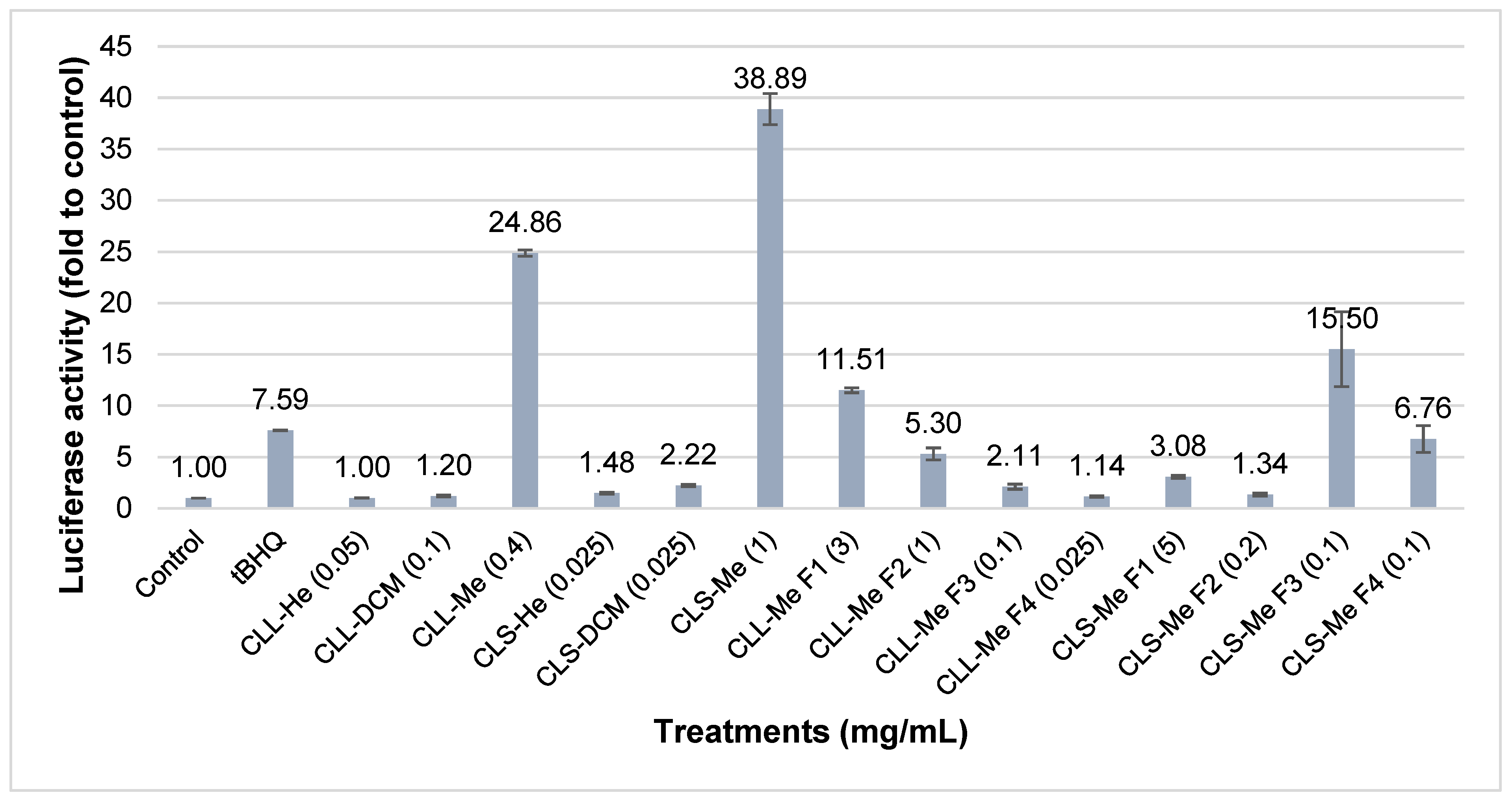
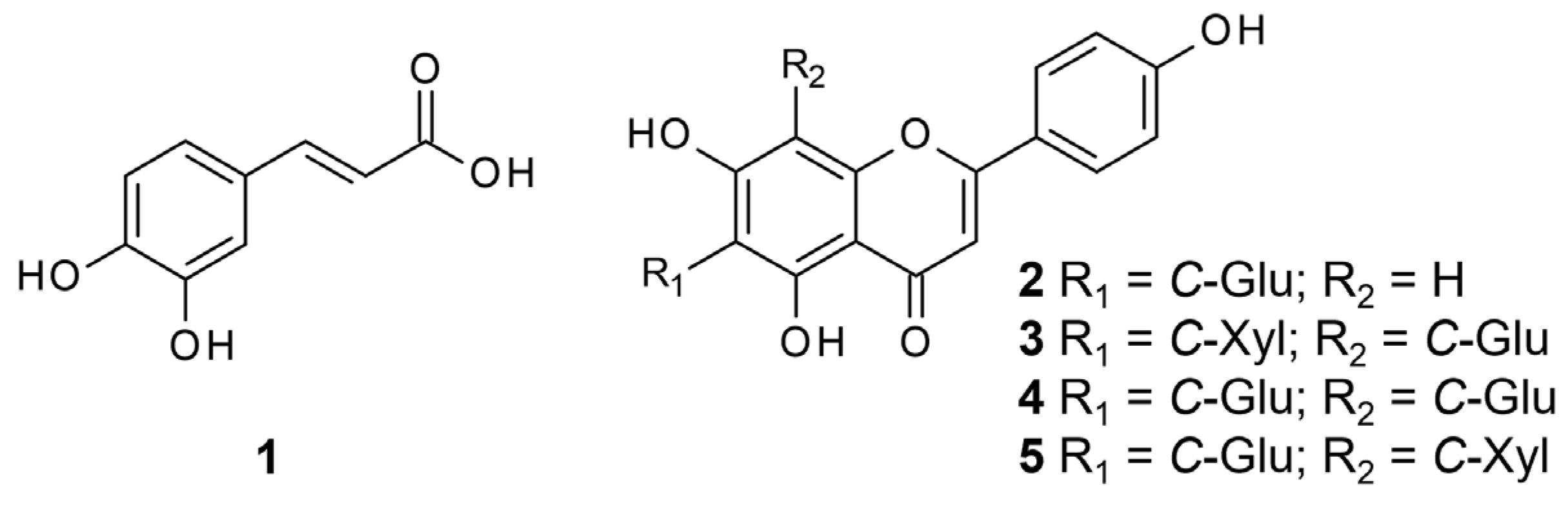
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals, Reagents, and Materials
3.2. General Experimental Procedures
3.3. Plant Materials
3.4. Extraction and Isolation of Compounds
3.5. Cell Culture
3.6. MTT Assay
3.7. Luciferase Reporter Assay
3.8. In Silico Studies for Bioactivity of Selected C-Glycosyl Flavones
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, M.T.; Alam Tumpa, M.A.; Yamin, M.; Islam, T.; Park, M.N.; Islam, M.R.; Rauf, A.; Sharma, R.; Cavalu, S.; et al. Exploring the recent trends in perturbing the cellular signaling pathways in cancer by natural products. Front. Pharmacol. 2022, 13, 950109. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. Cancer chemoprevention. In Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Avendaño, C., Menéndez, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 701–723. [Google Scholar]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Li, A. Plant natural products: Promising resources for cancer chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. Nrf2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Jeong, W.S.; Jun, M.; Kong, A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal 2006, 8, 99–106. [Google Scholar] [CrossRef]
- Ritchie, K.J.; Sarker, S.D. Cancer chemopreventive natural products. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Medicinal Natural Products—A Disease-focused Approach; Elsevier: London, UK, 2020; Volume 55, pp. 273–295. [Google Scholar]
- Zhao, C.R.; Gao, Z.H.; Qu, X.J. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010, 34, 523–533. [Google Scholar] [CrossRef]
- Wu, K.C.; McDonald, P.R.; Liu, J.; Klaassen, C.D. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med. 2014, 80, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Maneenoon, K.; Khuniad, C.; Teanuan, Y.; Saedan, N.; Prom-In, S.; Rukleng, N.; Kongpool, W.; Pinsook, P.; Wongwiwat, W. Ethnomedicinal plants used by traditional healers in Phatthalung province, peninsular Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Junsongduang, A.; Kasemwan, W.; Lumjoomjung, S.; Sabprachai, W.; Tanming, W.; Balslev, H. Ethnomedicinal knowledge of traditional healers in Roi Et, Thailand. Plants 2020, 9, 1177. [Google Scholar] [CrossRef]
- Middleton, J.D.; Armstrong, K.; Baba, Y.; Balslev, H.; Chayamarit, K.; Chung, R.C.K.; Conn, B.; Fernando, E.; Fujikawa, K.; Kiew, R.; et al. Progress on aoutheast asia’s flora projects. Gard. Bull. Singap. 2019, 71, 267–319. [Google Scholar] [CrossRef]
- Susila Rani, S.R.M.; Balakrishnan, N.P. A revision of the genus Claoxylon Adr. Jussieu (Euphorbiaceae) in India. Rheedea 1995, 5, 113–141. [Google Scholar]
- van Welzen, P.C.; Chayamarit, K. Flora of Thailand Euphorbiaceae. Available online: https://www.nationaalherbarium.nl/ThaiEuph/home.htm (accessed on 19 March 2025).
- Sutummawong, N.; Sampantamit, T.; Rungtawanrauengsri, S.; Ketmai, W.; Othong, S.; Tessana, N. Indigenous vegetables in Amphoe Kong Ra, Phatthalung province, Thailand. Thaksin J. 2011, 14, 67–78. [Google Scholar]
- Nakorn, W.; Chaymeang, C.; Chaison, C. Diversity and evenness of indigenous vegetables in Nakhon Si Thammarat province, Thailand. Int. J. Agric. Technol. 2018, 14, 1483–1494. [Google Scholar]
- Saupi, N.; Saidin, A.A.; Zakaria, M.H.; Sarbini, S.R.; Yusli, N.A. An ethnobotanical study of indigenous leafy vegetables among local communities in Bintulu, Sarawak, Malaysia. BJRST 2020, 10, 155–165. [Google Scholar] [CrossRef]
- Watcharachirasophol, R. Thai Traditional Medicine Practitioner. Interviewed by Chuanchom Khuniad. Phatthalung, Thailand. 3rd May 2021. [Google Scholar]
- Wardah, W.; Setyowati, F.M. Medicinal plant biodiversity in Dayak communities living in Kahayan Hulu Utara, Gunung Mas Regency, Central Kalimantan. In Proceedings of the International Conference on Medicinal Plants, Surabaya, Indonesia, 21–22 July 2010. [Google Scholar]
- Neamsuvan, O.; Tanthien, S.; Petchboon, S. A survey of medicinal plants for restoratives from Khao Phanom Bencha National Park, Krabi province. Thai Pharm. Health Sci. J. 2014, 9, 26–33. [Google Scholar]
- Othman, R.; Abdul Razak, N.I.; Ishak, N. Mandi serom or herbal traditional bath practices among traditional Malay midwives at the east coast of Malaysia. In Proceedings of the 5th International Conference on Natural Products for Health and Beauty (NATPRO 5), Phuket, Thailand, 6–8 May 2014. [Google Scholar]
- Goh, S.H.; Lee, K.H.; Ee, G.C.L.; Ong, H.C.; Geh, S.L.; Sylvester, R. A phytochemical study of Borneo. J. Herbs Spices Med. Plants 1996, 3, 55–69. [Google Scholar] [CrossRef]
- Kam, T.S. Alkaloids from Malaysian flora. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Pergamon: Amsterdam, The Netherlands, 1999; pp. 285–435. [Google Scholar]
- Hu, X.Q.; Peng, C.Z.; Jiang, J.H.; Wang, W.J.; Zhang, Y.; Chen, Y.G. Phenolics from Claoxylon longifolium. Chem. Nat. Compd. 2013, 49, 509–510. [Google Scholar] [CrossRef]
- McMahon, M.; Campbell, K.H.; MacLeod, A.K.; McLaughlin, L.A.; Henderson, C.J.; Wolf, C.R. HDAC inhibitors increase NRF2-signaling in tumour cells and blunt the efficacy of co-adminstered cytotoxic agents. PLoS ONE 2014, 9, e114055. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Lim, E.K.; Higgins, G.S.; Li, Y.; Bowles, D.J. Regioselectivity of glucosylation of caffeic acid by a UDP-glucose: Glucosyltransferase is maintained in planta. Biochem. J. 2003, 373 Pt 3, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Merugu, R. Shikimic acid as a major compound from Ludwigia alternifolia Linn. J. Pharmacogn. 2012, 3, 121–122. [Google Scholar]
- Ganbaatar, C.; Mishig, D.; Regdel, D.; Schmidt, A.; Knölker, H.J. Flavonoid glycosides from the aerial parts of Polygonatum odoratum (Mill.) Druce growing in Mongolia. Open Nat. Prod. J. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Yasukawa, K.; Kaneko, T.; Yamanouchi, S.; Takido, M. Studies on the constituents in the water extracts of crude drugs. V. On the leaves of Desmodium styracifolium Merr. Yakugaku Zasshi 1986, 106, 517–519. [Google Scholar] [CrossRef]
- Velozo, L.S.; Ferreira, M.J.; Santos, M.I.; Moreira, D.L.; Guimarães, E.F.; Emerenciano, V.P.; Kaplan, M.A. C-glycosyl flavones from Peperomia blanda. Fitoterapia 2009, 80, 119–122. [Google Scholar] [CrossRef]
- Hooper, A.M.; Caulfield, J.C.; Hao, B.; Pickett, J.A.; Midega, C.A.O.; Khan, Z.R. Isolation and identification of Desmodium root exudates from drought tolerant species used as intercrops against Striga hermonthica. Phytochemistry 2015, 117, 380–387. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Lu, F.; Jiang, X.; Friesen, J.B.; Pauli, G.F.; Chen, S.N.; Li, D.P. Preparation of flavone di-C-glycoside isomers from Jian-Gu injection (Premna fulva Craib.) using recycling counter-current chromatography. J. Chromatogr. A 2019, 16, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ajoku, G.A.; Okwute, S.K.; Okogun, J.I. Isolation of hexadecanoic acid methyl ester and 1,1,2-ethanetricarboxylic acid- 1-hydroxy-1, 1-dimethyl ester from the calyx of green Hibiscus sabdariffa (Linn). Nat. Prod. Chem. Res. 2015, 3, 169. [Google Scholar]
- Krishnamoorthy, K.; Subramaniam, P. Phytochemical profiling of leaf, stem and tuber parts of Solena amplexicaulis (Lam.) Gandhi using GC-MS. Int Sch. Res Not. 2014, 2014, 567409. [Google Scholar]
- Chinnasamy, P.S.; Parimala, S.; Kandhasamy, M. Phytochemical evaluation of seed and fruit pulp extracts of Passiflora foetida L. World J. Pharm. Res. 2018, 7, 1924–1932. [Google Scholar]
- Yu, F.R.; Lian, X.Z.; Guo, H.Y.; McGuire, P.M.; Li, R.D.; Wang, R.; Yu, F.H. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci. 2005, 8, 528–535. [Google Scholar]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef]
- Shoge, M.; Amusan, T. Phytochemical, antidiarrhoeal activity, isolation and characterisation of 11-octadecenoic acid, methyl ester isolated from the seeds of Acacia nilotica Linn. J. Biotechnol. Immunol. 2020, 2, 1–12. [Google Scholar]
- Manilal, S.J.; Shakir, C.; Kiran, G.S. Antibacterial activity of Falkenbergia hillebrandii (Born) from the Indian coast against human pathogens. Phyton Int. J. Exp. Bot. 2009, 78, 161–166. [Google Scholar]
- Latwal, S.; Sharma, M.; Rao, A. GC-MS analysis of volatile phytochemical compounds from the whole plant methanolic extract of Entodon rubicundus (Mitt.) A. Jaeger. and Thyridium fasciculatum (Hook. & Grev.). Mitten. Biol. Forum 2023, 15, 647–653. [Google Scholar]
- Lakani, S.A.; Hedjazi, S.; Abdulkhani, A. Chemical analysis and antioxidant activities of bark extracts from four endemic species of Hyrcanian forests in Iran. Holzforschung 2019, 73, 287–294. [Google Scholar] [CrossRef]
- Farag, S.M.; Essa, E.E.; Alharbi, S.A.; Alfarraj, S.; Abu El-Hassan, G.M.M. Agro-waste derived compounds (flax and black seed peels): Toxicological effect against the West Nile virus vector, Culex pipiens L. with special reference to GC-MS analysis. Saudi J. Biol. Sci. 2021, 28, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.; Tabassum, N.; Ganai, B.A. Phytochemical screening and antibacterial activity of Skimmia anquetilia N.P. Taylor and Airy Shaw: A first study from Kashmir Himalaya. Front. Plant Sci. 2022, 13, 937946. [Google Scholar] [CrossRef]
- Celik, S.; Albayrak, A.T.; Akyuz, S.; Ozel, A.E.; Sigirci, B.D. Synthesis, antimicrobial activity, molecular docking and ADMET study of a caprolactam-glycine cluster. J. Biomol. Struct. Dyn. 2020, 39, 2376–2386. [Google Scholar] [CrossRef]
- Singh, T.; Wahla, V. GC-MS analysis of antifungal compounds derived from soil actinobacteria. Int. Res. J. Phar. 2018, 9, 81–84. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs on fragrance raw materials: Isopropyl myristate. Food Cosmet. Toxicol. 1976, 14, 323–325. [Google Scholar] [CrossRef]
- Cardoso, V.M.; Solano, A.G.R.; Prado, M.A.F.; Nunan, E.D.A. Investigation of fatty acid esters to replace isopropyl myristate in the sterility test for ophthalmic ointments. J. Pharm. Biomed. Anal. 2006, 42, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.A.A.; Ismail, K.S.; Mashraqi, A.; Albishi, S.; Al-Namazi, A.A.; Masrahi, Y.S. GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates. Open Chem. 2023, 21, 20220325. [Google Scholar] [CrossRef]
- Demisie, S.; Oh, D.C.; Wolday, D.; Rinke de Wit, T.F.; Abera, A.; Tasew, G.; Shenkutie, A.M.; Girma, S.; Tafess, K. Diversity of culturable bacterial isolates and their potential as antimicrobial against human pathogens from Afar region, Ethiopia. Microbiol. Spectr. 2024, 12, e0181024. [Google Scholar] [CrossRef]
- Wagh, P.; Rai, M.; Deshmukh, S.; Durate, M. Bio-activity of oils of Trigonella foenum-graecum and Pongamia pinnata. Afr. J. Biotechnol. 2007, 6, 1592–1596. [Google Scholar]
- Zhang, Y.; Wei, D.; Guo, S.; Zhang, X.; Wang, M.; Chen, F. Chemical Components and antioxidant activity of the volatile Oil from Cassia tora L. Seed Prepared by Supercritical Fluid Extraction. J. Food Lipids 2007, 14, 411–423. [Google Scholar] [CrossRef]
- Rehman, Y.U.; Iqbal, A.; Ali, G.; Alotaibi, G.; Ahmed, A.; Ayaz, M. Phytochemical analysis, radical scavenging and glioblastoma U87 cells toxicity studies of stem bark of buckthorn (Rhamnus pentapomica R. Parker). BMC Complement. Med. Ther. 2024, 24, 12. [Google Scholar] [CrossRef]
- Momodu, I.; Okungbowa, E.S.; Agoreyo, B.; Maliki, M.M. Gas chromatography-mass spectrometry identification of bioactive compounds in methanol and aqueous seed extracts of Azanza garckeana fruits. Nig. J. Biotech. 2022, 38, 25–38. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Ramalho, S.D.; Pinto, M.E.F.; Ferreira, D.; Bolzani, V.S. Biologically active orbitides from the Euphorbiaceae family. Planta Med. 2018, 84, 558–567. [Google Scholar] [CrossRef]
- Coy-Barrera, C.A.; Galvis, L.; Rueda, M.J.; Torres-Cortés, S.A. The Croton genera (Euphorbiaceae) and its richness in chemical constituents with potential range of applications. Phytomed Plus 2025, 5, 100746. [Google Scholar] [CrossRef]
- Kgosiemang, I.K.R.; Lefojane, R.; Adegoke, A.M.; Ogunyemi, O.; Mashele, S.S.; Sekhoacha, M.P. Pharmacological significance, medicinal use, and toxicity of extracted and isolated compounds from Euphorbia species found in southern Africa: A review. Plants 2025, 14, 469. [Google Scholar] [CrossRef]
- Satyamitra, M.; Mantena, S.; Nair, C.; Chandna, S.; Dwarakanath, B.; Uma, P. The antioxidant flavonoids, orientin and vicenin enhance repair of radiation-induced damage. SAJ Pharm. Pharm. 2014, 1, 105. [Google Scholar]
- Kandhare, A.D.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Acute and repeated doses (28 days) oral toxicity study of vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice. Regul. Toxicol. Pharmacol. 2016, 81, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Renyou, G.; Huabin, L.; Mingxuan, Y.; Julian, M.D.; Ruichang, G.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother. 2023, 162, 114687. [Google Scholar] [CrossRef]
- Popoola, T.D.; Guetchueng, S.T.; Ritchie, K.J.; Awodele, O.; Dempster, N.M.; Akinloye, O.; Sarker, S.D.; Fatokun, A.A. Potent Nrf2-inducing, antioxidant, and anti-inflammatory effects and identification of constituents validate the anti-cancer use of Uvaria chamae and Olax subscorpioidea. BMC Complement. Med. Ther. 2021, 21, 234. [Google Scholar] [CrossRef]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.; Barakat, A.; Boraei, A.T.A. Discovery of new apoptosis-inducing agents for breast cancer based on ethyl 2-amino-4,5,6,7-tetra hydrobenzo[b] thiophene-3-carboxylate: Synthesis, in vitro, and in vivo activity evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef] [PubMed]
- Romanick, M.; Holt, A. Topological Polar Surface Area in An ABC of PK/PD; University of Alberta Library: Edmonton, AB, Canada, 2023. [Google Scholar]
- Linus Pauling Institute, Oregon State University. Flavonoids. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids#metabolism-bioavailability (accessed on 25 March 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Melançon, C.E., 3rd; Liu, H.W. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Ed. Engl. 2008, 47, 9814–9859. [Google Scholar] [CrossRef]
- Spriha, S.E.; Rahman, S.M.A. A review on biological activities of sugars and sugar derivatives. Dhaka Univ. J. Pharm. Sci. 2022, 20, 381–394. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, M. Advances in immunosuppressive agents based on signal pathway. Front. Pharmacol. 2022, 13, 917162. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A. Natural products as cancer chemopreventive agents: Where we stand. Nat. Prod. Commun. 2022, 17, 1934578X221144579. [Google Scholar]
- Latif, Z.; Sarker, S.D. Isolation of natural products by preparative high-performance liquid chromatography (prep-HPLC). In Natural Products Isolation, 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Humana Press: New York, NY, USA, 2012; pp. 27–41. [Google Scholar]
- Reid, R.G.; Sarker, S.D. Isolation of natural products by low-pressure column chromatography. In Natural Products Isolation, 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Humana Press: New York, NY, USA, 2012; pp. 155–188. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, X.J.; Hayes, J.D.; Wolf, C.R. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of Nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006, 66, 10983–10994. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Singha, S.; Nath, D.; Das, G.; Patra, J.K.; Talukdar, A.D. Phytochemicals from Allium tuberosum Rottler ex Spreng Show potent inhibitory activity against B-Raf, EGFR, K-Ras, and PI3K of non-small cell lung cancer targets. Appl. Sci. 2022, 12, 11749. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Computational phytochemistry: An overview. In Computational Phytochemistry, 2nd ed.; Sarker, S.D., Nahar, L., Eds.; Elsevier: London, UK, 2024; pp. 1–58. [Google Scholar]

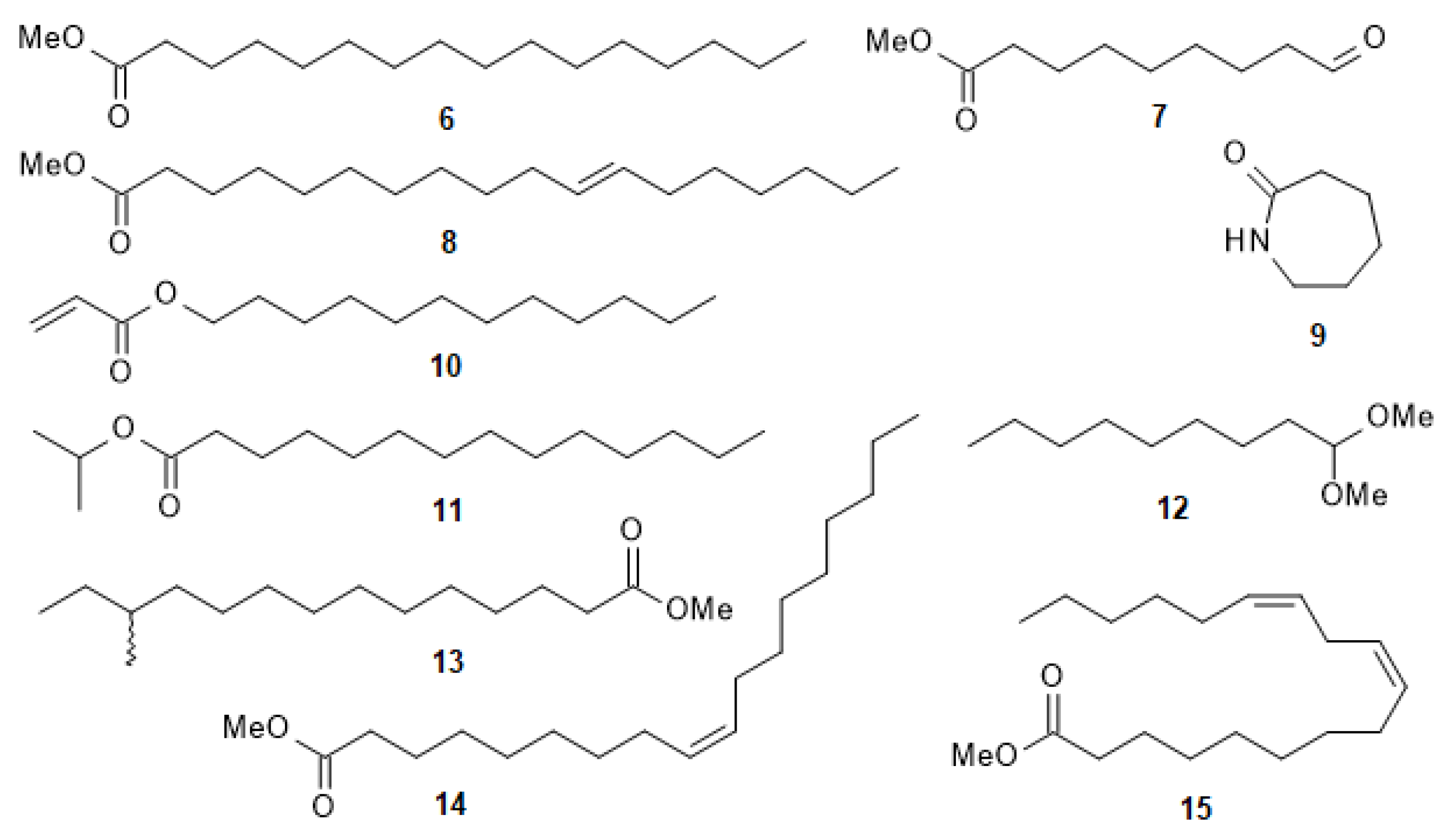
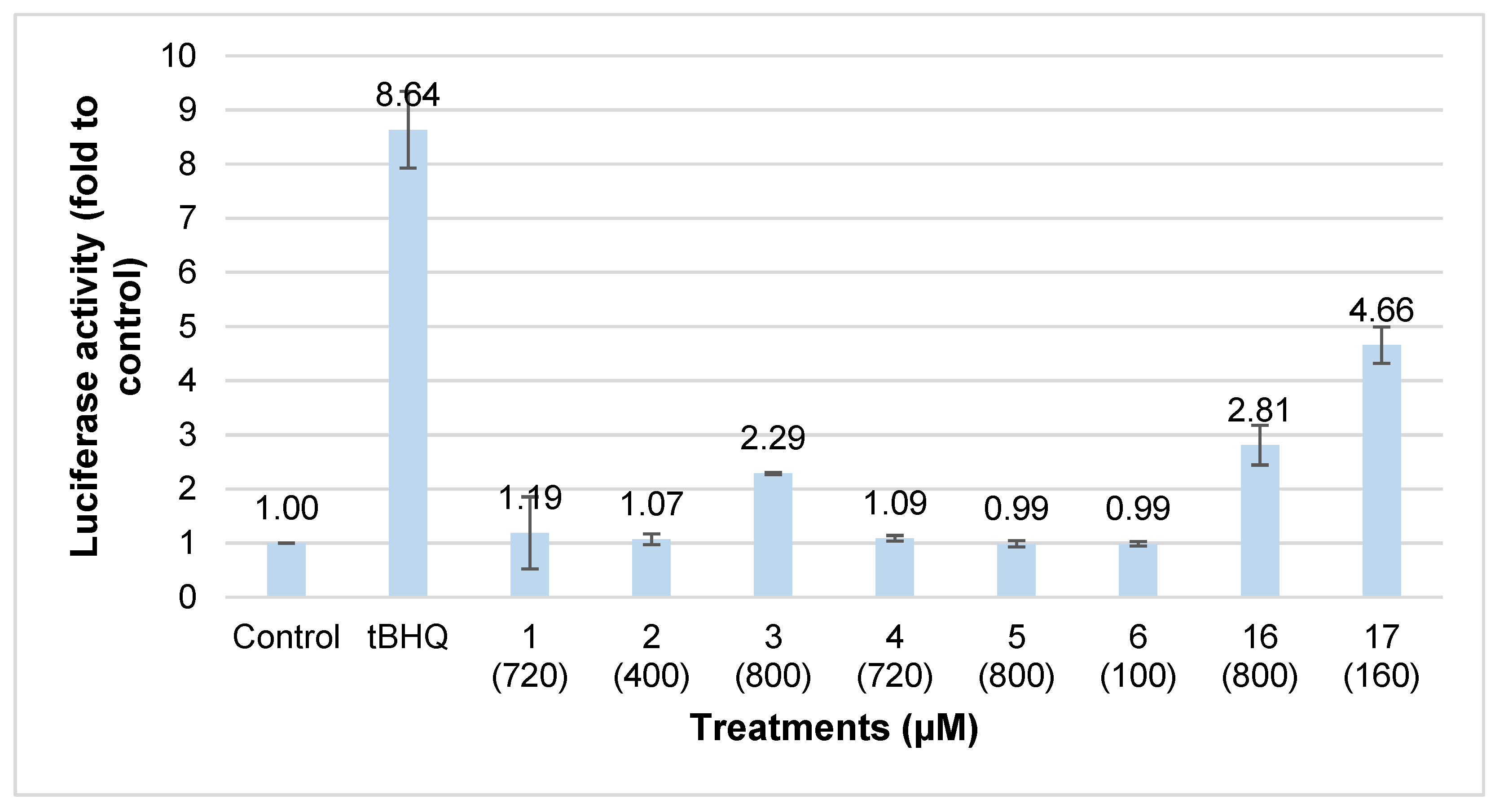
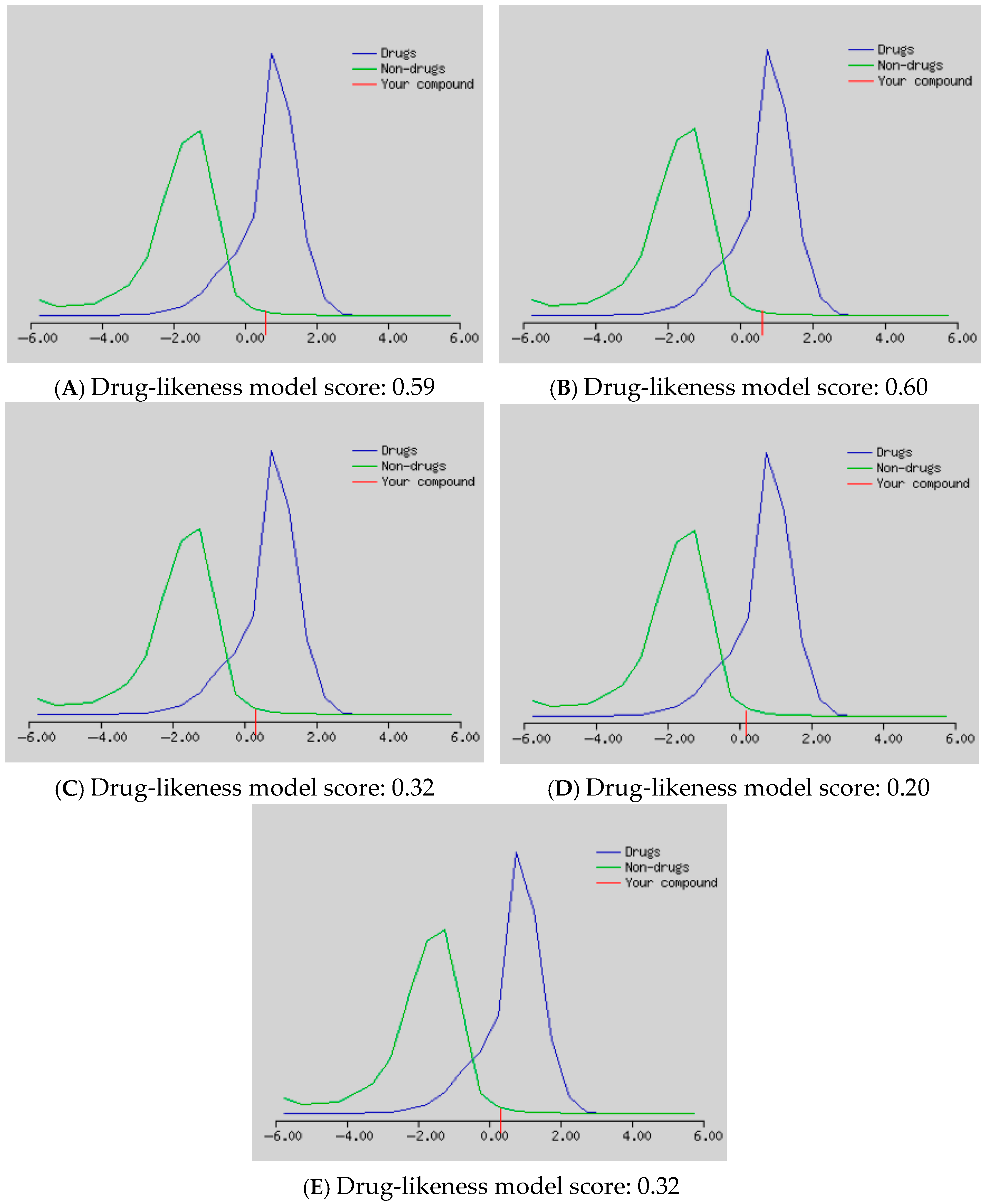
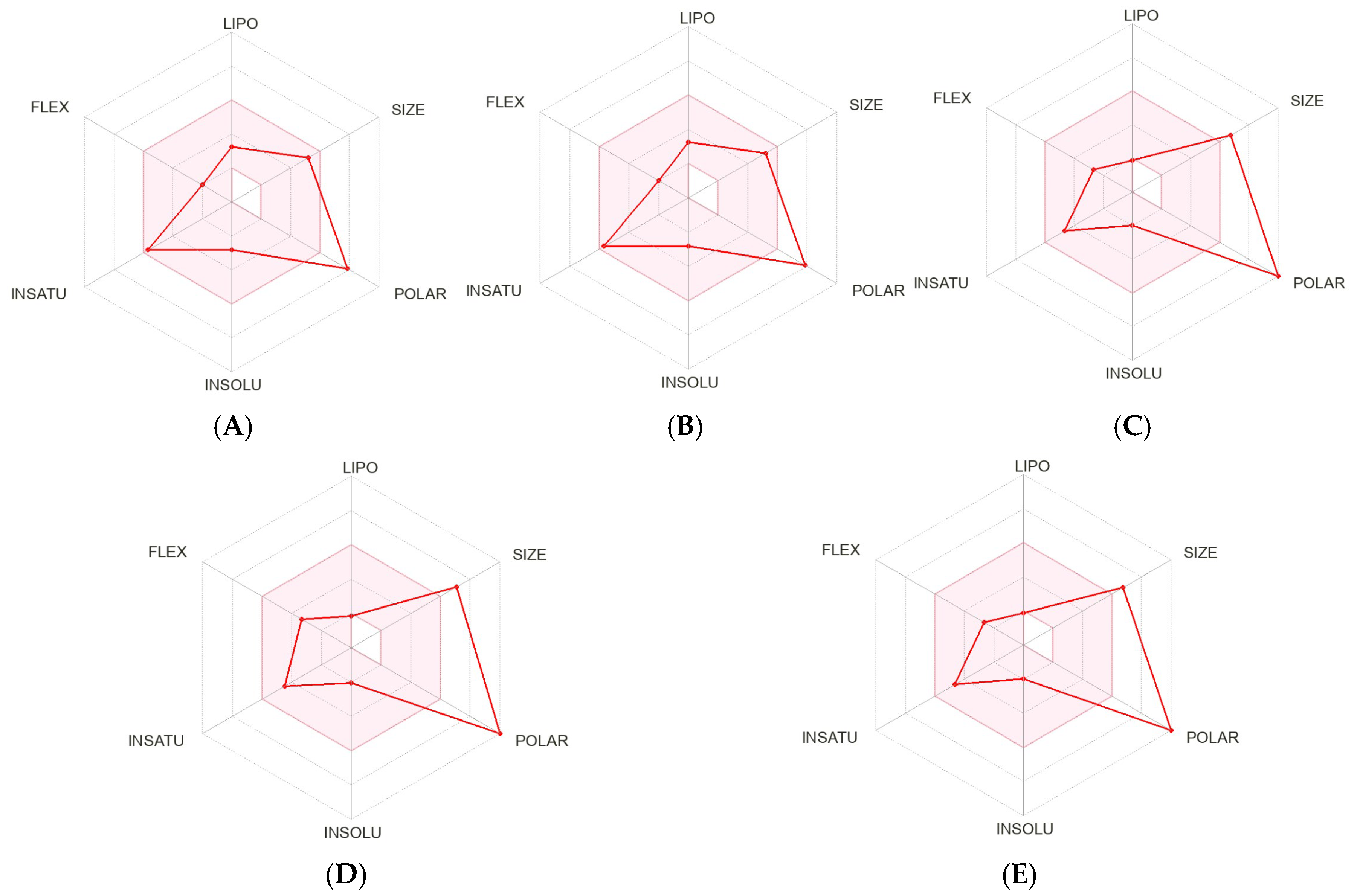
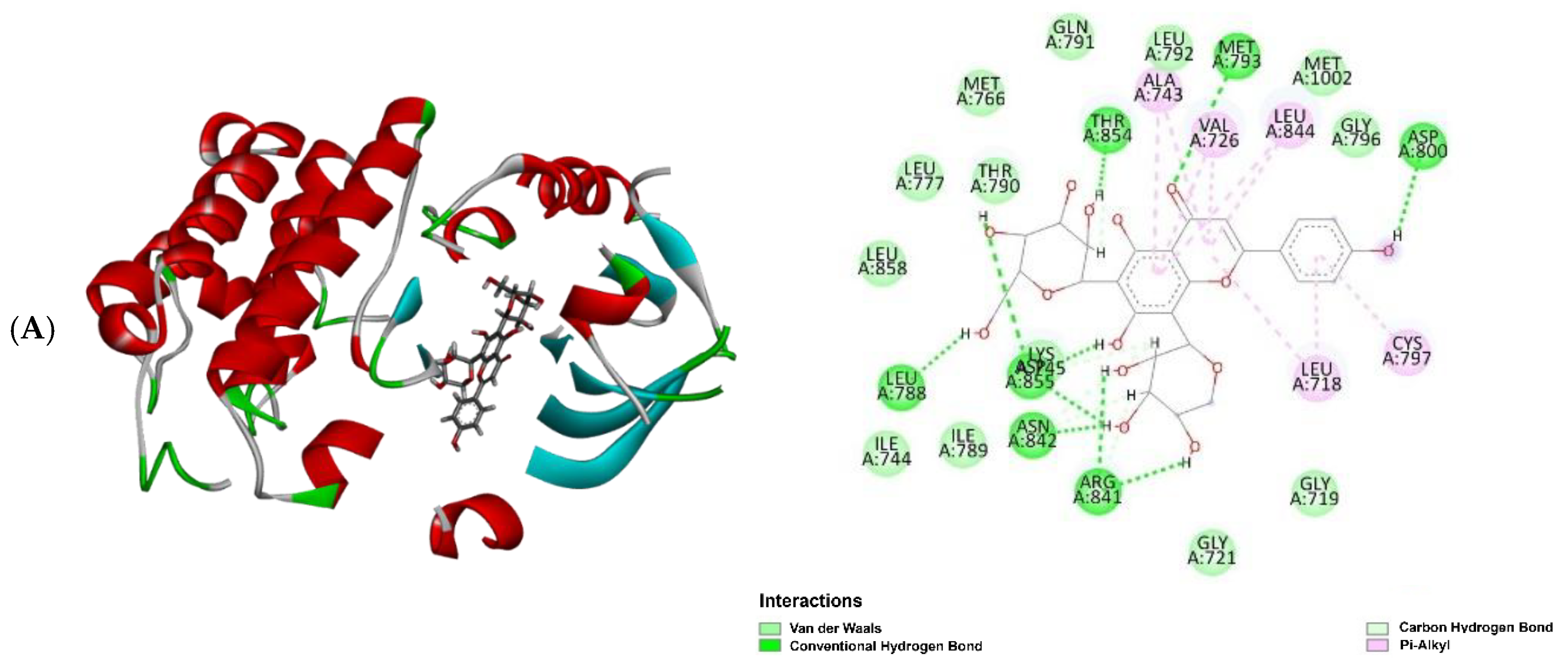

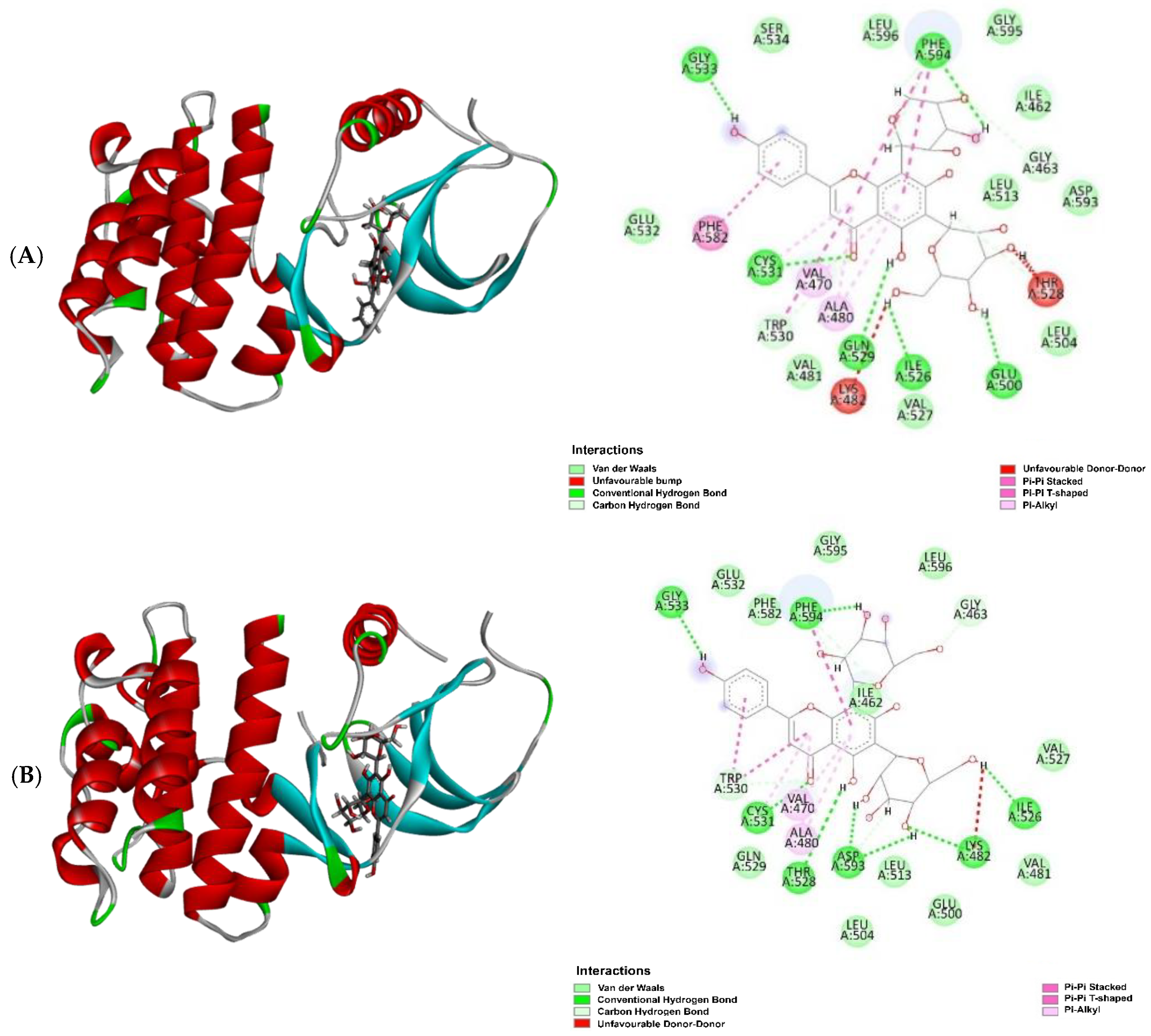


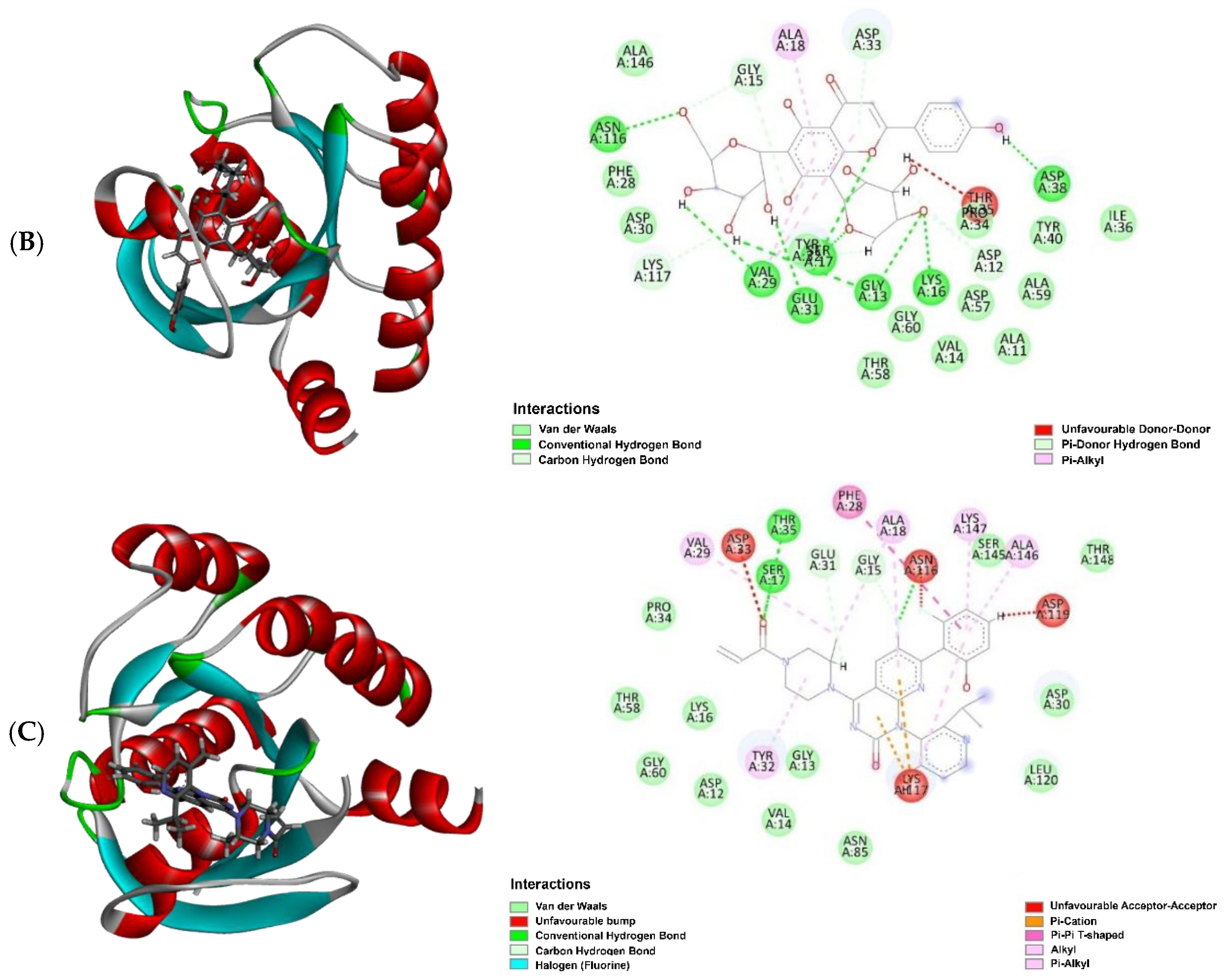
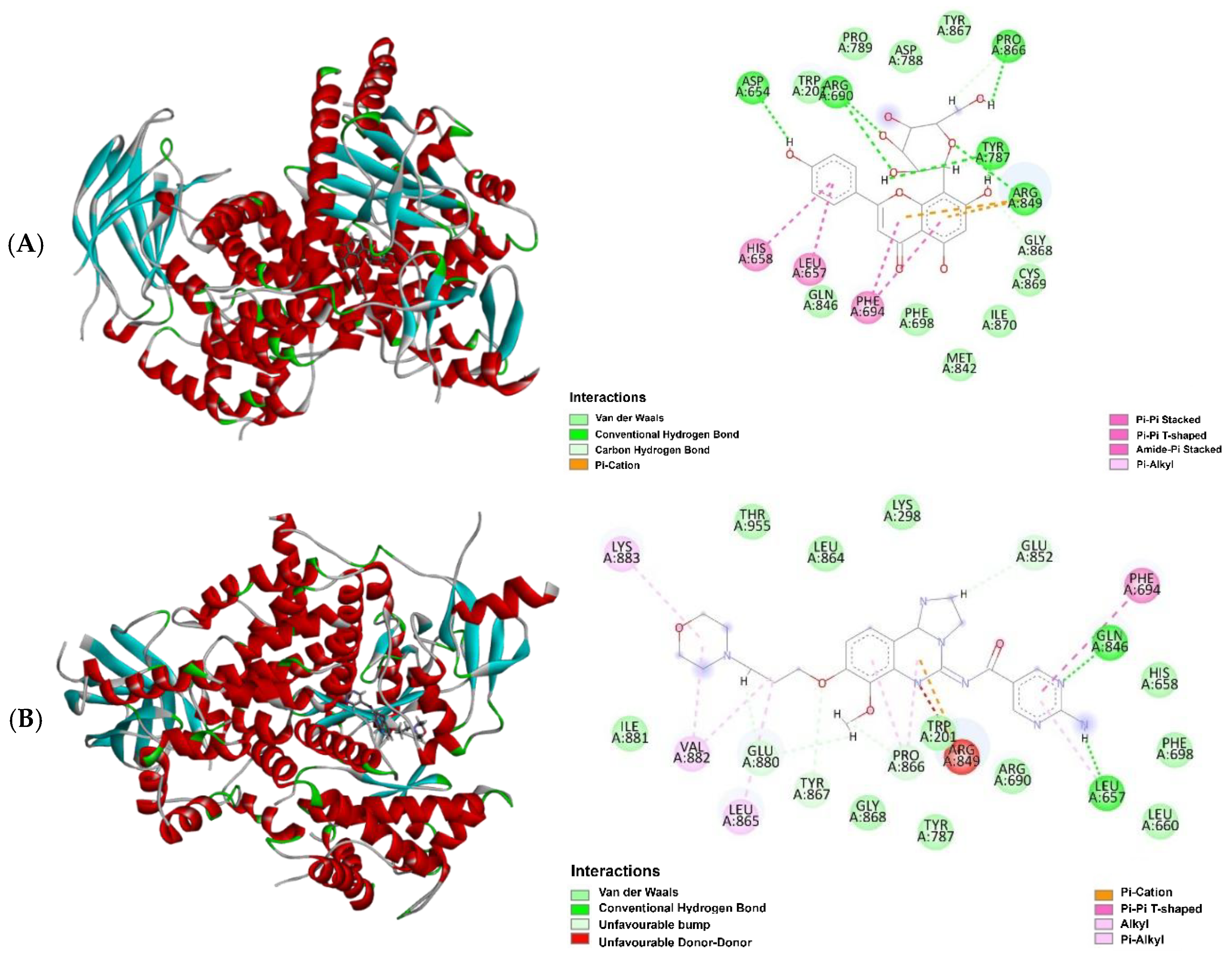
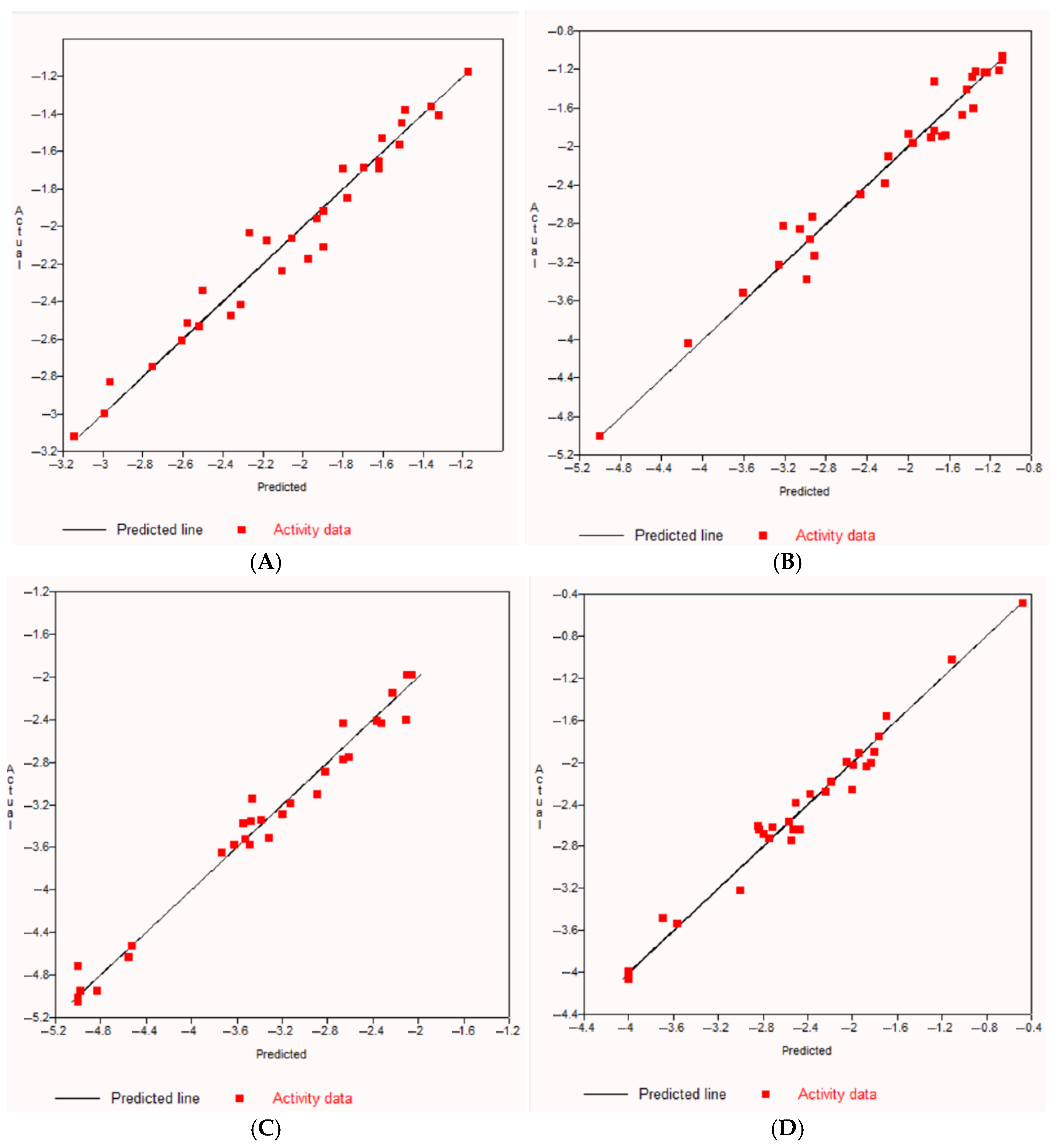
| Sample | Retention Time (tR) in min | Molecular Formula | Molecular Weight | Compound Name | Peak Area (%) |
| CLS-Me F3-P3 | 6.03 | C15H28O2 | 240.21 | Dodecyl acrylate (10) | 50.47 |
| CLS-Me F4-P6 | 2.60 | C6H11NO | 113.08 | Caprolactam (9) | 51.97 |
| 3.97 | C10H18O3 | 186.13 | 9-Oxononanoic acid methyl ester (7) | 13.69 | |
| 5.32 | C11H24O2 | 188.18 | Nonanal dimethyl acetal (12) | 13.07 | |
| 6.26 | C16H32O2 | 256.24 | 12-methyltetradecanoic acid methyl ester (13) | 6.29 | |
| 6.91 | C17H34O2 | 270.26 | Isopropyl myristate (11) | 4.72 | |
| CLS-Me F4-P7 | 3.98 | C10H18O3 | 186.13 | 9-Oxononanoic acid methyl ester (7) | 8.32 |
| 6.93 | C17H34O2 | 270.26 | Isopropyl myristate (11) | 21.93 | |
| 8.42 | C19H34O2 | 294.26 | (Z,Z)-9,12-octadecadienoic acid methyl ester (15) | 33.84 | |
| Compound 6 | 7.50 | C17H34O2 | 270.26 | Hexadecanoic acid methyl ester (6) | 100.00 |
| CLS-Me F4-P9 | 2.63 | C6H11NO | 113.08 | Caprolactam (9) | 13.68 |
| 6.93 | C17H34O2 | 270.26 | Isopropyl myristate (11) | 4.44 | |
| 7.51 | C17H34O2 | 270.26 | Hexadecanoic acid methyl ester (6) | 10.15 | |
| 8.49 | C19H36O2 | 296.27 | (Z)-9-octadecenoic acid methyl ester (14) | 61.56 | |
| 8.49 (overlapping peak on right) | C19H36O2 | 296.27 | 11-Octadecenoic acid methyl ester (8) |
| Compound Name | Biological Activity | References |
|---|---|---|
| 9-Oxononanoic acid methyl ester (7) | Anti-acne, antifungal, antihyperpigmentative, anti-inflammatory, antileukemic, antimicrobial, antioxidant, antiproliferative, antitumor, cytotoxic and larvicidal | [39,46] |
| 11-Octadecenoic acid methyl ester (8) | 5α-Reductase inhibitor, anti-acne, antiandrogenic, Antiarthritic, antibacterial, anticoronary, antidiarrheal, antieczemic, antihypercholesterolemia, anti-inflammatory, cancer preventive and hepatoprotective | [39,42] |
| Caprolactam (9) | Antibacterial | [48] |
| Dodecyl acrylate (10) | Antibacterial, anticancer, antifungal and antioxidant | [41,43,45,49] |
| Hexadecanoic acid methyl ester (6) | 5α-Reductase inhibitor, anti-acne, anti-androgenic, antiarthritic, antibacterial, anticancer, anticoronary, antieczemic, antihistaminic, anti-inflammatory, antioxidant, hepatoprotective, hypocholesterolemic, insecticide and nematicide | [38,47] |
| Isopropyl myristate (11) | Antifungal and antimicrobial | [50,51] |
| Nonanal dimethyl acetal (12) | Antibacterial | [52] |
| 12-Methyltetradecanoic acid methyl ester (13) | Antifungal | [53] |
| (Z)-9-Octadecenoic acid methyl ester (14) | 5α-Reductase inhibitor, anemiagenic anti-androgenic, antidiarrheal, anti-inflammatory, antimicrobial, cancer preventive, dermatitigenic, hypocholesterolemic and insectifuge | [38,44,54,55,56,57] |
| (Z,Z)-9,12-Octadecadienoic acid methyl ester (15) | Anti-arthritic, anticancer, anti-eczemic, antifungal, antihistaminic, anti-inflammatory, antioxidant, antitumour, hepatoprotective and hypocholesterolemic |
| Predicted Bioactivities | Isovitexin (2) | Vitexin (16) | Vicenin 1 (3) | Vicenin 2 (4) | Vicenin 3 (5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| Anticarcinogenic | 0.856 | 0.004 | - | - | 0.872 | 0.003 | 0.831 | 0.004 | 0.872 | 0.003 |
| Antimutagenic | 0.795 | 0.004 | 0.820 | 0.004 | - | - | - | - | - | - |
| Antineoplastic | 0.800 | 0.012 | 0.836 | 0.008 | 0.852 | 0.007 | 0.833 | 0.008 | 0.852 | 0.007 |
| Apoptosis agonist | 0.706 | 0.014 | 0.737 | 0.012 | - | - | - | - | - | - |
| Chemopreventive | - | - | - | - | - | - | 0.859 | 0.003 | 0.905 | 0.002 |
| Proliferative disease treatment | - | - | 0.723 | 0.005 | 0.720 | 0.005 | - | - | 0.720 | 0.005 |
| Compounds | 2 | 3 | 4 | 5 | 16 |
|---|---|---|---|---|---|
| Physicochemical properties | |||||
| Molecular weight (g/mol) | 432.38 | 564.49 | 594.52 | 564.49 | 432.38 |
| Heavy atoms | 31 | 40 | 42 | 40 | 31 |
| Aromatic heavy atoms | 16 | 16 | 16 | 16 | 16 |
| Fraction Csp3 | 0.29 | 0.42 | 0.44 | 0.42 | 0.29 |
| Rotatable bonds | 3 | 4 | 5 | 4 | 3 |
| H-bond acceptors | 10 | 14 | 15 | 14 | 10 |
| H-bond donors | 7 | 10 | 11 | 10 | 7 |
| Molar refractivity (MR) | 106.61 | 133.26 | 139.23 | 133.26 | 106.61 |
| TPSA (Å2) | 181.05 | 250.97 | 271.2 | 250.97 | 181.05 |
| Lipophilicity | |||||
| Log Po/w (iLOGP) | 1.94 | 1.41 | 1.27 | 1.63 | 1.38 |
| Log Po/w (XLOGP3) | 0.21 | −2.19 | −2.26 | −2.19 | 0.21 |
| Log Po/w (WLOGP) | −0.23 | −2.4 | −3.04 | −2.4 | −0.23 |
| Log Po/w (MLOGP) | −2.02 | −3.97 | −4.51 | −3.97 | −2.02 |
| Log Po/w (Silicos-IT) | 0.33 | 0.33 | −1.8 | 0.33 | 0.33 |
| Consensus Log P | −0.07 | −1.7 | −2.07 | −1.65 | 0.05 |
| Water solubility | |||||
| Log S (ESOL) | −2.84 | −1.99 | −2.05 | −1.99 | −2.84 |
| ESOL solubility (mg/mL) | 6.29 × 10−1 | 5.75 | 5.25 | 5.75 | 6.29 × 10−1 |
| ESOL solubility (mol/L) | 1.46 × 10−3 | 1.02 × 10−2 | 8.83 × 10−3 | 1.02 × 10−2 | 1.46 × 10−3 |
| ESOL class | Soluble | Very soluble | Soluble | Very soluble | Soluble |
| Log S (Ali) | −3.57 | −2.55 | −2.9 | −2.55 | −3.57 |
| Ali solubility (mg/mL) | 1.16 × 10−1 | 1.59 | 7.46 × 10−1 | 1.59 | 1.16 × 10−1 |
| Ali solubility (mol/L) | 2.68 × 10−4 | 2.82 × 10−3 | 1.26 × 10−3 | 2.82 × 10−3 | 2.68 × 10−4 |
| Ali class | Soluble | Soluble | Soluble | Soluble | Soluble |
| Log S (Silicos-IT) | −2.38 | −0.71 | −0.27 | −0.71 | −2.38 |
| Silicos-IT solubility (mg/mL) | 1.81 | 1.10 × 102 | 3.19 × 102 | 1.10 × 102 | 1.81 |
| Silicos-IT solubility (mol/L) | 4.20 × 10−3 | 1.95 × 10−1 | 5.36 × 10−1 | 1.95 × 10−1 | 4.20 × 10−3 |
| Silicos-IT class | Soluble | Soluble | Soluble | Soluble | Soluble |
| Pharmacokinetics | |||||
| GI absorption | Low | Low | Low | Low | Low |
| BBB permeant | No | No | No | No | No |
| P-gp substrate | No | Yes | No | Yes | No |
| CYP1A2 inhibitor | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No |
| Log Kp (cm/s) | −8.79 | −11.3 | −11.53 | −11.3 | −8.79 |
| Drug likeness | |||||
| Lipinski violations | 1 | 3 | 3 | 3 | 1 |
| Ghose violations | 0 | 3 | 4 | 3 | 0 |
| Veber violations | 1 | 1 | 1 | 1 | 1 |
| Egan violations | 1 | 1 | 1 | 1 | 1 |
| Muegge violations | 2 | 4 | 4 | 4 | 2 |
| Bioavailability score | 0.55 | 0.17 | 0.17 | 0.17 | 0.55 |
| Medicinal chemistry | |||||
| PAINS alerts | 0 | 0 | 0 | 0 | 0 |
| Brenk alerts | 0 | 0 | 0 | 0 | 0 |
| Leadlikeness violations | 1 | 1 | 1 | 1 | 1 |
| Synthetic accessibility | 4.99 | 6.18 | 6.4 | 6.18 | 5.12 |
| Target Protein: EGFR | Target Protein: BRAF | ||||
| Ligand | MolDock Score | HBond | Ligand | MolDock Score | HBond |
| Vicenin 3 (5) | −142.57 | −14.34 | Vicenin 3 (5) | −162.63 | −14.88 |
| Gefitinib * | −141.80 | −3.22 | Vicenin 2 (4) | −144.19 | −12.17 |
| Vicenin 1 (3) | −141.69 | −15.00 | Vicenin 1 (3) | −143.07 | −7.57 |
| Vicenin 2 (4) | −138.86 | −9.31 | Dabrafenib * | −137.99 | −3.27 |
| Vitexin (16) | −138.07 | −12.85 | Vitexin (16) | −134.15 | −4.44 |
| Isovitexin (2) | −109.20 | −14.82 | Isovitexin (2) | −132.29 | −10.80 |
| cin: KRAS | Target protein: PI3K | ||||
| Ligand | MolDock score | HBond | Ligand | MolDock score | HBond |
| Vicenin 2 (4) | −154.39 | −18.82 | Vitexin (16) | −133.27 | −12.62 |
| Vicenin 3 (5) | −153.37 | −17.17 | Copanlisib * | −126.30 | −6.37 |
| Sotorasib * | −150.41 | −3.61 | Isovitexin (2) | −85.47 | −11.64 |
| Vicenin 1 (3) | −149.67 | −17.55 | Vicenin 1 (3) | −75.69 | −13.35 |
| Vitexin (16) | −149.03 | −11.27 | Vicenin 2 (4) | −71.60 | −14.93 |
| Isovitexin (2) | −140.77 | −12.91 | Vicenin 3 (5) | −67.12 | −15.75 |
| Compounds | Calculated IC50 | |||
|---|---|---|---|---|
| EGFR (nM) | BRAF (nM) | KRAS (µM) | PI3K (nM) | |
| 2 | 15.85 | 28.18 | 169.82 | 245.47 |
| 3 | 7.08 | 11.75 | 186.21 | 64.57 |
| 4 | 2.45 | 58.88 | 229.09 | 363.08 |
| 5 | 7.08 | 11.75 | 186.21 | 64.57 |
| 16 | 18.62 | 60.26 | 165.96 | 213.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuniad, C.; Nahar, L.; Talukdar, A.D.; Nath, R.; Ritchie, K.J.; Sarker, S.D. Cancer Chemopreventive Potential of Claoxylon longifolium Grown in Southern Thailand: A Bioassay-Guided Isolation of Vicenin 1 as the Active Compound and In Silico Studies on Related C-Glycosyl Flavones. Molecules 2025, 30, 3173. https://doi.org/10.3390/molecules30153173
Khuniad C, Nahar L, Talukdar AD, Nath R, Ritchie KJ, Sarker SD. Cancer Chemopreventive Potential of Claoxylon longifolium Grown in Southern Thailand: A Bioassay-Guided Isolation of Vicenin 1 as the Active Compound and In Silico Studies on Related C-Glycosyl Flavones. Molecules. 2025; 30(15):3173. https://doi.org/10.3390/molecules30153173
Chicago/Turabian StyleKhuniad, Chuanchom, Lutfun Nahar, Anupam D. Talukdar, Rajat Nath, Kenneth J. Ritchie, and Satyajit D. Sarker. 2025. "Cancer Chemopreventive Potential of Claoxylon longifolium Grown in Southern Thailand: A Bioassay-Guided Isolation of Vicenin 1 as the Active Compound and In Silico Studies on Related C-Glycosyl Flavones" Molecules 30, no. 15: 3173. https://doi.org/10.3390/molecules30153173
APA StyleKhuniad, C., Nahar, L., Talukdar, A. D., Nath, R., Ritchie, K. J., & Sarker, S. D. (2025). Cancer Chemopreventive Potential of Claoxylon longifolium Grown in Southern Thailand: A Bioassay-Guided Isolation of Vicenin 1 as the Active Compound and In Silico Studies on Related C-Glycosyl Flavones. Molecules, 30(15), 3173. https://doi.org/10.3390/molecules30153173









